Abstract
Objective:
To conduct a randomized trial to test the primary hypothesis that once-daily tadalafil, administered orally for 48 weeks, lessens the decline in ambulatory ability in boys with Duchenne muscular dystrophy (DMD).
Methods:
Three hundred thirty-one participants with DMD 7 to 14 years of age taking glucocorticoids were randomized to tadalafil 0.3 mg·kg−1·d−1, tadalafil 0.6 mg·kg−1·d−1, or placebo. The primary efficacy measure was 6-minute walk distance (6MWD) after 48 weeks. Secondary efficacy measures included North Star Ambulatory Assessment and timed function tests. Performance of Upper Limb (PUL) was a prespecified exploratory outcome.
Results:
Tadalafil had no effect on the primary outcome: 48-week declines in 6MWD were 51.0 ± 9.3 m with placebo, 64.7 ± 9.8 m with low-dose tadalafil (p = 0.307 vs placebo), and 59.1 ± 9.4 m with high-dose tadalafil (p = 0.538 vs placebo). Tadalafil also had no effect on secondary outcomes. In boys >10 years of age, total PUL score and shoulder subscore declined less with low-dose tadalafil than placebo. Adverse events were consistent with the known safety profile of tadalafil and the DMD disease state.
Conclusions:
Tadalafil did not lessen the decline in ambulatory ability in boys with DMD. Further studies should be considered to confirm the hypothesis-generating upper limb data and to determine whether ambulatory decline can be slowed by initiation of tadalafil before 7 years of age.
Clinicaltrials.gov identifier:
Classification of evidence:
This study provides Class I evidence that tadalafil does not slow ambulatory decline in 7- to 14-year-old boys with Duchenne muscular dystrophy.
Dystrophin mutations cause the most common muscular dystrophy, Duchenne muscular dystrophy (DMD), a devastating x-linked muscle-wasting disease.1,2 Boys are diagnosed as toddlers, and most lose ambulation by 12 to 15 years of age. Average life expectancy is ≈30 years due to respiratory failure and cardiomyopathy.1 Corticosteroids can prolong ambulation by 2 to 3 years, reduce the risk of scoliosis, and temper pulmonary and possibly cardiac decline, but they cause well-known side effects.1 Thus, a more effective therapy is an urgent need.
Dystrophin is both a structural sarcolemma-stabilizing protein and a scaffolding protein that targets partner proteins to the sarcolemma.3,4 Among these is a muscle-specific variant of neuronal nitric oxide (NO) synthase (nNOSμ), the main enzymatic source of NO in skeletal muscle.5 Sarcolemmal nNOSμ increases its enzymatic activity and thus NO–cyclic guanosine monophosphate (cGMP) signaling during exercise6 and is implicated in muscle blood flow regulation and mitochondrial biogenesis, microtubule organization, and sarcolemmal targeting of other dystrophin-associated proteins.7–10 The NO generated modulates adrenergic vasoconstriction in exercising skeletal muscle, thereby optimizing perfusion (a protective mechanism called functional sympatholysis).11–13 In DMD, dystrophin deficiency disrupts sarcolemma targeting of nNOSμ,5 contributing to dystrophic muscle pathology7–10,14 and causing defective blood flow regulation.15–17 Consequently, repeated bouts of functional muscle ischemia could accelerate use-dependent injury of skeletal muscle fibers already weakened by loss of dystrophin.18
A compelling body of preclinical research in mouse, zebrafish, and dog models of DMD implicates the NO-cGMP pathway as a putative new drug target for DMD.6,10,19–30 Short-term dosing of tadalafil, a phosphodiesterase type 5 (PDE5) inhibitor that boosts defective NO-cGMP signaling in skeletal muscle microvessels, prevents exercise-induced muscle ischemia, injury, and fatigue in mdx mice.23,24 Short-term dosing of tadalafil also ameliorates forearm muscle ischemia during handgrip exercise in boys with DMD15 and men with Becker muscular dystrophy,31 a milder form of dystrophinopathy. Whether these effects translate to a meaningful clinical benefit is unknown.
Accordingly, we conducted a phase 3 clinical trial to test the primary hypothesis that once-daily tadalafil, administered orally for 48 weeks, lessens the decline in ambulatory ability in boys with DMD. In addition, we explored the effect on upper limb function due to its slower decline.32
METHODS
Standard protocol approvals, registrations, and patient consents.
The study protocol and consent forms were approved by the institutional review/ethics boards at each participating medical center and conducted in accordance with the Declaration of Helsinki and other international ethics guidelines. Written informed consent was obtained from parents or guardians, and written assent was obtained from participants. This trial is registered at ClinicalTrials.gov (NCT01865084).
Participants.
The diagnostic criteria for DMD were onset of clinical signs or symptoms before 6 years of age, markedly high serum creatine kinase level, and deletion or duplication dystrophin mutation or nearly absent dystrophin protein on muscle biopsy.1
Inclusion criteria were male with proven DMD, age of 7 to 14 years, 6-minute walk distance (6MWD) between 200 and 400 m, 2 baseline 6MWDs differing by <20%, left ventricular ejection fraction ≥50%, and systemic corticosteroid therapy for ≥6 months with a stable regimen ≥3 months. Exclusion criteria were heart failure; rhythm other than sinus or abnormal conduction on ECG; continuous mechanical ventilation; medication other than corticosteroids affecting muscle strength; other conditions affecting muscle performance; history of renal insufficiency, retinal disorder, hypotension, or uncontrolled hypertension; or cytochrome P450 3A4 inhibitor treatment.
Study design.
We conducted a phase 3 randomized, placebo-controlled, parallel 3-arm trial of tadalafil in patients with DMD in 63 sites in 15 countries.
Randomization and blinding.
Randomization was 1:1:1 (computer-generated random sequence) to placebo, tadalafil 0.3 mg/kg (low dose), or tadalafil 0.6 mg/kg (high dose). Randomization was stratified by site and baseline 6MWD <300 or ≥300 m. Siblings were assigned to the same group. Total daily dose was determined by baseline weight. All participants received the same number of identical-appearing tablets. Patients, parents/guardians, investigators, evaluators, and other study staff were blinded to group assignment.
Procedures.
Evaluation of the primary outcome and other efficacy measures.
The primary efficacy measure was the 6MWD assessed with a 6-minute walk test modified for boys with DMD (standardized verbal encouragement and a safety chaser).33 The 6MWD was assessed twice at baseline and once every 12 weeks thereafter.
Secondary efficacy measures included the North Star Ambulatory Assessment (NSAA), a 17-item evaluation of standing ability and other timed function tests.34 The total raw NSAA score was transformed to a linear 0-to-100 scale. Data are expressed as time to perform each test, the physical therapist's score, and velocity.
Exploratory efficacy measures included the Performance of Upper Limb (PUL), pulmonary function, and resting heart rate. Total PUL scale (0–72 points) is a functional assessment for upper limb activities of daily living that includes a total of 22 individual tests of motor performance with subscores at the level of the shoulder (high level, 0–16 points), elbow (midlevel, 0–32 points), and hand/fingers (distal level, 0–24 points). A prespecified subgroup analysis was done for boys with DMD >10 years of age, the age at which the total PUL scale begins to decline, with a proximal-to-distal gradient.32 Substudies aimed to explore leg muscle fat fraction by MRI at 4 centers and functional sympatholysis at 1 center.
Evaluators were trained physical therapists/physiotherapists. Assessments were performed in the same order (NSAA, 4-stair climb/descend, PUL, and 6MWD) generally at the same time of day.
The Pediatric Outcomes Data Collection Instrument was the main quality-of-life assessment.33
Drug exposures.
Population pharmacokinetic modeling was used to estimate steady-state tadalafil exposures. Blood samples were collected at various time intervals after dosing. Data are expressed as the mean area under the concentration-time curve (AUC).
Safety.
Safety measures included adverse events, laboratory data, vital signs, physical growth, ECGs, and echocardiograms.
Statistics.
Assuming an SD of 60 m, 102 patients per group provided 90% estimated power to detect a between-group difference of 30 m in change in 6MWD, which is clinically meaningful.33
Efficacy analyses on the full dataset were by intention to treat. A mixed-effects model with repeated measurements (MMRM) was used to test the primary null hypothesis of no difference between tadalafil and placebo in mean change in 6MWD at 48 weeks. The model included a continuous fixed effect for baseline 6MWD and fixed categorical effects of group assignment, visit, group-by-visit interaction, and country. Secondary and exploratory efficacy measures also were analyzed by MMRM. Fisher exact test was applied to categorical safety measures to flag potential treatment differences.
A multiplicity adjustment controlled for global type I error. Tests of the primary hypothesis and those conducted under the multiplicity adjustment are considered scientifically confirmatory, while all others are considered hypothesis generating.
Prespecified subgroup analyses were conducted on the primary outcome by baseline 6MWD <300 or ≥300 m and PUL scores/subscores by baseline age ≤10 or >10 years. Subgroup analyses used MMRM with addition of subgroup, subgroup-by-treatment interaction, and subgroup-by-treatment-by-visit interaction. For each subgroup analysis, a significant interaction was defined at the 0.10 level.
Primary research question.
Does daily tadalafil treatment lessen the decline in ambulatory ability in boys with DMD?
Classification of evidence.
This study provides Class I evidence that tadalafil does not slow ambulatory decline in 7- to 14-year old boys with DMD who are taking corticosteroids.
RESULTS
The first patient enrolled in September 2013, and the last patient visit for the double-blind trial was in December 2015. An open-label extension study was stopped in January 2016 because tadalafil had no effect on the primary and secondary efficacy endpoints of the trial. Here, we report the major outcomes of the double-blind trial.
Patient characteristics.
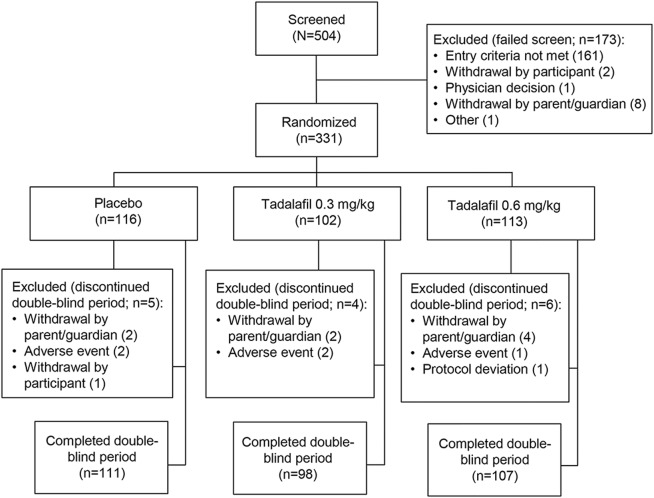
In total, 331 patients were randomized and 316 patients completed the trial (figure 1). One patient randomized to high-dose tadalafil was excluded from the safety analysis because he discontinued before the first dose, but his data were included in the efficacy analysis.
Figure 1. Patient disposition.
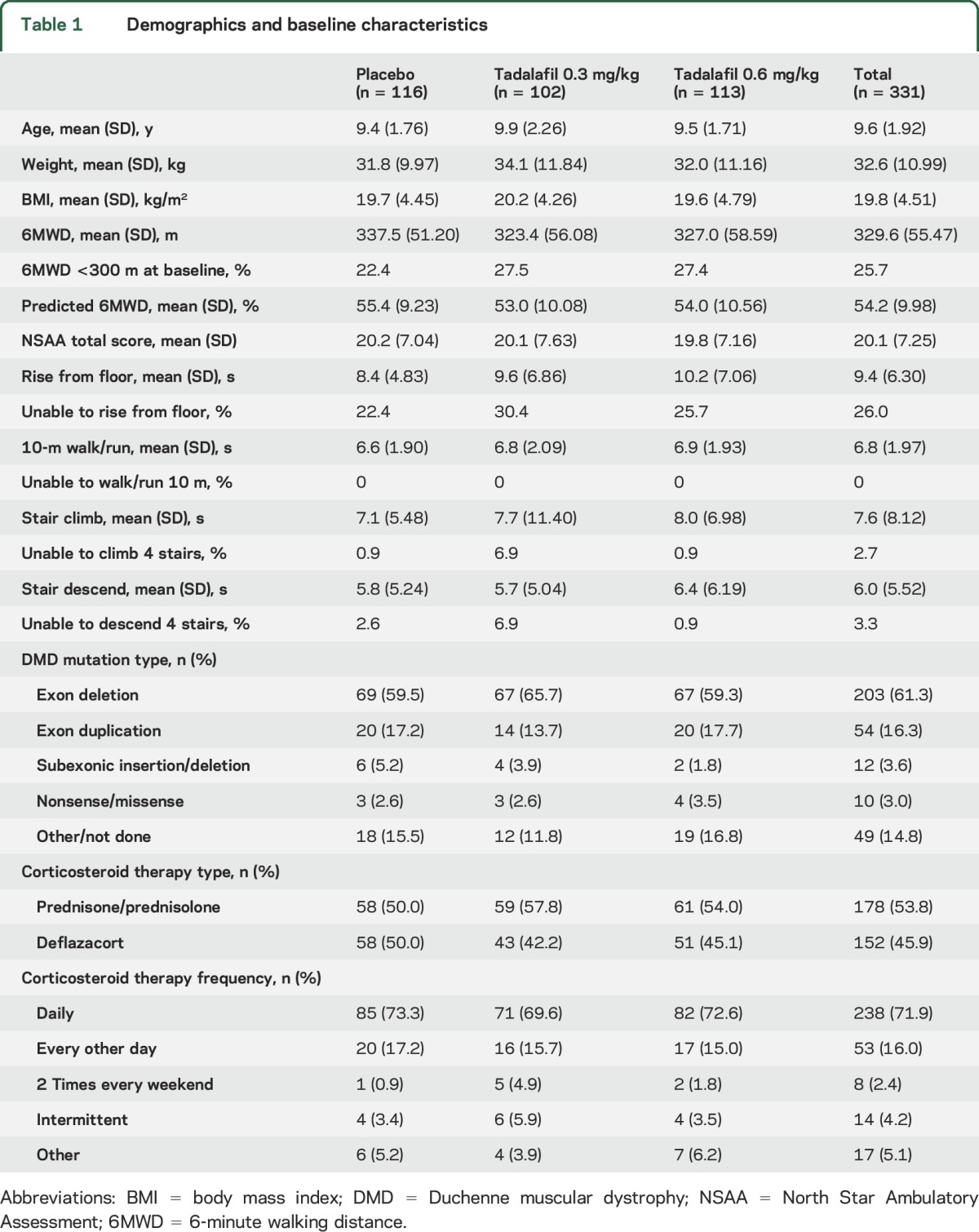
Baseline characteristics were similar across treatment groups, with some exceptions (table 1). On average, patients in the placebo group were younger and had a higher baseline 6MWD compared to those in the tadalafil groups, and a smaller proportion of patients in the placebo group had a baseline 6MWD <300 m. The proportion of patients unable to rise from floor independently and unable to perform the 4-stair climbing and descending tasks was higher in the low-dose tadalafil group compared with the placebo and high-dose tadalafil groups. A higher proportion of boys in the placebo group were taking deflazacort (table 1).
Table 1.
Demographics and baseline characteristics
Primary efficacy measure.
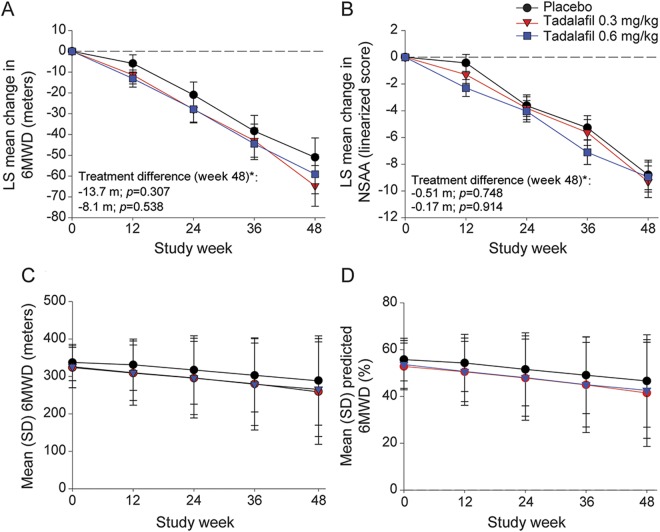
Tadalafil had no effect on the primary outcome. After 48 weeks, 6MWD declined by −51.0 ± 9.3 m with placebo vs −64.7 ± 9.8 m with low-dose tadalafil (p = 0.307 vs placebo) and −59.1 ± 9.4 m with high-dose tadalafil (p = 0.538 vs placebo) (figure 2). Baseline values of 6MWD and percent of predicted 6MWD were slightly less in the tadalafil groups; however, both measures declined in parallel regardless of group assignment (figure 2).
Figure 2. Mean change in efficacy measures.
(A) Mean change from baseline to week 48 in 6MWD. (B) Mean change from baseline to week 48 in NSAA (linearized score on scale of 0–100). (C) Mean 6MWD (meters), baseline to week 48. (D) Mean percent of predicted 6MWD, baseline to week 48. *Primary efficacy endpoint. Analyses for each efficacy parameter included all patients with nonmissing baseline and at least 1 nonmissing postbaseline value (for 6MWD: n = 113 in placebo, 101 in tadalafil 0.3 mg/kg, and 111 for tadalafil 0.6 mg/kg; for NSAA: n = 116 in placebo, 102 in tadalafil 0.3 mg/kg, and 112 for tadalafil 0.6 mg/kg). LS = least squares; NSAA = North Star Ambulatory Assessment; 6MWD = 6-minute walk distance.
After 48 weeks, 37.9% of participants in the placebo group experienced persistent 10% worsening in 6MWD compared with 37.3% in the low-dose tadalafil group (p = 1.000) and 44.2% in the high-dose tadalafil group (p = 0.350); 7 (6.0%) participants in the placebo group lost ambulation compared to 16 (15.7%) in the low-dose tadalafil group (p = 0.027) and 9 (8.0%) in the high-dose tadalafil group (p = 0.613).
As expected, 6MWD declined more steeply in patients with baseline 6MWD <300 m. However, tadalafil had no effect on either subgroup (figure e-1 at Neurology.org).
Secondary efficacy measures.
Tadalafil had no effect on secondary outcomes. NSAA data are shown in figure 2B and figure e-2, and timed function test data are given in tables e-1 and e-2 and figure e-3. The only statistically positive finding was a smaller decline in velocity of the rise from floor with low-dose tadalafil, but the difference compared with placebo was trivial (figure e-3).
Tadalafil had no effect on the decline in parent-rated quality of life (figure e-4). Data are not reported for adolescent self-rated data because the numbers of adolescent participants were too small (7–11 per group).
Exploratory efficacy measures.
PUL.
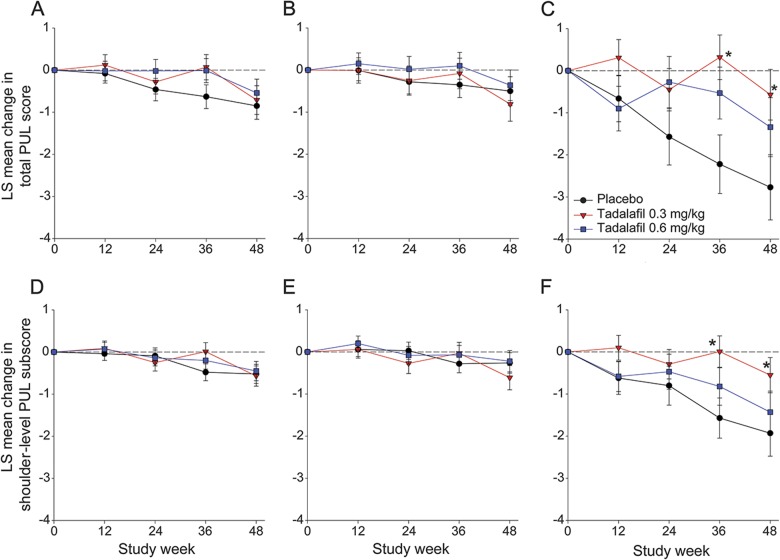
As expected, PUL declined only in the prespecified subgroup of boys >10 years of age (n = 75). Their total PUL score and shoulder subscore declined less with low-dose tadalafil (n = 33) than placebo (n = 20) (figure 3). After 48 weeks, total PUL score declined by 4% with placebo vs 1% with low-dose tadalafil (p = 0.02) and shoulder subscore by 14% with placebo vs 4% with low-dose tadalafil (p = 0.04). Total PUL score and shoulder subscore also tended to decline less (p > 0.1) with high-dose tadalafil (n = 22) than placebo. The age-by-treatment interaction p value was 0.056 and 0.060 for the total PUL score and shoulder subscore, respectively. Elbow and hand/finger scores were unchanged with placebo and tadalafil even in older boys (figure e-5).
Figure 3. Mean change in total and shoulder level PUL score.
(A) Total PUL score in the total population (n = 114 in placebo, 99 in tadalafil 0.3 mg/kg, and 107 in tadalafil 0.6 mg/kg). (B) Total PUL score in patients ≤10 years of age (n = 95 in placebo, 68 in tadalafil 0.3 mg/kg, and 85 in tadalafil 0.6 mg/kg). (C) Total PUL score in patients >10 years of age (n = 19 in placebo, 31 in tadalafil 0.3 mg/kg, and 22 in tadalafil 0.6 mg/kg). (D) Shoulder-level PUL subscore in the total population (n = 116 in placebo, 101 in tadalafil 0.3 mg/kg, and 111 in tadalafil 0.6 mg/kg). (E) Shoulder-level PUL subscore in patients ≤10 years of age (n = 96 in placebo, 69 in tadalafil 0.3 mg/kg, and 89 in tadalafil 0.6 mg/kg). (F) Shoulder-level PUL subscore in patients >10 years of age (n = 20 in placebo, 32 in tadalafil 0.3 mg/kg, and 22 in tadalafil 0.6 mg/kg). Changes in total PUL score are from a total score range of 0 to 72; changes in shoulder-level PUL are from a total score range of 0 to 16. Higher score represents better function. Analyses for each efficacy parameter included all patients with nonmissing baseline and at least 1 nonmissing postbaseline value. *p < 0.05. LS = least squares; PUL = Performance of Upper Limb.
Pulmonary function.
Pulmonary function tests showed little, if any, decline, as expected for this age group, with no treatment effect (table e-3).
Resting heart rate.
Resting heart rate by ECG tended to be slightly lower with tadalafil treatment, but this tendency was not statistically significant. After 48 weeks, heart rate tended to decline by 1.6 bpm from baseline with placebo compared with 3.8 bpm with low-dose tadalafil (p = 0.182 vs placebo) and 2.4 bpm with high-dose tadalafil (p = 0.635 vs placebo).
Substudies.
Data are not reported because too few participants completed either the leg fat MRI substudy (n = 9 per group) or the sympatholysis substudy (n = 2 or 3 per group).
Compliance and drug concentration measurements.
Mean compliance in all groups exceeded 97% at every visit. Tadalafil steady-state mean AUCs were 6550 ng ·h/mL and 9380 ng · h/mL during dosing with 0.3 and 0.6 mg/kg, respectively. Participant-specific data showed no association between tadalafil AUC values and changes in 6MWD (figure e-6).
Safety.
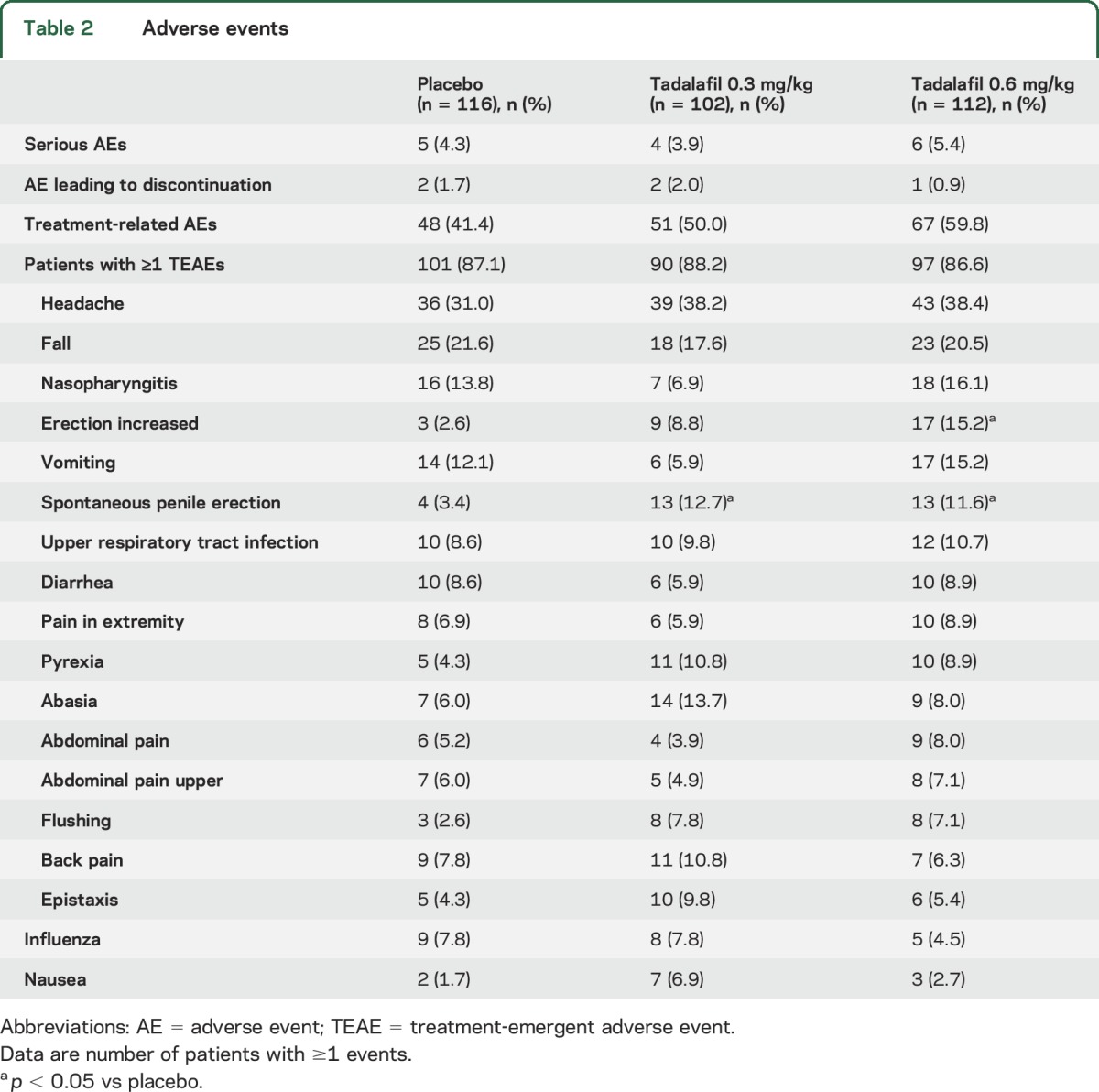
Serious adverse events (SAEs), treatment-emergent adverse events, or adverse events leading to discontinuation were similar across groups (table 2). Adverse events related to study drug were reported in 41.4%, 50.0%, and 59.8% of patients in the placebo, low-dose, and high-dose tadalafil groups, respectively; the difference between high-dose tadalafil and placebo was significant (p = 0.006). The treatment-emergent adverse events of “erection increased” with high-dose tadalafil (15.2%, p < 0.001) and “spontaneous penile erection” with low-dose tadalafil (12.7%, p = 0.012) and high-dose tadalafil (11.6%, p = 0.023) were reported at a higher frequency than with placebo. Individual SAEs (table e-4) were consistent with the type of events expected for patients with DMD receiving corticosteroid therapy; the most frequently reported SAEs were falls, fractures, and infections. No deaths occurred.
Table 2.
Adverse events
Changes in left ventricular ejection fraction were small with no group differences (table e-5). There were no differences between the tadalafil and placebo groups in safety monitoring data by laboratory parameters, vital signs, height, body weight, or ECG.
DISCUSSION
In the largest DMD clinical trial to date and the first to explore upper limb performance, 48 weeks of tadalafil did not lessen the decline in 6MWD or other measures of ambulatory ability in boys with DMD 7 to 14 years of age receiving standard glucocorticoid therapy. Yet, in a prespecified subgroup analysis of boys >10 years of age, tadalafil was associated with a lesser decline in upper limb function.
That tadalafil caused no discernible stabilization of ambulatory ability stands in marked contrast to the solid preclinical foundation of the trial, involving 3 different models. In the mdx mouse, multiple genetic and pharmacologic strategies that boost NO-cGMP signaling, including short- or long-term dosing of PDE5 inhibitors, markedly ameliorate many features of the dystrophic phenotype.6,10,19,20,23,24,26–30,35 In mdx mice, even short-term dosing of either tadalafil or sildenafil abrogates muscle ischemia, injury, and fatigue after downhill (eccentric) treadmill exercise23 and doubles horizontal walking distance, the mouse equivalent of 6MWD.24 Long-term sildenafil rescues dystrophic skeletal and cardiac muscle and prolongs survival in dystrophin-deficient zebrafish,22 and long-term tadalafil improves skeletal muscle histopathology and delays the onset of cardiomyopathy in the virulent large-animal golden retriever model.21
A short-term dosing study conducted in boys with DMD 8 to 13 years of age taking corticosteroids informed the tadalafil doses for this clinical trial.15 Tadalafil improved blood flow regulation in exercising forearm muscle in a dose-dependent manner without affecting blood flow in resting forearm muscle. The oral tadalafil was dosed short term at 0.5 or 1.0 mg/kg (approximating maximum adult doses for erectile dysfunction or pulmonary hypertension, respectively). For transition to once-daily administration in the trial, doses are 30% lower because tadalafil has a long elimination half-life and thus accumulates in plasma.36
Several other strengths of the trial design include the unprecedented DMD sample size and outstanding cohort retention and medication compliance with pharmacokinetic proof that the study achieved desired levels of 24-hour drug exposure. In our pediatric DMD participants, the 2 daily tadalafil doses produced steady-state drug concentrations that were comparable to those achieved when healthy adults are given the maximal allowable daily doses36,37 but unrelated to individual differences in the primary study outcome of 6MWD. Thus, the negative primary and secondary outcomes were not due to insufficient statistical power or drug exposure. We do not believe the negative outcomes are explained by the somewhat lower mean baseline values of 6MWD in the tadalafil groups because baseline 6MWD was a covariate in the primary analysis and no tadalafil effect was seen when the analysis was stratified by baseline 6MWD >30 or 300 m.
In the placebo group, the decline in total PUL score, mainly from a decline in shoulder function, confirms natural history data in DMD showing progressive decline in PUL after, but not before, age 10 with a proximal-to-distal gradient.32 In older boys, the slower decline in shoulder function and thus total PUL score with low-dose tadalafil should be interpreted cautiously and considered hypothesis generating. The lack of significant treatment effect with high-dose tadalafil may be due to this smaller sample of the subgroup. Thus, without further study, the treatment effect seen with low-dose tadalafil is of uncertain clinical significance.
However, the PUL data raise 2 potential explanations for the negative primary outcome of the trial. Because DMD progresses faster in lower than in upper extremities, the boys in this trial may not have engaged in enough daily ambulation and fatiguing leg exercise for the drug to prevent use-dependent leg muscle injury. In DMD, the therapeutic target of tadalafil, defective NO-cGMP signaling in skeletal muscle microvessels, regulates muscle blood flow only when the muscles are active.15–17 Because PDE5 levels were found in 1 study to be lower than normal in leg muscle biopsy tissue of 4 adult men with Becker muscular dystrophy,38 another possibility is that the cellular target of the drug (PDE5) was lacking in DMD leg muscle microvessels.
While PDE5 inhibitors improve cardiac function in preclinical models of DMD,19,21,22 a cardiac MRI study reported that systolic function possibly declined more rapidly in 8 adult patients with DMD cardiomyopathy treated with sildenafil than in 7 patients treated with placebo.39 While those results might be related to sample size or more advanced cardiomyopathy at baseline in the sildenafil group,39 we augmented cardiac safety monitoring of our pediatric patients with serial echocardiograms, which showed no adverse effect of tadalafil on cardiac function. Overall, safety monitoring revealed the expected increase in penile erections with tadalafil without priapism.
Our study has some important limitations in methodology. Despite the expertise and training of the physical therapists who conducted the outcome evaluations using state-of-the-art clinical trial methodology,33 the 6-minute walk test and other current tests used to assess ambulatory (and upper limb) ability in DMD all are invariably influenced by the boys' volition. MRI measurement of muscle fat fraction is a more sensitive nonvolitional index of DMD disease progression40; however, our exploratory leg muscle MRI substudy sample was too small to be informative. We could not document the extent to which tadalafil improved muscle blood flow regulation during the trial because the sympatholysis substudy sample was too small. However, our previous study strongly suggests that these doses of tadalafil should have improved muscle blood flow regulation at least during arm exercise.15
On the basis of the present findings, it remains unknown whether defective blood flow regulation during exercise in dystrophic human skeletal muscle is merely a reliable biomarker of defective nNOSµ signaling or remains a viable therapeutic target. Further studies should be considered both to confirm the hypothesis-generating upper limb data and to determine whether ambulatory decline in DMD can be slowed by initiation of PDE5 inhibition before 7 years of age.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the participants and their families, all the site investigators (coinvestigator list is available at Neurology.org), and staff for their dedication and commitment to the trial. They also thank the physiotherapist training team (Lindsay Alfano, PT; Kristy Rose Cocayne, PhD; Michelle Eagle, PhD; Julaine Florence, DPT; Meredith James, Linda Lowes, PhD; Anna Mayhew, PhD; Elena Mazzone, PT; and Leslie Nelson, MPT), Ann Martin, MS, GSC from DuchenneConnect, and Stephen Lynn, PhD, from TREAT-NMD for recruitment assistance, David Small, PhD, and Lisa Ferguson-Sells (Global PK/PD, Eli Lilly and Company) for analysis of tadalafil exposure data, Deborah D'Souza, PhD, MBA (InVentiv Clinical, LLC), for formatting assistance under the direction of the authors, Yvonne Kobayashi, PhD (research scientist, Eli Lilly and Company), for helpful scientific discussions, and Theresa Bauer (Clinical Trial Management, Eli Lilly and Company), whose tireless commitment was essential to the successful implementation and completion of this trial.
GLOSSARY
- AUC
area under the concentration-time curve
- cGMP
cyclic guanosine monophosphate
- DMD
Duchenne muscular dystrophy
- MMRM
mixed-effects model with repeated measurement
- NO
nitric oxide
- nNOSμ
muscle-specific splice variant of neuronal nitric oxide synthase
- NSAA
North Star Ambulatory Assessment
- PDE5
phosphodiesterase type 5
- PUL
Performance of Upper Limb
- SAE
serious adverse event
- 6MWD
6-minute walk distance
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Tadalafil DMD Study Group, Hoda Abdel-Hamid, Susan Apkon, Richard Barohn, Elena Belousova, Enrico Bertini, John Brandsema, Claudio Bruno, William Burnette, Russell Butterfield, Barry Byrne, Craig Campbell, Jose Carlo, Jong-Hee Chae, Saleel Chandratre, Giacomo Comi, Anne Connolly, Imelda De Groot, Nicolas Deconinck, Joseph Dooley, Alberto Dubrovsky, Julien Durigneux, Erika Finanger, Richard Finkel, L. Matthew Frank, Nathalie Goemans, Amy Harper, Ayako Hattori, Ozlem Herguner, Susan Iannaccone, Joanne Janas, Yuh-Jyh Jong, JanBerd Kirschner, Hirofumi Komaki, Nancy Kuntz, Wang-Tso Lee, Edward Leung, Jean Mah, Katherine Mathews, Craig McDonald, Eugenio Mercuri, Hugh McMillan, Wolfgang Mueller-Felber, Adolfo Lopez de Munain, Akinori Nakamura, Erik Niks, Katsuhisa Ogata, Samuel Pascual, Elena Pegoraro, Yann Pereon, Ben Renfroe, Ratna Bhavaraju Sanka, Jens Schallner, Ulrike Schara, Kathryn Selby, Isabel Illa Sendra, Laurent Servais, Edward Smith, Susan Sparks, Haluk Topaloglu, Ron Victor, Juan Jose Vilchez, Matthew Wicklund, Ekkehard Wilichoswki, and Brenda Wong
AUTHOR CONTRIBUTIONS
R.G. Victor contributed to the conception and design of the study, chaired the Steering Committee, served as the global principal investigator and a site investigator, participated in the interpretation of the data, wrote the first draft of the manuscript, wrote and approved the final version of the manuscript, had access to all the data, and takes overall responsibility for the data and accuracy of the data analysis. H.L. Sweeney participated in the conception and design of the study, was a member of the Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. R. Finkel participated in the conception and design of the study, was a site investigator and member of the trial Steering Committee for which he chaired the Publications Subcommittee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. C.M. McDonald participated in the conception and design of the study, was a site investigator and a member of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during development, and approved the final manuscript. B. Byrne was a site investigator and was a member of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. M. Eagle led the physiotherapist training team and oversaw implementation of the functional tests in the trial, was a member of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. N. Goemans was a site investigator and a member of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. K. Vandenborne contributed to the design of the MRI substudy, was a member of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. A.L. Dubrovsky and H. Topaloglu were site investigators, participated in the interpretation of the data, critically reviewed the manuscript during development, and approved the final manuscript. M.C. Miceli and P. Furlong were members of the trial Steering Committee, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final manuscript. J. Landry participated in the study design, supervised the statistical analyses, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. R. Elashoff contributed to the conception and design of the study, participated in the interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript. D. Cox participated in the design of the study and the analysis and interpretation of the data, critically reviewed the manuscript during its development, and approved the final version of the manuscript.
STUDY FUNDING
This study was funded by Eli Lilly and Company.
DISCLOSURE
R. Victor has had contracted research with Eli Lilly, Capricor, and Catabasis; he has received travel compensation from Eli Lilly and has received a consulting fee from Eli Lilly, which reimburses Cedars-Sinai Medical Center for Dr. Victor's time and effort serving as the global principal investigator for the phase 3 tadalafil trial. H. Sweeney provided consulting as a member of the trial Steering Committee but received no payments for this activity. R. Finkel has served on advisory boards and/or received travel support and compensation from Ionis, Biogen, Roche, AveXis, Catabasis, Summit, and PTC. C. McDonald reports clinical trial support from Acceleron Pharma, Bristol Myers Squibb, Cardero Therapeutics, Eli Lilly and Company, Halo, Novartis, NS Pharma, and ReveraGen BioPharma; consulting and research support from BioMarin; consulting and clinical trial support from Italfarmaco; consulting, clinical trial, and research support from Pfizer, PTC Therapeutics, and Santhera Therapeutics; consulting, clinical trials, advisory board, or research support from Marathon Pharmaceuticals and Sarepta Therapeutics; consulting for Catabasis Pharmaceuticals; speaking and research support from Parent Project Muscular Dystrophy; speaking support from Collaborative Clinical Trajectory Project, CureDuchenne, Duchenne Regulatory Science Consortium, and TREAT-NMD; and research support from Muscular Dystrophy Association. B. Byrne reports no disclosures relevant to the manuscript. M. Eagle has disclosures for paid consulting with PTC, Summit, Biomarin, Bristol Myers Squibb, Italfarmaco, Santhera, Sarepta, Catabasis, and Capricor. N. Goemans has served on advisory boards for Summit, Biogen, PTC, and BioMarin; she has received travel support and compensations as a speaker at symposia from PTC, BioMarin, and Biogen. K. Vandenborne reports grants from Eli Lilly, Sarepta Therapeutics, Catabasis Pharmaceuticals, Pfizer, Italfarmaco SpA, and Summit Therapeutics. A. Dubrovsky disclosures that he is a member of the Global Advisory Board for Pompe Disease from Genzyme; he received research grants and speaker honoraria from Genzyme (Sanofi); and he received travel expenses and speaker honoraria from PTC and BioMarin. H. Topaloglu reports no disclosures relevant to the manuscript. M. Miceli serves on the Solid Therapeutic Scientific Advisory Board for Gene Therapy and has patents issued or pending (US2012/053157 national phase/PCT patent filed: Identification of small molecules that enhance therapeutic exon skipping, Nelson/Miceli/Moran; UC- 2014-258-1, provisional patent filed, Calmodulin inhibitors enhance DMD exon 51 skipping in a patient cell line, Miceli/Nelson/Kendell). P. Furlong reports no disclosures relevant to the manuscript. J. Landry is an employee of Eli Lilly and possesses shares of Eli Lilly stock. R. Elashoff reports no disclosures relevant to the manuscript. D. Cox is an employee and stockholder of Eli Lilly and Company. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kinnett K, Rodger S, Vroom E, Furlong P, Aartsma-Rus A, Bushby K. Imperatives for DUCHENNE MD: a simplified guide to comprehensive care for Duchenne muscular dystrophy. PLoS Curr Epub 2015 Aug 7. [DOI] [PMC free article] [PubMed]
- 2.Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord 2014;24:482–491. [DOI] [PubMed] [Google Scholar]
- 3.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990;345:315–319. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. [DOI] [PubMed] [Google Scholar]
- 5.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 1995;82:743–752. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y, Thomas GD, Yue Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 2009;119:624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Palma C, Morisi F, Pambianco S, et al. Deficient nitric oxide signalling impairs skeletal muscle growth and performance: involvement of mitochondrial dysregulation. Skelet Muscle 2014;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froehner SC, Reed SM, Anderson KN, Huang PL, Percival JM. Loss of nNOS inhibits compensatory muscle hypertrophy and exacerbates inflammation and eccentric contraction-induced damage in mdx mice. Hum Mol Genet 2015;24:492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon Y, Balke JE, Madorma D, et al. Nitric oxide regulates skeletal muscle fatigue, fiber type, microtubule organization, and mitochondrial ATP synthesis efficiency through cGMP-dependent mechanisms. Antioxid Redox Signal 2017;26:966–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebolledo DL, Kim MJ, Whitehead NP, Adams ME, Froehner SC. Sarcolemmal targeting of nNOSµ improves contractile function of mdx muscle. Hum Mol Genet 2016;25:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 1996;98:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson MD, Rosenberry R, Barresi R, et al. Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J Physiol (London) 2015;593:5183–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hind limb muscle. Am J Physiol 1994;266:H920–H929. [DOI] [PubMed] [Google Scholar]
- 14.Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev 2016;96:253–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson MD, Rader F, Tang X, et al. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology 2014;82:2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sander M, Chavoshan B, Harris SA, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2000;97:13818–13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 1998;95:15090–15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech 2001;55:223–235. [DOI] [PubMed] [Google Scholar]
- 19.Adamo CM, Dai DF, Percival JM, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2010;107:19079–19083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One 2007;2:e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammers DW, Sleeper MM, Forbes SC, Shima A, Walter GA, Sweeney HL. Tadalafil treatment delays the onset of cardiomyopathy in dystrophin-deficient hearts. J Am Heart Assoc 2016;5:e003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawahara G, Karpf JA, Myers JA, Alexander MS, Guyon JR, Kunkel LM. Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2011;108:5331–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 2008;456:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi YM, Rader EP, Crawford RW, Campbell KP. Endpoint measures in the mdx mouse relevant for muscular dystrophy pre-clinical studies. Neuromuscul Disord 2012;22:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol 2012;228:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res 2003;92:554–560. [DOI] [PubMed] [Google Scholar]
- 27.Uaesoontrachoon K, Quinn JL, Tatem KS, et al. Long-term treatment with naproxcinod significantly improves skeletal and cardiac disease phenotype in the mdx mouse model of dystrophy. Hum Mol Genet 2014;23:3239–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet 2005;14:1921–1933. [DOI] [PubMed] [Google Scholar]
- 29.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 2001;155:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Yue Y, Li L, et al. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet 2013;22:3720–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin EA, Barresi R, Byrne BJ, et al. Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Sci Transl Med 2012;4:162ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pane M, Mazzone ES, Fanelli L, et al. Reliability of the performance of upper limb assessment in Duchenne muscular dystrophy. Neuromuscul Disord 2014;24:201–206. [DOI] [PubMed] [Google Scholar]
- 33.Henricson E, Abresch R, Han JJ, et al. The 6-minute walk test and person-reported outcomes in boys with Duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr 2013;5:ecurrents.md.9e17658b007eb79fcd6f723089f79e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayhew AG, Cano SJ, Scott E, et al. Detecting meaningful change using the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1046–1052. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GD, Ye J, De Nardi C, Monopoli A, Ongini E, Victor RG. Treatment with a nitric oxide-donating NSAID alleviates functional muscle ischemia in the mouse model of Duchenne muscular dystrophy. PLoS One 2012;7:e49350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forgue ST, Patterson BE, Bedding AW, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2006;61:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrishko RE, Dingemanse J, Yu A, et al. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol 2008;48:610–618. [DOI] [PubMed] [Google Scholar]
- 38.Witting N, Kruuse C, Nyhuus B, et al. Effect of sildenafil on skeletal and cardiac muscle in Becker muscular dystrophy. Ann Neurol 2014;76:550–557. [DOI] [PubMed] [Google Scholar]
- 39.Leung DG, Herzka DA, Thompson WR, et al. Sildenafil does not improve cardiomyopathy in Duchenne/Becker muscular dystrophy. Ann Neurol 2014;76:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willcocks RJ, Arpan IA, Forbes SC, et al. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul Disord 2014;24:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.