Abstract
Objective:
To test whether decline in specific cognitive domains associated with Alzheimer disease neuropathologic change (ADNC) is modified by co-occurrence of other neuropathologies such as Lewy body disease (LBD) or vascular brain injury (VBI).
Methods:
Data came from 1,603 autopsied participants evaluated at US Alzheimer's Disease Centers. Standardized z scores in memory, attention, language, and executive function were derived from neuropsychological test scores assessed at each annual visit. Multivariable linear mixed-effects models assessed associations between neuropathologies and longitudinal trajectories of domain scores.
Results:
Compared to other participants, those with ADNC + LBD generally had worse cognitive trajectories, particularly lower initial executive function and faster attention decline. Participants with ADNC + VBI typically had less impairment and slower decline. Interactions were significant between LBD and ADNC for memory (p = 0.046) and between VBI and ADNC for language (p = 0.03); decline was slower than expected if these neuropathologies acted additively on the rate of decline. In secondary models, these interactions were limited to those with high ADNC (but not intermediate ADNC). In a subset of 260 participants with data on microinfarct location, cortical and subcortical microinfarcts were associated with decline in memory, language, and executive function in those without ADNC, but this effect was reduced among those with ADNC.
Conclusions:
ADNC + LBD (but not ADNC + VBI) was associated with poorer executive function and attention compared to other pathology groupings. However, the effect of co-occurring pathologies on cognitive trajectories may depend on the severity of ADNC. Future studies using antemortem biomarkers should seek to replicate these neuropathologic observations.
Co-occurrence of Alzheimer disease neuropathologic change (ADNC), Lewy body disease (LBD), and vascular brain injury (VBI) is prevalent in autopsied older adults.1–4 These common neuropathologies are associated with premortem decline in specific cognitive domains.5,6 It is unclear whether neuropathologies interact to associate with cognitive decline in those with multiple co-occurring, namely, mixed neuropathologies.
In prior studies, ADNC as measured by Braak stage of neurofibrillary tangles, LBD as manifest by cortical Lewy bodies, and VBI as represented by gross and microscopic infarcts have been associated with lower cognitive function or cognitive decline in multiple domains.5,7 Two studies that reported testing interactions between ADNC and LBD or ADNC and VBI did not find significant interactions in their associations with cognitive decline.8,9 Prior studies may have been too small to detect significant interactions. In a large multicenter sample, we previously found that LBD and VBI interacted with ADNC in associations with overall clinical progression.10
In the present study, we used data from the National Alzheimer's Coordinating Center (NACC) on participants previously enrolled at US Alzheimer's Disease Centers (ADCs). We evaluated whether autopsied older adults with ADNC and co-occurring LBD or VBI had faster progression of impairment compared to those with single neuropathologies in 4 cognitive domains: memory, attention, language, and executive function. We tested whether the relationships between cognition and LBD or VBI were modified by co-occurrence of ADNC.
METHODS
Data sources and study populations.
Data came from the NACC Uniform Data Set on participants who had been prospectively evaluated, died, and were autopsied at 1 of ≈30 ADCs between September 2005 and September 2015. Participants were examined annually in person with a standard protocol, described in detail elsewhere.11,12 Neuropathologic data were collected following a standardized form on participants who died and consented to autopsy.
Autopsied participants were excluded on the basis of the following criteria: (1) rare cause of dementia such as Down syndrome, autosomal dominant genetic diseases, or frontotemporal lobar degeneration; (2) missing information on covariates or neuropathologic information on ADNC, LBD, or VBI; (3) no ADNC, LBD, or VBI but presence of other major pathologic burden such as hippocampal sclerosis, Braak stage V/VI with no/sparse neuritic plaques, frequent neuritic plaques but Braak stage 0 to II, or white matter disease; (4) presence of Braak stage V/VI with no/sparse neuritic plaques in those with LBD or VBI; and (5) no clinical visit proximal to death (last visit >2 years before death). Because participants with advanced dementia may be unable to complete neuropsychological tests, we excluded those with severe dementia at baseline, defined as a Clinical Dementia Rating Sum of Boxes score of 16 to 18.13,14 Participants missing all neuropsychological test scores at all visits also were excluded. Thus, 1,603 participants with at least 1 cognitive domain score remained for analyses (figure e-1 at Neurology.org).
Multiple microinfarcts may be the strongest vascular determinant of cognitive impairment,15,16 but number of infarcts was not collected for most participants. We abstracted additional data on the number of microinfarcts from neuropathology reports and conducted a subanalysis in ADC participants seen at the Oregon Health & Science University (OHSU) (n = 190) and University of Washington (UW) (n = 70) ADCs to address this limitation. These centers have a joint agreement to follow the same neuropathologic assessment protocol as part of the Pacific Northwest Dementia and Aging Neuropathology Group (PANDA). In this study, OHSU and UW ADCs are collectively referred to as PANDA ADCs hereafter.
Standard protocol approvals, registrations, and patient consents.
Participants provided written informed consent, and each ADC received institutional review board approval. This study was approved by the UW institutional review board.
Neuropathologic features.
ADCs follow consensus guidelines but conduct neuropathologic assessments according to their own protocols. Neuritic plaque density was defined by the Consortium to Establish a Registry for Alzheimer's Disease with scores of none, sparse, moderate, or frequent.17 Tau neurofibrillary pathology was measured with Braak stage: none, I to II, III/IV, and V/VI.18 ADNC was defined as moderate/frequent plaques and Braak stage III to VI. In a secondary analysis, we further categorized level of ADNC as intermediate (Braak stage III/IV) and high (Braak stage V/VI) among those with moderate/frequent plaques. Lewy bodies assessment and classification of LBD subtype followed guidelines.19 We categorized LBD as present in any brain region examined or absent. Presence of any VBI was defined as any gross infarcts or cortical microinfarcts. In NACC, the presence of cortical microinfarcts (infarcts in the cortex only seen microscopically), gross infarcts, or both was recorded regardless of the age of the infarcts. In the PANDA ADCs, the number of cortical and subcortical microinfarcts was recorded separately as 0, 1, 2, 3, or 4 or more following methods developed in the Honolulu Asia Aging Study.20 Other pathologies recorded included hippocampal sclerosis, white matter disease (subcortical leukoencephalopathy and/or white matter rarefaction), and severity (none, mild, moderate, severe) of cerebral amyloid angiopathy, atherosclerosis, and arteriolosclerosis. Participants were defined as having a low level of neuropathology if they were without ADNC, LBD, VBI, or other major neuropathologic burden.
Cognitive function in specific domains.
The Uniform Data Set neuropsychological test battery comprised 8 tests (12 measures) administered at each in-person visit.21 Cognitive function was quantified in 4 previously characterized domains: memory (episodic memory), attention (and working memory), language, and executive function.22 Memory tests included the Logical Memory Story A immediate and delayed recall.23 The attention domain included Digit Span Forward and Backward tests.23 Language tests included animals and vegetables list generation24 and the Boston Naming Test.25 The executive function domain was measured by the Digit Symbol26 and Trail Making Test Parts A and B.27 To create domain-specific scores, tests were converted into z scores and averaged. The z scores were calculated by subtracting individual raw scores from the mean and dividing by the SD of scores at the initial visit for all autopsied and nonautopsied participants. Scores for each domain can be interpreted as the units of SDs from the baseline average score in all NACC participants. If ≥1 test was missing, the corresponding domain was considered missing for that participant's visit.
Covariates.
Demographic characteristics included in the analyses were age, sex, education, and race/ethnicity, as well as ADC. History of comorbidities such as stroke was evaluated during each clinical visit. APOE genotyping was performed on consenting participants. APOE ε4 allele status was classified as at least 1 or none. At all ADCs, either a single clinician or a consensus group of clinicians made a diagnosis at each visit of normal cognition, impaired but not mild cognitive impairment, mild cognitive impairment, or dementia.
Statistical analyses.
To model longitudinal trends in cognitive domains and to test associations with neuropathologies, we used multivariable regression modeling via linear mixed-effects models following methods previously described.10 Briefly, the primary outcome was the z score for each cognitive domain at each visit, modeled as a continuous measure. Models included random intercepts for both participants and ADC and random slopes for participants. Primary predictors were dichotomous variables for ADNC, LBD, VBI, and time. Interaction terms between time and each primary neuropathology term and 3-way interactions of ADNC × LBD × time and ADNC × VBI × time investigated differences in mean annual rate of change in each domain score by neuropathology groupings. Empiric plots with natural cubic splines for time suggested that change in domain scores over time was approximately linear. Because of concern that trends were driven by those with dementia at enrollment but may differ in less severe clinical stages, we also examined estimates of decline in those without dementia at baseline (n = 704). Because the findings did not differ substantially and the sample size was small, we present findings for the overall sample. Primary models included adjustment for potential confounders: age at death, sex, race/ethnicity, education, and interval between last visit and death. In a secondary analysis, we examined whether any significant interactions between ADNC and LDB or VBI differed by severity of ADNC. In this model, interactions with LBD or VBI were assessed for intermediate and high ADNC separately. In PANDA participants, we reran models with number of cortical and subcortical microinfarcts separately as primary predictors in place of the dichotomous measure of VBI. Finally, because participants may have missing data due to severe impairment, we conducted a sensitivity analysis to evaluate the effect of missing data on our primary findings. We reran primary analyses with missing test scores imputed with the worst possible score if the participant had severe dementia (Clinical Dementia Rating Sum of Boxes score = 16–18) or if a cognitive or behavior problem was listed as the reason for missing data. In an attempt to account for potential selection bias due to studying only autopsied participants, we used inverse probability weighting.28 This allowed us to generalize findings back to the overall study sample.29 Bias-corrected and accelerated bootstrap confidence intervals were calculated to reflect uncertainty attributable to the estimated weights.30 Analyses were conducted with R (version 3.2.1).31 All tests were 2 sided with α = 0.05.
RESULTS
Participant characteristics.
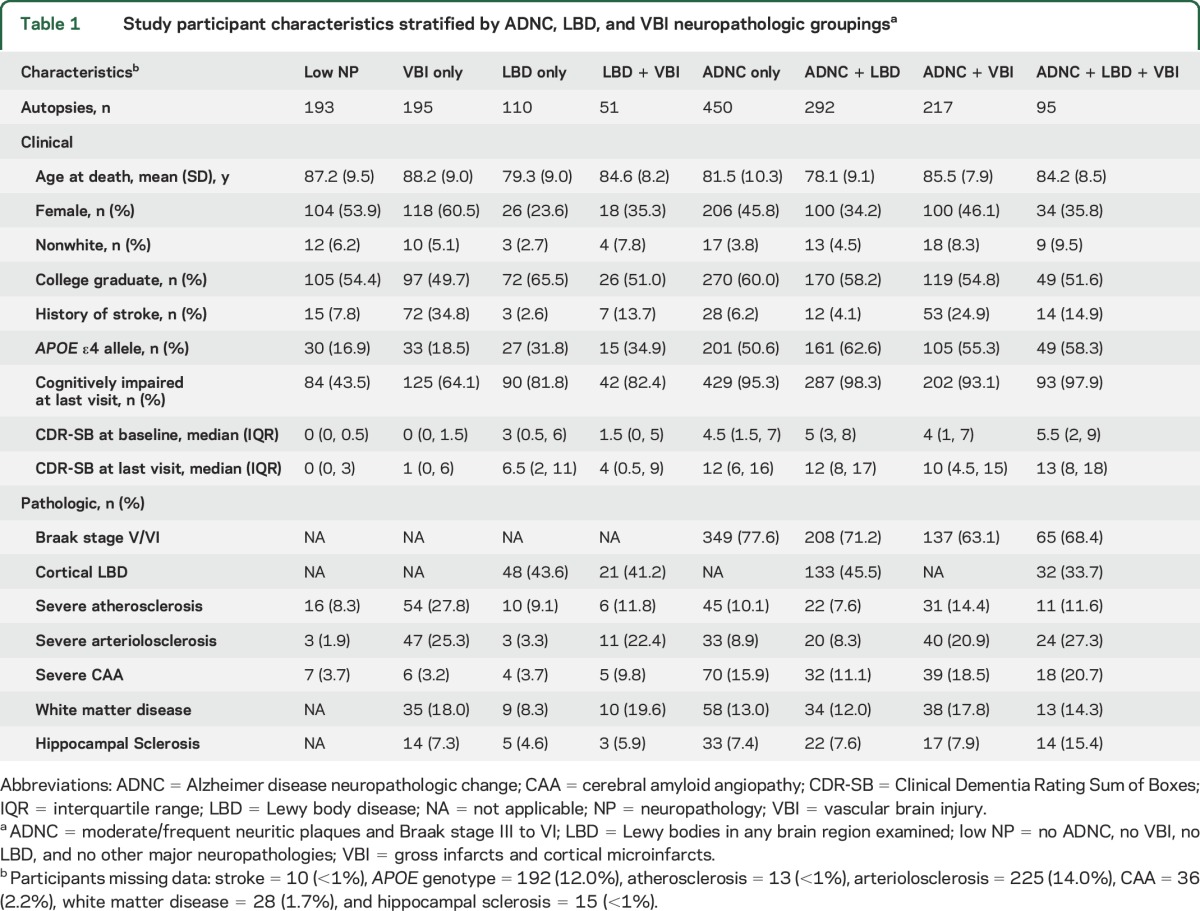
Among 1,603 autopsied Uniform Data Set participants, the mean age at death was 83.0 years (SD 9.9 years), the average interval between last clinical visit and death was 9.4 months (SD 6.1 months), and the median follow-up of participants with ≥2 clinical visits (n = 1,374) was 3.3 years (interquartile range 2.1–5.1 years). The number of participant visits that contributed to analyses for each cognitive domain score is shown from the last visit backward (table e-1). ADNC, LBD, and VBI and their co-occurrence were prevalent findings at autopsy (figure 1); 65.8% of participants had ADNC, of whom 36.7% had co-occurring LBD and 29.6% had co-occurring VBI. Characteristics of participants grouped by ADNC, LBD, and VBI neuropathologies are described in table 1. Unadjusted initial and last visit cognitive domain scores differed significantly by neuropathology grouping (all p < 0.001 with Kruskal-Wallis tests). Relatively few participants had LBD + VBI or ADNC + LBD + VBI; these groupings were not examined separately in analytic models.
Figure 1. Co-occurrence of Alzheimer disease neuropathologic change (ADNC), Lewy body disease (LBD), and vascular brain injury (VBI) in study participants (n = 1,603).
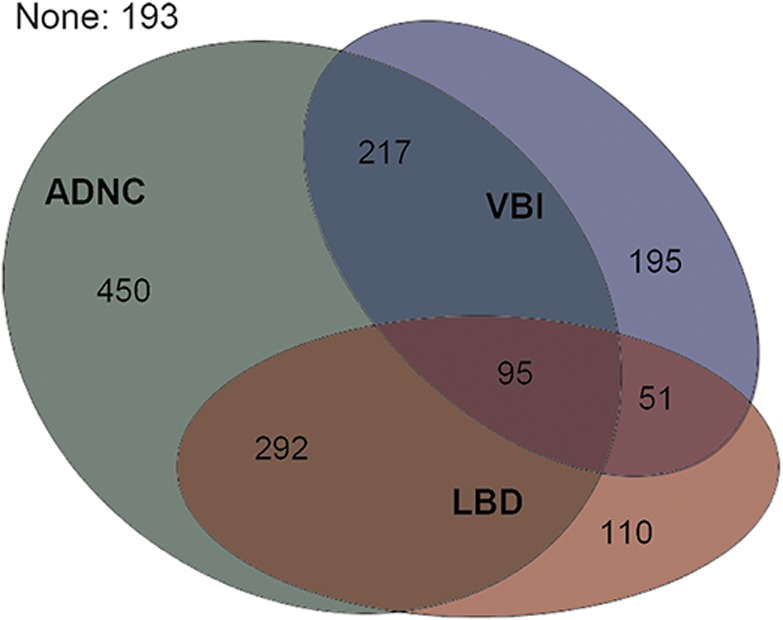
ADNC = moderate/frequent neuritic plaques and Braak stage III to VI; LBD = Lewy bodies in any brain region examined; VBI = gross infarcts and cortical microinfarcts.
Table 1.
Study participant characteristics stratified by ADNC, LBD, and VBI neuropathologic groupingsa
Associations with cognitive domains.
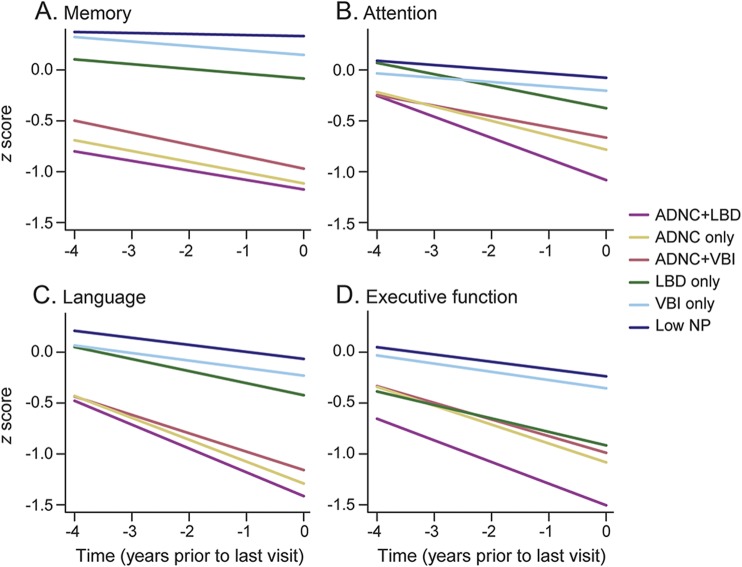
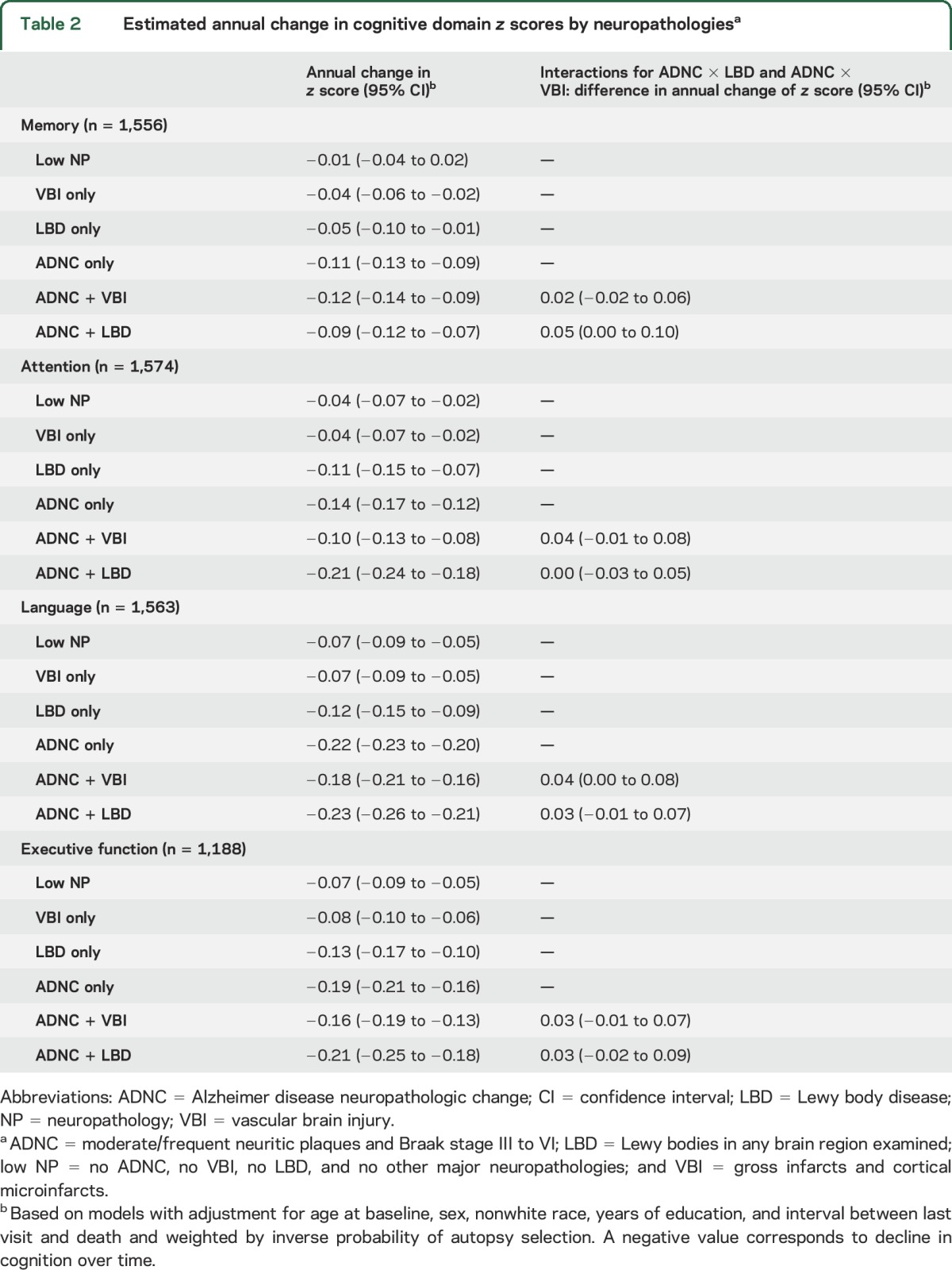
Participants with ADNC + LBD were generally the most impaired across follow-up in all domains, especially for attention and executive function (figure 2); differences in memory and executive function were present even 4 years before death. Participants with ADNC + VBI had trajectories similar to those with ADNC only, although they were slightly less impaired by the last visit in memory, attention, and language (figure 2); differences in memory were present up to 4 years before death. Trajectories across follow-up were worse for those with any ADNC (single or mixed) compared to those without ADNC. Rates of decline by neuropathology grouping and estimated interactions between ADNC and LBD or VBI are shown in table 2 for each cognitive domain. Rates of decline were the fastest for ADNC + LBD in all domains but memory. Participants with ADNC + LBD and ANDC + VBI tended to have slower estimated rate of decline than would be expected in an additive model in which each pathology contributes to decline independently. Only the interactions between ADNC and LBD for memory and ADNC and VBI for language were significant. In secondary models using a categorical measure for ADNC level, these interactions were found for those with high ADNC (p = 0.03 for ADNC × LBD for memory and p = 0.06 for ADNC × VBI for language), not intermediate ADNC (p = 0.5 for ADNC × LBD for memory and p = 0.6 for ADNC × VBI for language). Cognitive and behavior problems were listed as the primary reason for missing neuropsychological test scores (table e-2). Findings were similar in models that did not include weighting for autopsy selection and in models with missing neuropsychological test scores imputed as the worst score, although rates of decline tended to be slightly faster than in primary models (table e-3).
Figure 2. Model-based population mean trajectories of cognitive domain z scores: (A) memory, (B) attention, (C) language, and (D) executive function.
There were significant interactions (A) between LBD and ADNC for memory (p = 0.046) and (C) between VBI and ADNC for language (p = 0.03) in which decline was slower than expected if these neuropathologies acted additively on the rate of decline. ADNC = Alzheimer disease neuropathologic change (moderate/frequent neuritic plaques and Braak III–VI); LBD = Lewy body disease (Lewy bodies in any brain region examined); low NP = low neuropathology (no ADNC, no VBI, no LBD, and no other major neuropathologies); VBI = vascular brain injury (any gross infarcts or cortical microscopic infarcts).
Table 2.
Estimated annual change in cognitive domain z scores by neuropathologiesa
Microinfarcts and cognitive domains in PANDA ADCs.
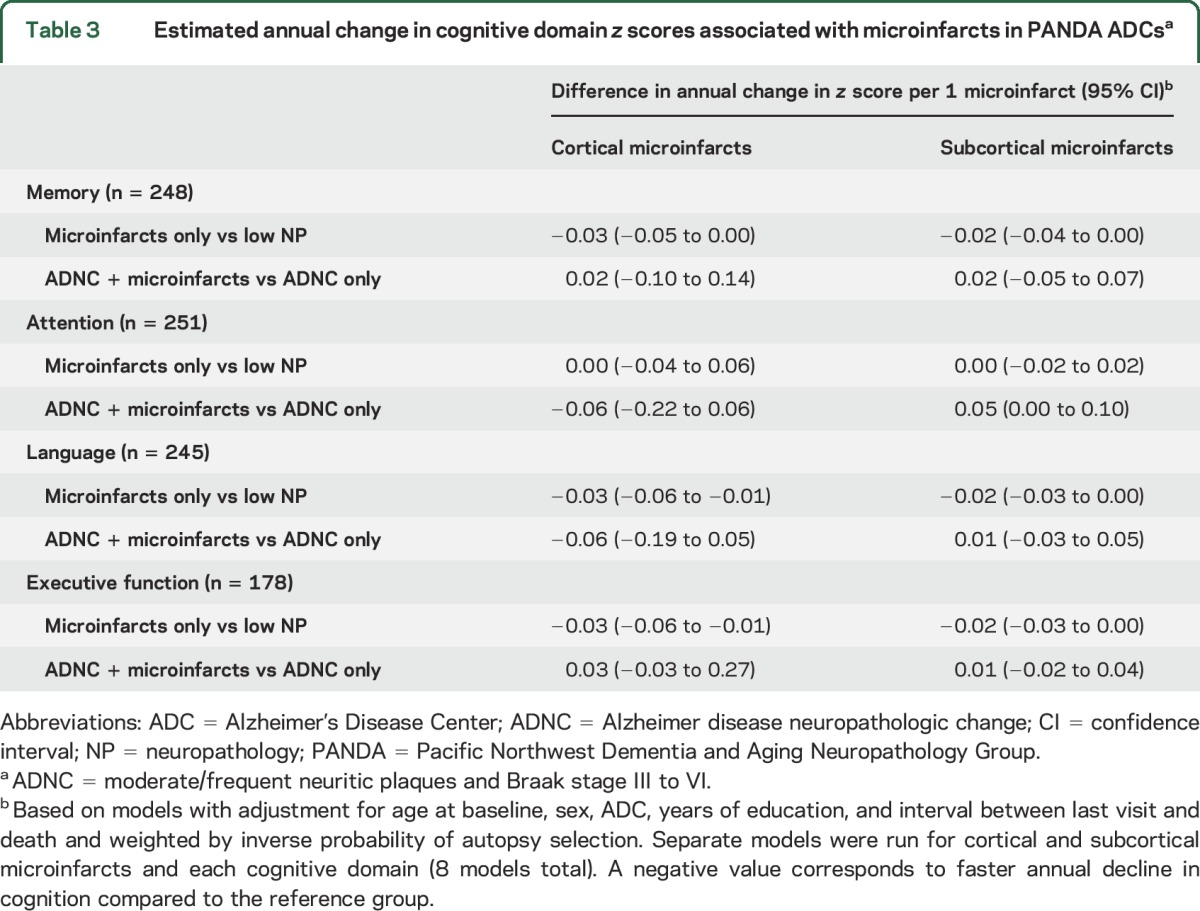
Autopsied PANDA ADC participants (n = 260) were slightly older with a lower prevalence of dementia than other NACC participants but a higher prevalence of VBI (table e-4). ADNC was present in 48.0% of participants; 19.5% had co-occurring cortical microinfarcts, and 33.3% had co-occurring subcortical microinfarcts. Decline in memory, language, and executive function (but not attention) was faster for those with increasing number of cortical and subcortical microinfarcts without ADNC compared to those with low neuropathology (table 3). Among those participants with ADNC, microinfarcts generally were not associated with faster decline. There was a trend for faster decline in those with ANDC + cortical microinfarcts compared to those with ADNC only for attention and language.
Table 3.
Estimated annual change in cognitive domain z scores associated with microinfarcts in PANDA ADCsa
DISCUSSION
We examined late-life trajectories of memory, attention, language, and executive function in participants with single and mixed neuropathologies. We allowed for interactions between neuropathologies in estimating trajectories, and we focused on combinations of ADNC, LBD, and VBI. Impairment preceding death was greatest for those with ADNC + LBD in all domains, especially executive function and attention. We found significant antagonistic interactions between ADNC and LBD in association with decline in memory and between ADNC and VBI in association with decline in language. These interactions, in which decline was slower than expected by an additive model, were limited to those with high ADNC in secondary models. Pathologies were associated in an additive manner for those with intermediate ADNC. Increasing number of both cortical and subcortical microinfarcts was associated with faster decline in memory, executive function, and language compared to those with low neuropathology. However, trajectories of those with ADNC + VBI or ADNC + microinfarcts tended to be similar or slightly slower than the trajectories for those with ADNC only.
Together, these findings suggest that although ADNC, VBI, and LBD are individually associated with decline in multiple cognitive domains, co-occurring pathologies do not always act additively on the rate of decline in those with multiple pathologies. Similar to our previous findings on overall clinical progression,10 we found unexpected antagonistic interactions between ADNC and LBD or VBI. In secondary models, interactions were limited to those with high ADNC, and trajectories were diverged further between those with mixed and single pathologies in those with intermediate ADNC. Although we cannot infer whether LBD or VBI biologically interacts with ADNC, our findings may suggest that ADNC clinically overwhelms the effect of additional neuropathologies in those with high ADNC. In other studies involving participants with dementia and high ADNC, decline was similar in those with single or mixed pathologies.32,33 Community-based studies that reported testing interactions have not found significant interactions between ADNC and LBD8 or ADNC and VBI,9 perhaps because of smaller sample sizes or study sample differences.
We found several patterns that differed between single and mixed pathologies. All participants with ADNC were more severely impaired across follow-up than those with low neuropathology, VBI only, or LBD only. Those with ADNC + LBD had worse impairment, especially in attention and executive function. Meanwhile, those with ADNC + VBI had slower decline in attention and language and less impairment in memory than those with ADNC only or ADNC + LBD, suggesting that they had a less severe clinical disease course proximal to death. This may be the result of selection against those with significant cerebrovascular disease at enrollment and because those with co-occurring VBI tended to have lower levels of ADNC.33 Findings in PANDA ADC participants and other studies7,20,34 suggest that associations with decline may be detectable only in those with multiple VBIs, particularly multiple microinfarcts. However, in this study, rates of decline between those with and without microinfarcts generally were similar among those with ADNC. Additional research may be needed to further investigate the effect of cumulative VBI in ADNC. Community-based studies have found mixed neuropathologies, in general, to be positively associated with dementia.1,2,4,35
This study has limitations. Level of cognitive impairment differed between pathology groupings at enrollment, but because time in our analysis was modeled backward from the last visit, we were limited in ability to account for these differences. Most participants enrolled with dementia, but we did not find evidence for different trends in those without dementia at baseline. However, studies with follow-up from symptom onset may show further divergence in rates of decline, similar to findings in those with intermediate ADNC, or nonlinear trends, as found in another study.36 However, trajectories can be interpreted with respect to last visit because all participants are anchored to death. We used composite domain scores; this reduces but does not eliminate potential for floor/ceiling effects in estimated rates of decline. In addition, because the number of observations differed between domain scores, comparisons of specific values between domains may not be appropriate. Many participants were missing data on neuropsychological test scores once they developed severe dementia. However, we used a modeling approach, linear mixed-effects modeling, which is valid when missingness can be predicted entirely on observed variables (i.e., missing at random).37,38 In addition, results of our sensitivity analyses were similar to those of primary analyses. Because neuropathologic assessments are conducted at autopsy, findings may not reflect the burden of neuropathology when clinical progression was measured; participants without a clinical visit proximal to death were excluded, which may help reduce this issue.
The study also has strengths. We used a large dataset of standardized clinical evaluations that enhanced power to detect statistical interactions. Our modeling approach allowed us to characterize average differences in overall trajectories before death between participants with mixed neuropathology and those with individual neuropathologies. We used standardized composite measures of cognitive domains to identify patterns of functioning associated with different neuropathology groupings. We conducted a variety of sensitivity analyses to assess model assumptions and to explore potential biases. We also investigated associations of microinfarcts in PANDA ADC participants whose brains underwent the same neuropathologic assessment protocols. Finally, we attempted to account for potential selection bias due to autopsy sampling.
Rates of decline in memory, attention, language, and executive function were associated with specific neuropathologic groupings. Trajectories of those with single and mixed neuropathologies differed from those of individuals with ADNC only across multiple domains. Those with ADNC + LBD tended to have faster decline and impairment across follow-up in attention and executive function. Baseline performance was worse for those with ADNC + LBD, more in executive function compared to other domains, which could have affected estimated trajectories. Future studies using antemortem biomarkers are needed to verify these patterns. In some domains, decline in those with mixed ADNC was slower than would be expected by additive or independent models, especially in those with high ADNC. The effects of additional neuropathologic burden may vary across the continuum of ADNC. These findings highlight complex but broad associations of common neuropathologies with cognitive decline in individuals with single and mixed neuropathologies.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to all the study participants, clinicians, and other workers at the ADCs and NACC who made this research possible. They also thank NACC, UW, and OHSU staff, including Allison Beller, Robin Guariglia, Lilah Besser, and Mark Bollenbeck, for help obtaining data. Preliminary versions of this work were included in a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy from the Department of Epidemiology at UW (W.D.B.). Analyses were run using the computer simulation cluster at the UW Center for Studies in Demography and Ecology.
GLOSSARY
- ADC
Alzheimer's Disease Centers
- ADNC
Alzheimer disease neuropathologic change
- LBD
Lewy body disease
- NACC
National Alzheimer's Coordinating Center
- OHSU
Oregon Health & Science University
- PANDA
Pacific Northwest Dementia and Aging Neuropathology Group
- UW
University of Washington
- VBI
vascular brain injury
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Willa D. Brenowitz: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis, study supervision. Rebecca A. Hubbard: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, study supervision. C. Dirk Keene: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Stephen E. Hawes: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. W.T. Longstreth, Jr: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Randy L. Woltjer: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data. Walter A. Kukull: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
The NACC database is funded by NIA/NIH grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). PANDA ADCs are supported by NIH P50 AG005136 (UW ADC); NIH grants P30 AG008017, R01 AG024059, M01 RR000334, and UL1 RR024140; Intel Corp; and Department of Veterans Affairs (OHSU ADC).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 2.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology 2015;85:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 2014;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging studies. Neurology 2016;86:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol 2011;68:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA. Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci 2013;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholerton B, Larson EB, Baker LD, et al. Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis 2013;36:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 2012;135:3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement 2017;13:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18:270–277. [PubMed] [Google Scholar]
- 12.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–258. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.O'Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's Research Consortium Study. Arch Neurol 2008;65:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 2007;62:406–413. [DOI] [PubMed] [Google Scholar]
- 16.Corrada MM, Sonnen JA, Kim RC, Kawas CH. Microinfarcts are common and strongly related to dementia in the oldest-old: the 90+ Study. Alzheimers Dement 2016;12:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 20.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 21.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden KM, Jones RN, Zimmer C, et al. Factor structure of the National Alzheimer's Coordinating Centers uniformdataset neuropsychological battery: an evaluation of invariance between and within groups over time. Dis Assoc Disord 2011;25:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 24.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S, Goodglass H. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 26.Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale–Revised. New York: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 27.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 28.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiol 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1994. [Google Scholar]
- 31.R Core Team. R: A Language and Environment for Statistical Computing, version 3.2.1 [online]. Vienna: R Foundation for Statistical Computing; 2015. Available at: http://www.R-project.org/. Accessed June 2015. [Google Scholar]
- 32.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet 1999;354:919–920. [DOI] [PubMed] [Google Scholar]
- 33.Pillai JA, Butler RS, Bonner-Jackson A, Leverenz JB. Impact of Alzheimer's disease, Lewy body and vascular co-pathologies on clinical transition to dementia in a national autopsy cohort. Dement Geriatr Cogn Disord 2016;42:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montine TJ, Sonnen JA, Montine KS, Crane PK, Larson EB. Adult Changes in Thought Study: dementia is an individually varying convergent syndrome with prevalent clinically silent diseases that may be modified by some commonly used therapeutics. Curr Alzheimer Res 2012;9:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013;74:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis, 2nd ed. Hoboken: Wiley; 2012. [Google Scholar]
- 38.Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York: Wiley; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.