Abstract
Evaluations of the ‘real-world’ efficacy and safety of tyrosine kinase inhibitors in patients with chronic myeloid leukemia are scarce. A nationwide, population-based, chronic myeloid leukemia registry was analyzed to evaluate (deep) response rates to first and subsequent treatment lines and eligibility for a treatment cessation attempt in adults diagnosed between January 2008 and April 2013 in the Netherlands. The registry covered 457 patients; 434 in chronic phase (95%) and 15 (3%) in advanced disease phase. Seventy-five percent of the patients in chronic phase were treated with imatinib and 25% with a second-generation tyrosine kinase inhibitor. At 3 years 44% of patients had discontinued their first-line treatment, mainly due to intolerance (21%) or treatment failure (19%). At 18 months 73% of patients had achieved a complete cytogenetic response and 63% a major molecular response. Deep molecular responses (MR4.0 and MR4.5) were achieved in 69% and 56% of patients, respectively, at 48 months. All response milestones were achieved faster in patients treated upfront with a second-generation tyrosine kinase inhibitor, but ultimately patients initially treated with imatinib also reached similar levels of responses. The 6-year cumulative incidence of eligibility for a tyrosine kinase cessation attempt, according to EURO-SKI criteria, was 31%. Our findings show that in a ‘real-world’ setting the long-term outcome of patients treated with tyrosine kinase inhibitors is excellent and the conditions for an attempt to stop tyrosine kinase inhibitor therapy are met by a third of the patients.
Introduction
Multiple, randomized controlled trials have provided solid evidence for the efficacy and safety of tyrosine kinase inhibitors (TKI) as treatment for chronic myeloid leukemia (CML), but analyses from observational studies, gathered in patients who did not participate in clinical trials (the ‘real-world’) are scarce. Clinical trials use tight inclusion criteria, strict rules for monitoring and treatment algorithms and may, therefore, not fully reflect results in the general treatment population.1–3 Moreover, randomized controlled trials mainly focus on the outcome of the core study treatment, while some patients will not be able to continue their initial study treatment and are subsequently switched to an alternative treatment outside the trial.4,5
To study treatment choices and patients’ outcome across different treatment lines, real-world data contain important information for clinical practice. Incidence and survival have been the main focuses of the published reports of nationwide population-based registries. The large European population-based EUTOS registry was the first to provide insight into real-world first- and second-line treatment patterns in relation to cytogenetic and molecular response.6 However, this report did not cover deep molecular responses or the proportion of patients meeting the criteria to attempt cessation of TKI treatment. Discontinuing TKI therapy is a novel opportunity in CML for patients with a durable deep molecular remission, of whom approximately half may successfully stop their TKI treatment while retaining a ‘treatment-free remission’.7,8
In the current article, we report findings from a nationwide population-based CML registry in the Netherlands capturing data from newly diagnosed CML patients in the majority of hospitals in our country. Detailed information was collected on the patients’ characteristics and their treatment, both at baseline and during follow-up. Importantly, all TKI are available in the Netherlands and the Dutch health care system includes mandatory health care insurance which covers all CML care making it accessible to all patients.
The aim of the current study was to provide a detailed overview of all aspects of CML care including responses to first and subsequent treatment lines with a specific focus on the impact of first-line treatment with imatinib compared to that of the second-generation TKI, dasatinib and nilotinib. We also sought to evaluate what proportion of patients become eligible to attempt to stop their TKI treatment.
Methods
Data sources
Data from two complementary Dutch population-based registries on CML patients (PHAROS-CML and Hemobase) were combined to cover the nationwide population of adult (≥18 years) CML patients diagnosed between January 2008 and April 2013 in all 12 Dutch provinces. PHAROS-CML is an extension of the Dutch Cancer Registry and consists of real-world data collected by trained data managers from medical records of patients newly diagnosed with CML between January 2008 and April 2013, covering the Dutch population in 11 out of 12 provinces.9 Approval for this comprehensive data collection was obtained by the individual hospital boards. The PHAROS-CML registry is a joint initiative of the Dutch-Belgian Hemato-Oncology Group (HOVON), the Institute of Medical Technology Assessment at the Erasmus University Rotterdam and the Netherlands Comprehensive Cancer Organization. Hemobase is a multidisciplinary web-based electronic patients’ record in the north-eastern part of the Netherlands covering the one province that was not included in the PHAROS-CML registry, which is the province of Friesland. The data in Hemobase were registered by physicians and laboratory employees10 and extracted from Hemobase to be combined with the PHAROS-CML registry by the first author (IG) who verified each record to ensure comparability. Together, data on all new CML patients in 75 of approximately 90 hospitals in the Netherlands were available. Additional molecular data were retrieved from all 15 Dutch molecular laboratories performing BCR-ABL1 diagnostic testing. Data on vital status and causes of death were obtained from the Netherlands Cancer Registry with a follow-up until the 1st February, 2016. The Medical Ethics Committee of the Erasmus Medical Center in Rotterdam authorized this study and the exemption from informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Definitions and end-points
Disease phase according to the European LeukemiaNet criteria,11 Sokal risk score,12 EUTOS long term survival (ELTS) score13 and Charlson Comorbidity index14 were calculated as described in the original publications. Complete cytogenetic response was defined as the absence of Philadelphia chromosome-positive metaphases examined by chromosome banding. BCR-ABL1 levels of ≤ 0.1%, ≤0.01% and ≤0.0032% on the international scale or ≥3 log, ≥4 log and ≥4.5 log reduction of BCR-ABL1 mRNA transcripts from baseline (in molecular laboratories not able to report on the international scale at the time) were defined as the molecular response end-points major molecular response (MMR), MR4.0 and MR,4.5 respectively. Undetectable BCR-ABL1 levels were classified as a MMR when control gene numbers were not available to determine the sensitivity of the test.
In the case of a switch in TKI therapy, the clinical chart was reviewed for the reason why the treating physician had changed the therapy (‘treatment failure’ or ‘TKI intolerance’). As a proxy for effective and tolerable first-line treatment ‘effective first-line treatment’ was reached when patients continued their first-line TKI for at least 1 year after achieving MMR. For the determination of eligibility to stop TKI, the inclusion criteria for the EURO-SKI trial (ClinicalTrials.gov Identifier: NCT01596114) were applied. In short, this required TKI treatment for a minimum of 3 years, MR4.0 for at least 1 year and no history of TKI switch for a less than optimal treatment response. Disease progression was defined according to European LeukemiaNet criteria.11 Death due to CML was defined as death preceded by disease progression.
Data analysis
Descriptive statistics were used to compare baseline characteristics between treatment groups. Patients treated upfront with nilotinib and dasatinib were clustered in the second-generation TKI group for comparison with imatinib-treated patients. Overall survival was analyzed using the Kaplan-Meier method with log-rank test. All other time-dependent analyses were performed using the cumulative incidence competing risks method. Death and progression were always considered a competing risk. Additional competing risks per specific analysis are shown in Online Supplementary Table S1. A P-value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS version 24 and R-software15 version 3.2.4.
Results
Population description
The registries covered 457 patients newly diagnosed with CML between January 2008 and April 2013 in the Netherlands. The 75 out of 90 Dutch hospitals participating in the registry differed in size and type (academic versus non-academic) and therefore provided an accurate representation of the performance of CML treatment in the Netherlands. At diagnosis, 434 patients were in chronic phase (95%), eight patients (2%) in accelerated phase and seven patients (1%) in blast crisis. Disease phase was unknown for eight patients (2%). For all 457 patients, follow-up information on survival status and death due to CML was available until February 1st, 2016. Disease-specific follow-up (treatment and/or response) for more than 1 year was available for 413 patients. Disease-specific follow-up was not available for five patients (1%) and for 39 patients (9%) the disease-specific follow-up was less than 1 year (26 died, 13 were lost to follow-up).
First-line treatment
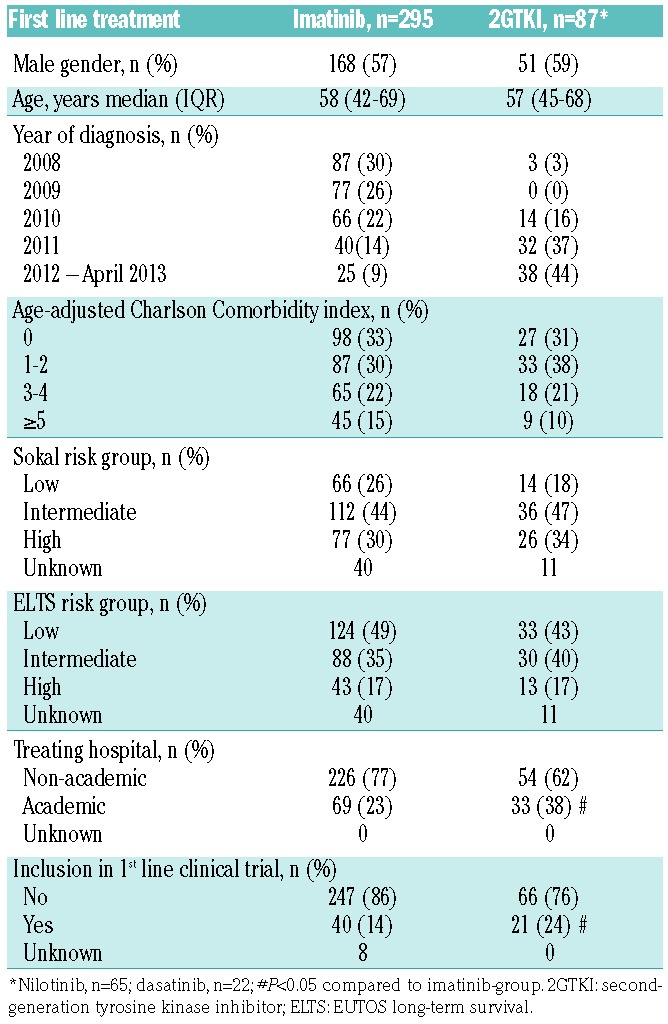
Of the patients with detailed treatment information available, 382 patients (97%) were treated with first-line TKI therapy (imatinib n=295, 75%; nilotinib n=65, 17%; dasatinib n=22, 6%) (Table 1), of whom 43% had received hydroxyurea prior to or simultaneous with the start of first-line TKI treatment. Hydroxyurea was the only reported treatment in six patients (2%) and two elderly patients with major comorbidities did not receive any treatment at all (0.5%). One patient was pregnant at diagnosis and was therefore treated first-line with interferon. One patient was in chronic phase at diagnosis, but was initially treated with leukapheresis and daunorubicin because of a hyperviscosity syndrome due to hyperleukocytosis at presentation (leukocytes 525×109/L). This patient died 1 day after diagnosis. Leukapheresis was performed in two other patients prior to imatinib treatment with white blood cell counts of 421×109/L and 606×109/L.
Table 1.
Patients’ characteristics at diagnosis for the sub-analysis of patients with chronic-phase-CML treated with a TKI.
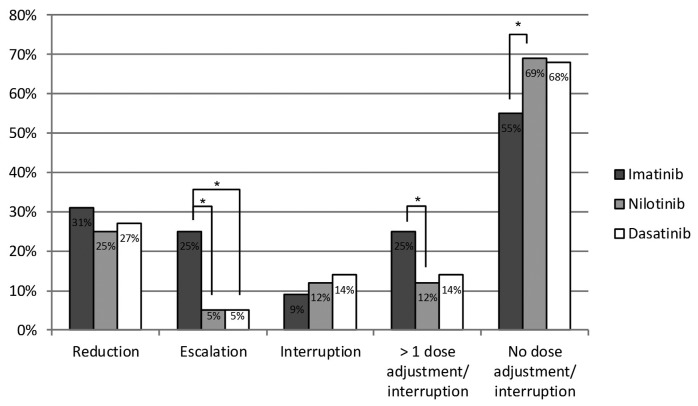
The majority of patients received the first-line TKI at standard doses: imatinib 400 mg QD (82%), nilotinib 300 mg BID (89%) and dasatinib 100 mg QD (95%). First-line dose adjustments were most frequently observed during imatinib treatment, especially dose escalations and sequential dose adjustments and/or interruptions (Figure 1).
Figure 1.
Dose adjustments of first-line tyrosine kinase inhibitors. *P<0.05.
Response
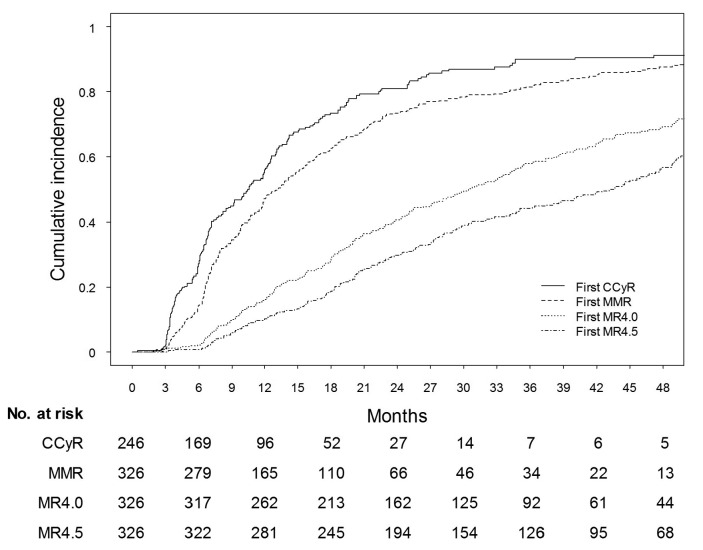
Cytogenetic response data were available for 246 out of 434 chronic-phase-CML patients (57%) and molecular response data for 326 out of 434 chronic-phase-CML patients (75%). Patients with no cytogenetic and/or no molecular response data available were significantly older, had a higher comorbidity index, were less frequently included in first-line clinical trials and more frequently treated in non-academic hospitals. The median time to first complete cytogenetic response was 10 months. The cumulative incidence of complete cytogenetic response was 55% (95% CI: 49–62%) at 12 months and 73% (95% CI: 67–79%) at 18 months (Figure 2). The median time to first MMR was 13 months. The cumulative incidence of MMR was 47% (95% CI: 42–52%) at 12 months and 63% (95% CI: 57–68%) at 18 months (Figure 2). Deeper molecular responses were achieved after a median treatment duration of 30.5 months (MR4.0) and 43 months (MR4.5) with cumulative incidence rates of 41% (95% CI: 35–46%) and 69% (95% CI: 63–74%) for MR4.0 after 24 months and 48 months, respectively, and cumulative incidence rates of 30% (95% CI: 25–35%) and 56% (95% CI: 50–62%) for MR4.5 after 24 months and 48 months, respectively (Figure 2).
Figure 2.
Achievement of cytogenetic and molecular response milestones. Cumulative incidence of first and unconfirmed achievement of CCyR, MMR, MR4.0 and MR4.5. CCyR: complete cytogenetic response; MMR: major molecular response; MR4.0: 0.01% on international scale; MR4.5: 0.0032% on international scale.
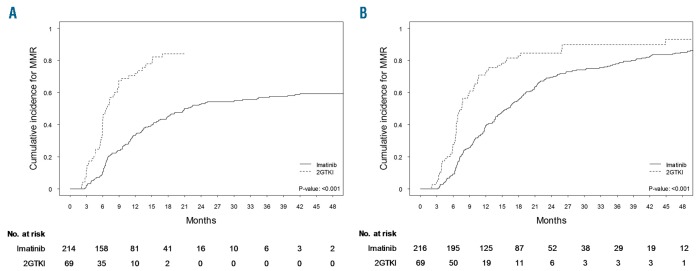
Both cytogenetic and molecular response milestones were achieved faster in patients treated upfront with a second-generation TKI (Figure 3, Online Supplementary Figures S1–S3). On initial treatment cumulative incidence rates of the achievement of all response milestones were significantly lower in patients treated with first-line imatinib (Figure 3A, Online Supplementary Figures S1A, S2A and S3A), but eventually, after switching TKI treatment, the same response rates were achieved (Figure 3B, Online Supplementary Figures S1B, S2B and S3B).
Figure 3.
Achievement of major molecular response on frontline imatinib and second-generation tyrosine kinase inhibitors. (A) Cumulative incidence of MMR on initial treatment. (B) Cumulative incidence of overall MMR achievement (including patients who switched TKI). MMR: major molecular response; 2GTKI: second-generation tyrosine kinase inhibitors.
Switching/discontinuation of tyrosine kinase inhibitor treatment and time to effective treatment
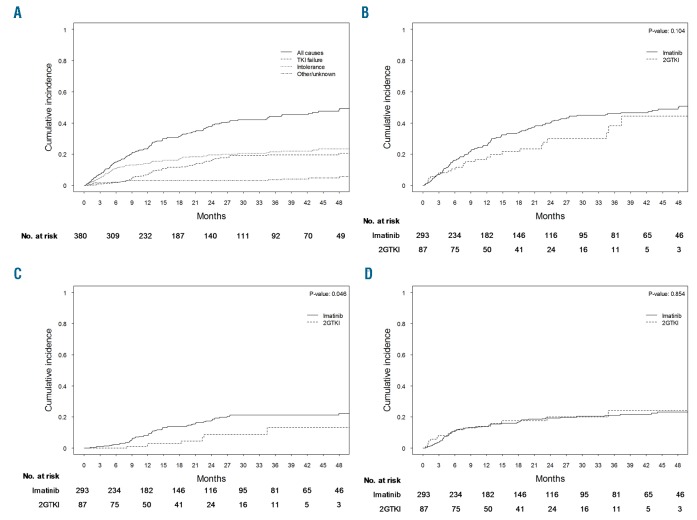
Within the first 3 years after diagnosis, 44% (95% CI: 38–50%) of the 382 patients on first-line TKI treatment discontinued their first-line treatment; 21% (95% CI: 17–26%) due to TKI intolerance, 19% (95% CI: 15–24%) due to treatment failure and 3% (95% CI: 1–5%) for other or unknown reasons (Figure 4A). The most frequently observed other reasons for first-line TKI discontinuation were trial inclusion within 4 months after starting treatment (n=5), inclusion in a TKI discontinuation trial (n=3) and pregnancy (n=3).
Figure 4.
Discontinuation of first-line tyrosine kinase inhibitors. (A) Cumulative incidence of all causes of TKI discontinuation. (B) Cumulative incidence of TKI discontinuation, a comparison of imatinib with 2GTKI. (C) Cumulative incidence of TKI discontinuation due to TKI failure. (D) Cumulative incidence of TKI discontinuation due to TKI intolerance. 2GTKI: second-generation tyrosine kinase inhibitor.
The 3-year cumulative incidence of TKI discontinuation was not different between patients treated first-line with imatinib or second-generation TKI (46% versus 38%, P=0.104) (Figure 4B). TKI discontinuation due to TKI failure was significantly more common in patients treated with first-line imatinib (21% versus 13%, P=0.046) (Figure 4C). TKI discontinuation due to TKI intolerance did not differ according to whether the first-line treatment was with imatinib or a second-generation TKI (21% versus 24%, P=0.854) (Figure 4D). In total, up to five subsequent treatment switches were observed (Online Supplementary Figure S4). An overview of the reported types of intolerance on all treatment lines and the actions that followed as a result of the intolerance can be found in the Online Supplementary Results section and Online Supplementary Table S2.
The median time to sustained effective and tolerable first-line treatment was 33 months and the cumulative incidence was 56% (95% CI: 49–64%) after 4 years of treatment: 51% (95% CI: 43–59%) with first-line imatinib treatment and 77% (95% CI: 57–97%) with first-line, second-generation TKI treatment (P<0.001).
Progression during tyrosine kinase inhibitor therapy
The median observation period for progression of TKI-treated chronic-phase-CML patients was 27 months (range, 0–82 months). A total of 17 patients progressed within 3 years, for a cumulative incidence of 3% after 1 year and 6% after 3 years. Progression occurred in 16 patients treated with first-line imatinib whereas it was observed in only one patient treated upfront with a second-generation TKI; the cumulative incidence rates for progression after 3 years were 7% versus 1% (P=0.193) in patients treated with frontline imatinib and frontline second-generation TKI, respectively.
Survival
The median observation time for survival of patients in chronic phase at diagnosis was 5 years and 7 months (range, 34–97 months). Eighty-two patients died during follow-up (19%). Overall survival rates after 1, 2 and 4 years were 96% (95% CI: 94–98%), 92% (95% CI: 90–95%) and 85% (95% CI: 81–88%), respectively. No significant differences in overall survival were observed when overall survival was stratified by type of first-line TKI treatment (P=0.244). The cumulative incidence of death due to CML was 1% after 1 year, 2% after 2 years and 3% after 4 years. Again, death due to CML was not significantly different between patients treated first-line with imatinib or a second-generation TKI (P=0.208).
Tyrosine kinase inhibitor stop eligibiity
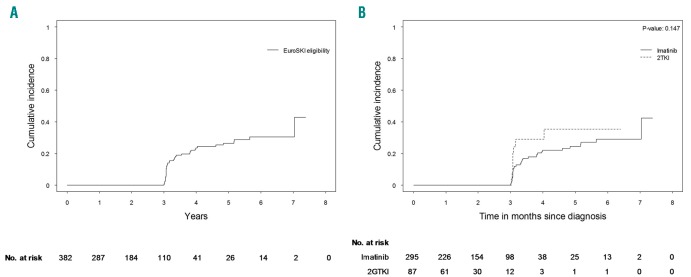
Eligibility for a TKI stop attempt according to the EURO-SKI criteria was evaluated for all 382 patients who started first line TKI treatment. A total of 43 patients (11%) met the eligibility criteria. During follow up 131 patients (34%) experienced a competing risk making them ineligible for a (EURO-SKI) stop attempt: first-line TKI failure (43%), less than 3 years on first- and second-line TKI (29%), death (20%) and progression (8%). Censoring occurred for 208 patients (55%): lost to disease-specific follow-up within 3 years (80%), not (yet) achieved 3× MR4.0 in 1 year (19%), emigration (1%) and unknown reason for TKI switch (0.5%). The cumulative incidences of stop attempt eligibility after 4 and 6 years were 24% (95% CI, 17–30%) and 31% (95% CI, 23–38), respectively (Figure 5A). The cumulative incidence of TKI stop eligibility after first line 2GTKI treatment was numerically higher than after imatinib treatment, but this did not reach statistical significance (P=0.147) (Figure 5B).
Figure 5.
Eligibility to attempt cessation of tyrosine kinase inhibitor therapy according to EURO-SKI criteria. (A) Cumulative incidence of overall eligibility of patients with chronic-phase-CML treated first-line with a TKI. (B) Comparison of cumulative incidences in patients treated upfront with imatinib or 2GTKI. 2GTKI: second-generation tyrosine kinase inhibitor.
Treatment and outcome of patients presenting in advanced-phase-chronic myeloid leukemia
The 15 patients who presented in advanced disease phase at diagnosis (8 accelerated phase and 7 blast crisis) were mainly treated upfront with a TKI (6 accelerated phase and 4 blast crisis). One patient in blast crisis was started on chemotherapy + TKI treatment and treatment was unknown for two other patients in blast crisis. Allogeneic stem cell transplantation was performed in three patients with accelerated-phase-CML and three with blast crisis. The median survival after diagnosis was 1.4 years in patients with accelerated-phase-CML and not reached in the blast crisis patients. The 5-year overall survival rates were 38% and 57%, respectively.
Discussion
Our nationwide, population-based study confirms the excellent results of TKI treatment in CML patients observed in randomized controlled trials outside clinical trials and adds insights into the patterns of TKI switching and patients’ outcome. Moreover, this study presents the first real-world data on the proportion of patients becoming eligible for an attempt to achieve treatment-free remission.
The baseline characteristics of the Dutch CML population are comparable to those of patients in other population-based CML registries.6,16,17 In contrast, patients included in the ENESTnd and DASISION randomized controlled trials18,19 were 10 years younger than those in our real-world cohort. Since nilotinib and dasatinib were both registered for first-line treatment in December 2010, the majority of patients treated with second-generation TKI were diagnosed in the second half of the inclusion period and therefore experienced a shorter follow-up period. Because of the relatively low number of individual patients treated upfront with nilotinib and dasatinib, it was decided to combine these two groups of patients for comparisons with the imatinib-treated patients.
Our real-world observations show that TKI therapy is effective and tolerable in the majority of patients, but frequent dose adjustments and temporary treatment interruptions are required (45% of patients treated upfront with imatinib and 31–32% of patients treated with a second-generation TKI). Only half (54%) of all imatinib-treated patients with chronic-phase-CML were estimated to remain on first-line TKI 3 years after treatment initiation in our observational cohort. This rate of TKI treatment persistence is notably lower than the rates observed in the ENESTnd and DASISION randomized controlled trials in which 62% and 69% of patients were still on their first-line imatinib treatment after 3 years.20,21 For nilotinib and dasatinib these rates were 71–74% and 71%, respectively,20,21 while we observed a 3-year treatment persistence rate of only 62% in patients treated with second-generation TKI. These differences were mainly due to the higher TKI discontinuation rates due to TKI intolerance observed in our real-world setting. It can be hypothesized that the threshold for TKI switching due to intolerance for both physicians and patients is lower in subjects treated outside a clinical trial. In concordance with our study, Castagnetti et al. reported a treatment persistence rate of 59% in patients treated with imatinib after a median of 48 months follow-up in a real-world setting in Italy, also because of frequent TKI switches due to intolerance.22
The observations of earlier and higher rates of achievement of the important response milestones, complete cytogenetic response and MMR, in patients treated with first-line, second-generation TKI in our real-world cohort confirm the reproducibility of the superior efficacy results of first-line, second-generation TKI found in the two large randomized controlled trials.4,5 Of note, the 3-year MMR rate achieved in our real-world cohort with first-line, second-generation TKI (84%) is even higher than the rates recorded in the randomized controlled trials (69–73%). Differences in methodology and study design of our observational study and the randomized controlled trials may have contributed to the variation in findings. A limitation to population-based cohort studies is that patients are not monitored as strictly as during the closely supervised clinical follow-up in trials. Furthermore, in our registry, patients with more favorable baseline characteristics more often had cytogenetic and molecular response analyses performed. This may in part explain the relatively high remission rates we observed.
Real-world data not only give insight into responses on first-line treatments, but also provide information on the overall response on subsequent treatment lines, in contrast to data from the currently published clinical trials. For example, the data from our nationwide registry demonstrate that 91% of all chronic-phase-CML patients treated with a TKI eventually achieved complete cytogenetic response and 88% reached a MMR after 4 years, whereas the results from randomized controlled trials on core treatment were significantly lower at this time point. The observational data on overall response do, therefore, reflect relevant patients’ outcomes much better than the clinical trial results do. Our overall response rates are comparable to those observed in other population-based registries.16,22,23 The observation that patients treated upfront with imatinib or a second-generation TKI eventually achieved comparable complete cytogenetic response and MMR rates, whether or not preceded by one or more treatment switches, has also been recognized before.23 An analysis of long-term molecular response on first-line treatment even showed that patients treated with imatinib 400 mg QD only can reach MMR rates nearly similar to those treated with imatinib 800 mg QD, nilotinib and dasatinib after 5 years.24
To our knowledge, this is the first observational study comparing deep molecular responses achieved on imatinib with deep molecular responses achieved on second-generation TKI. Of note, comparative analyses were hampered in our study by a relatively low number of patients receiving second-generation TKI and their shorter follow-up period. Despite this, we were still able to show that MR4.0 and MR4.5 were achieved significantly faster in the real-world when treatment was initiated with a second-generation TKI than when the initial treatment was imatinib, independently of subsequent treatment lines. In the randomized controlled trials both higher24 and lower4,5 cumulative response rates were observed than in our real-life cohort. These deep responses are especially interesting in the light of eligibility for an attempt to stop TKI therapy. Together with the duration of TKI treatment and switching history, a durable deep molecular remission is the main selection criterion currently used in trials investigating treatment-free remission. In a previous analysis of patients in first-line clinical trials, after 8 years of imatinib treatment, the cumulative incidence of stable (≥ 24 months) MR4.5, determined on an intent-to-treat basis, was 36.5%, suggesting that this proportion may be eligible for treatment-free remission.25 In our population-based study, the eligibility criteria of the largest treatment-free remission trial to date, EURO-SKI, were used to evaluate this endpoint and showed a cumulative incidence of 31% after 6 years. Although we observed a higher eligibility rate in patients treated upfront with a second-generation TKI than in those treated with imatinib, the difference did not reach statistical significance possibly because of the relatively low number of patients who had started on a second-generation TKI and had a follow-up beyond 3 years.
In conclusion, this population-based analysis showed overall favorable treatment responses compared to those in randomized controlled trials, which could be attributed to dose adjustments and subsequent treatment lines in the real-world setting. It also showed that the long-term outcome of patients initially treated with imatinib is excellent when these patients are switched to second-generation TKI when needed. The cumulative incidence of patients eligible to attempt to stop their TKI to achieve treatment-free remission was 31% after 6 years when the EURO-SKI criteria were applied.
Supplementary Material
Acknowledgments
The authors would like to thank Peter Huijgens, currently chairman of the Netherlands Comprehensive Cancer Organisation (IKNL) for initiating the Pharos registry. We thank Tom Wiggers, Wencke de Jager, Sanne Nijssen and Jolie Cheung for entering patients’ data into the Pharos database. We thank Marianne van der Mark from the IKNL for retrieving survival data from the Netherlands Cancer Registry and all the hospitals and molecular laboratories in the Netherlands that participated in the Pharos and Hemobase registries.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1842
References
- 1.Pulte D, Gondos A, Redaniel MT, Brenner H. Survival of patients with chronic myelocytic leukemia: comparisons of estimates from clinical trial settings and population-based cancer registries. Oncologist. 2011;16(5):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latagliata R, Carmosino I, Vozella F, et al. Impact of exclusion criteria for the DASISION and ENESTnd trials in the front-line treatment of a ‘real-life’ patient population with chronic myeloid leukaemia. Hematol Oncol. 2017;35(2):232–236. [DOI] [PubMed] [Google Scholar]
- 3.Mauro MJ, Davis C, Zyczynski T, Khoury HJ. The role of observational studies in optimizing the clinical management of chronic myeloid leukemia. Ther Adv Hematol. 2015;6(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20): 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29(6):1336–1343. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17–23. [DOI] [PubMed] [Google Scholar]
- 8.Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huijgens P, Posthuma E, Coebergh J, van de Poll-Franse L, Uyl-de Groot C, Sonneveld P. A ‘population based registry’ for hemato-oncology. Ned Tijdschr Hematol. 2010;7(8):321–325. [Dutch] [Google Scholar]
- 10.Hoogendoorn M, Joosten P, Storm H, Kibbelaar R. Hemobase: an intelligent electronic patient file as aid for hemato-oncology care. Ned Tijdschr Hematol. 2009;6(3):104–110. [Dutch] [Google Scholar]
- 11.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokal JE, Gomez GA, Baccarani M, et al. Prognostic significance of additional cytogenetic abnormalities at diagnosis of Philadelphia chromosome-positive chronic granulocytic leukemia. Blood. 1988;72(1):294–298. [PubMed] [Google Scholar]
- 13.Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48–56. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 15.Team RC. R: A Language and Environment for Statistical Computing. 2016. [Google Scholar]
- 16.Hoglund M, Sandin F, Hellstrom K, et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood. 2013;122(7):1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beinortas T, Tavoriene I, Zvirblis T, Gerbutavicius R, Jurgutis M, Griskevicius L. Chronic myeloid leukemia incidence, survival and accessibility of tyrosine kinase inhibitors: a report from population-based Lithuanian haematological disease registry 2000–2013. BMC Cancer. 2016;16:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. [DOI] [PubMed] [Google Scholar]
- 20.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castagnetti F, Di Raimondo F, De Vivo A, et al. A population-based study of chronic myeloid leukemia patients treated with imatinib in first line. Am J Hematol. 2017;92(1):82–87. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann VS, Baccarani M, Hasford J, et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia. 2017;31(3):593–601. [DOI] [PubMed] [Google Scholar]
- 24.Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol. 2015;2(3):e118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branford S, Yeung DT, Ross DM, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121(19): 3818–3824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.