Abstract
Objective:
To explore the association between metabolic syndrome and the Unified Parkinson’s Disease Rating Scale (UPDRS) scores and, secondarily, the Symbol Digit Modalities Test (SDMT).
Methods:
This is a secondary analysis of data from 1,022 of 1,741 participants of the National Institute of Neurological Disorders and Stroke Exploratory Clinical Trials in Parkinson Disease Long-Term Study 1, a randomized, placebo-controlled trial of creatine. Participants were categorized as having or not having metabolic syndrome on the basis of modified criteria from the National Cholesterol Education Program Adult Treatment Panel III. Those who had the same metabolic syndrome status at consecutive annual visits were included. The change in UPDRS and SDMT scores from randomization to 3 years was compared in participants with and without metabolic syndrome.
Results:
Participants with metabolic syndrome (n = 396) compared to those without (n = 626) were older (mean [SD] 63.9 [8.1] vs 59.9 [9.4] years; p < 0.0001), were more likely to be male (75.3% vs 57.0%; p < 0.0001), and had a higher mean uric acid level (men 5.7 [1.3] vs 5.3 [1.1] mg/dL, women 4.9 [1.3] vs 3.9 [0.9] mg/dL, p < 0.0001). Participants with metabolic syndrome experienced an additional 0.6- (0.2) unit annual increase in total UPDRS (p = 0.02) and 0.5- (0.2) unit increase in motor UPDRS (p = 0.01) scores compared with participants without metabolic syndrome. There was no difference in the change in SDMT scores.
Conclusions:
Persons with Parkinson disease meeting modified criteria for metabolic syndrome experienced a greater increase in total UPDRS scores over time, mainly as a result of increases in motor scores, compared to those who did not. Further studies are needed to confirm this finding.
ClinicalTrials.gov identifier:
Metabolic syndrome is a combination of conditions—hypertension, hyperglycemia, hyperlipidemia, and increased waist circumference—that, when occurring together, escalate a person's risk for heart disease, stroke, and diabetes mellitus. Recent studies suggest that the syndrome is also associated with increased risk of other diseases,1–8 including Parkinson disease (PD).1,9 However, studies on the association of metabolic syndrome10 or its components,11–20 e.g., hyperglycemia or diabetes mellitus, and PD have yielded inconsistent results. For example, 2 recent meta-analyses of the association of diabetes mellitus and the risk of developing PD had opposite conclusions: one that diabetes mellitus increases the risk of PD21 and another that it does not.22 Higher body mass index (BMI) in midlife, i.e., >25 kg/m2, has been associated with an increased risk of PD in multiple studies16,18,23 but not in others.19,24 A recent study found that patients with PD with increasing BMI had slower PD progression than those with a stable or declining BMI25 as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS). Another study reported that diabetes mellitus was associated with more rigidity and a parkinsonian-type gait in aging persons without a diagnosis of PD or dementia.26
To the best of our knowledge, the effect of metabolic syndrome on PD progression has not previously been studied. The aim of this study was to investigate the relationship between metabolic syndrome and progression of PD using change in UPDRS. Because metabolic syndrome may have a role in driving cognitive impairment in PD, we also explored the association of metabolic syndrome and a cognitive measure. Using data from the National Institute of Neurological Disorders and Stroke Exploratory Trials in PD Long-Term Study 1 (NET-PD LS 1),27 we compared the progression of PD in those who had metabolic syndrome throughout the first 3 years of the trial to those who were without evidence of metabolic syndrome.
METHODS
Participants.
NET-PD LS 1 was a large, multicenter, placebo-controlled, randomized, double-blind trial of 10 mg creatine monohydrate vs placebo and was conducted from March 2007 to September 2013. The study was terminated early, when an interim analysis determined that creatine had no effect on progression of PD.28 All participants had early-stage PD and were on dopaminergic therapy at randomization. The study design and characteristics of the population have been reported previously.27
The NET-PD LS 1 study enrolled a total of 1,741 participants. Each participant was categorized as having metabolic syndrome or not at baseline and 1-, 2-, and 3-year visits. As a result of missing data at any of these visits, we were unable to assess the metabolic syndrome status of 319 participants. Furthermore, only those who maintained the same metabolic syndrome status for consecutive visits were included. Four hundred participants experienced a change in their metabolic syndrome status during the first 3 years of the study and were excluded from the analysis. Therefore, of the 1,741 participants at baseline, 1,022 were included in the final analysis.
Standard protocol approvals, registrations, and patient consents.
NET-PD LS 1 is registered on clinicaltrials.gov with identifier NCT00449865. The institutional review boards of each institution that participated approved the study, and all participants signed informed consent.
Outcome measures.
As part of NET-PD LS-1, UPDRS scores for parts I, II, and III were assessed over time. Three outcome measures were used for this study from baseline to the 3-year visit. The primary outcome measure was the change in the total UPDRS score (which we define here as parts I + II + III) from the baseline (randomization) to the 3-year visit. The secondary outcome measure was the change in the motor UPDRS (part III) over the same time range. An additional outcome measure was the Symbol Digit Modalities Test (SDMT) score,29 which was the only cognitive test collected at annual visits.
Exposure.
We categorized participants on the basis of the commonly agreed-on criteria from the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), which was updated in 2005.30 The NCEP criteria require 3 or more of the following to be diagnosed with metabolic syndrome: (1) waist circumference >35 in for women and >40 in for men, (2) serum triglycerides ≥150 mg/dL (1.7 mmol/L) or medication therapy for high triglycerides, (3) serum high-density lipoprotein cholesterol <50 mg/dL (1.3 mmol/L) in women and <40 mg/dL (1 mmol/L) in men or medication treatment for low high-density lipoprotein cholesterol, (4) blood pressure ≥130/85 mm Hg or on medication therapy for high blood pressure, and (5) fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or on medication therapy for high glucose.
Because the protocol for NET-PD LS 1 did not include waist measurements or collection of cholesterol, triglyceride, or fasting glucose levels, we adapted the ATP III criteria30 for use in our study.30 Metabolic syndrome was defined as having 2 or more of the following criteria: (1) BMI >30 kg/m2; (2) on statin medication; (3) systolic blood pressure >130 mm Hg, diastolic blood pressure >85 mm Hg, or on antihypertensive medication; or (4) random blood sugar >120 mg/dL or on antihyperglycemic medication. Criteria were the same for both sexes. Participants who no longer fulfilled our modified criteria at subsequent visits were excluded from the analysis to avoid misclassification bias. Using these modified ATP III criteria, participants were classified into 2 groups: participants who had metabolic syndrome throughout the 3 years of the study and participants who were without evidence of metabolic syndrome across all 3 years.
Statistical methods.
Descriptive statistics (mean, SD, frequency, and percentages) were used to summarize the demographics for 2 groups: metabolic syndrome and no metabolic syndrome. Differences in the mean or proportions between these 2 groups were checked by t test or χ2 test.
The association of metabolic syndrome with the change in the total and motor UPDRS and SDMT scores across time was estimated with a linear mixed model. Because the randomization process included blocking by site, both site and treatment assignment (creatine vs placebo) were used as covariates.27
Metabolic syndrome, time in years, treatment group, and the interaction term of metabolic syndrome and time in years were tested in this model after adjustment for confounding variables: baseline age, baseline total UPDRS, sex, handedness, race, uric acid levels, and disease duration at baseline. Similarly, a second model was adjusted for motor UPDRS score, and the only difference was the covariate baseline motor UPDRS instead of total UPDRS score as stated before. A locally weighted scatterplot smoothing plot for change in total UPDRS across time is presented to show the differences by group. The SDMT analysis, using change in SDMT from baseline as the response variable, predictors of baseline SDMT, metabolic syndrome status, time in years, and the interaction term of metabolic syndrome and time in years, was adjusted for confounding variables of baseline age, total UPDRS score, sex, handedness, race, uric acid levels, and disease duration.
All statistical analyses were conducted with SAS statistical software (version 9.4, SAS Institute Inc, Cary, NC).
Sensitivity analyses.
We considered alternative ways of analyzing the current dataset. We looked at the entire NET-PD LS1 cohort, dividing the metabolic vs no metabolic syndrome groups according to their status at baseline, and followed them for their entire participation in the study, up to 5 years, without regard to whether they changed status at any annual visit. We also ran the analyses with a stricter definition of metabolic syndrome, defining metabolic syndrome as having 3 or more of the 4 criteria rather than 2 or more as in the presented data. Furthermore, we ran the analyses with different criteria for metabolic syndrome, considering that if participants were taking an antihypertensive, antihyperlipidemia, or antihypergylcemic medication, they would not meet that criterion for metabolic syndrome because the indication was adequately treated. Each of these sensitivity analyses produced the same results. Because the results were the same as those presented here, they are not shown, but they are available on request from the authors.
RESULTS
Table 1 shows the demographic characteristics of the participants for this study according to whether participants were categorized as having metabolic syndrome. Baseline mean age and mean uric acid levels were significantly different in these 2 groups, as well as the proportion of men and women in these 2 groups. Participants with metabolic syndrome were more likely to be men, to be older, and to have a higher mean uric acid level compared to those without metabolic syndrome.
Table 1.
Baseline characteristics of participants (n = 1,022) by metabolic syndrome status
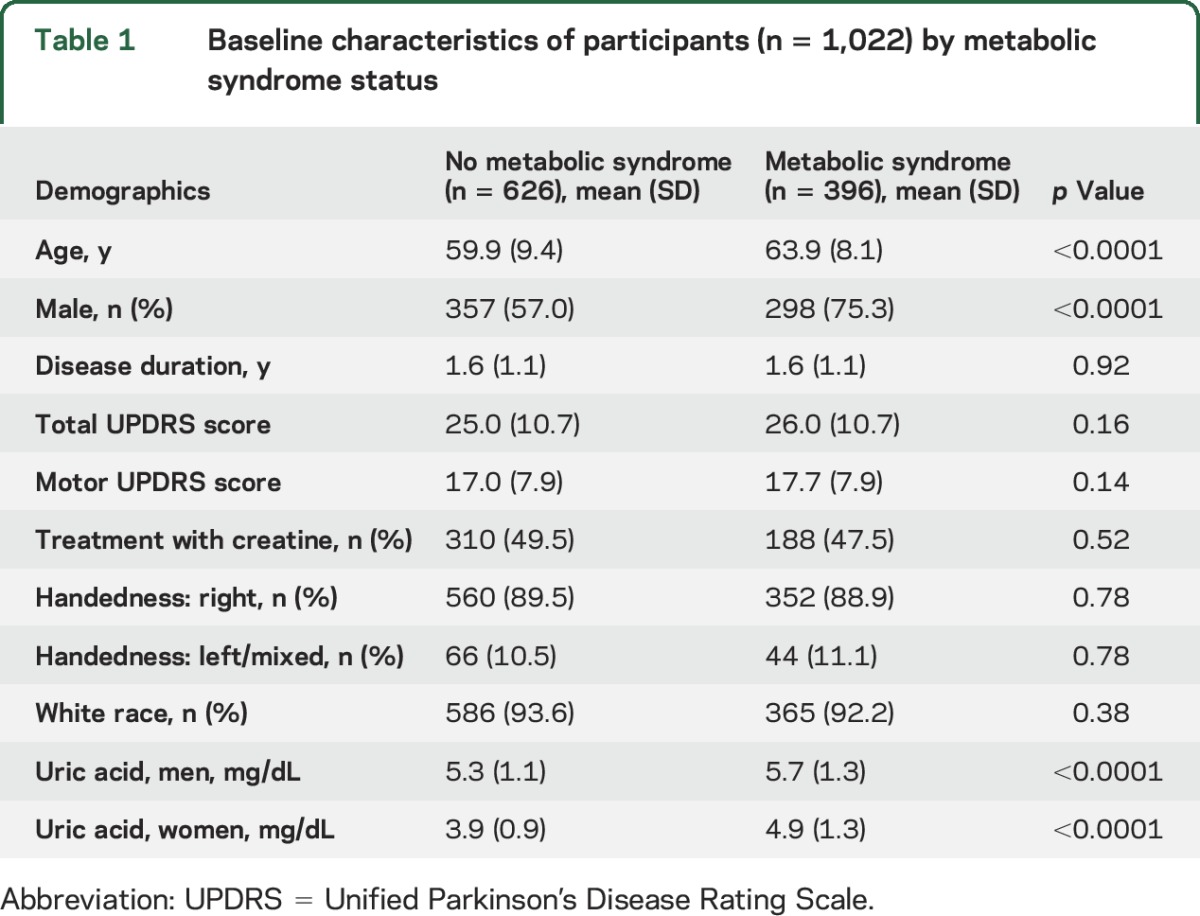
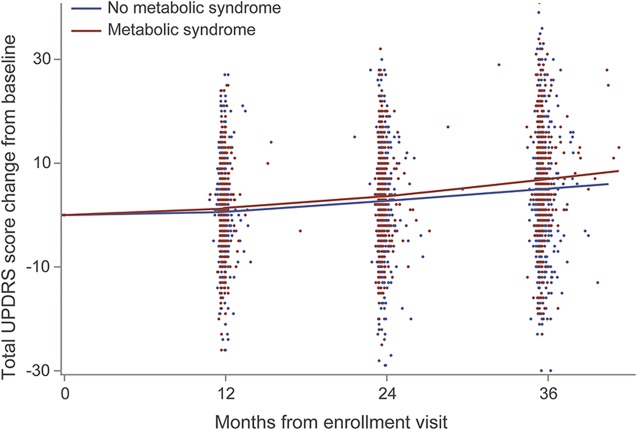
Table 2 shows the change in total UPDRS over 3 years compared between the metabolic syndrome and no metabolic syndrome groups. On average, participants without metabolic syndrome experienced a 1.7-unit annual increase in total UPDRS score from their baseline values, while participants with metabolic syndrome experienced a 2.3- (1.7 + 0.6) unit annual increase in total UPDRS change from baseline after controlling for covariates. This information is also presented in the figure, which demonstrates that participants with metabolic syndrome were more likely to have increases in total UPDRS scores, especially in the third year of study.
Table 2.
Change in total UPDRS
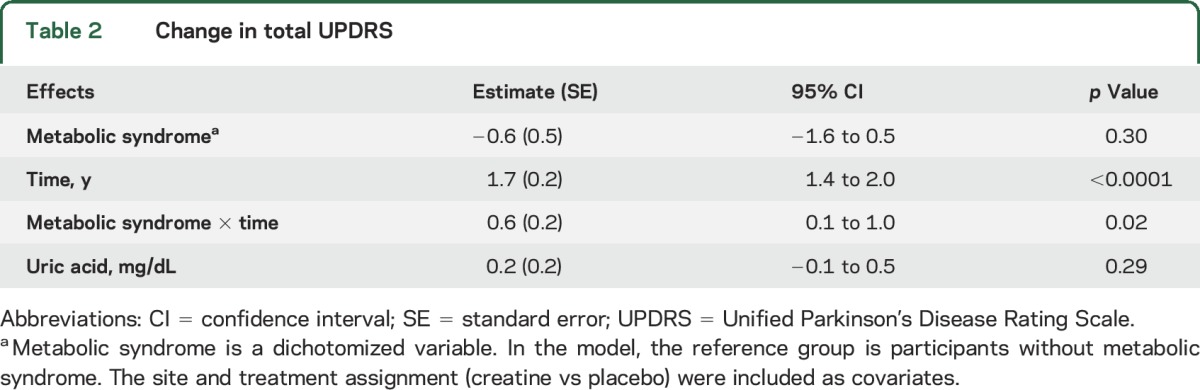
Figure. Locally weighted scatterplot smoothing plot for change in total UPDRS.
Sample size: 1,022 (assuming that the duration of 1 month is 30.5 days). UPDRS = Unified Parkinson’s Disease Rating Scale.
Table 3 shows the change in motor UPDRS (part III) score, the secondary outcome measure, over 3 years compared between the 2 groups. On average, participants without metabolic syndrome experienced a 0.8-unit annual increase in their motor UPDRS score, while participants with metabolic syndrome experienced a 1.3- (0.8 + 0.5) unit increase per year.
Table 3.
Change in motor UPDRS
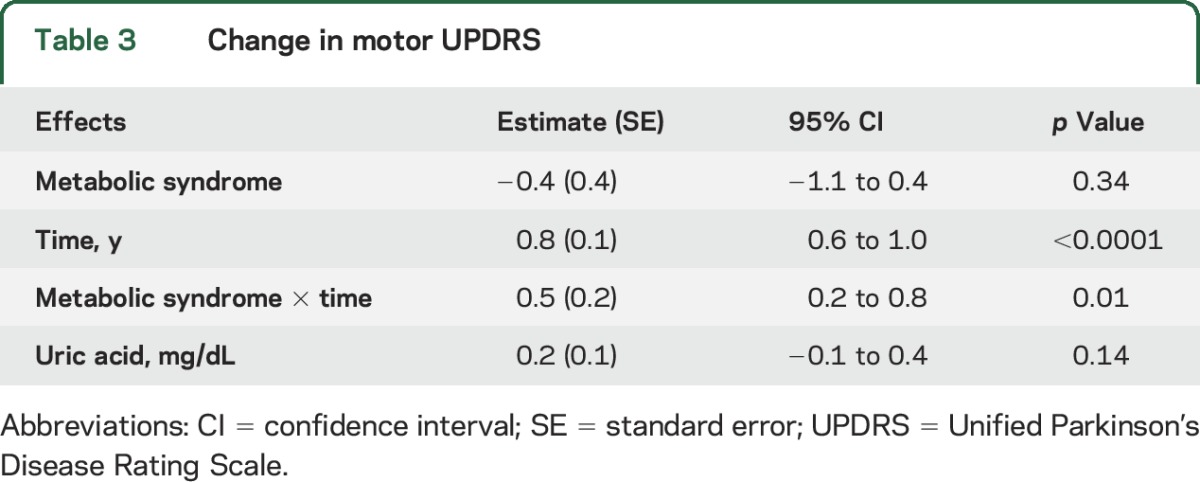
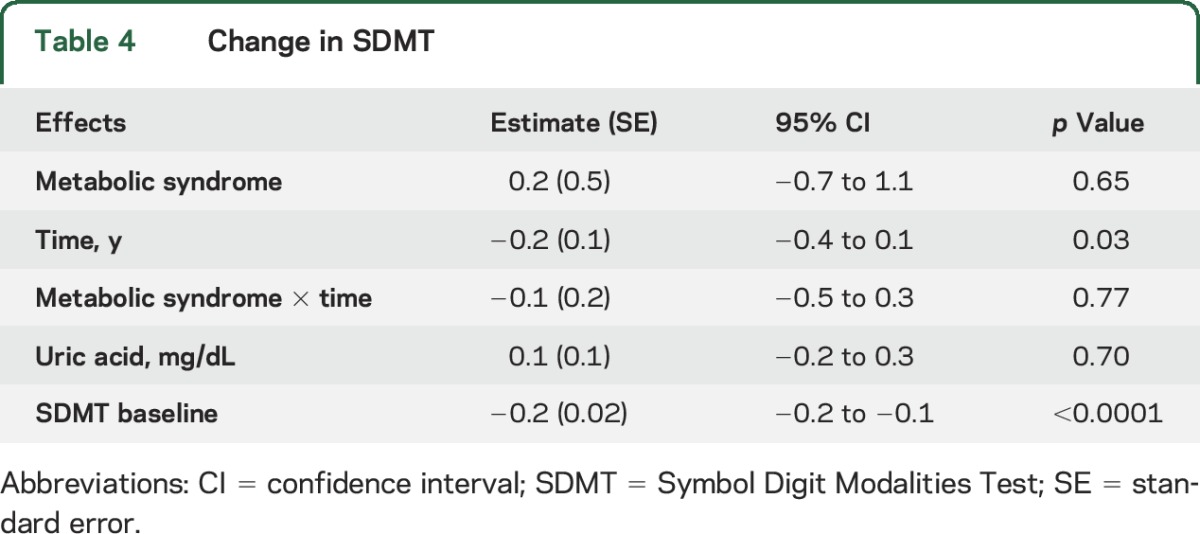
Table 4 shows the change in SDMT. While the SDMT declined 0.2 units (standard error = 0.1) per year (p = 0.03), there was no significant difference, 0.3- vs 0.2-unit annual decline, between those with vs without metabolic syndrome (p = 0.77), respectively.
Table 4.
Change in SDMT
DISCUSSION
This study shows that participants with early-stage, treated PD who met modified criteria for metabolic syndrome had more rapid progression as measured by both the total and motor UPDRS scores over time compared to those without metabolic syndrome. This finding is consistent with prior studies suggesting that the presence of metabolic syndrome is associated with increased risk of developing PD, Alzheimer disease, cognitive decline, and other diseases.1–8 We could find no other study showing an association between metabolic syndrome and progression of PD, so this finding needs confirmation. If confirmed, this would raise the possibility that improved treatment of metabolic syndrome could offer a novel approach to slowing PD progression.
How metabolic syndrome might accelerate PD progression is not known. Because metabolic syndrome is a combination of conditions, each of these conditions could contribute to the association. A recent analysis of the NET-PD population found that an increase in BMI was associated with a slower increase in UPDRS scores, so it seems unlikely that the high BMI component of metabolic syndrome contributes strongly to the association of metabolic syndrome and increasing UPDRS scores. Another metabolic syndrome condition, hypertension, could drive faster PD progression if it caused those affected to have more CNS ischemia or strokes. Brain imaging data were not collected in the NET-PD study, so this theory cannot be substantiated with our data. Regarding blood trigyceride,23 glucose,23,31 and high-density lipoprotein cholesterol levels, the literature to date is conflicting, lacking, or not informative. Insulin resistance and inflammation underlie metabolic syndrome,32 and these pathologic mechanisms also contribute to the progressive loss of dopaminergic cells that results in PD.33,34 The association found between metabolic syndrome and PD may therefore be attributed to common pathophysiologic pathways.
The faster progression of motor signs in our participants with metabolic syndrome compared to those without could be related to the accumulating evidence of brain abnormalities in the expression of insulin and insulin growth factors and their related receptors and CNS insulin resistance being reported in PD. Activities of insulin growth factors include support of neuronal growth and survival. Recent literature suggests that these insulin-related CNS abnormalities may increase sensitivity to neurotoxins and the accumulation of α-synuclein.35,36 While further studies are needed to judge whether these brain abnormalities correlate with clinical longitudinal signs, given that patients with PD have CNS insulin-related abnormalities that normally play a protective role, concurrent metabolic syndrome is likely to exacerbate these baseline abnormalities and to enhance the progression of disease.
While uric acid levels are not a defined component of metabolic syndrome, the syndrome is associated with higher uric acid levels. Studies to date suggest that higher uric acid levels are associated with more slowly increasing UPDRS scores.37,38 In this study, however, the metabolic syndrome group had higher uric acid levels and had faster increasing UPDRS scores.
The association of metabolic syndrome and cognitive function was explored in this study. In NET-PD, the only cognitive measure captured at annual visits was the SDMT score. There was no significant difference: 0.3- vs 0.2-unit annual decline in participants with metabolic syndrome vs without (p = 0.77).
However, this analysis was limited by the minimal decline in SDMT scores that occurred in this early treated PD group.39 Furthermore, the SDMT evaluates attention and not other cognitive domains or global cognitive function.40
A strength of this study is that it is derived from a relatively large and well-characterized cohort. In addition, the results of the sensitivity analyses consistently generated the same result: those with metabolic syndrome had greater increasing UPDRS scores over time than those without metabolic syndrome. However the conclusions are limited by the use of a modified definition of metabolic syndrome. Therefore, additional studies incorporating stricter measurements of the components of metabolic syndrome are required to confirm the findings of this initial study.
In NET-PD LS1, participants meeting criteria for metabolic syndrome experienced a greater increase in total UPDRS scores, mainly due to increases in motor scores, compared to those not meeting these criteria. If confirmed, future work should determine whether treatment of metabolic syndrome results in a slower increase in UPDRS scores over time.
Supplementary Material
GLOSSARY
- ATP III
Adult Treatment Panel III
- BMI
body mass index
- NCEP
National Cholesterol Education Program
- NET-PD LS 1
National Institute of Neurological Disorders and Stroke Exploratory Trials in PD Long-Term Study 1
- PD
Parkinson disease
- SDMT
Symbol Digit Modalities Test
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Editorial, page 1760
AUTHOR CONTIBUTIONS
Maureen Leehey, MD: study concept and design, interpretation of data, drafting/revising manuscript. Sheng Luo, PhD: analysis and interpretation of data, statistical analysis. Saloni Sharma, MBBS: study concept and design, interpretation of data, manuscript review. Anne-Marie A. Wills, MD, MPH: interpretation of data, revision of manuscript. Jacquelyn L. Bainbridge, BSPharm, PharmD, FCCP: study concept and design, interpretation of the data. Pei Shieen Wong, PharmD, BCPS: study concept and data, interpretation of data. David K. Simon, MD, PhD: interpretation of data and review of manuscript. Jay Schneider, PhD: drafting/revising manuscript. Yunxi Zhang, MS: analysis and interpretation of data, statistical analysis. Adriana Pérez, MS, PhD: review and critique of statistical analyses, review and critique of manuscript. Rohit Dhall, MD, MSPH: interpretation of data and revising manuscript. Chadwick W. Christine, MD: interpretation of data, review of manuscript. Carlos Singer, MD: interpretation of data and revising manuscript. Franca Cambi, MD, PhD: study design, writing of manuscript. James T. Boyd, MD: study concept and design, data collection, interpretation of the data.
STUDY FUNDING
NET-PD was funded by the National Institute of Neurological Disorders and Stroke: U01NS043127, U01NS043128, and U10NS44415-44555.
DISCLOSURE
M. Leehey reports no disclosures relevant to the manuscript. S. Luo received grants from the NIH, International Parkinson and Movement Disorder Society, and CHDI Foundation. S. Sharma reports no disclosures relevant to the manuscript. A. Wills serves as a consultant for Accordant and Sage Bionetworks and has received research support from the ALS Association, Pfizer, and Acorda. J. Bainbridge, P. Wong, D. Simon, J. Schneider, Y. Zhang, A. Pérez, R. Dhall, C. Christine, C. Singer, and F. Cambi report no disclosures relevant to the manuscript. J. Boyd served as a consultant and/or scientific advisor for AbbVie Inc, Auspex, Lundbeck, and Medical Education Resources and received research support from the Michael J. Fox Foundation, NIH/National Institute of Neurological Disorders and Stroke, Auspex, Biotie, CHDI Foundation, NeuroDerm, Chrono Therapeutics, and Vaccinex. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson's disease. Oxid Med Cell Longev 2014;2014:729194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhindi B, Xie WY, Kulkarni GS, et al. Influence of metabolic syndrome on prostate cancer stage, grade, and overall recurrence risk in men undergoing radical prostatectomy. Urology 2016;93:77–85. [DOI] [PubMed] [Google Scholar]
- 3.Bil E, Dilbaz B, Cirik DA, Ozelci R, Ozkaya E, Dilbaz S. Metabolic syndrome and metabolic risk profile according to polycystic ovary syndrome phenotype. J Obstet Gynaecol Res 2016;42:837–843. [DOI] [PubMed] [Google Scholar]
- 4.Bueloni-Dias FN, Spadoto-Dias D, Delmanto LR, Nahas-Neto J, Nahas EA. Metabolic syndrome as a predictor of endometrial polyps in postmenopausal women. Menopause 2016;23:759–764. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Pena V, Toral-Rios D, Becerril F, et al. Metabolic syndrome as a risk factor for Alzheimer's disease: is Abeta a crucial factor in both pathologies? Antioxid Redox Signal 2017;26:542–560. [DOI] [PubMed] [Google Scholar]
- 6.Hishikawa N, Fukui Y, Sato K, et al. Cognitive and affective functions in Alzheimer's disease patients with metabolic syndrome. Eur J Neurol 2016;23:339–345. [DOI] [PubMed] [Google Scholar]
- 7.Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Ferri C, Falaschi P. Recent advances in the role of cortisol and metabolic syndrome in age-related degenerative diseases. Aging Clin Exp Res 2016;28:17–23. [DOI] [PubMed] [Google Scholar]
- 8.Ng TP, Feng L, Nyunt MS, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore Longitudinal Ageing Study cohort. JAMA Neurol 2016;73:456–463. [DOI] [PubMed] [Google Scholar]
- 9.Laudisio A, Lo Monaco MR, Vetrano DL, et al. Association of metabolic syndrome with falls in patients with Parkinson's disease. Clin Nutr 2017;36:559–563. [DOI] [PubMed] [Google Scholar]
- 10.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 2011;26(suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- 11.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu C, Hu G, Kivipelto M, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension 2011;57:1094–1100. [DOI] [PubMed] [Google Scholar]
- 13.Vikdahl M, Backman L, Johansson I, Forsgren L, Haglin L. Cardiovascular risk factors and the risk of Parkinson's disease. Eur J Clin Nutr 2015;69:729–733. [DOI] [PubMed] [Google Scholar]
- 14.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson's disease. Am J Epidemiol 2006;164:998–1002. [DOI] [PubMed] [Google Scholar]
- 15.Palacios N, Gao X, McCullough ML, et al. Obesity, diabetes, and risk of Parkinson's disease. Mov Disord 2011;26:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson's disease. Neurology 2002;59:1051–1057. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson's disease. Am J Epidemiol 2004;159:547–555. [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology 2006;67:1955–1959. [DOI] [PubMed] [Google Scholar]
- 19.Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Body mass index and risk of Parkinson's disease: a prospective cohort study. Am J Epidemiol 2007;166:1186–1190. [DOI] [PubMed] [Google Scholar]
- 20.Saaksjarvi K, Knekt P, Mannisto S, et al. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol 2014;29:285–292. [DOI] [PubMed] [Google Scholar]
- 21.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson's disease. Mov Disord 2013;28:257. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson's disease: an updated meta-analysis of case-control studies. PLoS One 2014;9:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saaksjarvi K, Knekt P, Mannisto S, Lyytinen J, Heliovaara M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson's disease. Parkinsonism Relat Disord 2015;21:1148–1155. [DOI] [PubMed] [Google Scholar]
- 24.van der Marck MA, Dicke HC, Uc EY, et al. Body mass index in Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord 2012;18:263–267. [DOI] [PubMed] [Google Scholar]
- 25.Wills AM, Perez A, Wang J, et al. Association between change in body mass index, Unified Parkinson's Disease Rating Scale scores, and survival among persons with Parkinson disease: secondary analysis of longitudinal data from NINDS Exploratory Trials in Parkinson Disease Long-Term Study 1. JAMA Neurol 2016;73:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–666. [DOI] [PubMed] [Google Scholar]
- 27.Elm JJ; NINDS NET-PD Investigators. Design innovations and baseline findings in a long-term Parkinson's trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson's Disease Long-Term Study-1. Mov Disord 2012;27:1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators, Kieburtz K, Tilley BC, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 2015;313:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test: Manual. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol 2005;4:198–203. [DOI] [PubMed] [Google Scholar]
- 31.Arvanitakis Z, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology 2004;63:996–1001. [DOI] [PubMed] [Google Scholar]
- 32.Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism 2010;59:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson's disease, insulin resistance and novel agents of neuroprotection. Brain 2013;136:374–384. [DOI] [PubMed] [Google Scholar]
- 34.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurology 2012;78:1507–1511. [DOI] [PubMed] [Google Scholar]
- 35.Athauda D, Foltynie T. Insulin resistance and Parkinson's disease: a new target for disease modification? Prog Neurobiol 2016;145–146:98–120. [DOI] [PubMed] [Google Scholar]
- 36.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis 2005;7:45–61. [DOI] [PubMed] [Google Scholar]
- 37.Ascherio A, LeWitt PA, Xu K, et al. ; Parkinson Study Group DATATOP Investigators. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzschild MA, Marek K, Eberly S, et al. ; Parkinson Study Group PRECEPT Investigators. Serum urate and probability of dopaminergic deficit in early “Parkinson's disease.” Mov Disord 2011;26:1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wills AA, Elm JJ, Ye R, et al. Cognitive function in 1736 participants in NINDS Exploratory Trials in PD Long-Term Study-1. Parkinsonism Relat Disord 2016;33:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser RA, Li R, Perez A, et al. ; NINDS NET-PD Investigators. Longer duration of MAO-B inhibitor exposure is associated with less clinical decline in Parkinson's disease: an analysis of NET-PD LS1. J Parkinsons Dis 2017;7:117–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.