Abstract
Candida chorioamnionitis is rare but can lead to neonatal infection, high mortality, and neurodevelopmental impairment. We aimed to investigate maternal clinical features and perinatal outcomes and discuss future management strategies. We reviewed the medical records of women with Candida chorioamnionitis at our hospital over a 10-year period (n = 9) and previous published case reports and case series. The most prevalent Candida species was C. albicans (71.3% of the all cases). The most prevalent predisposing condition was preterm premature rupture of membranes (31/123, 25.2%), followed by pregnancy with a retained intrauterine contraceptive device (26/123, 21.1%) and pregnancy after in vitro fertilization (25/123, 20.3%). Preterm labor was the most common symptom (52/123, 42.3%), and only 13% of cases involved fever. Of the infants, 27% of the singletons and 23.8% of the twins were born before 22 gestational weeks, while 60% of the singletons and 76.2% of the twins were born at 22–36 weeks. The median birth weight of the babies born after 22 weeks was 1230 g. The mortality rates of the singletons and twins born after 22 weeks of gestation in the year 2000 or later were 28.6% and 52.4%, respectively. Antenatal treatment for Candida chorioamnionitis has not been established.
1. Introduction
Despite the high incidence of vulvovaginal candidiasis during pregnancy (13–20%) [1, 2], Candida species rarely cause chorioamnionitis. A recent neonatal study demonstrated that chorioamnionitis is an important risk factor of invasive early-onset candidiasis in extremely low-birth-weight infants, which leads to high mortality (71%) and neurodevelopmental impairment rates (86%) [3]; thus, the establishment of antenatal management is crucial. Since the first case of chorioamnionitis caused by Candida albicans that resulted in preterm birth and neonatal death was reported in 1958 [4], many case reports and short case series have been published. However, owing to the rarity of the disease, only two studies involving small groups of patients, a case series of 32 patients [5] and a case control study of 18 patients [6], have been published to date. Therefore, the clinical features of Candida chorioamnionitis are not well understood and management of the disease has not been established yet. Here, we present a case series of Candida chorioamnionitis in the past 10 years at our hospital and review the previously published case reports and case series. This study aimed to investigate the maternal clinical features and perinatal outcomes of these cases and discuss future management strategies.
2. Materials and Methods
We reviewed the medical records of women with Candida chorioamnionitis who attended the University of Miyazaki Hospital, a tertiary medical center in Miyazaki, Japan, between 2007 and 2016. The ethics committee of the Faculty of Medicine of University of Miyazaki approved this study (registration number O-0135). Candida chorioamnionitis is diagnosed on the basis of one or more of the following criteria: (1) Candida species are isolated from amniotic fluid obtained using transabdominal amniocentesis; (2) clinical or histological chorioamnionitis along with fetal/neonatal/placental culture test results positive for Candida species; or (3) histological chorioamnionitis (defined by polymorphonuclear leukocytes in the chorion or chorioamnion) and/or funisitis (defined by polymorphonuclear leukocytes in the wall of a blood vessel in the umbilical cord or the chorionic plate) involving yeast forms with pseudohyphae on periodic acid-Schiff staining, which is characteristic of Candida species. Cases of neonatal congenital candidiasis without any signs of maternal clinical/histological chorioamnionitis were excluded owing to the possibility of a birth canal infection during delivery rather than an in utero infection. In our hospital, we routinely perform transabdominal amniocentesis in women with preterm labor with or without preterm premature rupture of membranes (pPROM) to exclude intra-amniotic infection after obtaining their informed consent, unless other comorbid conditions such as placental position, inadequate amniotic space, or large bag protrusion into the vagina are present since we have reported that management with amniocentesis for women with preterm labor with intact membranes might improve neonatal outcome born between 22 and 28 weeks of gestation [7]. Umbilical cord blood cultures were immediately collected after delivery from all the women with preterm delivery and from those with term delivery who had signs of clinical chorioamnionitis, such as fever, leukocytosis, and maternal/fetal tachycardia. Histopathological examinations of the placentas/umbilical cords from these women were also performed. Periodic acid-Schiff staining was additionally performed after hematoxylin and eosin staining when a Candida infection was suspected.
We searched Medline, PubMed, and Google Scholar for case reports and case series in the English literature that were published till December 2016, using the terms “chorioamnionitis,” “intra-amniotic infection,” “Candida species,” “Candida albicans,” “Candida glabrata,” “Torulopsis glabrata,” and “congenital candidiasis”. We also searched the references of the published case series. We used the above-mentioned criteria for Candida chorioamnionitis and excluded duplicate cases and reports without adequate information about the maternal clinical course.
3. Results
3.1. Case Series
Between January 2007 and December 2016, 2717 deliveries were performed in our hospital. During this period, we had nine cases (0.3%) of Candida chorioamnionitis (Table 1). The isolated organisms were C. albicans in six women, C. glabrata in two women, and C. famata in one woman. Eight cases were antenatally diagnosed using transabdominal amniocentesis. One case, in which a transabdominal amniocentesis was not conducted, was diagnosed as histological chorioamnionitis and funisitis with Candida infection using an umbilical blood culture. This case involved dichorionic diamniotic (Di-Di) twins, of whom only the first infant had C. albicans infection.
Table 1.
Clinical features of nine cases of Candida chorioamnionitis in our hospital.
| Case | Age (y) | GA at delivery | Species | Positive culture | Predisposing conditions | Clinical signs | BW (g) | CCC | Neonatal outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | 21 w 6 d | Candida albicans | Amniotic fluid | IVF | Preterm labor and afebrile WBC: 16,500/mm3 CRP: 0.51 mg/dL |
490 | No | Artificial abortion |
| 2 | 31 | 22 w 6 d | C. glabrata | Amniotic fluid | IVF | Preterm labor and afebrile WBC: 11,600/mm3 CRP: 1.68 mg/dL |
594 | Yes | Death on day 59 |
| 3 | 28 | 23 w 0 d | C. famata | Amniotic fluid | Smoking | Preterm labor and afebrile WBC: 16,400/mm3 CRP: 0.68 mg/dL |
498 | No | Hydrocephalus after IVH |
| 4 | 35 | 23 w 3 d | C. albicans | Amniotic fluid | IVF Gestational diabetes |
Preterm labor and afebrile WBC: 11,700/mm3 CRP: 3.71 mg/dL |
580 | Yes | Neonatal death |
| 5 | 28 | 23 w 4 d | C. albicans | Amniotic fluid | Pregestational diabetes | Preterm labor and afebrile WBC: 12,600/mm3 CRP: 3.8 mg/dL |
536 | No | Brain atrophy and CLD |
| 6 | 33 | 25 w 2 d | C. albicans | Amniotic fluid | None | Preterm labor and afebrile WBC: 15,400/mm3 CRP: 7.51 mg/dL |
786 | No | PDA ligation |
| 7 | 26 | 26 w 6 d | C. albicans | Amniotic fluid | None | Preterm labor and afebrile WBC: 9,800/mm3 CRP: 3.05 mg/dL |
924 | Yes | CLD |
| 8 | 36 | 28 w 2 d | C. albicans | Umbilical blood | Di-Di twins | Preterm labor and afebrile WBC: 9,500/mm3 CRP: 3.34 mg/dL |
1,290/1,168 | No | Normal Only one twin had the infection |
| 9 | 38 | 33 w 3 d | C. glabrata | Amniotic fluid | pPROM | Afebrile, WBC: 10,200/mm3 CRP: 2.68 mg/dL |
1,912 | No | Normal |
GA, gestational age; BW, birth weight; CCC, congenital cutaneous candidiasis; IVF, in vitro fertilization; Di-Di twin, dichorionic diamniotic twin; WBC, white blood cell; CRP, C-reactive protein; IVH, intraventricular hemorrhage; CLD, chronic lung disease; PDA, patent ductus arteriosus; pPROM, preterm premature rupture of membranes.
3.1.1. Maternal Clinical Features
The mean maternal age was 32 years (range, 26–38 years). Three cases of pregnancy after in vitro fertilization (IVF) embryo transfer and one case of pPROM occurred. One woman had pregestational diabetes, and another had gestational diabetes; both conditions were well controlled. None of the patients had an intrauterine contraceptive device (IUCD) or a cervical cerclage. Eight patients were hospitalized for preterm labor. None of the patients were febrile. In three patients, the white blood cell counts reached >15,000/mm2 and the C-reactive protein (CRP) levels ranged from 0.51 to 7.51 mg/dL (mean, 3.1 mg/dL). One patient had an induced abortion at 21 weeks of gestation. In seven women diagnosed using transabdominal amniocentesis, delivery was immediately induced by oxytocin or performed via cesarean section after diagnosis. The woman with Di-Di twins who did not undergo transabdominal amniocentesis had a spontaneous delivery. No women received antenatal corticosteroids.
3.1.2. Fetal/Neonatal Outcome
The case of induced abortion was excluded from the analysis. The mean gestational age at delivery was 24.5 weeks (range, 22–33 weeks). The mean birth weight was 690 g (range, 498–1912 g). One of the twins, neither of whom had been diagnosed prenatally, had a positive umbilical blood culture, and the other three infants had cutaneous candidiasis. All the infants received intravenous fluconazole or micafungin and did not test positive in the following blood and surface cultures. Two infants died at the neonatal intensive care unit, one of liver hemorrhage and the other of intestinal perforation. Two infants were at risk of severe neurological impairment at the time of discharge.
3.2. Literature Review
We identified 123 cases (102 singletons [4, 8–76]/21 twins [49, 62, 69, 77–90]) with Candida chorioamnionitis, including our nine cases for whom adequate maternal clinical data were available.
3.2.1. Microbiological Characteristics
Cultural identification was conducted in 101 cases, while the remaining 22 cases were diagnosed using histopathological placenta/umbilical findings without cultural identification. The most prevalent species was C. albicans (71.3% [72/101]), followed by C. glabrata (21.7% [22/101]), C. tropicalis (3% [3/101]), C. lusitaniae (2% [2/101]), C. parapsilosis (2% [2/101]), C. famata (1% [1/101]), and C. kefyr (1% [1/101]; Table 2). Coinfection with C. albicans and C. parapsilosis was found in two cases [56, 83], one of which was a twin pregnancy. C. albicans was identified in one infant, while C. parapsilosis was identified in another [83].
Table 2.
Species identified in the literature review excluding 22 unidentified cases (n = 101).
| Species | n (%) |
|---|---|
| Candida albicans | 72/101 (71.3) |
| C. albicans alone | 70/101 (69.3) |
| Coinfection with C. parapsilosis | 2/101 (2) |
| C. glabrata | 22/101 (21.7) |
| C. tropicalis | 3/101 (3) |
| C. lusitaniae | 2/101 (2) |
| C. famata | 1/101 (1) |
| C. kefyr | 1/101 (1) |
3.2.2. Maternal Clinical Features
The maternal clinical features are shown in Table 3. The median maternal age was 29 years (range, 16–47 years). Five patients had pregestational or gestational diabetes, and one patient received prednisolone for suspected immunological miscarriages. None of the patients had human immunodeficiency virus infection or other immunosuppressive diseases. The most prevalent predisposing condition was pPROM in 25.2% (31/123) of the patients, followed by pregnancy with a retained IUCD (21.1% [26/123]), pregnancy after IVF (20.3% [25/123]), a history of transabdominal amniocentesis during the current pregnancy (8.9% [11/123]), or cervical cerclage (6.5% [8/123]). Of the cases, 11.4% (14/123) had a history of treatment for vulvovaginal candidiasis during the current pregnancy. Preterm labor was the most common symptom (42.3% [52/123]), while only 13% (16/123) of the cases involved fever. Cervical dilatation without uterine contraction was found in 8.9% (11/123) of the cases, while 9.8% (12/123) had no symptoms. Laboratory data were unavailable in most of the reports. No cases of maternal death occurred.
Table 3.
Maternal clinical features in the literature review.
| n (%) | |
|---|---|
| Maternal age, years; median (range) | 29 (16–47) |
| Singleton/twins | 102/21 |
| Predisposing condition | |
| pPROM | 31/123 (25.2) |
| IUCD | 26/123 (21.1) |
| IVF | 25/123 (20.3) |
| History of amniocentesis during current pregnancy | 11/123 (8.9) |
| Cervical cerclage | 8/123 (6.5) |
| Pregestational or gestational diabetes | 5/123 (4.1) |
| History of treatment for vaginal candidiasis during current pregnancy | 14/123 (11.4) |
| Symptoms | |
| Preterm labor with intact membranes | 52/123 (42.3) |
| Fever | 16/123 (13) |
| Cervical dilatation | 11/123 (8.9) |
| Abdominal pain | 7/123 (5.7) |
| Vaginal bleeding | 6/123 (4.9) |
| Reduced fetal movement | 2/123 (1.6) |
| None | 12/123 (9.8) |
pPROM, preterm premature rupture of membranes; IUCD, intrauterine contraceptive device; IVF, in vitro fertilization.
3.2.3. Fetal/Neonatal Outcome
The distribution of the gestational ages at delivery is shown in Table 4. Two singletons were excluded because of missing information on gestational age. Birth before 22 weeks occurred in 27% (27/100) of the singletons and 23.8% (5/21) of the twins, while birth between 22 and 36 weeks occurred in 60% (60/100) of the singletons and 76.2% (16/21) of the twins. The distributions of the singletons and twin births were as follows: between 22 and 23 weeks, 11% (11/100) and 9.5% (2/21), respectively; between 24 and 27 weeks, 24% (24/100) and 19% (4/21), respectively. The median birth weight of the babies born after 22 weeks was 1,230 g (range, 425–4350 g).
Table 4.
Gestational age at delivery in the literature review.
| Gestational age (weeks) | Singletons (n = 100) | Twins (n = 21) |
|---|---|---|
| <22 | 27/100 (27%) | 5/21 (23.8%) |
| 22–36 | 60/100 (60%) | 16/21 (76.2%) |
| 22-23 | 11/100 (11%) | 2/21 (9.5%) |
| 24–27 | 23/100 (23%) | 4/21 (19%) |
| 28–31 | 13/100 (13%) | 7/21 (33.3%) |
| 32–36 | 13/100 (13%) | 3/21 (14.3%) |
| ≥37 | 13/100 (13%) | 0/21 (0%) |
2 singletons with unknown gestational age were excluded.
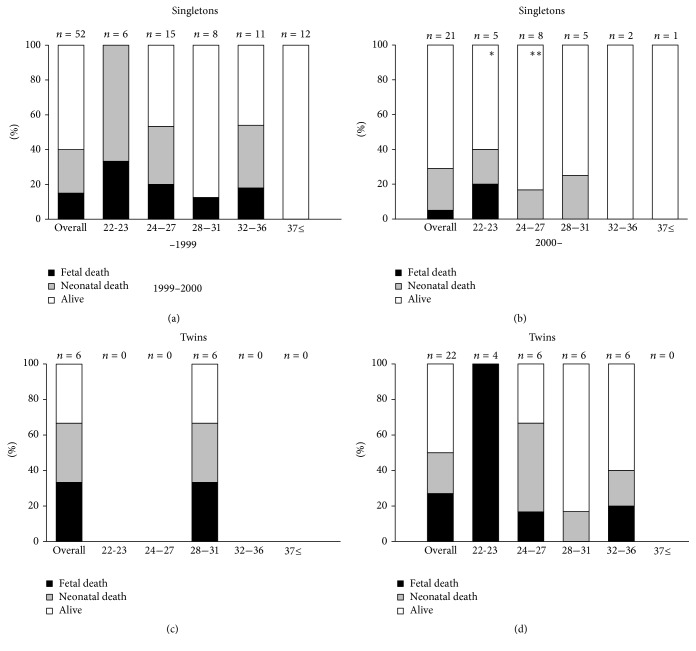
The mortality rates of the singletons and twins born after 22 weeks are shown in Figure 1. Since the description of the clinical features of the infants varied among the reports, we were unable to obtain sufficient data to evaluate the prevalence of congenital candidiasis caused by Candida chorioamnionitis. Therefore, we analyzed all infants associated with Candida chorioamnionitis. The mortality rate of the singletons born after 22 weeks since 2000 was 29%, while that of the twins was 50%. In five cases of twins, only one twin was associated with Candida chorioamnionitis, and another twin had no signs of chorioamnionitis or funisitis. These five infants without chorioamnionitis or funisitis were excluded from the analysis. The overall mortality rate of the singletons since the year 2000 improved to 28.6% from 40.4% before the year 2000. The overall mortality rate of the twins since 2000 was 52.4%, higher than that of the singletons.
Figure 1.
Perinatal mortality rate of infants with Candida chorioamnionitis born after 22 weeks' gestation in the literature review. (a) Singletons born before 2000; (b) singletons born in 2000 or later; (c) twins born before 2000; (d) twins born in 2000 or later. Black bar: fetal death; gray bar: neonatal death; white bar: alive for the first 28 days of life. ∗One infant died on day 59. ∗∗One infant died on day 42. One twin of five twins without chorioamnionitis was excluded from analysis.
3.2.4. Antenatal Treatment
Antenatal treatment was conducted in 13 cases, including four of the twins (Table 5) [41, 44, 64, 71, 73–76, 81, 87–89]. All the cases were diagnosed using amniotic fluid culture obtained through transabdominal amniocentesis at a median gestational age of 22 weeks (range, 19–26 weeks). Fluconazole was the most prevalent agent used in six cases, followed by amphotericin B in five cases and ketoconazole and micafungin in one case each. The administration routes included oral, intravenous, vaginal, transabdominal intra-amniotic, and transcervical intra-amniotic. The prolongation period varied from 0 days to 8 weeks. Six of 13 cases with antenatal treatment succeeded in having alive infants.
Table 5.
Antenatal treatment for Candida chorioamnionitis.
| GA at diagnosis (weeks) | GA at delivery (weeks) | Species | Antifungal agent | Administration route | Fetal/neonatal outcomes | Reference |
|---|---|---|---|---|---|---|
| 19 | 19 | Candida albicans | Fluconazole | Intravenous | Stillbirth | [74] |
| 19 | 21 | C. albicans | Fluconazole | Oral | Stillbirth | [75] |
| 21 | 22 | C. albicans | Fluconazole | Oral | Stillbirth | [64] |
| 21 | 27 | C. albicans | Fluconazole | Transabdominal intra-amniotic, oral, and vaginal | Alive | [71] |
| 23 | 31 | C. albicans | Fluconazole | Transabdominal intra-amniotic, oral, and vaginal | Alive | [71] |
| 26 | 29 | C. glabrata | Fluconazole | Intravenous | Alive (PVL)/alive | [81] |
| 18 | 23 | C. glabrata | Amphotericin B | Intravenous | Stillbirth | [88] |
| 21 | 24 | C. glabrata | Amphotericin B | Intravenous | Stillbirth/stillbirth | [87] |
| 24 | 24 | C. glabrata | Amphotericin B | Intravenous | Died on day 42 | [76] |
| 24 | 28 | C. glabrata | Amphotericin B | Intravenous | Alive/alive | [89] |
| 27 | 29 | C. albicans | Amphotericin B | Transcervical intra-amniotic | Alive | [44] |
| 21 | 21 | C. albicans | Ketoconazole | Not described | Stillbirth | [91] |
| 26 | 28 | C. glabrata | Micafungin | Intravenous | Alive | [41] |
GA, gestational age; PVL, periventricular leukomalacia.
4. Discussion
4.1. Epidemiology
The prevalence of Candida chorioamnionitis at our institution was 0.3% of all pregnant women. This finding is consistent with the 0.5% prevalence reported in a previous study [6]. However, considering that both institutions are regional referral centers, we suspect that the true prevalence is less frequent than 0.3–0.5%.
4.2. Maternal Clinical Features
The presence of an IUCD during pregnancy, an established risk factor of intra-amniotic Candida infection [92, 93], showed a high incidence (21.1% [26/123]) in this review. Contaminated foreign body caused direct insemination of Candida species into the uterus. Some authors suspect that cervical cerclage, another foreign body during pregnancy, is also a risk factor [6, 91]. However, the most prevalent predisposing condition was pPROM, which leads to an ascending infection of Candida species colonized in the vagina. The incidence of Candida species in the amniotic fluid of patients with pPROM was reportedly 5% using polymerase chain reaction and 3.2% using culture-based methods [93], which are higher than those of patients with preterm labor with intact membranes (1.2% and 0.6%, resp.) [94]. However, whether cervical cerclage and pPROM are risk factors is unclear because cervical incompetence, which is the indication for cervical cerclage, and pPROM could result from chorioamnionitis. Case reports of chorioamnionitis with C. glabrata associated with IVF have been increasing recently. A recent review reported that 65% of cases of C. glabrata chorioamnionitis were associated with IVF [74]. Our study showed that 20.3% (25/123) of cases with Candida chorioamnionitis were associated with IVF and that C. glabrata, C. albicans, C. parapsilosis, C. kefyr, and C. famata were identified. Several steps of the IVF procedure can contaminate the fertilized embryo or introduce the pathogen into the uterine cavity, although the risk of chorioamnionitis after IVF has not been well studied yet [95]. A case report showed that the eradication of Candida species in the vagina led to the birth of a healthy infant after stillbirth due to Candida chorioamnionitis [85]. The insemination of Candida species colonized in the skin through transabdominal amniocentesis could be another risk factor, as two authors suspected that the amniocentesis procedure itself led to the Candida chorioamnionitis [26, 57]. Amniocentesis provides an early diagnosis and an opportunity for aggressive management, including antenatal treatment, as described later. Candida chorioamnionitis may manifest as preterm labor, pPROM, or cervical incompetence without fever, while 39% (48/123) of cases in this study were antenatally diagnosed using amniotic fluid culture obtained through transabdominal amniocentesis. A recent case report also showed that the collection of amniotic fluid sludge succeeded to detect C. albicans [96]. Thus, we recommend performing transabdominal amniocentesis under strict aseptic conditions in cases of preterm labor or pPROM.
Since the information of Candida colonization in vagina was missing in most cases of the literature review, we were unable to evaluate the effect of Candida colonization in vagina on Candida chorioamnionitis in this study. A recent study, which showed recurrent vaginal colonization with C. albicans in early pregnancy was a risk factor for preterm delivery and low birth weight, indicates that the routine screening and consequent treatment for Candida colonization can be useful to improve pregnancy outcomes [96]. Although it is still unknown that vaginal colonization with Candida is associated with Candia chorioamnionitis, we also suggest that the screening and treatment for Candida colonization in vagina in early pregnancy is a reasonable strategy to prevent ascending infection.
4.3. Fetal/Neonatal Outcome
Almost 30% of singletons and 50% of twins born after 22 weeks died, even after the year 2000. Immaturity and severe fetal inflammation could be causes of the high mortality rate. As shown in Table 4, among the singletons delivered after 22 weeks of gestation, almost half were born before 28 weeks.
4.4. Antenatal Treatment
Antenatal treatment for Candida chorioamnionitis is challenging. Although the maternal administration of various antifungal agents via various routes has been conducted, only half of cases resulted in the delivery of live infants. Fluconazole was the most popular agent used in seven cases. However, fluconazole has less activity against C. glabrata, the second most prevalent species, and whether fluconazole crosses the placenta is unknown [97]. The use of high-dose systemic fluconazole during the first trimester has the potential risk of teratogenic effects on the fetus [97, 98], although a cohort study showed that low-dose fluconazole was not associated with birth defect [99]. Thus, amphotericin B is recommended as the first-line treatment in cases of invasive candidiasis in pregnant women and neonatal candidiasis [98]. The advantages of amphotericin B include that it crosses the placenta, has no reported adverse effects in humans [97], and is generally effective against C. glabrata. One case report showed that intravenous amphotericin B treatment resulted in pregnancy extension by 4 weeks and the delivery of live infants with no signs of inflammatory changes in the placenta or umbilical cord [89], although three other cases resulted in stillbirth [76, 87, 88]. Micafungin was used in one case, although its efficacy and safety in pregnant women are unclear, and whether it crosses the placenta remains unknown. However, its high molecular weight (approximately 1292 for the sodium salt), low lipid solubility, and very high protein binding ability should limit its transfer to the fetus [97]. Some cases of successful treatment with intra-amniotic administration were also reported [49, 71]. Transcervical intra-amniotic amphotericin B administration succeeded in extending a pregnancy by 2 weeks and resulted in the delivery of a healthy infant [49]. Transabdominal intra-amniotic fluconazole treatment with oral and vaginal administration successfully extended pregnancy by 6–8 weeks and resulted in the delivery of live infants [71]. Sheep studies showed that intra-amniotic fluconazole treatment prevented systemic inflammation and cerebral inflammation and injury [100, 101]. These reports indicate that intra-amniotic treatment is an antenatal treatment to be considered. We suggest amphotericin B should be chosen as a first-line agent and fluconazole should be avoided due to the possibility of adverse outcomes for the fetus [97].
5. Conclusion
Candida chorioamnionitis may manifest as preterm labor, pPROM, or cervical incompetence without fever. Therefore, Candida chorioamnionitis should be considered in pregnant women with these symptoms, even in the absence of fever, especially in those with early gestation with a retained IUCD, pregnancy after IVF, a history of amniocentesis, or cervical cerclage and preterm delivery. The preterm birth and fetal/neonatal mortality rates are high, and antenatal treatment has yet to be established.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Kiss H., Petricevic L., Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. British Medical Journal. 2004;329(7462):371–374. doi: 10.1136/bmj.38169.519653.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts C. L., Rickard K., Kotsiou G., Morris J. M. Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: an open-label pilot randomized controlled trial. BMC Pregnancy and Childbirth. 2011;11, article 18 doi: 10.1186/1471-2393-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton M., Shen A., OBrien K., Robinson JL., Davies HD., Simpson K. Early onset invasive candidiasis in extremely low birth weight infants: perinatal acquisition predicts poor outcome. Clin Infect Dis. 2017;64(7):921–927. doi: 10.1093/cid/cix001. [DOI] [PubMed] [Google Scholar]
- 4.Benirschke K., Raphael S. I. Candida albicans infection of the amniotic sac. American Journal of Obstetrics & Gynecology. 1958;75(1):200–202. doi: 10.1016/0002-9378(58)90572-6. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi F., Jacques S. M., Bendon R. W., et al. Candida funisitis: a clinicopathologic study of 32 cases. Pediatric and Developmental Pathology. 1998;1(2):118–124. doi: 10.1007/s100249900014. [DOI] [PubMed] [Google Scholar]
- 6.Whyte R. K., Hussain Z., DeSa D. Antenatal infections with Candida species. Archives of Disease in Childhood. 1982;57(7):528–535. doi: 10.1136/adc.57.7.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki Y., Furukawa S., Kodama Y., Sameshima H., Ikenoue T. Amniocentesis for threatened preterm labor with intact membranes and the impact on adverse outcome in infants born at 22 to 28 weeks of gestation. Early Human Development. 2015;91(5):333–337. doi: 10.1016/j.earlhumdev.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Belter L. F. Thrush of the umbilical cord. Obstet Gynecol. 1959;14:796–798. [PubMed] [Google Scholar]
- 9.Galton M., Benirschke K. The implication of candida albicans infection of the amniotic sac. BJOG: An International Journal of Obstetrics & Gynaecology. 1960;67(4):644–645. doi: 10.1111/j.1471-0528.1960.tb09228.x. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak A. M., Gavaller B. Congenital systemic candidiasis, report of a case. The New England Journal of Medicine. 1966;274(10):540–543. doi: 10.1056/NEJM196603102741003. [DOI] [PubMed] [Google Scholar]
- 11.Abaci F., Aterman K. Monilial infection of the umbilical cord. Obstetrics & Gynecology. 1966;27(6):845–849. [PubMed] [Google Scholar]
- 12.Albarracin N. S., Jr., Patterson W. S., Haust M. D. Candida albicans infection of the placenta and fetus, Report of a case. Obstetrics & Gynecology. 1967;30(6):838–841. [PubMed] [Google Scholar]
- 13.Schweid A. I., Hopkins B. G. Monilial chorionitis associated with an intrauterine contraceptive device. Obstetrics & Gynecology. 1968;31(5):719–721. doi: 10.1097/00006250-196805000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Rhatigan R. M. Congenital cutaneous candidiasis. American Journal of Diseases of Children. 1968;116(5):545–546. doi: 10.1001/archpedi.1968.02100020549018. [DOI] [PubMed] [Google Scholar]
- 15.Lopez E., Aterman K. Intra-Uterine Infection by Candida. American Journal of Diseases of Children. 1968;115(6):663–670. doi: 10.1001/archpedi.1968.02100010665005. [DOI] [PubMed] [Google Scholar]
- 16.Ho C.-Y., Aterman K. Infection of the fetus by Candida in a spontaneous abortion. American Journal of Obstetrics & Gynecology. 1970;106(5):705–710. doi: 10.1016/0002-9378(70)90394-7. [DOI] [PubMed] [Google Scholar]
- 17.Misenhimer H. R., Garcia-Bunuel R. Failure of intrauterine contraceptive device and fungal infection in the fetus. Obstetrics & Gynecology. 1969;34(3):368–372. [PubMed] [Google Scholar]
- 18.Schirar A., Rendu C., Vielh J. P., Gautray J. P. Congenital mycosis (Candida albicans) Neonatology. 1974;24(5-6):273–288. doi: 10.1159/000240658. [DOI] [PubMed] [Google Scholar]
- 19.Brandsma M. A. C., Braaksma J. T., van der Harten J. J. Immature delivery after intrauterine Candida albicans infection. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1975;5(6):331–335. doi: 10.1016/0028-2243(75)90062-3. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph N., Tariq A. A., Reale M. R., Goldberg P. K., Kozinn P. J. Congenital cutaneous candidiasis. JAMA Dermatology. 1977;113(8):1101–1103. doi: 10.1001/archderm.113.8.1101. doi: 10.1001/archderm.113.8.1101. [DOI] [PubMed] [Google Scholar]
- 21.Quirke P., Hwang W. S., Validen G. C. Congenital Torulopsis glabrata infection in man. American Journal of Clinical Pathology. 1980;73(1):137–140. doi: 10.1093/ajcp/73.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan R., Sworn M. J., Noble A. D. Abortion associated with intrauterine infection by candida albicans case report. BJOG: An International Journal of Obstetrics & Gynaecology. 1979;86(9):741–744. doi: 10.1111/j.1471-0528.1979.tb11278.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D. E., Thompson T. R., Ferrieri P. Congenital Candidiasis. American Journal of Diseases of Children. 1981;135(3):273–275. doi: 10.1001/archpedi.1981.02130270065023. [DOI] [PubMed] [Google Scholar]
- 24.Nagata K., Nakamura Y., Hosokawa Y., et al. Intrauterine candida infection in premature baby. Acta Pathologica Japonica. 1981;31(4):695–699. doi: 10.1111/j.1440-1827.1981.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 25.Delprado W. J., Baird P. J., Russell P. Placental candidiasis: Report of three cases with a review of the literature. Pathology. 1982;14(2):191–195. doi: 10.3109/00313028209061293. [DOI] [PubMed] [Google Scholar]
- 26.Delaplane D., Wiringa K. S., Shulman S. T., Yogev R. Congenital mucocutaneous candidiasis following diagnostic amniocentesis. American Journal of Obstetrics & Gynecology. 1983;147(3):342–343. doi: 10.1016/0002-9378(83)91126-2. [DOI] [PubMed] [Google Scholar]
- 27.Bittencourt A. L., dos Santos W. L. C., de Oliveira C. H. Placental and fetal candidiasis - Presentation of a case of an abortus. Mycopathologia. 1984;87(3):181–187. doi: 10.1007/BF00436906. [DOI] [PubMed] [Google Scholar]
- 28.Honoré L. H. Placental candidiasis: Report of two cases, one associated with an IUCD in situ. Contraception. 1984;30(6):555–560. doi: 10.1016/0010-7824(84)90005-2. [DOI] [PubMed] [Google Scholar]
- 29.Romero R., Reece E. A., Duff G. W., Coultrip L., Hobbins J. C. Prenatal diagnosis of Candida albicans chorioamnionitis. American Journal of Perinatology. 1985;2(2):121–122. doi: 10.1055/s-2007-999928. [DOI] [PubMed] [Google Scholar]
- 30.Mamlok R. J., Joan Richardson C., Mamlok V., Nichols M. M., Goldblum R. M. A case of intrauterine pulmonary candidiasis. Pediatric Infectious Disease. 1985;4(6):692–693. doi: 10.1097/00006454-198511000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Spaun E., Klünder K. Candida Chorioamnionitis and Intra‐Uterine Contraceptive Device. Acta Obstetricia et Gynecologica Scandinavica. 1986;65(2):183–184. doi: 10.3109/00016348609158377. [DOI] [PubMed] [Google Scholar]
- 32.Bruner J. P., Elliott J. P., Kilbride H. W., Garite T. J., Knox G. E. Candida chorioamnionitis diagnosed by amniocentesis with subsequent fetal infection. American Journal of Perinatology. 1986;3(3):213–218. doi: 10.1055/s-2007-999870. [DOI] [PubMed] [Google Scholar]
- 33.Faix R. G., Naglie R. A., Barr M., Jr. Intrapleural inoculation of candida in an infant with congenital cutaneous candidiasis. American Journal of Perinatology. 1986;3(2):119–122. doi: 10.1055/s-2007-999846. [DOI] [PubMed] [Google Scholar]
- 34.Smith C. V., Horenstein J., Platt L. D. Intraamniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: A case report. American Journal of Obstetrics & Gynecology. 1988;159(1):123–124. doi: 10.1016/0002-9378(88)90505-4. [DOI] [PubMed] [Google Scholar]
- 35.Bider D., Ben-Rafael Z., Barkai G., Mashiach S. Intrauterine fetal death apparently due to Candida chorioamnionitis. Archives of Gynecology and Obstetrics. 1989;244(3):175–177. doi: 10.1007/BF00931296. [DOI] [PubMed] [Google Scholar]
- 36.Morgan M. A., Pippitt C. H., Thurnau G. R. Antenatal diagnosis of candida chorioamnionitis. Southern Medical Journal. 1989;82(2):p. 276. doi: 10.1097/00007611-198902000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Donders G. G. G., Moerman P., Caudron J., Van Assche F. A. Intra-uterine Candida infection: a report of four infected fetusses from two mothers. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1991;38(3):233–238. doi: 10.1016/0028-2243(91)90298-Y. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz D. A., Reef S. Candida albicans placentitis and funisitis: Early diagnosis of congenital candidemia by histopathologic examination of umbilical cord vessels. The Pediatric Infectious Disease Journal. 1990;9(9):661–664. [PubMed] [Google Scholar]
- 39.Arbegast K. D., Lamberty L. F., Koh J. K., Pergram J. M., Braddock S. W. Congenital candidiasis limited to the nail plates. Pediatric Dermatology. 1990;7(4):310–312. doi: 10.1111/j.1525-1470.1990.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 40.Mazor M., Chaim W., Pak I., Goldstein D. Intraamniotic infection with Candida albicans associated with a retained intrauterine device: a case report. Obstetrics, Gynaecology and Reproductive Medicine. 1992;37(11):950–952. [PubMed] [Google Scholar]
- 41.Chaim W., Mazor M., Meril T., Peleg R., Maor E. Late miscarriage and intraamniotic candidiasis in a woman with a retained intrauterine contraceptive device. Archives of Gynecology and Obstetrics. 1993;253(3):157–160. doi: 10.1007/BF02767335. [DOI] [PubMed] [Google Scholar]
- 42.Mazor M., Chaim W., Shinwell E., Glezerman M. Asymptomatic amniotic fluid invasion with Candida albicans in preterm premature rupture of membranes: Implications for obstetric and neonatal management. Acta Obstetricia et Gynecologica Scandinavica. 1993;72(1):52–54. doi: 10.3109/00016349309013351. [DOI] [PubMed] [Google Scholar]
- 43.Ng P. C., Siu Y. K., Lewindon P. J., Wong W., Cheung K. L., Dawkins R. Congenital Candida pneumonia in a preterm infant. Journal of Paediatrics and Child Health. 1994;30(6):552–554. doi: 10.1111/j.1440-1754.1994.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 44.Shalev E., Battino S., Romano S., Blondhaim O., Ben-Ami M. Intraamniotic infection with Candida albicans successfully treated with transcervical amnioinfusion of amphotericin. American Journal of Obstetrics & Gynecology. 1994;170(5 I):1271–1272. doi: 10.1016/S0002-9378(94)70140-7. [DOI] [PubMed] [Google Scholar]
- 45.Van Winter J. T., Ney J. A., Ogburn P. L., Jr., Johonson R. V. Preterm labor and congenital candidiasis, A case report. J Reprod Med. 1994;39(12):987–990. [PubMed] [Google Scholar]
- 46.Jin Y., Endo A., Shimada M., et al. Congenital systemic candidiasis. The Pediatric Infectious Disease Journal. 1995;14(9):818–820. doi: 10.1097/00006454-199509000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Nichols A., Khong T. Y., Crowther C. A. Candida tropicalis chorioamnionitis. American Journal of Obstetrics & Gynecology. 1995;172(3):1045–1047. doi: 10.1016/0002-9378(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 48.Raval D. S., Barton L. L., Hansen R. C., Kling P. J. Congenital cutaneous candidiasis: Case report and review. Pediatric Dermatology. 1995;12(4):355–358. doi: 10.1111/j.1525-1470.1995.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 49.Khambadkone S. M., Dixit K. M., Divekar A., Joshi S. M., Irani S. F., Desai M. Congenital candidiasis. Indian Pediatrics. 1996;33(6):512–516. [PubMed] [Google Scholar]
- 50.DiLorenzo D. J., Wong G., Ludmir J. Candida lusitaniae chorioamnionitis in a bone marrow transplant patient. Obstetrics & Gynecology. 1997;90(4):702–703. doi: 10.1016/S0029-7844(97)00304-9. [DOI] [PubMed] [Google Scholar]
- 51.Engelhart C. M., Van De Vijver N. M. A., Nienhuis S. J., Hasaart T. H. M. Fetal Candida sepsis at midgestation: A case report. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1998;77(1):107–109. doi: 10.1016/S0301-2115(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 52.Berry D. L., Olson G. L., Wen T. S., Belfort M. A., Moise K.J. J. Candida chorioamnionitis: A report of two cases. The Journal of Maternal-Fetal Medicine. 1997;6(3):151–154. doi: 10.1002/(SICI)1520-6661(199705/06)6:3<151::AID-MFM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 53.Rivasi F., Gasser B., Bagni A., Ficarra G., Negro R. M., Philippe E. Placental candidiasis: Report of four cases, one with villitis. APMIS-Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1998;106(12):1165–1169. doi: 10.1111/j.1699-0463.1998.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 54.Pradeepkumar V. K., Rajadurai V. S., Tan K. W. Congenital Candidiasis: Varied Presentations. Journal of Perinatology. 1998;18(4):311–316. [PubMed] [Google Scholar]
- 55.Roqué H., Abdelhak Y., Young B. K. Intra amniotic candidiasis. Case report and meta-analysis of 54 cases. Journal of Perinatal Medicine. 1999;27(4):253–262. doi: 10.1515/JPM.1999.036. [DOI] [PubMed] [Google Scholar]
- 56.Waguespack-LaBiche J., Chen S.-H., Yen A. Disseminated congenital Candidiasis in a premature infant. JAMA Dermatology. 1999;135(5):510–512. doi: 10.1001/archderm.135.5.510. [DOI] [PubMed] [Google Scholar]
- 57.Rode M. E., Morgan M. A., Ruchelli E., Forouzan I. Candida chorioamnipnitis after serial therapeutic amniocenteses: a possible association. Journal of Perinatology. 2000;20(5):335–337. doi: 10.1038/sj.jp.7200381. [DOI] [PubMed] [Google Scholar]
- 58.Horn L.-C., Nenoff P., Ziegert M., Höckel M. Missed abortion complicated by Candida infection in a woman with rested IUD. Archives of Gynecology and Obstetrics. 2001;264(4):215–217. doi: 10.1007/s004040000117. [DOI] [PubMed] [Google Scholar]
- 59.Segal D., Gohar J., Huleihel M., Mazor M. Fetal death associated with asymptomatic intrauterine Candida albicans infection and a retained intrauterine contraceptive device. Infectious Diseases. 2001;33(1):77–78. doi: 10.1080/003655401750064158-1. [DOI] [PubMed] [Google Scholar]
- 60.Barth T., Broscheit J., Bussen S., Dietl J. Maternal sepsis and intrauterine fetal death resulting from Candida tropicalis chorioamnionitis in a woman with a retained intrauterine contraceptive device. Acta Obstetricia et Gynecologica Scandinavica. 2002;81(10):981–982. doi: 10.1034/j.1600-0412.2002.811014.x. [DOI] [PubMed] [Google Scholar]
- 61.Diana A., Epiney M., Ecoffey M., Pfister R. E. "White dots on the placenta and red dots on the baby": Congential cutaneous candidiasis - A rare disease of the neonate. Acta Paediatrica. 2004;93(7):996–999. doi: 10.1080/08035250410028093. [DOI] [PubMed] [Google Scholar]
- 62.Matsuzawa S., Ohyama M., Kawataki M., et al. Congenital candida clabrata infection without specific nodules on the placenta and umbilical cord. The Pediatric Infectious Disease Journal. 2005;24(8):744–745. doi: 10.1097/01.inf.0000173611.59475.30. [DOI] [PubMed] [Google Scholar]
- 63.Freydiere A. M., Piens M. A., Andre J. M., Putet G., Picot S. Successful treatment of Candida glabrata peritonitis with fluconazole plus flucytosine in a premature infant following in vitro fertilization. European Journal of Clinical Microbiology & Infectious Diseases. 2005;24(10):704–705. doi: 10.1007/s10096-005-0034-6. [DOI] [PubMed] [Google Scholar]
- 64.Crawford J. T., Pereira L., Buckmaster J., Gravett M. G., Tolosa J. E. Amniocentesis results and novel proteomic analysis in a case of occult candidal chorioamnionitis. The Journal of Maternal-Fetal and Neonatal Medicine. 2006;19(10):667–670. doi: 10.1080/14767050600738289. [DOI] [PubMed] [Google Scholar]
- 65.Meizoso T., Rivera T., Fernández-Aceñero M. J., Mestre M. J., Garrido M., Garaulet C. Intrauterine candidiasis: Report of four cases. Archives of Gynecology and Obstetrics. 2008;278(2):173–176. doi: 10.1007/s00404-007-0554-7. [DOI] [PubMed] [Google Scholar]
- 66.Lee H. S. J., Lo A. W. I. Placental and fetal candidiasis associated with intrauterine contraceptive device in situ. Pathology. 2011;43(3):276–277. doi: 10.1097/PAT.0b013e328343ca18. [DOI] [PubMed] [Google Scholar]
- 67.Canpolat F. E., Çekmez F., Tezer H. Chorioamnionitis and neonatal sepsis due to Candida tropicalis. Archives of Gynecology and Obstetrics. 2011;283(4):919–920. doi: 10.1007/s00404-010-1677-9. [DOI] [PubMed] [Google Scholar]
- 68.Huang M., Cham E. M., Eppes C. S., Gerber S. E., Reed K. D., Ernst L. M. Placental and fetal findings in intrauterine Candida lusitaniae infection following in vitro fertilization and embryo transfer. Pediatric and Developmental Pathology. 2012;15(2):127–131. doi: 10.2350/11-04-1019-CR.1. [DOI] [PubMed] [Google Scholar]
- 69.Özer E., Ünlü M., Erşen A., Gülekli B. Intrauterine fetal loss associated with Candida glabrata chorioamnionitis: Report of two cases. Turk Patoloji Dergisi/Turkish Journal of Pathology. 2013;29(1):77–79. doi: 10.5146/tjpath.2013.01154. [DOI] [PubMed] [Google Scholar]
- 70.Ito F., Okubo T., Yasuo T., et al. Premature delivery due to intrauterine Candida infection that caused neonatal congenital cutaneous candidiasis: A case report. Journal of Obstetrics and Gynaecology Research. 2013;39(1):341–343. doi: 10.1111/j.1447-0756.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- 71.Bean L. M., Jackson J. R., Dobak W. J., Beiswenger T. R., Thorp J. A. Intra-amniotic fluconazole therapy for Candida albicans intra-amniotic infection. Obstetrics & Gynecology. 2013;121(2):452–454. doi: 10.1097/AOG.0b013e31827566ca. [DOI] [PubMed] [Google Scholar]
- 72.Iwatani S., Murakami Y., Mizobuchi M., et al. Successful management of an extremely premature infant with congenital candidiasis. American Journal of Perinatology Reports. 2014;4(01):005–008. doi: 10.1055/s-0033-1358766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alfei A., Rizzo A., Cavanna C., Lallitto F., Spinillo A. Candida glabrata and pre-term premature rupture of membrane complicating in vitro pregnancy: case report and confirmation of mother to neonate transmission. Archives of Gynecology and Obstetrics. 2014;290(2):211–214. doi: 10.1007/s00404-014-3222-8. [DOI] [PubMed] [Google Scholar]
- 74.Ganer Herman H., Mevorach Zussman N., Krajden Haratz K., Bar J., Sagiv R. Candida glabrata chorioamnionitis following in vitro fertilization: Review of the literature. Gynecologic and Obstetric Investigation. 2015;80(3):145–147. doi: 10.1159/000431221. [DOI] [PubMed] [Google Scholar]
- 75.Poliquin V., Al-Sulmi E., Menticoglou S. Intra-amniotic infection involving Candida albicans subsequent to emergency cerclage: a case series. Canadian Journal of Infectious Diseases & Medical Microbiology. 2015;26(5):245–246. doi: 10.1155/2015/589078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Flores J., Cruceyra M., Cañamares M., Garicano A., Nieto O., Tamarit I. Candida chorioamnionitis: Report of two cases and review of literature. Journal of Obstetrics & Gynaecology. 2016;36(7):843–844. doi: 10.1080/01443615.2016.1196479. [DOI] [PubMed] [Google Scholar]
- 77.Levin S., Zaidel L., Bernstein D. Intrauterine infection of fetal brain by candida. American Journal of Obstetrics & Gynecology. 1978;130(5):597–599. doi: 10.1016/0002-9378(78)90092-3. [DOI] [PubMed] [Google Scholar]
- 78.Sfameni S. F., Talbot J. M., Chow S. L., Brenton L. A., Scurry J. P. Candida G lab rata chorioamnionitis following in vitro fertilization and embryo transfer. ANZJOG. 1997;37(1):88–91. doi: 10.1111/j.1479-828X.1997.tb02225.x. [DOI] [PubMed] [Google Scholar]
- 79.Donders G. G. G., Gordts S., Cornelis A., Moerman P. Intrauterine candidiasis in a twin pregnancy after myomectomy, in vitro fertilization and embryo transfer. Archives of Gynecology and Obstetrics. 1997;259(4):201–204. doi: 10.1007/BF02505333. [DOI] [PubMed] [Google Scholar]
- 80.Friebe-Hoffmann U., Bender D. P., Sims C. J., Rauk P. N. Candida albicans chorioamnionitis associated with preterm labor and sudden intrauterine demise of one twin: a case report. Obstetrics, Gynaecology and Reproductive Medicine. 2000;45(4):354–356. [PubMed] [Google Scholar]
- 81.Arai H., Goto R., Matsuda T., et al. Case of congenital infection with Candida glabrata in one infant in a set of twins. Pediatrics International. 2002;44(4):449–450. doi: 10.1046/j.1442-200X.2002.01565.x. [DOI] [PubMed] [Google Scholar]
- 82.Ibara A. S., Marcorelles P., Le Martelot M. T., et al. Two cases of systemic candida glabrata infection following in vitro fertilization and embryo transfer. European Journal of Clinical Microbiology & Infectious Diseases. 2004;23(1):53–56. doi: 10.1007/s10096-003-1051-y. [DOI] [PubMed] [Google Scholar]
- 83.Krallis N., Tzioras S., Giapros V., et al. Congenital candidiasis caused by different candida species in a dizygotic pregnancy. The Pediatric Infectious Disease Journal. 2006;25(10):958–959. doi: 10.1097/01.inf.0000235683.57619.05. [DOI] [PubMed] [Google Scholar]
- 84.Carmo K. B., Evans N., Isaacs D. Congenital candidiasis presenting as septic shock without rash. Archives of Disease in Childhood. 2007;92(7):627–628. doi: 10.1136/adc.2007.115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asemota O. A., Nyirjesy P., Fox R., Sobel J. D. Candida glabrata complicating in vitro pregnancy: Successful management of subsequent pregnancy. Fertility and Sterility. 2011;95(2) doi: 10.1016/j.fertnstert.2010.07.1095. [DOI] [PubMed] [Google Scholar]
- 86.Pineda C., Kaushik A., Kest H., Wickes B., Zauk A. Maternal sepsis, chorioamnionitis, and congenital Candida kefyr infection in premature twins. The Pediatric Infectious Disease Journal. 2012;31(3):320–322. doi: 10.1097/INF.0b013e31823eee1a. [DOI] [PubMed] [Google Scholar]
- 87.Jackel D., Lai K. Candida glabrata sepsis associated with chorioamnionitis in an in vitro fertilization pregnancy: Case report and review. Clinical Infectious Diseases. 2013;56(4):555–558. doi: 10.1093/cid/cis936. [DOI] [PubMed] [Google Scholar]
- 88.Akhanoba F., MacDougall J., Mathur R., Hassan W. Severe systemic candidiasis following immunomodulation therapy in in vitro fertilisation-embryo transfer (IVF-ET) BMJ Case Reports. 2014;2014 doi: 10.1136/bcr-2013-203202.203202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan S. Q., Ng O. T., Khong C. C. Candida glabrata sepsis associated with chorioamnionitis in an IVF twin pregnancy: should we deliver? Journal of Obstetrics and Gynaecology Research. 2015;41(6):962–966. doi: 10.1111/jog.12656. [DOI] [PubMed] [Google Scholar]
- 90.Chen W., Chen S., Tsai S., Tsao P., Tang R., Soong W. Congenital systemic fungus infection in twin prematurity—a case report and literature review. American Journal of Perinatology Reports. 2015;05(01):e046–e050. doi: 10.1055/s-0035-1548730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaim W., Mazor M., Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Archives of Gynecology and Obstetrics. 1992;251(1):9–15. doi: 10.1007/BF02718273. [DOI] [PubMed] [Google Scholar]
- 92.Kim S. K., Romero R., Kusanovic J. P., et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD) Journal of Perinatal Medicine. 2010;38(1):45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DiGiulio D. B., Romero R., Kusanovic J. P., et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. American Journal of Reproductive Immunology. 2010;64(1):38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiGiulio D. B., Romero R., Amogan H. P., et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0003056.e3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McDonald S. D., Murphy K., Beyene J., Ohlsson A. Perinatel outcomes of singleton pregnancies achieved by in vitro fertilization: a systematic review and meta-analysis. Journal of Obstetrics and Gynaecology Canada. 2005;27(5):449–459. doi: 10.1016/S1701-2163(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 96.Farr A., Kiss H., Holzer I., Husslein P., Hagmann M., Petricevic L. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(9):989–996. doi: 10.1111/aogs.12697. [DOI] [PubMed] [Google Scholar]
- 97.Briggs G. G., Freeman R. K. Drug in pregnancy and lactation. 10th. Wolters Kluwer; 2015. [Google Scholar]
- 98.Pappas P. G., Kauffman C. A., Andes D. R., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clinical Infectious Diseases. 2016;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mølgaard-Nielsen D., Pasternak B., Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. The New England Journal of Medicine. 2013;369(9):830–839. doi: 10.1056/NEJMoa1301066. [DOI] [PubMed] [Google Scholar]
- 100.Ophelders D. R. M. G., Gussenhoven R., Lammens M., et al. Neuroinflammation and structural injury of the fetal ovine brain following intra-amniotic Candida albicans exposure. Journal of Neuroinflammation. 2016;13(1, article 29) doi: 10.1186/s12974-016-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maneenil G., Payne M. S., Senthamarai Kannan P., et al. Fluconazole treatment of intrauterine Candida albicans infection in fetal sheep. Pediatric Research. 2015;77(6):740–748. doi: 10.1038/pr.2015.48. [DOI] [PubMed] [Google Scholar]