Abstract
Recruitment of neutrophil granulocytes to sites of infectious tissue damage is an early event in innate immune responses. Following chemotactic signals neutrophils establish a first line of defense in a swarm-like manner. Intracellular pathogens such as Leishmania major can, however, evade neutrophil-mediated killing and survive inside neutrophils. To achieve this the parasites evolved potent evasion mechanisms. Since neutrophils are a major source of inflammation regulating lipid mediators, we hypothesized that intracellular infection modifies the release of pro- and anti-inflammatory lipid mediators like leukotriene B4 (LTB4) and lipoxin A4 (LXA4), respectively. In the present study, we demonstrated in vitro that L. major-infected primary human neutrophils release an increased amount of LTB4, whereas LXA4 liberation is reduced during the first hours of infection. To investigate whether lipid mediator modulation is a common feature in intracellular infections, we tested the impact of an infection with Anaplasma phagocytophilum. Similarly to L. major, neutrophil infection with A. phagocytophilum led to an enhanced release of LTB4 and decreased LXA4 production. Together, our findings indicate that intracellular infections modulate the lipid mediator profile of neutrophils. This effect is likely to contribute to the survival of the pathogens in neutrophils and to the outcome of the infections.
1. Introduction
As the most numerous cell type in early acute inflammation, polymorphonuclear neutrophil granulocytes (PMN) can be central choreographers of inflammation [1–4]. During this phase, they occupy an outstanding position in the regulation of local lipid mediators, a family of mainly arachidonic-acid-derived signal molecules with potent effects on leukocyte recruitment and activity [5, 6]. The proinflammatory lipid mediator leukotriene B4 (LTB4) works as an amplifier for localized inflammatory signals and was shown to be critical for sufficient PMN recruitment, termed swarming, in vivo [7]. In addition, LTB4 was shown to enhance phagocytic activity, activation, degranulation, and killing of internalized pathogens [8–11]. Since PMN are the predominant source of LTB4, they promote acute inflammation in a feed-forward manner [12, 13]. In addition to their proinflammatory functions neutrophils also contribute to the resolution of inflammation. To inhibit overwhelming inflammation, the LTB4-precursor LTA4 is used for the synthesis of proresolving lipid mediators (SPMs). This process, termed class switch, is characterized by the production of lipoxin A4 (LXA4), a prototype member of SPMs [5, 14]. LXA4 is known to inhibit leukocyte chemotaxis, transmigration, ROS-generation, NF-kB activation, and synthesis of proinflammatory cytokines [15, 16]. LXA4 is mainly produced by transcellular dual lipoxygenation. In this process PMN-derived LTA4 is used as a substrate of 12- and 15-LO expressed in epithelial cells [17, 18]. Therefore, neutrophils not only produce the proinflammatory lipid mediator LTB4 but also contribute to the production of the anti-inflammatory mediator LXA4. Upon exposure to the calcium ionophore ionomycin the LTB4 and LXA4 production by neutrophils can be regarded as a measurement of the cells' capacity for the synthesis of these lipid mediators. By using LPS + fMLP information regarding the LTB4 release by neutrophils in an infected/inflammatory environment can be obtained.
Certain pathogenic microorganisms such as the protozoan parasite Leishmania major (L. major) can evade destruction and survive inside neutrophils [19]. L. major is an obligatory intracellular parasite and causative agent of Old World cutaneous Leishmaniasis. During blood meal the parasites are transmitted into the skin of mammalian hosts by the bite of infected sandflies. Neutrophils are rapidly recruited to the site of Leishmania infection and phagocytose the parasites [20, 21]. In this context, L. major parasites were shown to exploit the early inflammatory response by using PMN as transient host cells [22–25]. Lipid mediators have been shown to be involved in the survival strategy of Leishmania parasites [26–29]. However, no data concerning the impact of L. major infection on neutrophil-derived lipid mediators is available.
In the present study, we investigated how intracellular infection with L. major affects the release of the pro- and anti-inflammatory lipid mediators LTB4 and LXA4 by primary human neutrophil granulocytes in vitro. In addition to L. major, we tested the impact of an infection with Anaplasma phagocytophilum (A. phagocytophilum) on LTB4- and LXA4-synthesis to investigate whether the neutrophil lipidome is a common target of intracellular pathogens. A. phagocytophilum is an obligate intracellular bacterium and causative agent of the tick-borne Human Granulocytic Anaplasmosis (HGA). It is critically dependent on neutrophils as its definitive host cells and well known for host cell modulations that lead to subversion of PMN antimicrobial defense mechanisms to ensure intracellular survival [30].
2. Materials and Methods
2.1. Ethics
Blood collection was conducted with the understanding and written consent of each participant and was approved by the ethical committee of the Medical Faculty of the University of Lübeck (05-124).
2.2. Isolation of Human Peripheral Blood Neutrophil Granulocytes
Peripheral blood was collected in lithium-heparin-containing tubes. Neutrophils were isolated in a combination of two density gradient centrifugations as described previously [31]. Using layered lymphocyte separation medium 1077 (PAA, Pasching, Austria) and Histopaque 1119 (Sigma-Aldrich, Deisenhofen, Germany) first, the separated granulocytes were purified in a discontinuous Percoll (Amersham Biosciences, Uppsala, Sweden) gradient centrifugation. After isolation neutrophils were resuspended in complete medium (RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum) and 50 μM β-mercaptoethanol (all from Sigma-Aldrich, Steinheim, Germany), 4 mM L-glutamine, and 10 mM HEPES (both Biochrom, Berlin, Germany). The obtained cell preparations contained >99% granulocytes according to morphological examination of cytocentrifuge slides stained with Diff Quik (Medion Diagnostics, Düdingen, Switzerland).
2.3. Leishmania Major Culture
The origin and propagation of the cloned virulent L. major strain MHOM/IL/81/FEBNI has been described elsewhere [32]. In short, L. major promastigotes were cultured on biphasic rabbit blood agar-containing microtiter plates at 26°C in humidified atmosphere containing 5% CO2 for 7 to 10 days and a maximum of five passages. Each plate well contained 100 μl liquid medium and 50 μl of a Novy-MacNeal-Nicolle (NNN) blood agar slant, which was prepared by supplementing 200 ml of Brain-Heart-Infusion agar base (Difco, Detroit, MI, USA) with 50 ml defibrinated fresh rabbit blood (Elocin-Lab, Oberhausen, Germany). The liquid medium consisted of RPMI-1640 medium supplemented with 5% heat-inactivated fetal calf serum and 25 μM β-mercaptoethanol (all from Sigma-Aldrich), 4 mM L-glutamine, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Biochrom). For infection L. major promastigotes were washed with complete medium for 10 minutes at 2600 ×g. After centrifugation the supernatant was discarded and the pellet was resuspended in complete medium. Promastigotes with active flagellar movement were counted in a hemocytometer with a chamber depth of 0.02 mm.
2.4. Preparation of Cell-Free Anaplasma phagocytophilum
The A. phagocytophilum Webster strain was a kind gift of Dr. J. S. Dumler, John Hopkins University, Baltimore, MD. The bacteria were propagated and cell-free A. phagocytophilum was prepared as described previously [33]. Briefly, infected HL-60 cells were centrifuged at 250 ×g for 10 min and resuspended in 2 ml of PBS. Subsequently, cells were passed through a 25 G needle followed by a 27 G needle for 10 times each and vortexed with sterile solid glass-beads for 1 minute. By centrifuging at 750 ×g for 10 min cellular debris was removed. The supernatant was collected and centrifuged at 2.500 ×g for 15 min. The obtained cell-free Anaplasma containing pellets were used to infect neutrophils.
2.5. HT-29 Cell Culture
The human adenocarcinoma epithelial-like cell line HT-29 was kept in DMEM medium supplemented with 10% heat-inactivated FCS (both Sigma-Aldrich), 2 mML-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Biochrom) under humidified conditions at 37°C and 5% CO2. Placed in a well of a 96-well flat bottom microplate 9 × 104 cells formed a confluent monolayer within 24 hours. Preliminary experiments showed that HT-29 cells do not release LTB4 nor LXA4 on their own (data not shown).
2.6. In Vitro Coincubation of Neutrophils with Pathogens and Determination of Infection Rate
Neutrophils (5 × 106 per ml) were coincubated with L. major promastigotes or cell-free A. phagocytophilum in complete medium for 300 min at 37°C in humidified atmosphere containing 5% CO2. The multiplicity of infection (MOI) for L. major was 5. For infection with A. phagocytophilum the infectious load was 1 : 1 meaning that one neutrophil was infected with A. phagocytophilum obtained from one infected HL-60 cell. Subsequently, neutrophils were washed three times (400 ×g, 10 min) with PBS to remove noningested pathogens. Immediately before induction of lipid mediator release, the cells were resuspended in FCS-free complete medium since preliminary studies indicated a high FCS induced background signal in the LTB4-ELISA assay. The infection rates for L. major and A. phagocytophilum were determined by morphological examination of >200 PMN after Diff Quik staining of cytocentrifuge slides. A. phagocytophilum bacteria were visualized in neutrophils by immunocytochemical staining with the use of a polyclonal anti-A. phagocytophilum antibody (a kind gift of Professor J. Stephen Dumler, John Hopkins University, Baltimore, MD).
2.7. Induction and Measurement of LTB4
Prior to the induction of LTB4 release, the cells were resuspended in FCS-free complete medium. Infected and noninfected PMN as well as pathogen controls (L. major promastigotes or cell-free A. phagocytophilum without neutrophils) were exposed to either the combination of LPS and fMLP (1 μg/ml LPS for 30 min followed by 0.5 μM fMLP 10 min; both Sigma-Aldrich) or ionomycin (0.2 μM, 10 min, Sigma-Aldrich). Control samples were left untreated. The induction was carried out in a 37°C water bath and terminated by cold centrifugation (4°C, precooled, 800 ×g, 10 min). The supernatants were collected and stored at −80°C. LTB4 was finally quantified by competitive enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2.8. Induction and Measurement of LXA4
Neutrophils were coincubated with L. major or with A. phagocytophilum for 300 min as described above. For the induction of LXA4 10 ng/ml GM-CSF (Peprotech, Hamburg, Germany) was added as priming agent [34] for the last 90 minutes of incubation. The same procedure was applied to the pathogen controls. All cells were then washed and resuspended in FCS-free complete medium. The cells were then transferred to a 96-well flat bottom microplate with wells containing a confluent monolayer of HT-29 epithelial cells. The ratio of HT-29 cells to PMN in the coculture was approximately 1 to 7.5. For the induction of LXA4, infected and noninfected PMN as well as pathogen controls were treated with 1 μM ionomycin (10 min or 360 min; Sigma-Aldrich) or left untreated. Stimulation was carried out at 37°C in humidified atmosphere containing 5% CO2 and terminated by centrifugation at 4°C (precooled, 800 ×g, 10 min). Supernatants were collected and stored at −80°C. LXA4 was finally quantified by ELISA (United States Biological, Salem, MA, USA) according to the manufacturer's instructions.
2.9. Statistical Analysis
Student's t-test for paired samples was performed in GraphPad Prism 7 software (La Jolla, CA, USA) for the comparison of lipid mediator content in the supernatants of infected and noninfected PMN after induction with ionomycin or LPS + fMLP. Data are presented as mean (±SD). Differences with a p value ≤ 0.05 were considered significant.
3. Results
3.1. Infection with Leishmania major Leads to Enhanced Production of LTB4 by Primary Human Neutrophils
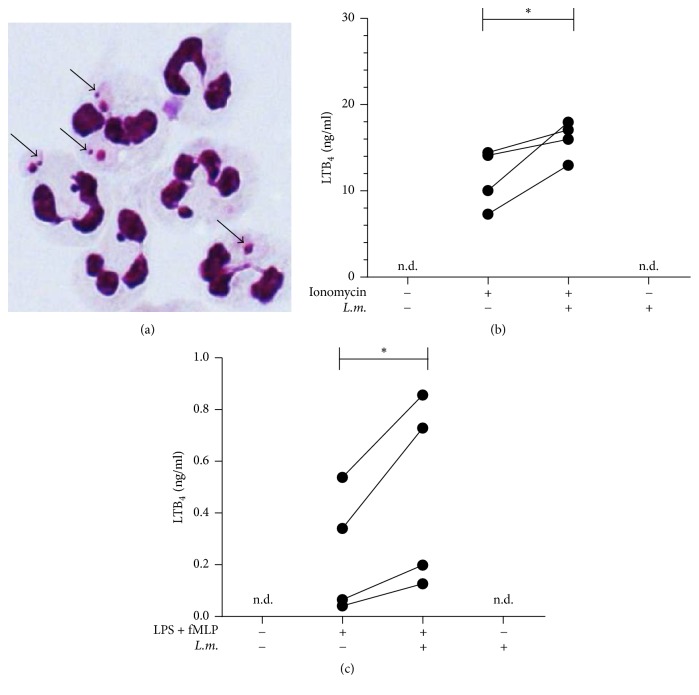
Primary human neutrophils were infected in vitro with stationary phase L. major promastigotes. After five hours of coincubation with the parasites the ratio of infected neutrophils was 65% ± 15% as determined by examination of Diff Quik-stained slides (Figure 1(a)). Treatment with ionomycin or the combination of LPS and fMLP resulted in the release of LTB4 (Figures 1(b) and 1(c)). Infection with L. major significantly (p = 0.048) enhanced the ionomycin-induced LTB4 release from 11.5 (±3.4) ng/ml to 16.0 (±2.1) ng/ml (Figure 1(b)). After infection with L. major the LTB4-release induced by LPS + fMLP was also significantly (p = 0.049) enhanced from 0.24 (±0.23) ng/ml to 0.48 (±0.37) ng/ml (Figure 1(c)). The infection with L. major itself, without additional stimulation, did not result in the release of LTB4 (Figures 1(b) and 1(c)).
Figure 1.
Infection with L. major leads to enhanced production of LTB4 by neutrophils. PMN were coincubated with L. major stationary phase promastigotes for 300 min at 37°C in humidified atmosphere containing 5% CO2 in complete medium. The multiplicity of infection (MOI) for L. major was 5. (a) The micrograph shows Diff Quik-stained neutrophils with internalized L. major promastigotes (arrows). (b) L. major-infected and uninfected neutrophils were treated with ionomycin (0.2 μM, 10 min). LTB4 content of the supernatants was measured by ELISA. (c) L. major-infected and uninfected neutrophils were exposed to LPS (1 μg/ml, 30 min) followed by fMLP (0.5 μM, 10 min). LTB4 content of the supernatants was measured by ELISA. n = 4, n.d.: not detectable, and ∗p ≤ 0.05.
3.2. Infection with Leishmania major Results in Decreased Release of LXA4 by Neutrophils
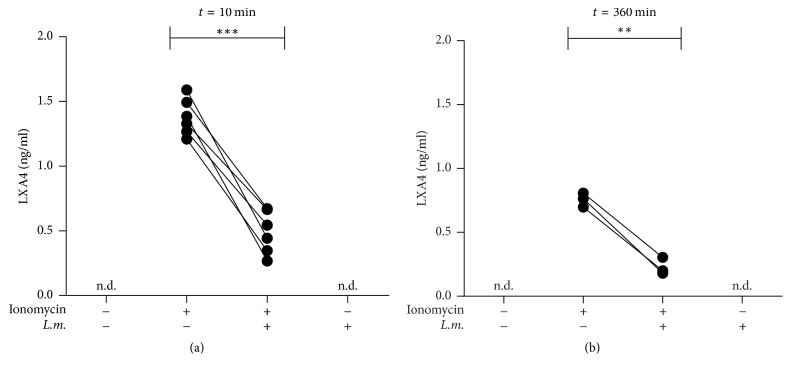
Neutrophil-mediated LXA4 production was assessed in a coculture assay with HT-29 epithelial cells. In this assay ionomycin induced a rapid production of LXA4 10 minutes after induction with ionomycin (Figure 2(a)). At this point in time, infection with L. major led to a significant (p = 0,0001) reduction of LXA4 production from 1.38 (±0.14) ng/ml to 0.49 (±0.17) ng/ml (Figure 2(a)). Also 360 minutes after induction with ionomycin the production of LXA4 was significantly (p = 0.0027) reduced from 0.76 (±0.05) ng/ml in noninfected PMN to 0.23 (±0.07) ng/ml in L. major-infected neutrophils (Figure 2(b)). The infection with L. major, without additional stimulation, did not result in the release of LXA4 at both points in time (Figures 2(a) and 2(b)).
Figure 2.
The release of LXA4 is reduced in Leishmania major-infected PMN. PMN were infected with L. major promastigotes for 300 minutes. During the last 90 min of infection 10 ng/ml GM-CSF was added. L. major-infected and uninfected neutrophils were coincubated with HT-29 cells and stimulated with ionomycin (1.0 μM) for 10 (a) or 360 (b) min. LXA4 content of the supernatants was determined by ELISA. (a) n = 6, (b) n = 3, n.d.: not detectable, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001.
3.3. Anaplasma phagocytophilum-Infected Primary Human Neutrophils Show Increased LTB4 and Decreased LXA4 Release
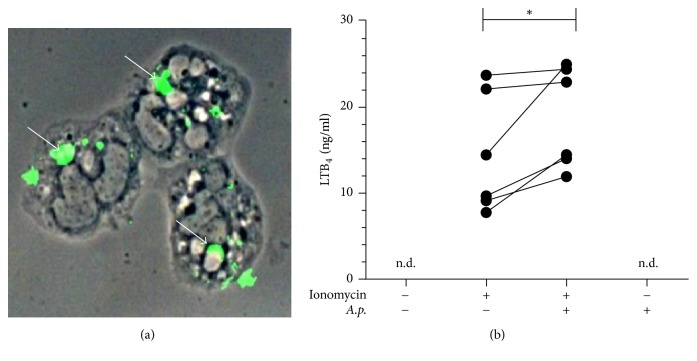
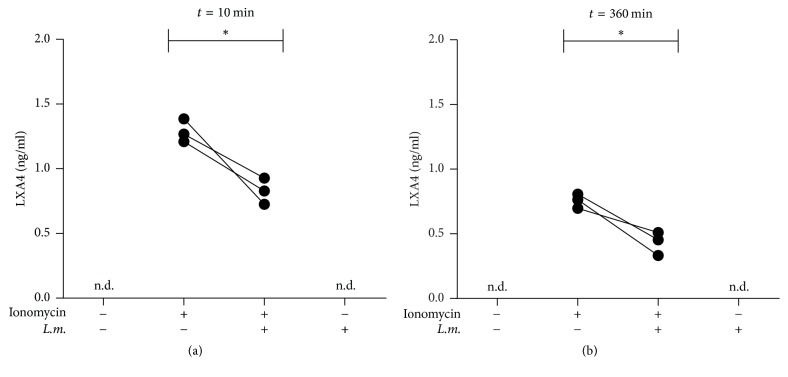
After coincubation with cell-free A. phagocytophilum for five hours, more than 95% of primary human neutrophils contained A. phagocytophilum bacteria (Figure 3(a)). Infection with A. phagocytophilum led to a significantly (p = 0.03) enhanced release of LTB4 by neutrophils 10 minutes after induction with ionomycin from 14.5 (±6.9) ng/ml to 18.8 (±5.9) ng/ml (Figure 3(b)). The release of LXA4 was significantly (p = 0.04) reduced in A. phagocytophilum-infected neutrophils at the same time point from 1.3 (±0.09) ng/ml to 0.8 (±0.1) ng/ml (Figure 4(a)). Six hours after induction with ionomycin a low LXA4 production was observed which was significantly (p = 0.04) inhibited by infection with A. phagocytophilum from a level of 0.76 (±0.05) ng/ml to 0.43 (±0.09) ng/ml (Figure 4(b)). The infection with A. phagocytophilum alone did not induce neither LTB4 nor LXA4 (Figures 3(b); 4(a), 4(b)).
Figure 3.
Infection with Anaplasma phagocytophilum leads to an enhanced release of LTB4. Primary human neutrophil granulocytes were coincubated with cell-free A. phagocytophilum for 300 min and extracellular bacteria were removed by washing. (a) Immunocytochemical staining of coincubated PMN reveals the presence of intracellular bacteria (arrows). (b) A. phagocytophilum-infected and noninfected neutrophils were treated with ionomycin (0.2 μM, 10 min). LTB4 content of the supernatants was measured by ELISA. n = 6, n.d.: not detectable, and ∗p ≤ 0.05.
Figure 4.
The release of LXA4 is reduced in Anaplasma phagocytophilum-infected PMN. Primary human neutrophil granulocytes were coincubated with cell-free A. phagocytophilum for 300 min and extracellular bacteria were removed by washing. During the last 90 min of infection 10 ng/ml GM-CSF was added. For transcellular lipoxin synthesis A. phagocytophilum-infected and uninfected neutrophils were coincubated with HT-29 cells and stimulated with ionomycin (1.0 μM) for 10 (a) or 360 (b) min. LXA4 content of the supernatants was determined by ELISA. n = 3 each, n.d.: not detectable, and ∗p ≤ 0.05.
4. Discussion
Lipid mediators are essential regulators of neutrophil granulocyte recruitment and activity [13, 35]. In this study we investigated the influence of intracellular infections on the early release of LTB4 and LXA4 by primary human neutrophils in vitro. We showed that infection with either L. major or A. phagocytophilum leads to an increased release of proinflammatory LTB4 whereas proresolving LXA4 is significantly reduced. Taken together, our data revealed a proinflammatory shift in PMN-derived lipid mediators during the early phase of intracellular infection with both pathogens. Limitations of this work are, however, the use of the epithelial tumor cell line HT-29 instead of primary cells, the limited numbers of experimental replications, and the in vitro approach itself.
With regard to the neutrophil-dependent establishment of productive L. major infections and the Trojan horse hypothesis [22, 23] our findings suggest that L. major parasites abuse infected PMN for augmented recruitment of neutrophils. By increasing neutrophil-derived LTB4 as a key recruitment factor [7] along with an upregulated IL-8 [36], L. major can ensure the sufficient presence of transient host cells for the subsequent infection of macrophages [22]. In accordance with this view, we could show a downregulation of the recruitment antagonist LXA4 during the first six hours of infection. As L. major parasites benefit from high doses of LXA4 in the later phase of inflammation [37], it seems likely that in the beginning of an infection an increased PMN recruitment induced by LTB4 has a higher priority in the Leishmania survival strategy than the downregulation of PMN effector functions by LXA4.
Previously, our group identified a Leishmania promastigote-derived lipid mediator termed Leishmania chemotactic factor (LCF) [36]. On the one hand, comparable to LTB4, this lipid selectively recruits PMN and induces PMN-derived IL-8 which forms an amplifying loop for neutrophil recruitment [36, 38]. On the other hand, LCF shows analogies to resolution-phase LXA4 by simultaneously deactivating PMN and increasing uptake and intracellular survival of Leishmania as well as mediating its effects via the lipoxin A4 receptor (ALX/FPRL-1) [37]. Summing up, LCF shares features of both LTB4 and LXA4 and illustrates the strong impact of Leishmania on the lipid mediator environment. In line with our present results, LCF completes the view that L. major actively influences the early inflammation phase by changing towards a proinflammatory lipid mediator milieu for sufficient PMN recruitment while simultaneously dampening their activation in a LXA4-like fashion. Consequently our in vitro findings support the view that the induction of neutrophil-derived LTB4 is a part of the parasites survival strategy which enables the initial establishment of Leishmania major infection.
Experiments with Anaplasma phagocytophilum confirmed the important role of lipid mediators in the early phase of infection with intracellular pathogens. Comparable to Leishmania major, the observed increase of PMN-derived LTB4 and decrease of LXA4 production could enable the recruitment of large numbers of host cells and are likely to contribute to the establishment of A. phagocytophilum infection.
Since early after infection the survival of both pathogens depends on the recruitment of sufficient number of host neutrophils, the enhanced induction of LTB4 production appears to be crucial to successfully establishing the infection of the host. Moreover, the enhancing effect of LTB4 on the neutrophil phagocytic capacity [39] likely contributes to the entry of the pathogens into neutrophils. However, LTB4 also leads to enhanced antimicrobial effector functions such as the production of reactive effect oxygen species and phagosome-lysosome fusion [40, 41]. Since these functions can be detrimental for both L. major and A. phagocytophilum, the pathogens must possess effective mechanisms to evade these antimicrobial effector functions. Indeed, both L. major and A. phagocytophilum can effectively inhibit both the production of ROS [42, 43] and acidification of the phagolysosomes [30, 44].
For their transmission both Leishmania and Anaplasma are dependent on vectors. Salivary gland extracts (SGE) of Lutzomyia longipalpis sandflies that are insect vectors for Leishmania parasites were found to inhibit LTB4 production and LTB4-mediated chemotaxis [45]. Furthermore, SGE induces PGE2 which in turn can promote LXA4 synthesis. Consequently, SGE was shown to facilitate survival of L. infantum [46]. Similar to sandflies, Ixodes ricinus, the tick vector of A. phagocytophilum, secretes a leukotriene binding protein termed Ir-LBP that works as a “scavenger” for LTB4. By this effect tick saliva can decrease the number and activation level of PMN located at the tick bite site in vivo [47]. In summary there seems to be opposite LTB4-signaling of vector and pathogen due to different intentions. Whereas sandflies and ticks aim to secure blood meals by dampening inflammation, the pathogens need inflammation for sufficient host cell influx.
Other intracellular pathogens that are not dependent on neutrophils as host cells show different approaches to influencing the balance of lipid mediators. For example, Mycobacterium tuberculosis inhibits proinflammatory PGE2 and enhances lipoxin synthesis in vivo [48]. Toxoplasma gondii has been shown to express its own 15-LOX leading to an increased lipoxin synthesis [49]. By induction of anti-inflammatory lipid mediators these pathogens seem to differ from the group of intracellular pathogens that use neutrophils as host cells. Nevertheless, these findings in concert with our data illustrate that manipulation of local lipid mediators by pathogens is a widespread phenomenon.
5. Conclusions
Taken together, our data support the view [50] that the neutrophil lipidome is a common target of intracellular parasites to modulate the influx and function of leukocytes at the site of infection. We showed that the vector-transmitted intracellular pathogens Leishmania major and Anaplasma phagocytophilum promote neutrophil-driven amplification of acute inflammation. This modulatory function likely contributes to the recruitment of host cells that are essential for the survival and multiplication of the pathogens. Further studies and in vivo models should confirm the impact of intracellular pathogens on host neutrophil lipidome. This could in turn provide a basis for therapeutic approaches to counteract the pathogens survival strategies at this level.
Acknowledgments
This work was funded by the German Research Foundation (DFG), represented in the IRTG1911 Project B4. The authors thank Professor Christian Sina (University of Lübeck, Germany) for providing them with the HT-29 colorectal adenocarcinoma cell line and Professor J. Stephen Dumler (John Hopkins University, Baltimore, MD) for providing the A. phagocytophilum strain and antibodies for the immunohistological detection of A. phagocytophilum. They thank Ms. Sonja Möller for expert technical assistance.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Mócsai A. Diverse novel functions of neutrophils in immunity, infammation, and beyond. The Journal of Experimental Medicine. 2013;210(7):1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaillon S., Galdiero M. R., Del Prete D., Cassatella M. A., Garlanda C., Mantovani A. Neutrophils in innate and adaptive immunity. Seminars in Immunopathology. 2013;35(4):377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 3.Sadik C. D., Kim N. D., Luster A. D. Neutrophils cascading their way to inflammation. Trends in Immunology. 2011;32(10):452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadik C. D., Kim N. D., Iwakura Y., Luster A. D. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(46):E3177–E3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan C. N., Chiang N., Dalli J., Levy B. D. Lipid mediators in the resolution of inflammation. Cold Spring Harbor Perspectives in Biology. 2015;7(2) doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basil M. C., Levy B. D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Reviews Immunology. 2016;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lämmermann T., Afonso P. V., Angermann B. R., et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 9.Lärfars G., Lantoine F., Devynck M.-A., Palmblad J., Gyllenhammar H. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93(4):1399–1405. [PubMed] [Google Scholar]
- 10.Serezani C. H. C., Aronoff D. M., Jancar S., Mancuso P., Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106(3):1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudreault E., Thompson C., Stankova J., Rola-Pleszczynski M. Involvement of BLT1 endocytosis and Yes kinase activation in leukotriene B4-induced neutrophil degranulation. The Journal of Immunology. 2005;174(6):3617–3625. doi: 10.4049/jimmunol.174.6.3617. [DOI] [PubMed] [Google Scholar]
- 12.Kienle K., Lämmermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunological Reviews. 2016;273(1):76–93. doi: 10.1111/imr.12458. [DOI] [PubMed] [Google Scholar]
- 13.Lämmermann T. In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues. Journal of Leukocyte Biology. 2016;100(1):55–63. doi: 10.1189/jlb.1MR0915-403. [DOI] [PubMed] [Google Scholar]
- 14.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunology. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 15.Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. The Journal of Immunology. 2000;164(4):1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 16.Chiang N., Serhan C. N., Dahlén S.-E., et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological Reviews. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 17.Sala A., Folco G., Murphy R. C. Transcellular biosynthesis of eicosanoids. Pharmacological Reports. 2010;62(3):503–510. doi: 10.1016/S1734-1140(10)70306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capra V., Rovati G. E., Mangano P., Buccellati C., Murphy R. C., Sala A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851(4):377–382. doi: 10.1016/j.bbalip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Laufs H., Müller K., Fleischer J., et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infection and Immunity. 2002;70(2):826–835. doi: 10.1128/IAI.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matte C., Olivier M. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. The Journal of Infectious Diseases. 2002;185(5):673–681. doi: 10.1086/339260. [DOI] [PubMed] [Google Scholar]
- 21.Müller K., Zandbergen G., Hansen B., et al. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Medical Microbiology and Immunology. 2001;190(1-2):73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- 22.Laskay T., Van Zandbergen G., Solbach W. Neutrophil granulocytes - Trojan horses for Leishmania major and other intracellular microbes? Trends in Microbiology. 2003;11(5):210–214. doi: 10.1016/S0966-842X(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 23.Peters N. C., Egen J. G., Secundino N., et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro-Gomes F. L., Peters N. C., Debrabant A., Sacks D. L. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathogens. 2012;8(2) doi: 10.1371/journal.ppat.1002536.e1002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salei N., Hellberg L., Köhl J., Laskay T. Enhanced survival of Leishmania major in neutrophil granulocytes in the presence of apoptotic cells. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0171850.e0171850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner N. E., Malemud C. J. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. The Journal of Immunology. 1985;134(1):556–563. [PubMed] [Google Scholar]
- 27.Morato C. I., da Silva I. A., Borges A. F., et al. Essential role of leukotriene B4 on Leishmania (Viannia) braziliensis killing by human macrophages. Microbes and Infection. 2014;16(11):945–953. doi: 10.1016/j.micinf.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Tavares N. M., Araújo-Santos T., Afonso L. Understanding the mechanisms controlling Leishmania amazonensis infection in vitro: the role of LTB4 derived from human neutrophils. Journal of Infectious Diseases. 2014;210(4):656–666. doi: 10.1093/infdis/jiu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araújo-Santos T., Rodríguez N. E., Moura-Pontes S., et al. Role of prostaglandin F2α production in lipid bodies from Leishmania infantum chagasi: insights on virulence. The Journal of Infectious Diseases. 2014;210(12):1951–1961. doi: 10.1093/infdis/jiu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clinical Microbiology Reviews. 2011;24(3):469–489. doi: 10.1128/CMR.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esmann L., Idel C., Sarkar A., et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. The Journal of Immunology. 2010;184(1):391–400. doi: 10.4049/jimmunol.0900564. [DOI] [PubMed] [Google Scholar]
- 32.Solbach W., Forberg K., Kammerer E., Bogdan C., Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. The Journal of Immunology. 1986;137(2):702–707. [PubMed] [Google Scholar]
- 33.Dumler J. S., Choi K.-S., Garcia-Garcia J. C., et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerging Infectious Diseases. 2005;11(12):1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiore S., Serhan C. N. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. The Journal of Experimental Medicine. 1990;172(5):1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serhan C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Zandbergen G., Hermann N., Laufs H., Solbach W., Laskay T. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infection and Immunity. 2002;70(8):4177–4184. doi: 10.1128/IAI.70.8.4177-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel A., Van Zandbergen G. Lipoxin A4 receptor dependent leishmania infection. Autoimmunity. 2009;42(4):331–333. doi: 10.1080/08916930902828239. [DOI] [PubMed] [Google Scholar]
- 38.Gainet J., Chollet-Martin S., Brion M., Hakim J., Gougerot-Pocidalo M.-A., Elbim C. Interleukin-8 production by polymorphonuclear neutrophils in patients with rapidly progressive periodontitis: an amplifying loop of polymorphonuclear neutrophil activation. Laboratory Investigation. 1998;78(6):755–762. [PubMed] [Google Scholar]
- 39.Mancuso P., Nana-Sinkam P., Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infection and Immunity. 2001;69(4):2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Ferrante A., Poulos A., Harvey D. P. Neutrophil oxygen radical generation: synergistic responses to tumor necrosis factor and mono/polyunsaturated fatty acids. The Journal of Clinical Investigation. 1996;97(7):1605–1609. doi: 10.1172/JCI118585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Showell H. J., Otterness I. G., Marfat A., Corey E. J. Inhibition of leukotriene B4-induced neutrophil degranulation by leukotriene B4-dimethylamide. Biochemical and Biophysical Research Communications. 1982;106(3):741–747. doi: 10.1016/0006-291X(82)91773-9. [DOI] [PubMed] [Google Scholar]
- 42.Bogdan C., Röllinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. International Journal for Parasitology. 1998;28(1):121–134. doi: 10.1016/S0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 43.Woldehiwet Z. Immune evasion and immunosuppression by Anaplasma phagocytophilum, the causative agent of tick-borne fever of ruminants and human granulocytic anaplasmosis. The Veterinary Journal. 2008;175(1):37–44. doi: 10.1016/j.tvjl.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Dermine J.-F., Scianimanico S., Privé C., Descoteaux A., Desjardins M. Leishmania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cellular Microbiology. 2000;2(2):115–126. doi: 10.1046/j.1462-5822.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- 45.Monteiro M. C., Nogueira L. G., Almeida Souza A. A., Ribeiro J. M. C., Silva J. S., Cunha F. Q. Effect of salivary gland extract of Leishmania vector, Lutzomyia longipalpis, on leukocyte migration in OVA-induced immune peritonitis. European Journal of Immunology. 2005;35(8):2424–2433. doi: 10.1002/eji.200526160. [DOI] [PubMed] [Google Scholar]
- 46.Araújo-Santos T., Prates D. B., França-Costa J., et al. Prostaglandin E2/Leukotriene B4 balance induced by Lutzomyia longipalpis saliva favors Leishmania infantum infection. Parasites & Vectors. 2014;7(1, article no. 601) doi: 10.1186/s13071-014-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaufays J., Adam B., Menten-Dedoyart C., et al. Ir-LBP, an Ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS ONE. 2008;3(12) doi: 10.1371/journal.pone.0003987.e3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietzold J., Gopalakrishnan A., Salgame P. Duality of lipid mediators in host response against Mycobacterium tuberculosis: good cop, bad cop. F1000Prime Reports. 2015;7, article no. 29 doi: 10.12703/P7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannenberg G. L., Aliberti J., Hong S., Sher A., Serhan C. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous Lipoxin A4 biosynthesis. The Journal of Experimental Medicine. 2004;199(4):515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rub A., Arish M., Husain S. A., Ahmed N., Akhter Y. Host-lipidome as a potential target of protozoan parasites. Microbes and Infection. 2013;15(10-11):649–660. doi: 10.1016/j.micinf.2013.06.006. [DOI] [PubMed] [Google Scholar]