Abstract
Allogeneic blood or marrow hematopoietic cell transplantation continues to be the most potent anti-leukemic treatment for adult patients with standard, high-risk, or chemo-refractory acute myeloid leukemia. Until recently, this procedure was generally limited to those recipients who had an available matched-sibling donor or matched-unrelated donor. Technical advances in graft cell processing and manipulation, control of bidirectional T cell alloreactivity, graft-versus-host disease prophylaxis, and other supportive measures in haploidentical transplantation now enable nearly all patients with acute myeloid leukemia to benefit from the graft-versus-leukemia effect with substantial reduction in procedure-related mortality. Over recent years, haploidentical donors have been increasingly adopted as a valid donor source in allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the absence of an HLA-matched donor. Among centers of the European Society for Blood and Marrow Transplantation, the use of haploidentical related donor transplantation has increased by 250% since 2010, and 291% since 2005. On behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation, we summarize recent utilization trends in haploidentical transplantation for acute myeloid leukemia and describe the transformative changes in haploidentical hematopoietic cell transplantation techniques over the past decade, which have led to the current widespread use of this procedure. Furthermore, we review the efficacy of haploidentical hematopoietic cell transplantation for acute myeloid leukemia from available studies, including preliminary comparative studies, and bring attention to remaining unanswered questions and directions for future research. We conclude this report with our recommendations for the role of haploidentical hematopoietic cell transplantation in acute myeloid leukemia.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for acute myeloid leukemia (AML), with 3- to 5-year overall survival (OS) rates ranging from 23% to 88%1–3 depending on the AML risk profile, stage, and the presence or absence of minimal residual disease (MRD). AML continues to be the primary indication for allogeneic HCT, and the number of these procedures performed for AML among the European Society for Blood and Marrow Transplantation (EBMT) centers has steadily increased over the past decade, with the most recent report showing a record number of 6,189 allogeneic HCT performed in 2015 compared to 3,946 in 2010.4,5 In addition to the development of reduced-intensity (RI) conditioning regimens, thereby extending the use of allogeneic HCT for AML patients above the age of 60 or for those with co-morbidities, the significant growth of allogeneic HCT for AML is a result of the increased availability of alternative donors, particularly haploidentical family donors.
While a HLA-matched sibling donor (MSD) remains the preferred donor choice for optimal transplant outcomes, in reality, approximately only 30% of patients from Western countries have such a donor, therefore 70% of patients require an unrelated donor source.6,7 Interestingly, a recent analysis using population data from the USA has challenged this well-accepted sibling match probability and describes a variation in rates ranging between 13% to 51%.8 Perhaps more alarming is the finding of the effect of a 40-year decline in USA birth rates on decreased availability of a MSD for transplant-eligible patients. As such, the current generation of young adults (18 to 44 years) will be 1.5 times less likely to find a MSD during the peak need for HCT (at around 61 years of age) compared to their current adult counterparts (aged 45 to 64 years).8 It is expected that a similar evolution in MSD accessibility is occurring in Western Europe as total fertility rates remain low.9 These changes highlight the upcoming demand for and utilization of alternative donor sources.
Alternative donor options include HLA-matched unrelated donors (MUD), partially HLA-mismatched unrelated donors (MMUD), single or double umbilical cord blood units (sUCB or dUCB), and haploidentical (haplo) family donors. While MUD have traditionally been considered to be the next preferred donor following a MSD, the success of finding an 8/8 HLA MUD depends on race. While Caucasians have an approximately 75% likelihood of finding an 8/8 MUD, the probability falls to less than 20% for patients of African descent or other ethnic minorities.7,10 Furthermore, differences in laws for donor selection and recruitment among different countries limit or delay the acquisition of a MUD.10 The use of an unrelated donor or UCB product with a mismatch at one or two HLA loci expands the accessibility of HCT to the vast majority of patients, however, this is at the cost of an increased risk of poor transplant outcomes and/or increased expense, particularly with the use of UCB cells.
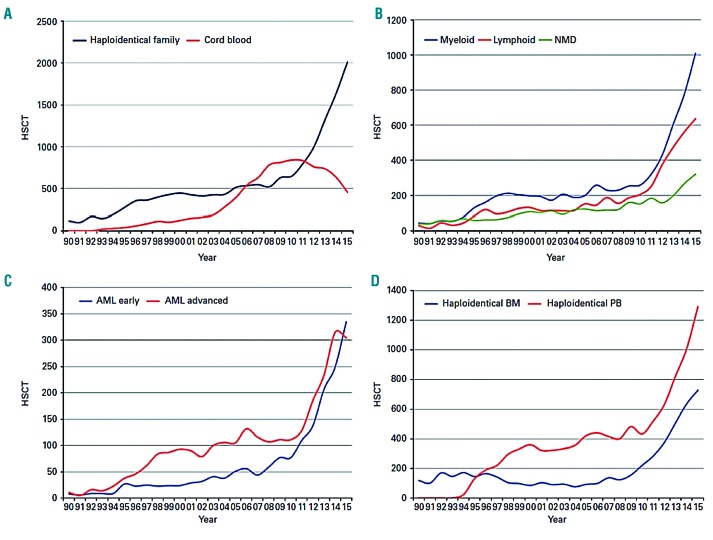
Over recent years, haploidentical donors have been increasingly adopted as a valid source of donor cells for allogeneic HCT of AML in the absence of an HLA-matched donor.4,11 A haploidentical related donor is defined by the sharing of one haplotype (or a single identical copy of chromosome 6) with the patient containing the HLA region involving class I and class II histocompatibility genes. However, a haploidentical family donor may be greater than half-matched and have common alleles on the unshared haplotype (mismatched related donor). The most recent EBMT activity survey report described haploidentical donors as a family member with two or more loci mismatch within the loci HLA-A, -B, -C, -DRB1 and- DQB1.4 Among centers of the EBMT, the use of haploidentical transplantation (haploHCT) for malignant and non-malignant disorders has surged by 250% since the year 2010, and by 291% since 2005. In 2010, 802 haploHCT were performed, and this number increased to 1,571 in 2013, followed by 2,012 haploHCT in 2015.4,11 The highest utilization for haploHCT in 2015 was seen in myeloid malignancies (n=1,008), and the majority of these patients had a diagnosis of AML (n=735), with an equal proportion of patients in first complete remission (CR1) or more advanced disease (Figure 1). In contrast, the utilization of unrelated umbilical cord blood transplantation (UCBT) has sharply declined for myeloid and lymphoid malignancies.4 This apparent preference for haploidentical donors is a result of improvements in conditioning regimens combined with new strategies to diminish the risk of graft-versus-host disease (GvHD) associated with one haplotype mismatched donors that have resulted in favorable clinical outcomes comparable to HLA-matched allogeneic HCT, compounded with the nearly universal and immediate availability of the donor and ease of recurrent stem cell collections for repeat cellular infusions. The ability to have rapid access to a haploidentical donor is a crucial benefit for patients with high-risk AML, as a delay in transplantation due to donor issues can result in poor outcomes.
Figure 1.
Trends in haploidentical HCT in Europe between 1990–2015. (A) Increasing use to haploidentical family HCT from cord blood HCT. (B) Increasing use of haploidentical HCT by main disease group. (C) Similar increase in rates of haploidentical HCT for AML early disease and AML advanced disease. (D) Haploidentical HCT by cell source; bone marrow (BM) versus peripheral blood (PB). Adapted from Passweg et al.4 used under theCreative Commons License. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplantation; NMD: non-malignant disorders.
On behalf of the Acute Leukemia Working Party (ALWP) of the EBMT, herein we aim to first describe the early strategies used in haploidentical transplantation and the pivotal developments that have made its use universal and available to nearly all patients requiring hematopoietic cell transplantation. We then summarize the evidence from available studies, evaluating its efficacy in AML, including preliminary non-comparative and comparative studies of haploHCT with other alternative donor transplants, and lastly, discuss future directions for research.
Early experiences in haploidentical transplantation
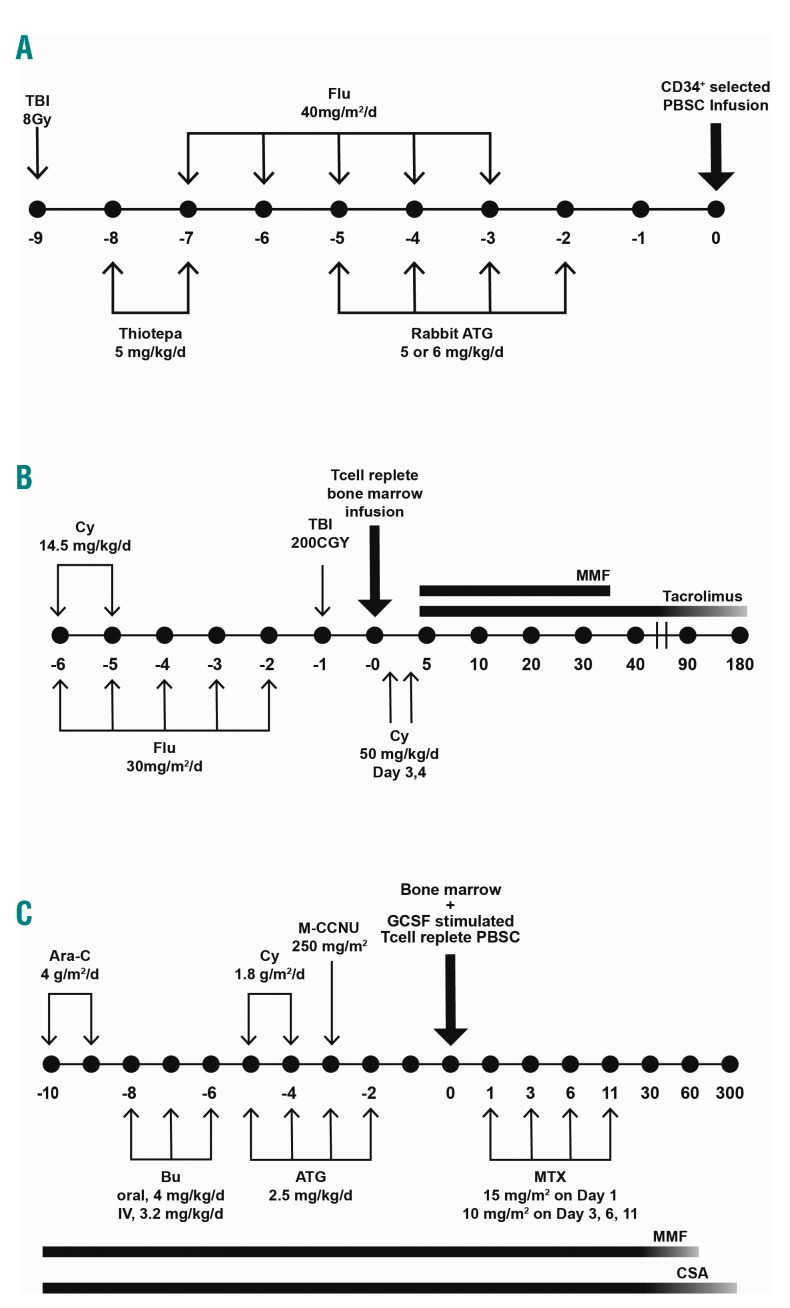
Initial experiences with unmodified bone marrow (BM) haploidentical HCT in acute leukemias generated poor outcomes as a consequence of intense bi-directional T cell alloreactivity associated with HLA-mismatches. Limited success was primarily related to delayed engraftment, graft failure, and acute graft-versus-host disease (aGvHD).12–15 In order to overcome these challenges, several alternative strategies were developed. In 1993, investigators from the University of Perugia pioneered a strategy of T cell-depletion (TCD) with ex vivo CD34+ cell selection and in vivo antithymocyte globulins (ATG) administration as sole prophylaxis for GvHD, accompanied by infusion of a large number of CD34+ cells following intensive myeloablative and immunosuppressive conditioning, with the rationale that this strategy would help promote engraftment and decrease graft failure (Figure 2A). “Mega-doses” of stem cells were obtained by combining TCD BM with granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cells (PBSC).16–18 With additional modifications, 95% of patients with acute leukemia (AL) achieved primary engraftment, and aGvHD and chronic GvHD (cGvHD) were minimal. With more than 15 years of follow-up, the relapse incidence (RI) was 17% in patients with AML who were transplanted in any complete remission (CR), while the 17-year disease-free survival (DFS) rate was 43%.19,20 In addition to a highly myeloablative regimen, the emergence of natural killer (NK) cell alloreactions following transplantation may explain the decreased incidence of relapse and improved survival.21–24 Despite the success of the anti-leukemic effects of this strategy, TCD haploHCT was associated with high transplant-related mortality (TRM) of up to 40% mainly due to a delay in immune recovery and life-threatening infections.18,19 Findings from an EBMT retrospective analysis of 173 adults with de novo AL who received a TCD haploidentical HCT in Europe showed similar outcomes, including high engraftment rate, negligible GvHD, and high TRM.25 To circumvent the pitfalls associated with TCD haploHCT, other forms of T-cell cellular therapy were exploited, including selective T-cell-depletion,26,27 adoptive transfer of donor T cells following transplant,28 selective T-cell add-backs29–32 and gene-modified donor T cells.33
Figure 2.
Commonly used platforms used in haploidentical-related transplantation.111 (A) University of Perugia: myeloablative conditioning and T cell-depletion with “megadose” CD34+ cell allografts. (B) Johns Hopkins: non-myeloablative conditioning with high-dose, post-transplantation cyclophosphamide. (C) Peking University: myeloablative conditioning and in vivo T cell modulation (GIAC protocol). Panel B was adapted from Luznik et al.44 used under the Creative Commons License. Ara-C: cytarabine; ATG: anti-thymocyte globulin; BM: bone marrow; Bu: busulfan; CSA: ciclosporin-A; Cy: cyclophosphamide; Flu: fludarabine; GCSF: granulocyte colony-stimulating factor; M-CCNU: semustine; MMF: mycophenolate mofetil; MTX: methotrexate; PBSC: peripheral-blood stem cell; TBI: total body irradiation.
Post-transplant cyclophosphamide: a pivotal point in haploidentical transplantation
The rationale behind the use of post-transplantation cyclophosphamide (PTCy) stems from early preclinical studies demonstrating its role in targeting alloreactive T cells and reducing GvHD when given within a narrow window following allografting.34–39 Furthermore, the finding of preserved hematopoietic stem and progenitor cells (and in later work, regulatory T cells40) when exposed to cyclophosphamide owing to the high expression of aldehyde dehydrogenase,41,42 gave rise to the first-in-human clinical trial at Johns Hopkins Hospital in 1999. Thirteen patients with high-risk hematologic malignancies underwent T cell-replete (TCR) haploidentical bone marrow transplantation (haploBMT) using a non-myeloablative (NMA) conditioning regimen consisting of fludarabine and low-dose total body irradiation (TBI), as well as 50 mg/kg of cyclophosphamide on day + 3 post-transplant, followed by tacrolimus and mycophenolic mofetil (MMF) on day + 4 for GvHD prophylaxis. Owing to a high rate of graft rejection (2 out of the first 3 patients), cyclophosphamide 14.5 mg/kg was introduced into the conditioning regimen. This adaptation resulted in an 80% primary engraftment rate (8 out of 10 patients), giving proof of concept to move into next phase studies.43
As the initial phase I study ultimately had a high cumulative incidence of graft failure and severe GvHD at 6 months post-transplant, Luznik et al.44 modified the regimen by adding an additional dose of cyclophosphamide 50mg/kg on day + 4 post-transplant (Figure 2B). In a collaborative phase 2 trial between Hopkins and Seattle, 68 patients with AML (n= 27) received the revised regimen, and results yielded an 87% engraftment rate, one-year non-relapse mortality (NRM) and relapse of 15% and 51%, respectively, and two-year OS and event-free survival (EFS) of 36% and 26%, respectively. Additionally, the cumulative incidences (CI) of grades II–IV and grade III–IV aGvHD by day 200 were 34% and 6%, respectively. A trend towards a lower incidence of extensive cGvHD with the use of 2 doses of PTCy as compared to one dose was noted (5% vs. 25%, P=0.05). In an updated analysis of 210 recipients of NMA haploBMT, the Hopkins group reported similar outcomes.45
Due to the early reports of success with unmanipulated haploidentical HCT and pioneering of PTCy for prevention of GvHD, other centers, mainly in Western Europe and the USA, have favored the use of TCR grafts over TCD haploHCT.10,46,47 Ciurea et al.46 reported significantly improved 1-year NRM (16% vs. 42%, P=0.02), OS (64% vs. 30%, P=0.02) and progression-free survival (PFS) (50% vs. 21%, P=0.02) in 65 consecutive patients treated with a myeloablative TCR haploBMT with PTCy (n=32), compared to a TCD PBSC graft with ATG followed by infusion of CD34+ selected cells and no other post-transplantation immunosuppression (n=33). Engraftment rate and grade II–IV aGvHD were not significantly different, whereas cGvHD was significantly lower in patients treated with a TCR graft. In conclusion, given the ease of donor acquisition and administration of PTCy-based protocols alongside the favorable results seen in patients with high-risk hematologic malignancies, more investigation into the role of haploHCT in the early steps of decisional algorithms for the treatment of acute leukemias is ongoing.
Comparative donor studies of haploidentical transplantion for acute myeloid leukemia
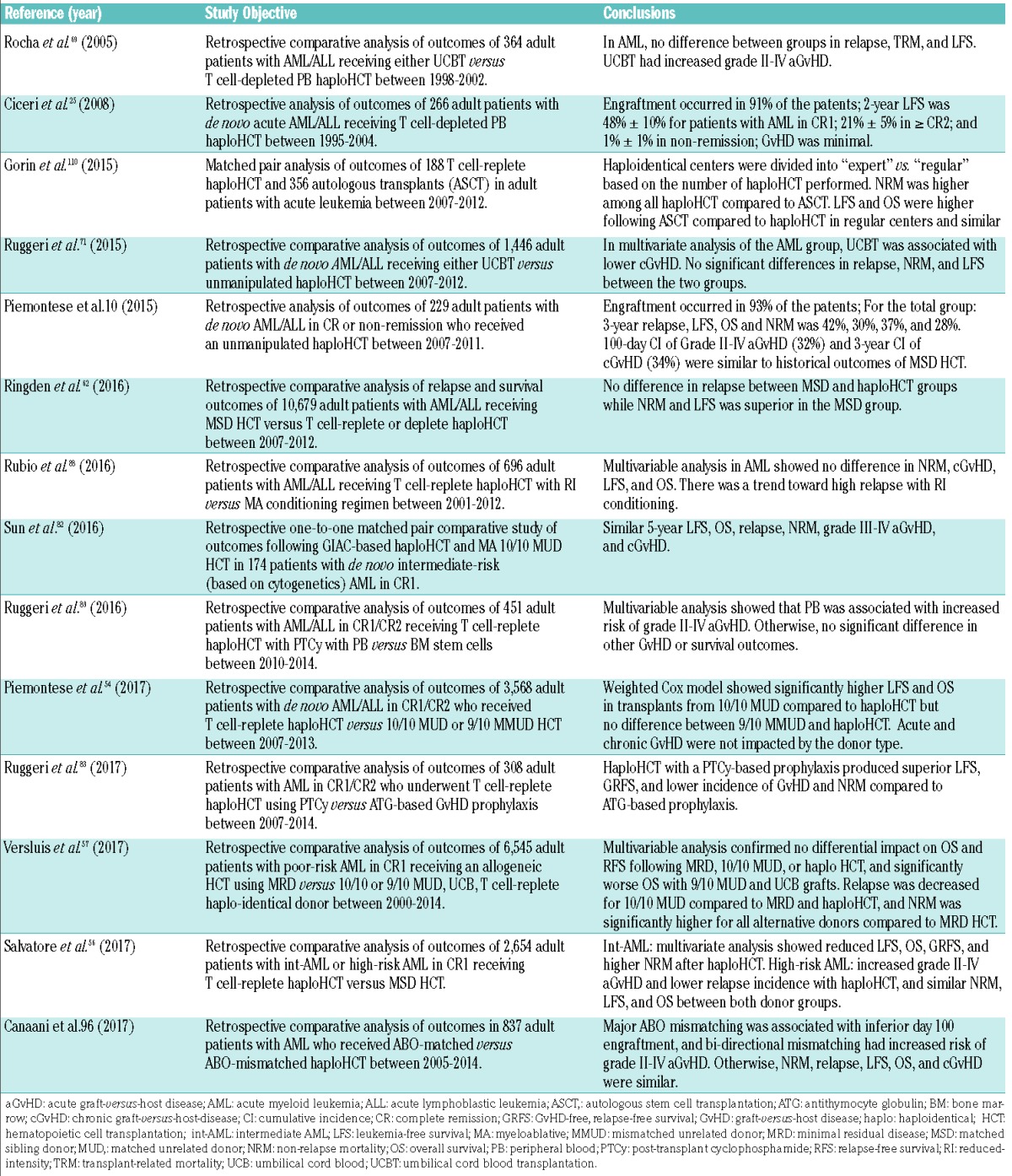
Haploidentical versus matched sibling or unrelated donor transplantation
At present there are no prospective randomized comparisons of transplantations using a haploidentical donor versus a MSD or MUD for AML. Based on several non-randomized comparative studies evaluating transplantation outcomes following haploidentical transplantation with post-transplant cyclophosphamide or other in vivo T cell-depletion methods,10,48–57 the combined data suggest similar outcomes for haploHCT compared with MSD and MUD HCT. Table 1 summarizes the available comparative studies of haploHCT with PTCy platform versus MSD or MUD HCT. In one of the earliest studies of haploHCT with PTCy, Bashey et al.49 demonstrated equivalent primary outcomes of 271 patients with a variety of hematologic malignancies (~ 34% AML), who contemporaneously underwent a T cell-replete haploidentical MSD or MUD transplant. However, post-relapse survival at 12 months was unexpectedly lower compared to a well-matched MSD or MUD HCT (17% vs. 67% vs. 63%, P<0.001). In an updated cohort of 475 patients (~ 36% AML) and median follow-up of 45 months, these investigators again reported non-significant differences between haplo, MSD, and MUD transplants in DFS (54% vs. 56% vs. 50%), OS (57% vs. 72% vs. 59%), CI of NRM (17% vs. 14% vs. 16%), and relapse (29% vs. 30% vs. 34%) at 2 years after transplantation. The CIs of grade II–IV aGvHD were not significantly different between haplo and MUD HCT, however, haploHCT was associated with a significantly higher incidence of aGvHD compared to MSD (P=0.005 for grade II–IV). The 2-year CI of moderate-severe cGvHD was also significantly lower in haploHCT than in MSD or MUD HCT recipients (31% vs. 44% vs. 47%, P=<0.05), and showed a similar trend for patients receiving a PBSC graft only.50 In another contemporaneously treated cohort of 227 patients with AML/myelodysplastic syndrome (MDS), Di Stasi et al.51 reported superimposable survival curves between haplo and 10/10 HLA MUD HCT, a non-significant improvement in outcomes with MSD HCT and a similar CI of GvHD across all donor groups.
Table 1.
Comparative studies of haploidentical HCT with PTCy versus matched donor HCT.
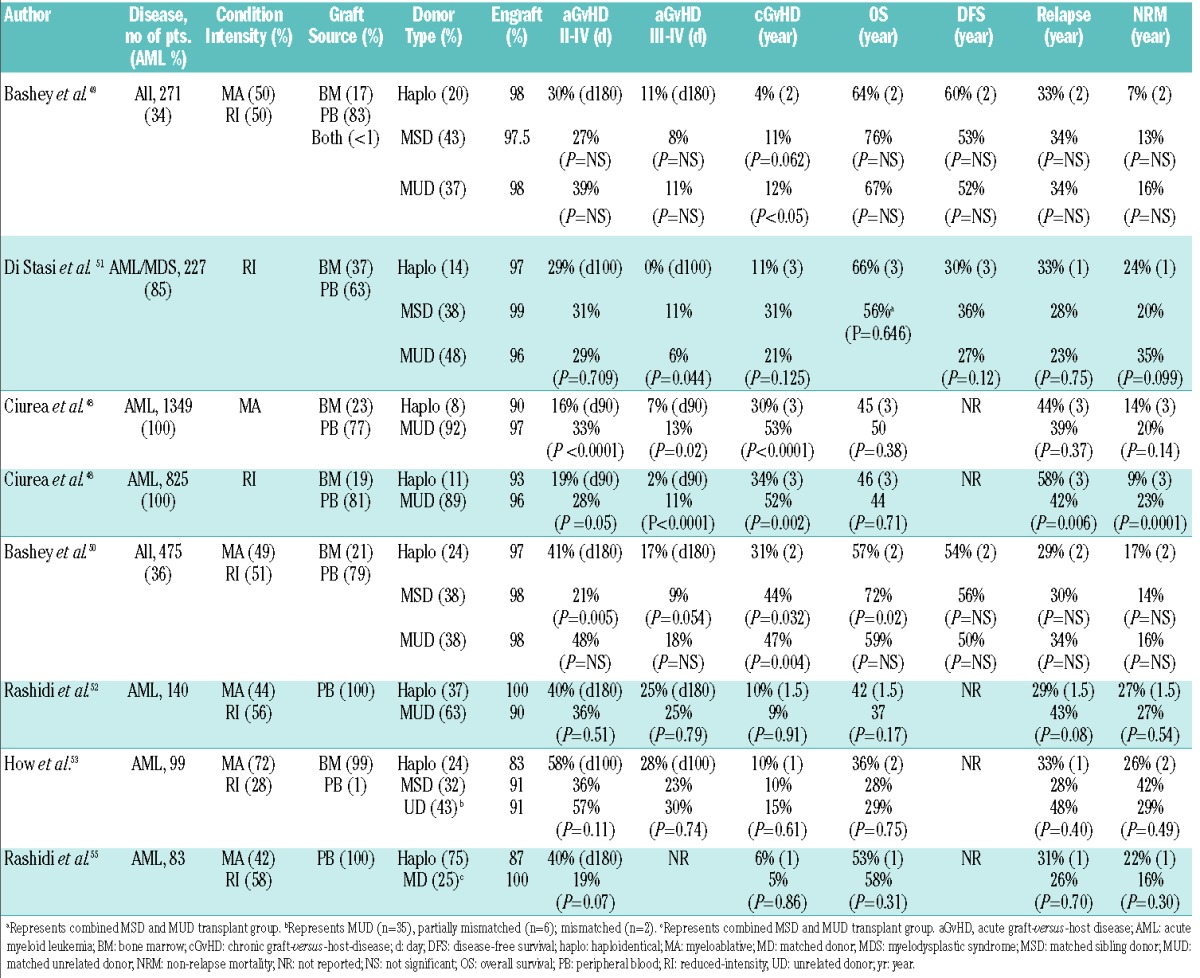
In the largest study carried out in the USA focusing on AML, Ciurea et al.48 utilized the Center for International Blood and Marrow Transplant Research (CIBMTR) registry data and reported comparable 3-year OS following haploidentical (n=192) and 8/8 HLA matched MUD HCT (n=1982) in patients with AML in various disease stages (CR1, CR2, and more advanced) who received either a myeloablative (MA) (45% vs. 50%, P=0.38) or RI (46% vs. 44%, P=0.71) conditioning regimen. Further subset analysis revealed no differences in 3-year NRM (14% vs. 20%, P=0.14) or relapse (44% vs. 39%, P=0.37) by donor type in the MA cohort, however, there was a significant decrease in 3-year NRM (9% vs. 23%, P=0.0001) and increase in relapse (58% vs. 42%, P=0.006) in the RI group. In both cohorts, 3-month grade II–IV and grade III–IV aGvHD, and 3-year cGvHD were lower after haploidentical compared with MUD transplants (MA: grade II–IV aGvHD: 16% vs. 33%, P<0.0001; grade III–IV aGvHD: 7% vs. 13%, P=0.02; cGvHD: 30% vs. 53%, P<0.0001; RI: grade II–IV aGvHD: 19% vs. 28%, P=0.05; grade III–IV aGvHD: 2% vs. 11%, P<0.0001; cGvHD: 33% vs. 52%, P=0.002). In this study, the majority of recipients of haploHCT received a BM graft, whereas PBSC were predominantly utilized in MUD HCT. Owing to the limitations inherent in an observational registry study, the investigators could not assess the impact of the donor source of stem cells on clinical outcomes. To address this question, Rashidi et al.52 reported results from a single-center retrospective analysis of 140 patients who underwent a haploHCT (n=52) or MUD HCT (n=88) with PBSC. This group showed a significantly faster neutrophil and platelet recovery in the MUD group, but no statistically significant difference in OS, NRM, aGvHD or cGvHD at 1.5 years. Lastly, the refined disease risk index (DRI) developed by Armand and colleagues58,59 in order to help stratify outcomes based upon disease risk and stage has been used to compare the effects of the graft-versus-tumor response mediated by NMA haploBMT with PTCy against historical outcomes in the setting of HLA-matched donor HCT following RI conditioning.60 Risk-stratified disease and their associated survival outcomes appeared similar between the two groups. For example, 3-year PFS estimates in the low-, intermediate-, and high/very high-risk patient groups following NMA haploBMT with PTCy were 65%, 37%, and 22%, respectively, and 66%, 31%, and 15% in the original DRI study cohort of recipients of RI HLA-matched donor transplantation.60
The viability of T cell-replete haploidentical HCT with post-transplantation cyclophosphamide in patients with active AML has also been described. How et al.53 compared outcomes of 99 patients who received either a MSD (n=32), unrelated donor (all unrelated, n=43; MUD, n=35), or a haploidentical related (n=24) donor transplantation for active AML, defined by ≥5% blasts in the pre-transplantation BM, persistent cytogenetics, or extramedullary disease. With a median follow-up of 18 months, no statistically significant differences between MSD, unrelated donor, and haploidentical donor HCT in 1- and 2-year OS were identified (1 yr: 28% vs. 41% vs. 45%; 2 yr: 28% vs. 29% vs. 36%, P=0.75), EFS (1 yr: 27% vs. 28% vs. 39%; 2 yr: 18% vs. 22% vs. 23%, P=0.93), TRM (1 yr: 42% vs. 23% vs. 26%; 2 yr: 42% vs. 29% vs. 26%, P=0.49), or 1-year relapse (28% vs. 48% vs. 33%, P=0.40). Similarly, the CI of grades III–IV aGvHD at day 100 (23% vs. 30% vs. 28%, P=0.74) and severe cGvHD at 1 year (10% vs. 15% vs. 10%, P=0.61) were comparable. Although not evaluated in a comparative donor study, RI T cell-replete haploHCT incorporating donor change and utilizing PTCy for postgrafting immunosuppression has also been successfully used for patients with AL relapsing after a first autologous or allogeneic transplantation.61 These results preliminarily support the decision to use a haploidentical related donor source in transplantation of patients with active AML or relapsed AML after first transplantation, as both of these patient populations have an urgent indication to proceed to transplantation and may have a readily available haploidentical family donor.
The ALWP of the EBMT have also published results of several large multi-center comparative studies using EBMT registry data (Table 2). In the first retrospective comparative analysis of 10,679 patients with AL who received allogeneic HCT from a MSD or a haploidentical donor, Ringden et al.62 sought to determine whether a stronger graft-versus-leukemia (GvL) effect is exerted with T cell-deplete or T cell-replete haploidentical transplantation due to the presence of mismatched major HLA antigens on leukemic cells. The investigators determined no difference in the probability of relapse between recipients of haploidentical and MSD grafts. In a more recent study, Salvatore et al.56 compared outcomes of T cell-replete haploHCT (n=185) to those from MSD HCT (n=2,469) among 2,654 adults with intermediate-/high-risk AML in first CR. GvHD prophylaxis consisted of PTCy in 74% of patients and ATG in 26%. In multivariate analyses of patients with intermediate-risk AML, haploHCT was associated with reduced 2-year leukemia-free survival (LFS), OS and GvHD-free, relapse-free survival (GRFS), and higher NRM as compared to MSD HCT. In high-risk AML patients, 2-year RI was lower in haploHCT, however, no other differences were observed in NRM, LFS, OS, and GRFS.56 In a separate registry study which focused on 6,545 patients with poor-risk AML in CR1, Versluis et al.57 reported similar 2-year OS following MSD (n=3,511) with 10/10 MUD (n=1,959) and haploHCT (n=193) (hazard ratio [HR], 0.99 and 1.12, respectively), whereas both 9/10 MUD (n =549) and UCB (n=333) grafts were associated with inferior OS (HR, 1.23, P=0.005; and HR, 1.54, P<0.001, respectively). Although the RI was decreased for 10/10 MUD (HR, 0.74, P<0.001) and haplo (HR, 0.60, P=0.001) compared with MSD HCT, NRM was significantly higher. Lastly, Piemontese et al.54 described clinical outcomes from T cell-replete haploHCT versus allogeneic transplants from 10/10 HLA matched and 9/10 HLA mismatched unrelated donors (MMUD) for adult patients with de novo AL in CR1/CR2. In this cohort, 265 patients (AML, n=176) received a haploHCT, 2,490 patients (AML, n=1,645) received a 10/10 MUD, and 813 patients (AML, n=510) received a MMUD transplant. Post-transplant cyclophosphamide was used as GvHD prophylaxis in 40% of haploHCT. Among patients with AML, 3-year LFS, OS, and NRM were significantly improved in 10/10 MUD compared to haploHCT, but there was no difference in GRFS, grade II–IV aGvHD or cGvHD. Further, no differences were found in GvHD or survival outcomes between 9/10 MMUD HCT and haploHCT. Based on the collective data, outcomes from haploidentical transplantation are encouraging, however, a larger cohort, longer follow-up period, and prospective comparative donor analyses are needed in order to firmly establish its place in the hierarchy of alternative donors. At this time, the ALWP-EBMT supports a 10/10 MUD as the best donor option in the absence of a MSD, and further supports the use of a haploidentical donor or 9/10 MMUD as equally viable alternatives in the absence of a fully matched donor, or in the case of the need for an urgent transplant.
Table 2.
Published ALWP-EBMT studies of haploidentical transplantation in adults with AML.
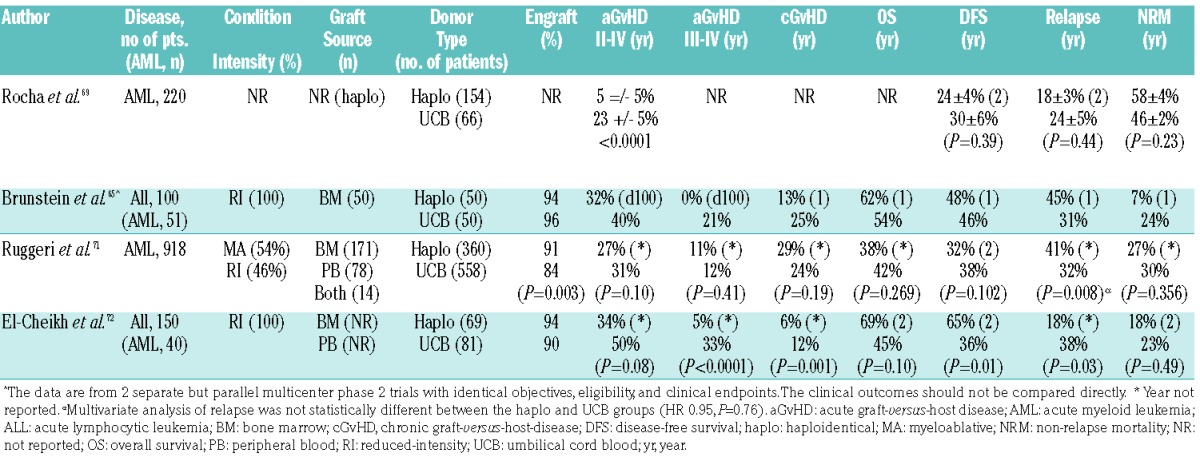
Haploidentical versus UCBT
Single- and multi-center studies have also shown the value of single or double UCBT for AML in the setting of an urgent need for transplant and lack of an HLA-matched sibling or an unacceptable unrelated donor.63–68 Therefore, early comparative studies of alternative donor sources focused on examining differences in clinical outcomes with the use of haploidentical or UCB as sources of stem cells (Figure 3).65,69–72 In the earliest retrospective comparative study, the Eurocord group, in collaboration with the ALWP-EBMT, reported outcomes on 220 adult recipients who received T cell-deplete haploHCT with PBSC (n=154) or unrelated single or double UCBT (n=66) for AML. The 2-year incidences of relapse, TRM and LFS were not statistically different after haploHCT or UCBT, however, UCBT was associated with delayed neutrophil recovery and a higher incidence of aGvHD.69 In another large EBMT observational study of 918 AML patients (haplo, n=158; UCBT, n=558), Ruggeri et al.71 demonstrated similar findings of a comparable RI (HR=0.95, P=0.76), NRM (HR=1.16, P=0.47), and LFS (HR=0.78, P=0.78) between unmanipulated haploHCT and UCBT. While grade II–IV and grade III–IV aGvHD were similar between the two groups, the CI of cGvHD was less in the UCBT cohort (HR=0.63, P=0.008).
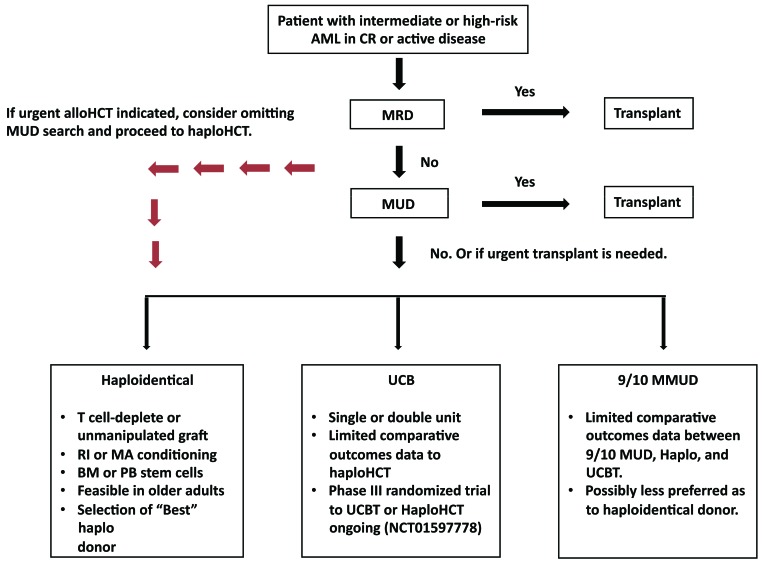
Figure 3.
Recommended donor choice algorithm for adults with intermediate or high-risk AML with an indication for allogeneic HCT. AML: acute myeloid leukemia; BM: bone marrow; CR: complete remission; alloHCT: allogeneic hematopoietic cell transplantation; haploHCT: haploidentical hematopoietic cell transplantation; MMUD: mismatched unrelated donor; MRD: matched related donor; MUD: matched unrelated donor; PB: peripheral blood; UCB: umbilical cord blood; UCBT: umbilical cord blood transplantation. RI: reduced-intensity; MA: myeloablative.
In order to study the reproducibility of the results found in retrospective analyses, the USA Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) conducted two parallel multicenter prospective clinical trials focused on outcomes associated with unmanipulated related haplo-BM graft with PTCy (n=50) and dUCBT (n=50) following identical RI conditioning regimens for patients with high-risk leukemia or lymphoma (AML: haplo, n=22; dUCB, n=29). Results from this trial successfully replicated those from single-center studies and showed no significant differences between the two modalities.65 Furthermore, both cohorts had comparable survival rates to patients with high-risk hematologic malignancies who underwent MUD HCT with blood or marrow after RI conditioning.73 An ongoing phase III multicenter randomized trial (clinicaltrials.gov Identifier: 01597778) is attempting to clarify the relative efficacies of double unrelated cord and haploidentical related BMT, and the estimated completion date of the trial is June 2019.
The Beijing experience
An alternative strategy for prevention of GvHD after T cell-replete haploidentical donor transplantation has incorporated pre-transplant ATG. The Peking University group in China pioneered an approach that used a combination of G-CSF-priming of the donor, intensified immunosuppression, ATG, and combination of T cell-replete BM plus peripheral blood as the stem cell source (GIAC protocol) (Figure 2C).74,75 An early trial in patients with acute leukemia, including 108 AML patients, suggested encouraging GvL effects with universal engraftment, low incidence of relapse following transplantation (13 out of 108 AML patients) and 3-year relapse probabilities of 11.9% and 20.2% in the standard- and high-risk AML groups, resulting in DFS rates of 71% and 56%, respectively. While TRM at D+100 was favorable in both risk groups, the 3-year TRM in standard-risk and high-risk AML groups was 19.4% and 29.4%. The CI of grade II–IV and grade III–IV aGvHD were 45.8% and 13.4% at D+100, respectively, while the 3-year CI of total cGvHD and extensive cGvHD were 53.9% and 22.6%.75 An updated trial including 756 patients with AL over a time period of 9 years confirmed their previous findings.76 A subsequent comparative study in patients with AML who received the GIAC haploHCT protocol revealed a similar CI of acute and chronic GvHD, TRM, 5-year relapse and OS rates when compared to MUD HCT, but a significantly reduced incidence of 5-year relapse (14.2% vs. 34%, P=0.008) compared to MSD HCT. A superior GvL effect for high-risk leukemia was also observed in haploHCT, as 5-year relapse rates were 15.4%, 28.2%, and 49.9% in haplo, MUD (P=0.07), and MSD HCT, respectively (P=0.002).77 Results from Wang et al.78 also suggested a superior GvL effect by haploHCT compared to a matched sibling HCT in patients with high-risk AL (50 AML out of 117), whereas other studies indicated no significant difference.79,80 In three of the four studies, grade II–IV aGvHD was significantly more frequent after haploHCT compared to MSD HCT. In the only prospective study comparing post-transplantation outcomes in 450 patients with intermediate- or high-risk AML in CR1 who received a haplo or MSD HCT, Wang et al.81 demonstrated a similar CI of relapse (15% vs. 15%, P=0.98), 3-year DFS (74% vs. 78%, P=0.34), NRM (13% vs. 8%, P=0.13), and OS (79% vs. 82%, P=0.36). The CI of 100-day aGvHD and 1-year cGvHD, including severe cGvHD, was significantly higher in the haploHCT group. Owing to the lack of randomization, this comparative study suggests haploidentical HCT as a valid alternative option for this patient population for whom no matched sibling donor is available.
Due to the reported high leukemia-free survival rates associated with the Beijing strategy, the ALWP of the EBMT performed a retrospective one-to-one matched pair comparative study of outcomes following GIAC-based haploidentical HCT and myeloablative (non-TBI based) 10/10 MUD HCT in patients with de novo intermediate-risk (based on cytogenetics) AML in CR1.82 Subjects were matched in age, time to transplant, and number of induction courses to reach CR1. Similar outcomes were observed between haploHCT and MUD HCT in terms of 5-year LFS (73.5% vs. 60.3%, P=0.15), OS (78.2% vs. 63.6%, P=0.15), relapse (12.7% vs. 24%, P=0.08), NRM (13.8% vs. 15.7%, P=0.96), grade III–IV aGvHD (9.2% vs. 9.4%, P=1), and cGvHD (42.5% vs. 34.9%, P=0.39). Based on this analysis, the authors concluded that the Beijing protocol is a feasible alternative to allogeneic transplantation with a 10/10 MUD.
Following several publications showing very low incidences of GvHD after ATG-based intensive immunosuppression established in the GIAC haploHCT protocol, Ruggeri et al.83 compared this GvHD prophylaxis regimen to the PTCy platform in the setting of unmanipulated haploHCT for patients with various-risk AML in CR1 or CR2. A total of 308 patients were studied (PTCy, n=193; ATG, n=115), and both groups were well matched in regards to recipient and donor age, AML disease risk, disease status at transplant, and conditioning intensity. Notably, a BM stem cell source was used more frequently in the PTCy group (60.1% vs. 39.9%, P=0.01), and that cohort also had shorter follow up (18 vs. 36 months, P<0.001). At day 100, similar outcomes in grade II–V aGvHD were observed between patients receiving PTCy versus ATG (31% vs. 21%, P=0.07), however, grade III–IV aGvHD was significantly lower in the PTCy group (4.7% vs. 12.5%, P=0.01). The incidence of 2-year cGvHD did not differ between the two groups (33.7% vs. 28.3%, P=0.33). Multivariate analysis of NRM, LFS, OS, and GRFS also significantly favored the PTCy regimen.
Although different haploHCT methods have not been prospectively compared in a randomized fashion, the available cumulative evidence demonstrates the feasibility of haploidentical transplantation and the benefit of having a readily accessible donor, regardless of the platform used.
Ongoing research in T cell-replete haploidentical transplantation
Since the demonstration of the safety and efficacy of NMA haploHCT with PTCy, there has been increasing research interest in optimizing clinical outcomes for different patient populations through modifications of the original platform. For example, some groups have explored optimizing the anti-leukemia effects of haploHCT, particularly in high-risk or advanced AML, by intensifying the conditioning regimen or substituting BM with PBSC as the stem cell graft source, due to the concern of high relapse rates associated with NMA haploHCT and PTCy. In the former setting, several single-center non-comparative studies have reported a low risk of acute and chronic GvHD and encouraging rates of TRM and OS with myeloablative conditioning.84–87 These observations were recently validated by the first large retrospective comparative analysis performed by the ALWP-EBMT showing similar OS, LFS, NRM, and cGvHD between MA and RI conditioning regimens in T cell-replete haploHCT, in particular for patients with AML in CR1.88 Multivariable analyses revealed a trend towards higher relapse incidence, with RI versus MA conditioning (HR 1.34, P=0.09), and when taken collectively the data supported the use of either high or low intensity conditioning haploHCT with PTCy in the first-line treatment of high-risk AML.88 In this study, there was an increased risk of grade II–IV aGvHD and cGvHD independent of the conditioning regimen intensity and of the use of PTCy with the use of a PBSC graft compared to BM, but no difference was seen with regard to the incidence of NRM and other survival outcomes.88 Ruggeri et al.89 also described the use of PBSC as the sole factor associated with an increased risk of grade II–IV aGvHD (HR 2.2, 95% CI 1.27–3.9, P=0.005) in patients with AL, the majority of whom were transplanted with a MA regimen for AML in CR1. Otherwise, the type of stem cell graft (PBSC vs. BM) proved to have no significant difference on grade III–IV aGvHD, cGvHD, relapse, or survival. In line with the attempts to exploit a PBSC source, Peccatori et al. developed a calcineurin inhibitor-free GvHD prophylaxis based on rapamycin, mycophenolate mofetil and ATG, with the aim of promoting a fast post-transplant immune recovery with a preferential accumulation of regulatory T cells.90 Recently, this sirolimus platform has been modified with the substitution of ATG by PTCy, which showed a significant reduction in cGvHD.91 In the NMA haploHCT setting, both Castagna et al.92 and O’ Donnell et al.93 reported comparable outcomes in acute and chronic GvHD, engraftment rates, NRM, and OS after haplo-BM or haplo-PBSC transplantation; however, the incidence of relapse at 1 to 3 years was significantly lower after haplo-PBSC transplant compared with haplo-BM transplants in the latter study. Other groups have also demonstrated the feasibility of NMA haploHCT with either PBSC or BM stem cells in older adults.94,95 The significance of ABO incompatibility on outcomes after haploHCT for AML have recently been published by the ALWP-EBMT, and preliminarily demonstrate a significantly increased risk of grade II–IV aGvHD with bi-directional ABO mismatching and a lower OS rate in patients with major ABO mismatching transplanted with BM grafts.96 Lastly, the impact of haploHCT for specific high-risk AML cytogenetic and molecular risk groups as well as the role of post-transplant cellular therapies are of interest.
Table 3.
Comparative studies of haploidentical HCT versus umbilical cord blood transplantation.
The significance of pre-transplant MRD as a poor prognostic and predictive factor of outcomes after allogeneic HCT in AML has been reported.3,97–99 For example, the Seattle group published inferior 3-year OS and relapse outcomes among AML patients receiving a myeloablative matched donor HCT with pre-transplant MRD-positive (morphologic remission) compared to MRD-negative (morphologic remission), and further demonstrated comparable outcomes to patients with active disease at the time of HCT.3 Several other groups have studied the significance of pre-transplant MRD on a more granular level and demonstrated that the level of pre-transplant MRD may differentially impact post-transplantation outcomes100,101 The significance of pre-transplant MRD has also been described in the setting of haploHCT.102,103 Wang et al.102 retrospectively evaluated outcomes of 255 patients with AML in CR1 or CR2. Multivariate analysis indicated failure of CR after 2 courses of induction therapy as the strongest independent prognostic factor for relapse and LFS. In subgroup analysis, positive pre-transplant MRD as compared to negative MRD also resulted in worse LFS at 3 years (76% vs. 52%, P=0.041) and CI of relapse at 2 years (10% vs. 35%, P=0.002). These results must be interpreted cautiously due to the limited patient sample (negative MRD, n=110; positive MRD, n=20). Conversely, other groups have reported no significant influence of MRD status (positive vs. negative) prior to haploHCT on PFS104 or relapse103 for patients with AML in CR1/CR2, and further hypothesize that while detectable MRD before HCT is a strong unfavorable prognostic factor, its adverse impact may be overcome by the potentially stronger GvL effects of unmanipulated haploHCT.103
Perspectives
Over the last two decades, the international BMT community have witnessed incredible advances in HLA-typing and alternative donor transplantation strategies, such that in the present day nearly all transplant-eligible patients with AML will have an available donor. Unmanipulated haploidentical related transplantation with post-transplant cyclophosphamide has emerged as a potentially powerful strategy for the cure of AML and is the dominant haploHCT platform in Europe.105 Other significant advantages of haploHCT with PTCy include its associated low non-relapse mortality and GvHD, ease of donor accessibility often leading to minimal length of time to transplantation, and low acquisition costs. The cost-effectiveness associated with haploHCT with PTCy may have the most appeal in developing countries, where economic resources are more limited.105 Unmanipulated haploidentical transplantation with post-transplantation immunosuppression also shows promise in decreasing post-transplant infections and death due to infections, however, further data on immune reconstitution, infections and their related complications (i.e., hemorrhagic cystitis) among different haploHCT strategies are warranted.106 The incidence of post-transplant cardiomyopathy secondary to GvHD prophylaxis with high-dose PTCy appears non-significant in the absence of severe infection,107 however, further research evaluating predictive factors for cardiomyopathy following PTCy based HCT are necessary. While there has been questioning of donor-derived malignancies (DDM) associated with PTCy, a recent retrospective study by the Hopkins group showed an extremely low proportion of patients with a DDM (4 out of 789) over a 10-year period, suggesting that PTCy does not appear to increase the risk of DDM.108 However, the authors acknowledge the short follow-up period of their study and report the need for continued close monitoring of DDMs over a longer follow-up time.
Another key issue arising in the setting of unmanipulated haploHCT is the selection of the “best” donor, as some patients will have multiple haploidentical donor candidates and donor selection may significantly impact GvHD, relapse, TRM, and survival outcomes. Owing to improved approaches of unmanipulated haploHCT with PTCy or ATG-based GvHD prophylaxis, the effects of HLA disparity have vanished, nonetheless, other donor-related variables should be considered. These include the selection of donors for whom there are no recipient donor-specific antibodies (DSA); alternatively, measures to remove DSA should be undertaken in the patient; the selection of a younger, male donor over an older, female donor due to the potential for superior survival, decreased risk of grade II–IV aGvHD and age-related clonal hematopoiesis leading to subsequent malignancies; and the selection of an ABO compatible donor, followed by a minor ABO mismatched and then a major ABO mismatched donor. Other factors to consider include donor and recipient cytomegalovirus (CMV) serostatus, NK cell alloreactivity and KIR haplotype matching, and non-inherited maternal HLA antigens (NIMA) mismatching.109 However, more research is needed as the significance of each of these factors may change depending on the haploHCT protocol or platform used, and indeed, may vanish, due to the emergence of new variables as haploHCT becomes increasingly utilized.
In conclusion, the growing body of literature has consistently demonstrated comparable outcomes of haploidentical donor HCT as compared to an UCB, matched sibling and unrelated donor transplantation for patients with AML. However, the available studies are nonrandomized, underpowered, and lack long-term follow-up data. Accordingly, the ALWP-EBMT endorses haploidentical transplantation as a valid post-remission therapy for high-risk AML in the absence of a matched donor or in the case of the need for an urgent transplant procedure (Figure 3). Further prospective studies randomizing haploHCT to UCBT (in the USA) or to MUD or MMUD HCT (in Europe) are ongoing, and will help to establish its position in the hierarchy of alternative donors.
Position statement from the ALWP- EBMT
Haploidentical donor transplantation is a valid option for patients with AML lacking a matched sibling or unrelated donor.
In certain clinical situations, especially in the case of a need for an urgent transplant procedure and lack of a MDS, a readily available haploidentical donor may be considered over initiating an unrelated donor search.
The evidence for the superiority of haploidentical vs. MMUD vs. UCBT is insufficient, but there is the potential for a cost benefit with regard to haploHCT.
There is insufficient evidence for the superiority of one haploidentical HCT platform over another. Economic factors, together with individual center experience, may be decisive.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1810
References
- 1.Zuckerman T, Rowe JM. Transplantation in acute myeloid leukemia. Hematol Oncol Clin North Am. 2014;28(6):983–994. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590. [DOI] [PubMed] [Google Scholar]
- 3.Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34(4):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52(6):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passweg JR, Baldomero H, Gratwohl A, et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012;47(7):906–923. [DOI] [PubMed] [Google Scholar]
- 6.Tiercy JM. How to select the best available related or unrelated donor of hematopoietic stem cells? Haematologica. 2016;101(6):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besse K, Maiers M, Confer D, Albrecht M. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: estimating the need for an unrelated donor source. Biol Blood Marrow Transplant. 2016;22(3):410–417. [DOI] [PubMed] [Google Scholar]
- 9.Employment, Social Affairs & Inclusion Eurostat Demography Report. European Commission Luxembourg: Publications Office of the European Union; 2015. [updated May 2015; cited 8 August 2017]. Available from: http://ec.europa.eu/eurostat/web/population-demography-migration-projections/overview [Google Scholar]
- 10.Piemontese S, Ciceri F, Labopin M, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(5):1069–1075. [DOI] [PubMed] [Google Scholar]
- 11.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765–771. [DOI] [PubMed] [Google Scholar]
- 13.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197–204. [DOI] [PubMed] [Google Scholar]
- 14.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29(2):79–91. [DOI] [PubMed] [Google Scholar]
- 15.Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1(8325):612–615. [DOI] [PubMed] [Google Scholar]
- 16.Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84(11):3948–3955. [PubMed] [Google Scholar]
- 17.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17): 1186–1193. [DOI] [PubMed] [Google Scholar]
- 18.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. [DOI] [PubMed] [Google Scholar]
- 19.Aversa F. Setting the standard in T-cell-depleted haploidentical transplantation and beyond. Best Pract Res Clin Haematol. 2011;24(3):325–329. [DOI] [PubMed] [Google Scholar]
- 20.Mancusi A, Ruggeri L, Velardi A. Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood. 2016;128(23):2616–2623. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. [PubMed] [Google Scholar]
- 22.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 23.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21(5):525–530. [DOI] [PubMed] [Google Scholar]
- 25.Ciceri F, Labopin M, Aversa F, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112(9):3574–3581. [DOI] [PubMed] [Google Scholar]
- 26.Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360(9327):130–137. [DOI] [PubMed] [Google Scholar]
- 27.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triplett BM, Shook DR, Eldridge P, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50(7):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111(2):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. [DOI] [PubMed] [Google Scholar]
- 32.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–644. [DOI] [PubMed] [Google Scholar]
- 33.Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10(5):489–500. [DOI] [PubMed] [Google Scholar]
- 34.Berenbaum MC, Brown IN. Prolongation of homograft survival in mice with single doses of cyclophosphamide. Nature. 1963;200:84. [DOI] [PubMed] [Google Scholar]
- 35.Berenbaum MC, Brown IN. Dose-response relationships for agents inhibiting the immune response. Immunology. 1964;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- 36.Santos GW, Owens AH. Production of graft-versus-host disease in the rat and its treatment with cytotoxic agents. Nature. 1966;210(5032):139–140. [DOI] [PubMed] [Google Scholar]
- 37.Mayumi H, Himeno K, Shin T, Nomoto K. Drug-induced tolerance to allografts in mice. VI. Tolerance induction in H-2-haplotype-identical strain combinations in mice. Transplantation. 1985;40(2):188–194. [DOI] [PubMed] [Google Scholar]
- 38.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. [DOI] [PubMed] [Google Scholar]
- 39.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8(3):131–138. [DOI] [PubMed] [Google Scholar]
- 40.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to post-transplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–1950. [PubMed] [Google Scholar]
- 42.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 43.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–386. [DOI] [PubMed] [Google Scholar]
- 44.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munchel A, Kesserwan C, Symons HJ, et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3 Suppl 2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devillier R, Bramanti S, Furst S, et al. T-replete haploidentical allogeneic transplantation using post-transplantation cyclophosphamide in advanced AML and myelodysplastic syndromes. Bone Marrow Transplant. 2016;51(2):194–198. [DOI] [PubMed] [Google Scholar]
- 48.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10): 1310–1316. [DOI] [PubMed] [Google Scholar]
- 50.Bashey A, Zhang X, Jackson K, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22(1):125–133. [DOI] [PubMed] [Google Scholar]
- 51.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12):1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rashidi A, DiPersio JF, Westervelt P, et al. Comparison of outcomes after peripheral blood haploidentical versus matched unrelated donor allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia: a retrospective single-center review. Biol Blood Marrow Transplant. 2016;22(9):1696–1701. [DOI] [PubMed] [Google Scholar]
- 53.How J, Slade M, Vu K, et al. T Cell-Replete T cell-replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide results in outcomes similar to transplantation from traditionally matched donors in active disease acute myeloid leukemia. Biol Blood Marrow Transplant. 2017;23(4):648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piemontese S, Ciceri F, Labopin M, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rashidi A, Slade M, DiPersio JF, Westervelt P, Vij R, Romee R. Post-transplant high-dose cyclophosphamide after HLA-matched vs haploidentical hematopoietic cell transplantation for AML. Bone Marrow Transplant. 2016;51(12):1561–1564. [DOI] [PubMed] [Google Scholar]
- 56.Salvatore D, Labopin M, Ruggeri A, et al. Outcomes of non T cell-depleted haploidentical HSCT versus HSCT from matched sibling donors in patients with acute myeloid leukemia in first complete remission, an ALWP-EBMT study. 22nd European Hematology Association Congress; 2017; Madrid, Spain; 2017. [Google Scholar]
- 57.Versluis J, Labopin M, Ruggeri A, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Advances. 2017;1:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armand P, Gibson CJ, Cutler C, et al. A dis ease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tischer J, Engel N, Fritsch S, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014;49(7):895–901. [DOI] [PubMed] [Google Scholar]
- 62.Ringden O, Labopin M, Ciceri F, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30(2):447–455. [DOI] [PubMed] [Google Scholar]
- 63.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8): 3064–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815–1822. [DOI] [PubMed] [Google Scholar]
- 67.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. [DOI] [PubMed] [Google Scholar]
- 68.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocha V, Aversa F, Labopin M, et al. Outcomes of unrelated cord blood and haploidentical stem cell transplantation in adults with acute leukaemia. Blood. 2005;106(11): 301. [Google Scholar]
- 70.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. [DOI] [PubMed] [Google Scholar]
- 71.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891–1900. [DOI] [PubMed] [Google Scholar]
- 72.El-Cheikh J, Crocchiolo R, Furst S, et al. Unrelated cord blood compared with haploidentical grafts in patients with hematological malignancies. Cancer. 2015;121(11): 1809–1816. [DOI] [PubMed] [Google Scholar]
- 73.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant. 2007;13(7):844–852. [DOI] [PubMed] [Google Scholar]
- 74.Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38(4):291–297. [DOI] [PubMed] [Google Scholar]
- 75.Huang XJ, Liu DH, Liu KY, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(2):257–265. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Liu DH, Liu KY, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119(5):978–985. [DOI] [PubMed] [Google Scholar]
- 77.Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Liu DH, Xu LP, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant. 2011;17(6):821–830. [DOI] [PubMed] [Google Scholar]
- 79.Chen XH, Gao L, Zhang X, et al. HLA-haploidentical blood and bone marrow transplantation with anti-thymocyte globulin: long-term comparison with HLA-identical sibling transplantation. Blood Cells Mol Dis. 2009;43(1):98–104. [DOI] [PubMed] [Google Scholar]
- 80.Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107(8):3065–3073. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–3962. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y, Beohou E, Labopin M, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair-matched comparative study of the Beijing approach with the EBMT database. Haematologica. 2016;101(8):e352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruggeri A, Sun Y, Labopin M, et al. Posttransplant cyclophosphamide versus anti-thymocyte globulin as graft- versus-host disease prophylaxis in haploidentical transplant. Haematologica. 2017;102(2):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1): 117–122. [DOI] [PubMed] [Google Scholar]
- 85.Bacigalupo A, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50 Suppl 2:S37–39. [DOI] [PubMed] [Google Scholar]
- 86.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12): 1859–1866. [DOI] [PubMed] [Google Scholar]
- 87.Solomon SR, Sizemore CA, Sanacore M, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21(7):1299–1307. [DOI] [PubMed] [Google Scholar]
- 88.Rubio MT, Savani BN, Labopin M, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruggeri A, Labopin M, Bacigalupo A, Gulbas Z, Koc YDB. Use of bone marrow or peripheral blood stem cell grafts in non T cell depleted haploidentical transplants using post-transplant cyclophosphamide, an ALWP-EBMT analysis. 58th American Society of Hematology; 2016; San Diego, CA; 2016. [Google Scholar]
- 90.Peccatori J, Forcina A, Clerici D, et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia. 2015;29(2):396–405. [DOI] [PubMed] [Google Scholar]
- 91.Cieri N, Greco R, Crucitti L, et al. Post-transplantation cyclophosphamide and sirolimus after conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant. 2015;21(8):1506–1514. [DOI] [PubMed] [Google Scholar]
- 92.Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20(5):724–729. [DOI] [PubMed] [Google Scholar]
- 93.O’Donnell PV, Eapen M, Horowitz MM, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant. 2016;51(12):1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blaise D, Furst S, Crocchiolo R, et al. Haploidentical T cell-replete transplantation with post-transplantation cyclophosphamide for patients in or above the sixth decade of age compared with allogeneic hematopoietic stem cell transplantation from a human leukocyte antigen-matched related or unrelated donor. Biol Blood Marrow Transplant. 2016;22(1):119–124. [DOI] [PubMed] [Google Scholar]
- 96.Canaani J, Savani BN, Labopin M, et al. Impact of ABO incompatibility on patients’ outcome after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia - a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017;102(6):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374(5):422–433. [DOI] [PubMed] [Google Scholar]
- 100.Anthias C, Dignan FL, Morilla R, et al. Pretransplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant. 2014;49(5):679–683. [DOI] [PubMed] [Google Scholar]
- 101.Buccisano F, Maurillo L, Piciocchi A, et al. Variable outcome of allogeneic stem cell transplant according to the different levels of pre-transplant minimal residual disease, in adult patients with acute myeloid leukemia. Blood. 2015;126(23):3230. [Google Scholar]
- 102.Wang Y, Liu DH, Liu KY, et al. Impact of pretransplantation risk factors on post transplantation outcome of patients with acute myeloid leukemia in remission after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(2):283–290. [DOI] [PubMed] [Google Scholar]
- 103.Zhao XS, Qin YZ, Liu YR, et al. The impact of minimal residual disease prior to unmanipulated haploidentical hematopoietic stem cell transplantation in patients with acute myeloid leukemia in complete remission. Leuk Lymphoma. 2017;58(5):1135–1143. [DOI] [PubMed] [Google Scholar]
- 104.Bachegowda LS, Saliba RM, Ramlal R, et al. Predictive model for survival in patients with AML/MDS receiving haploidentical stem cell transplantation. Blood. 2017;129(22):3031–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Apperley J, Niederwieser D, Huang XJ, et al. Haploidentical hematopoietic stem cell transplantation: a global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant. 2016;22(1):23–26. [DOI] [PubMed] [Google Scholar]
- 106.Aversa F, Prezioso L, Manfra I, Galaverna F, Spolzino A, Monti A. Immunity to infections after haploidentical hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis. 2016;8(1):e2016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin CJ, Vader JM, Slade M, DiPersio JF, Westervelt P, Romee R. Cardiomyopathy in patients after posttransplant cyclophosphamide-based hematopoietic cell transplantation. Cancer. 2017;123(10):1800–1809. [DOI] [PubMed] [Google Scholar]
- 108.Majzner RG, Mogri H, Varadhan R, et al. Post-transplantation cyclophosphamide after bone marrow transplantation is not associated with an increased risk of donor-derived malignancy. Biol Blood Marrow Transplant. 2017;23(4):612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol. 2016;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gorin NC, Labopin M, Piemontese S, et al. T-cell-replete haploidentical transplantation versus autologous stem cell transplantation in adult acute leukemia: a matched pair analysis. Haematologica. 2015;100(4): 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.