Abstract
Blastic plasmacytoid dendritic cell neoplasm is an aggressive hematologic malignancy with a poor prognosis. No consensus regarding optimal treatment modalities is currently available. Targeting the nuclear factor-kappa B pathway is considered a promising approach since blastic plasmacytoid dendritic cell neoplasm has been reported to exhibit constitutive activation of this pathway. Moreover, nuclear factor-kappa B inhibition in blastic plasmacytoid dendritic cell neoplasm cell lines, achieved using either an experimental specific inhibitor JSH23 or the clinical drug bortezomib, interferes in vitro with leukemic cell proliferation and survival. Here we extended these data by showing that primary blastic plasmacytoid dendritic cell neoplasm cells from seven patients were sensitive to bortezomib-induced cell death. We confirmed that bortezomib efficiently inhibits the phosphorylation of the RelA nuclear factor-kappa B subunit in blastic plasmacytoid dendritic cell neoplasm cell lines and primary cells from patients in vitro and in vivo in a mouse model. We then demonstrated that bortezomib can be associated with other drugs used in different chemotherapy regimens to improve its impact on leukemic cell death. Indeed, when primary blastic plasmacytoid dendritic cell neoplasm cells from a patient were grafted into mice, bortezomib treatment significantly increased the animals’ survival, and was associated with a significant decrease of circulating leukemic cells and RelA nuclear factor-kappa B subunit expression. Overall, our results provide a rationale for the use of bortezomib in combination with other chemotherapy for the treatment of patients with blastic plasmacytoid dendritic cell neoplasm. Based on our data, a prospective clinical trial combining proteasome inhibitor with classical drugs could be envisaged.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare malignancy derived from plasmacytoid dendritic cells and is classified among acute myeloid leukemias by the 2008 World Health Organization (WHO). BPDCN is associated with a poor prognosis with a median overall survival of 8–12 months in the largest series of patients.1–3 The diagnosis is made from the typical cutaneous lesions that rapidly progress (90%) to bone marrow and extramedullary sites. The diagnosis is mainly based on histopathological and phenotypic characterization of blastic cells in the peripheral blood or bone marrow expressing the following markers CD123, BDCA2 (CD303), BDCA4 (CD304) and TCL1 as analyzed by flow cytometry.1–3
There is currently no consensus regarding optimal treatment modalities. Classical treatments such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimens show disappointing results.4 While intensive chemotherapy regimens (including those for acute myeloid leukemia and acute lymphoblastic leukemia) followed by allogeneic hematopoietic cell transplantation have been reported to improve the survival beyond 30 months in young patients,5–10 elderly patients are not eligible for this approach. Altogether, this makes it necessary to evaluate new therapeutic strategies.
Recently, Sapienza et al. demonstrated a constitutive activation of the nuclear factor-kappa B (NF-κB) pathway in primary BPDCN cells which represents a potential therapeutic target.11 Using the proteasome inhibitor bortezomib, known to inhibit NF-κB activation,12 these authors demonstrated that treatment of the BPDCN cell line CAL-1 inhibits cell proliferation and induces a significant cytotoxic effect. More recently, Ceroi et al. confirmed this cons titutive activation of the NF-κB pathway in other primary BPDCN cells and demonstrated the induction of apoptosis of BPDCN cell lines (CAL-1 and GEN2.2) in vitro in response to the NF-κB p65 inhibitor, JSH23.13 Overall, targeting the NF-κB pathway by bortezomib would represent a promising, easily available therapeutic option for BPDCN patients if its efficacy were to be confirmed in vitro using primary BPDCN samples and in vivo in a preclinical BPDCN model. This was the goal of our work.
Methods
Patients’ cells, cell lines and culture
Two human BPDCN cell lines (CAL-1, Dr. Maeda, Nagasaki University, Japan and GEN 2.2, patent #0215927, EFS, France)14,15 and samples from seven BPDCN patients (Online Supplementary Table 1) from our French national network (authorization #DC2016-2791) fully diagnosed as BPDCN by their phenotype (CD123+, CD56+, CD123+, CD303+, CD304+, TCL1+)1,16–18 were used. This study was approved by the Besançon local ethic committee (CPPEST II, Besançon, France).
Primary blastic plasmacytoid dendritic cell neoplasm cell xenograft model
NOD/SCID/IL2Rγc-deficient (NSG) mice (6 to 8 weeks of age, The Jackson Laboratory, Sacramento, CA, USA) were irradiated (2.5 Gy) and inoculated intravenously with 2×106 primary BPDCN cells from patient #127. Mice were treated with bortezomib (0.25 mg/kg, intraperitoneally) once or twice a week for 2 or 4 weeks. Engraftment and quantification of the BPDCN cell line are described in the Online Supplement. These procedures were carried out in accordance with the guidelines for animal experimentation according to an approved protocol (protocol 11007R, Veterinary Services for Animal Health & Protection, issued by the Ministry for Agriculture, Paris, France).
Drugs
Bortezomib was tested at different concentrations from 10 to 75 nM for in vitro evaluation, as previously described,11 and at 20 nM when associated with other drugs. BPDCN cells were cultured at 106 cell/mL in RPMI-1640 glutamax medium (Invitrogen, Cergy Pontoise, France) supplemented with 10% fetal calf serum (Invitrogen) and 1% penicillin/streptomycin (PAA Laboratoires, Vélizy-Villacoublay, France) at 37°C under 5% CO2 for 24 or 48 h (Online Supplement). Bortezomib was injected intraperitoneally into mice at a dose of 0.25 mg/kg. The NF-κB p65 inhibitor, JSH-23 (Calbiochem-EMD Biosciences, Inc, San Diego, CA, USA) was used as a control at a dose of 40 mg/kg. Others drugs tested are described in the Online Supplement.
Cytotoxicity, proliferation and cell cycle assay by flow cytometry
A panel of monoclonal antibodies against CD123, CD45, CD56 and BDCA4 was used to gate BPDCN cells (Online Supplement). BPDCN cells from seven patients and BPDCN cell lines (CAL-1, GEN2.2) were incubated at 106 cells/mL at 37°C in 5% CO2 with bortezomib at various concentrations (10 – 50 nM) for 24 or 48 h. The cytotoxic effects of drugs were evaluated in vitro using annex-in-V and 7-amino actinomycin D (AV/7AAD, Beckman Coulter, Roissy, France) staining and flow cytometry.13,19 Cells were labeled by Dye eFluor® V450 (Ebioscience, San Diego, CA, USA) to assess cell proliferation.13 The percentage of cells in subG1, G1, S and G2 cell cycle phases was evaluated using CXP and MultiCycle software (Beckman Coulter).13
Nuclear factor-kappa B pathway activation
CAL-1 cells or PDX (patient derived xenograft) cells obtained in vivo from blood of mice, after treatment with bortezomib for 6 h followed by stimulation with a TLR7 agonist (R848, 1 μg/mL, Invivogen, Toulouse, France) for 45 min were investigated by phospho-flow staining using phosphorylated-NF-κB subunit RelA (pRelA) staining, as described as described in the Online supplement.
Statistical analysis
Statistical analyses were performed using the Student t-test or the Mann-Whitney test (GraphPad Prism software 5.0c, San Diego, CA, USA) (Online Supplement).
Results
Bortezomib is cytotoxic against blastic plasmacytoid dendritic cell neoplasm cell lines and primary cells
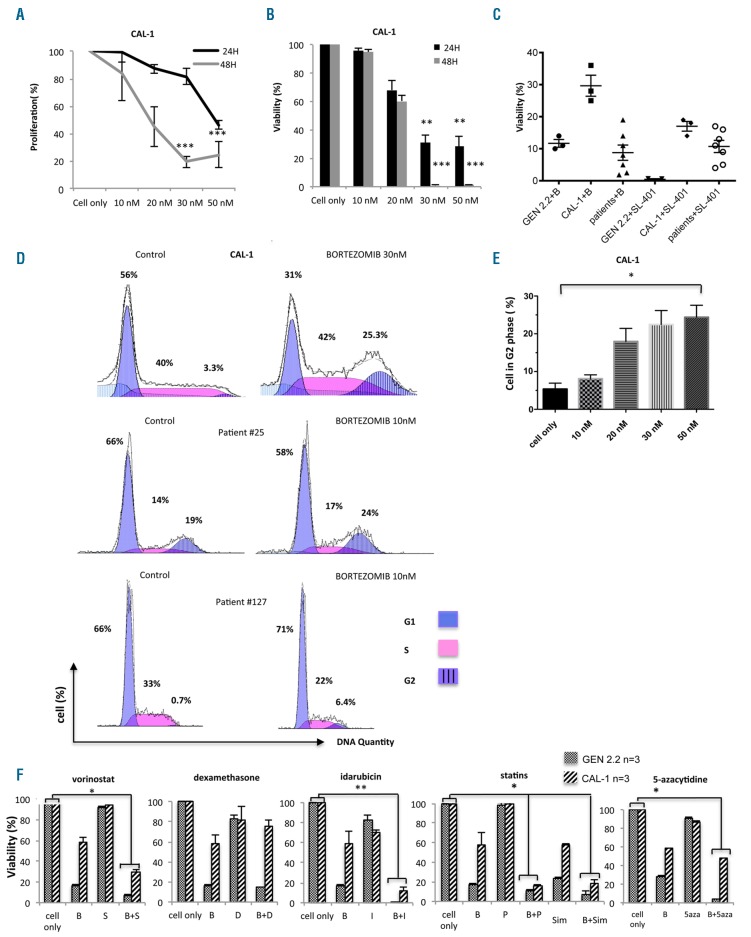
Treatment of CAL-1 cells with bortezomib for 24 h (n=5, 50 nM) markedly decreased cell proliferation (from 51.7±7% to 16.8±7.9%, P<0.001) (Figure 1A) and cell survival (from 89.2±1.5% to 26.6±6.5%, P<0.001) (Figure 1B). After 48 h of treatment with bortezomib (50 nM), cell proliferation was also significantly reduced from 43.4±9.8% to 22.4±9.4% (n=4, P<0.001) (Figure 1A) and viability was significantly decreased (from 51.7±7% to 16.8±7.9%) (Figure 1B). Bortezomib treatment (30 nM) induced robust cytotoxicity in vitro, similarly to SL-401 (used as the positive control) (P<0.01) (Figure 1C). Similar data showing significant bortezomib-induced cytotoxity were also obtained for the GEN2.2 BPDCN cell line and primary BPDCN cells from seven different patients (Figure 1C). Moreover, exposure of CAL-1 cells to bortezomib induced a significant accumulation of BPDCN cells in the G2 phase of the cell cycle (from 5.8±1.4% to 24.1±3.02% at 24 h, n=4, P<0.05) (Figure 1D,E). These data were confirmed using primary cells isolated from patients and treated in vitro with bortezomib [patient #25 (n=2) and patient #127 (n=1)] (Figure 1D). Subsequently, CAL-1 cells underwent apoptosis, as attested by an increase of cell arrest in the subG1 phase (from 18.8±7.3% to 60.8±7.6% at 24 h, n=4, P=NS) (Figure 1D). Overall, this demonstrates that, in vitro, bortezomib inhibits BPDCN cell proliferation and induces cell death.
Figure 1.
Bortezomib inhibits cell proliferation and survival of blastic plasmacytoid dendritic cell neoplasm cell lines and primary cells. Results are expressed as percentage ± SEM of (A) proliferation using the Dye eFluor® V450 dilution assay and (B) viable cells using AV−/7-AAD− staining of the CAL-1 cell line treated with bortezomib (10 – 50 nM) for 24 h (black) and 48 h (gray) (n=4). Untreated CAL-1 cells were arbitrarily assigned a value of 100%. (C) Percentage ± SEM of viable GEN 2.2 cells (n=3), CAL-1 (n=6) cells and primary BPDCN cells from seven patients was determined after incubation with bortezomib (30 nM), or SL-401 (365 pM) for 24 h. Untreated cells were considered as 100% viable. (D) One representative histogram showing the percentage of CAL-1 cells and primary cells from two patients (patient #25 and patient #127) in the different phases of the cell cycle: G1, S and G2 after treatment or not with bortezomib at 10 or 30 nM for 24 h. (E) Percentage of cells in the G2 phase in the CAL-1 cell line after treatment or not with bortezomib (10–50 nM) for 24 h (n=4). Histograms represent the mean ± SEM of four independent experiments, *P<0.05, **P<0.01, ***P<0.001 between bortezomib and untreated cells. (F) Percentage of viable CAL-1 (n=3) and GEN2.2 (n=3) cells after incubation with bortezomib (B, 20 nM) in association with: idarubicin (I) at 0.03 μM, dexamethasone (D) at 0.637 mM, vorinostat (S) at 1.25 μM, statins, such as pravastatin (P) and simvastatin (Sim) at 5 μM and 5-azacytidine (5-Aza) at 4 μM. Histograms represent the mean ± SEM of three independent experiments. *P<0.05, **P<0.01.
Bortezomib inhibits nuclear factor-kappa B pathway activation in blastic plasmacytoid dendritic cell neoplasm cells
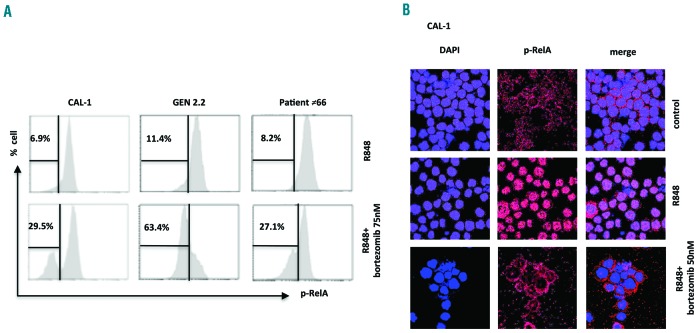
While bortezomib is a proteasome inhibitor with well-known anti-NF-κB properties and was used to treat BPDCN CAL-1 cells,11 inhibition of the NF-κB pathway in primary BPDCN cells was not demonstrated. Treatment of BPDCN cells with bortezomib (75 nM, 24 h) decreased R848-induced RelA phosphorylation in CAL-1 cells from 91.8±2.6% to 71 ±1.6% (n=4, P<0.05), in GEN 2.2 cells from 97.5±0.2% to 19.8±4.4% (n=3, P<0.05), and in five different primary BPDCN cells (#24, #25, #127, #66, #38) from 79.9±7.23% to 61.6±3.41%. The percentage of BPDCN cells (CAL-1, GEN 2.2 and BPDCN #66) exhibiting reduced pRelA expression increased after bortezomib treatment (from 6.9% to 29.5%, −11.4% to 63.4% and −8.2% to 27.1%, respectively, after treatment with bortezomib 75 nM) (Figure 2A). Moreover, pRelA analysis by confocal microscopy revealed a decrease of pRelA nuclear translocation in CAL-1 cells associated with a cytoplasmic retention of pRelA after bortezomib treatment (50 nM, 6 h, n=3) (Figure 2B). Similar results were also observed with the GEN 2.2 cell line (19±1% to 2±0.05%, n=3; data not shown).
Figure 2.
Bortezomib inhibits the nuclear factor-kappa B signaling pathway in blastic plasmacytoid dendritic cell neoplasm cell lines and primary cells. (A–B) BPDCN cell lines (GEN 2.2 and CAL-1 cells, n=3) and primary BPDCN cells from a patient were incubated with bortezomib (50 nM and 75 nM) or vehicle for 6 h before TLR7 stimulation for 45 min (R848, 1 μg/mL). One representative example of intracellular expression of NF-κBp-65 evaluated in CAL-1, GEN 2.2 cell lines and in primary BPDCN cells from patient #66 were analyzed by (A) flow cytometry and by (B) confocal microscopy in the CAL-1 cell line.
Association of bortezomib with others drugs increases its cytotoxic effect
Since limited treatment efficiency has been reported for BPDCN and no consensus exists on treatment modality, associations of bortezomib with other drugs were tested. Idarubicin was used since this drugs exerts potent in vitro cytoxicity on BPDCN,19 but idarubicin was used at a nontoxic concentration (0.03 μM). Since BPDCN has been shown to exhibit altered cholesterol metabolism,13 inhibitors of cholesterol synthesis (statins) were also tested. The viability of BPDCN cell lines (CAL-1 and GEN 2.2) treated with bortezomib (20 nM, a non-cytotoxic concentration) in association with other drugs was evaluated at 24 h (Figure 1F). The viability of CAL-1 cells was 51.2±4.8% (n=3) after treatment with suberoylanilide hydroxamic acid (SAHA) alone and decreased to 26.1±2.6% when bortezomib and SAHA were associated together. The viability of CAL-1 cells (n=3) was 61.6±2% with idarubicin alone and decreased to 10.8±3.1% when bortezomib and idarubicin were associated together. In the same way the viability of CAL-1 cells (n=3) was 50.1±1.8% with simvastatin alone and 91.1±1.7% with pravastatin alone and decreased to 16.3±3.4% or to 13.9±1.1% when bortezomib and simvastatin or pravastatin were associated together. The viability of CAL-1 cells (n=3) was 87.03±0.73% with 5-azacytidine alone and decreased to 47.9±0.85% when bortezomib and 5-azacytidine were associated together. Only the association of bortezomib with dexamethasone (65.7±14.4% to 78.8±5.1%) did not induce a synergistic effect. The viability of GEN 2.2 cells (n=3) was 83.2±0.7% after treatment with SAHA alone and decreased to 5.9±0.6% when bortezomib and SAHA were associated together. The viability of GEN 2.2 cells (n=3) was 68.3±5.4% after treatment with idarubicin alone and decreased to 0.16±0.03% when bortezomib and idarubicin were associated together (P<0.01). The viability of GEN 2.2 cells (n=3) was 19.1±1.3% after treatment with simva statin alone or 82±1.7% with pravastatin alone and decreased to 6±3% or to 8.5±1.5% when bortezomib and simvastatin or pravastatin were associated together. The viability of GEN 2.2 cells (n=3) was 68.6±4.3% after treatment with dexamethasone alone and decreased to11.8±0.6% when bortezomib and dexamethasone were associated together. The viability of GEN 2.2 cells (n=3) was 37.66±1.38% after treatment with 5-azacytidine alone and decreased to 1.4±0.3% when bortezomib and 5-azacytidine were associated together. Thus, the association of bortezomib with idarubicin, SAHA, 5-azacytidine or statins increases the cytotoxic effect of the proteasome inhibitor on the two BPDCN cell lines.
Luciferase-expressing CAL-1 cell xenograft in mice to assess antitumor efficacy
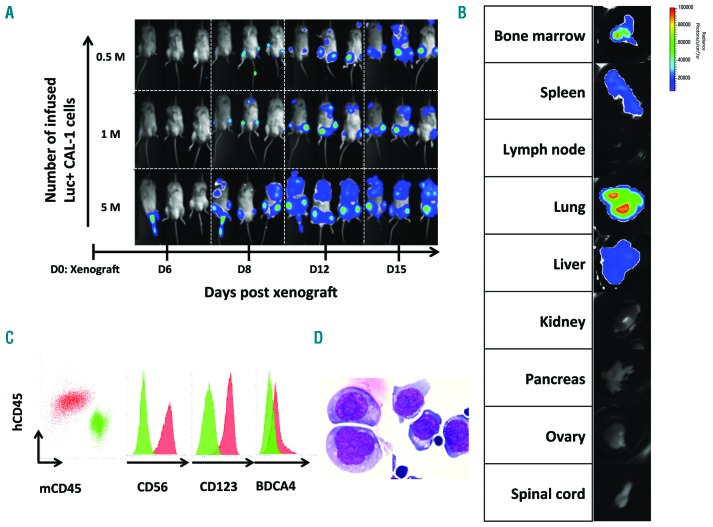
In a xenograft model using the CAL-1 cell line, wild-type leukemic cells remained faintly detectable in mouse blood.20 We, therefore, developed a Luc+ CAL-1 cell line to assess leukemic cell proliferation by non-invasive imaging of the luminescent leukemic cells allowing the monitoring of disease progression by bioluminescence. Luciferase-expressing CAL-1 cells obtained after retroviral transduction with a Luc-retroviral vector carrying luciferase (Luc+) and neomycin resistance (NeoR) genes were injected into NOG mice to develop a BPDCN xenograft mouse model. While disease progression was undetectable in the blood, injection of 0.5, 1 or 5×106 Luc+ CAL-1 cells into NOG mice provided a rapidly detectable total body bioluminescent imaging (BLI) signal (Figure 3A). Indeed, BLI signals were first detectable in the group injected with 5×106 at day 6, and at day 8 in the groups injected with 1×106 or 0.5×106 cells. By day 15, all mice that received 5×106 Luc+ CAL-1 cells were dead from leukemic progression. They developed paralysis of the lower limbs. The other six mice from groups given 0.5×106 and 1×106 cells were euthanized on day 15 and tissue infiltration was evaluated by BLI (Figure 3B). Luc+ CAL-1 cell infiltration was detectable in the bone marrow, spleen, lungs and liver whereas it remained undetectable in the lymph nodes, kidneys, pancreas, ovary and spinal cord. After sacrificing the mice, immunostaining of the spleen and bone marrow confirmed the presence of cells with a BPDCN phenotype (human CD45+, murine CD45−, CD56+, CD123+, BDCA4+) (Figure 2C) and cytological analysis showed, as previously described,21 large cells with blastic round or convoluted nuclei with slightly condensed chromatin, several nucleoli and a basophilic cytoplasm (Figure 3D). This model can be evaluated to assess the in vivo antitumor efficacy of bortezomib directly.
Figure 3.
Development of a luciferase-expressing CAL-1 cell xenograft model. Luc+ CAL-1 cells (0.5, 1 or 5×106) were injected intravenously into NOG mice and animals were imaged at days 6, 8, 12, and 15 after the xenograft. Luciferin was administered and images were obtained by integrating the bioluminescent signal. (A) In vivo kinetics of tumor cell growth following the Luc+ CAL-1 cell xenograft. A pseudocolor luminescent image from blue (least intense) to red (most intense) is depicted. (B) Representative analysis of bioluminescent organs at sacrifice at day 15 after the xenograft. This mouse was injected with 0.5×106 Luc+ CAL-1 cells. (C) One representative example of the immunostaining of circulating peripheral blood mononuclear cells performed at day 6 after engraftment. Murine cells (green) and human BPDCN cells (red) are distinguishable based on specific human or murine CD45 antibody expression. Human BPDCN cells express CD56, CD123, and BDCA4. (D) Analysis of circulating cells from blood (left) and spleen cells (right) after May Grünwald Giemsa staining (standard MGG, magnification ×1000). These cells were obtained at sacrifice from a mouse inoculated with Luc+ CAL-1 cells.
In vivo efficacy of bortezomib against primary blastic plasmacytoid dendritic cell neoplasm
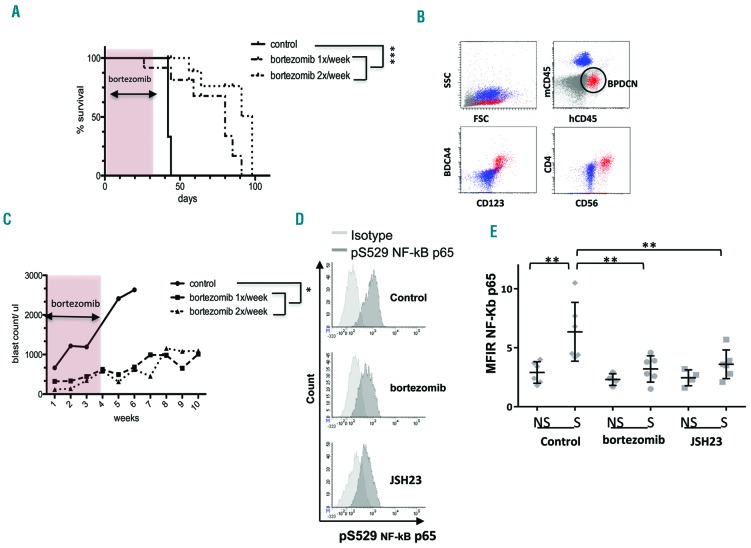
In order to assess the in vivo efficacy of bortezomib treatment in another way, a xenograft model was developed using primary BPDCN cells isolated from a patient. Weekly injections of bortezomib (4 weeks) significantly increased the overall survival of mice grafted with primary BPDCN cells compared to that of the same mice treated with phosphate-buffered saline (66±13 days versus 42±1 days, P<0.001). Twice-weekly injections of bortezomib further increased the overall survival of mice (77±11 days, P<0.001, n=3–4 mice/group in 2 independent experiments) (Figure 4A). Circulating human BPDCN cells identified in murine blood as human CD45+, CD123+, BDCA4+, CD4+, CD56+ cells decreased from 2640±220 cells/μL (phosphate-buffered saline control mice) to 680±298 cells/μL or 622±158 cells/μL (weekly or twice-weekly bortezomib-treated mice, respectively) at 5 weeks (P<0.01) (Figure 4B,C). We also monitored hemoglobin and platelet counts in mice to assess leukemic cell bone marrow infiltration without observing any major cytopenia in any conditions (data not shown). Thus, in this in vivo model, bortezomib extended mouse survival and reduced circulating blast cells. Furthermore, using measurements of mean fluorescent intensity ratio (MFIR), we confirmed in vivo that PDX cells (patient #127) extracted from the blood of mice treated with bortezomib for 6 h exhibited significantly reduced pRelA expression after bortezomib treatment (mean of MFIR: 3.2±1.1) compared to pRelA expression of PDX cells from untreated mice (mean of MFIR: 6.35±2.4). This in vivo inhibition of pRelA after bortezomib treatment was similar to that obtained with JSH23 treatment (mean of MFIR: 3.6±1.2) (Figure 2C).
Figure 4.
Bortezomib treatment is efficient at controlling tumor growth in a xenograft model using primary blastic plasmacytoid dendritic cell neoplasm cells. NSG mice were irradiated (2 Gy) and then inoculated intravenously with 1×106 to 2×106 primary BPDCN cells from patient #127 on day 0. Treatment was started on day 100 (J1) after the graft with bortezomib (0.25 mg/kg/mouse intraperitoneally) given one or twice weekly for 4 weeks (n=7 and n=4 mice, respectively). Mice injected with phosphate-buffered saline (PBS) over the 4 weeks were used as the control (n=3). (A) Overall survival of BPDCN inoculated-mice treated with bortezomib (dotted line) or with PBS (solid line) is shown. (B) One example of the immunostaining of peripheral blood performed at day 89 after engraftment. Murine cells (blue) and primary BPDCN (red) cells are distinguishable based on specific human or murine CD45 antibody expression. Human BPDCN cells express CD123, BDCA4, and CD4. (C) Mean of BPDCN cell counts in the blood of mice following treatment with bortezomib (dotted line) or PBS (solid line). (*P<0.05 and ***P<0.001). Intracellular expression of pRelA (pS529 NF-κB p65) was evaluated in PDX cells (BPDCN patient #127) obtained in mouse blood at day 1 and day 15 after in vivo treatment with bortezomib (0.25 mg/kg/mouse intraperitoneally) for 6 h (n=3 mice). JSH23 was used as a positive control (40 mg/kg, n=3 mice) and PBS (control, n=3 mice) as a negative control. PDX cells were stimulated ex vivo with TLR7 for 45 min (R848, 1 μg/mL) before staining. (D) Representative examples of intracellular expression of pRelA and isotype control staining after ex vivo TLR7 stimulation in these different conditions. (E) This histogram represents the mean fluorescence intensity ratio (MFIR) ± SEM of intracellular NF-κBp-65 in PDX cells obtained after treatment with bortezomib on day 1 and day 15, *P<0.05, **P<0.01. NS: unstimulated; S: stimulated with R848. The MFIR was obtained by dividing the mean fluorescence intensity (MFI) obtained with the anti-NF-κBp-65 antibody by the MFI of the respective isotype control antibody.
Discussion
BPDCN is an aggressive hematodermic neoplasia with a short-term survival.2,4 As there are no data supporting a particular regimen for this acute leukemia, treatments vary from chemotherapy based on a single agent used in B-cell lymphoma4,8 to poly-chemotherapy regimens similar to those given to patients with high-risk acute lymphocytic or acute myeloid leukemia,9,22 and allogeneic hematopoietic cell transplantation for consolidation.7,10,23–25 New approaches using more targeting therapies are needed for the majority of BPDCN patients unable to receive intensive chemotherapy regimens because of a median age of around 70 years at diagnosis.2,4 Bortezomib is a first-generation proteasome inhibitor approved by the Food and Drug Administration for the treatment of refractory multiple myeloma and mantle cell lymphoma.26 The efficacy of bortezomib is governed by its capacity to inhibit the NF-κB pathway, which plays an important role in the pathophysiology of BPDCN. Hirai et al. showed that bortezomib suppresses the survival and immunostimulatory functions of non-leukemic plasmacytoid dendritic cells by targeting intracellular trafficking of nucleic acid-sensing Toll-like receptors and altering endoplasmic reticulum homeostasis.27 Using a genomics approach, Sapienza et al. showed that the NF-κB pathway is aberrantly activated in BPDCN, and they reported inhibition of the cell cycle progression and survival of CAL-1 cells after bortezomib treatment.11 The percentage of viable CAL-1 cells decreased significantly by more than 50% when the cells were treated with 30 nM bortezomib for 24 h.11 We recently confirmed constitutive NF-κB activation in BPDCN cells with upregulation of the NF-κB p105 precursor-coding gene (NFKB1) in 12 primary BPDCN samples and demonstrated that inhibition of NF-κB p65 subunit translocation by the specific inhibitor JSH-23 is sufficient to induce BPDCN cell death in vitro.13 Here, our study confirmed in vitro that two BPDCN cell lines (CAL-1 and GEN2.2) and seven primary samples from BPDCN patients are sensitive to bortezomib treatment in terms of cell cycle arrest, cell proliferation inhibition, and cell death induction. Bortezomib has been shown to induce G2/M cell cycle arrest in different tumor cell models.28,29 We confirmed that exposure of CAL-1 and GEN2.2 cells to bortezomib caused a significant accumulation in the G2 phase and in sub-G1 phase (related to apoptotic cells). Furthermore, we demonstrated for the first time in a mouse model that in vivo treatment using bortezomib decreases pRelA. These results reinforce published data from Sapienza et al. obtained in the CAL-1 cell line.11 Moreover, we were able to show that bortezomib is effective in vivo at extending the survival of a primary BPDCN xenograft model. In this model, bortezomib − infused twice weekly for 4 weeks − increased survival for at least 6 additional weeks. Bortezomib is not a “conventional” cytotoxic agent and is used, for example, in multiple myeloma.31,32 For instance, synergistic effects with dexamethasone30 and histone deacetylase inhibitors31,32 have been previously reported. In multiple myeloma, bortezomib is currently used in association with thalidomide and dexamethasone.33 In our hands, we observed an in vitro synergistic effect of bortezomib and a histone deacetylase inhibitor (SAHA), idarubicin, simvastatin and 5-azacytidine in CAL-1 and GEN2.2 cells lines. Recently, Ceroi et al. demonstrated that cholesterol homeostasis is modified in BPDCN cells: cholesterol accumulation within leukemic cells is responsible for these cells’ high proliferative properties and can be normalized by treatment with LXR agonists.13 LXR stimulation in BPDCN exerts an anti-leukemic effect that can be enhanced by increasing cholesterol efflux. Cholesterol dependency of BPDCN cells was confirmed, since inhibition of the mevalonate pathway (i.e., cholesterol synthesis) by atorvastatin was sufficient to induce significant BPDCN cell death. Here, we extend these data. Indeed, we observed an important effect of statins against BPDCN cell lines and a synergistic effect with bortezomib mainly when associated with statins. Kim et al. recently observed a similar effect with simvastatin in combination with bergamottin − an inhibitor of some cytochrome P450 isoforms − that potentiates apoptosis through modulation of the NF-κB signaling pathway in human chronic myelogenous leukemia.34 These results suggest that statins could be a new approach for BPDCN treatment in combination with bortezomib.
In the CAL-1 xenograft model, engraftment is not clearly detectable in blood early after infusion, and we, therefore, developed another model to track BPDCN cells easily using evaluation of luciferase-expressing BPDCN cells by measuring the BLI signal. Luc+ CAL-1 cells were preferentially detectable in the bone marrow, spleen, lungs and liver as described in BPDCN patients who exhibit BDPCN cell involvement in many tissues, including the spleen, liver, central nervous system, tonsils, mucous membranes, lungs, kidneys, and muscle.3 Nevertheless, the xenograft model using primary BPDCN cells revealed that bortezomib treatment induced a significant (up to 2-fold) increase of mouse survival with a significant reduction of circulating BPDCN cells.
Although current treatment regimens for BPDCN can achieve complete responses, many patients relapse, even after allogeneic hematopoietic cell transplantation, underscoring the need for novel therapeutics. Bortezomib is effective at killing BPDCN cells in vitro and exerts an anti-leukemic effect in a xenograft mouse model of primary BPDCN. Given its low toxicity, it could be used in combination with other drugs, such as 5-azacytidine or simvastatin, in maintenance for several cycles of treatment to improve the response in elderly patients who cannot benefit from allogeneic hematopoietic cell transplantation. Several compounds of this proteasome inhibitor family are currently under development. Recently, carfilzomid, an irreversible and selective proteasome inhibitor, has shown superiority compared to bortezomib in a phase III myeloma clinical trial.35 Moreover, ixazomib is an orally bioavailable, reversible proteasome inhibitor, approved in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma.36 These molecules should be tested against BPDCN cells alone, or rather in combination with others drugs, such as hypomethylating agents like 5-azacytidine (tested here), which shows promising effects on patients with refractory acute myeloid leukemia,37 simvastatin (tested here), or lenalidomide, which has demonstrated efficacy in a xenograft mouse model of human BPDCN.20 The synergistic effect of these molecules can be evaluated in PDX mouse models.
In conclusion, our preclinical results provide a rationale for the use of bortezomib in combination with classical chemotherapy for the treatment of BPDCN patients. A prospective clinical trial combining proteasome inhibitor with cytotoxic drugs should now be performed to prospectively validate these results.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1861
Funding
This study was supported by grants from the Agence Nationale de la Recherche (LabEx LipSTIC, ANR-11-LABX-0021), the Conseil Régional de Bourgogne Franche-Comté (LabEx LipSTIC 2016 to PS), the DGOS and INCa (National PHRC #PHRC-K 16-93 and PRT INCA 2015 #PRT-K15-175), as well as the Ligue Régionale Contre le Cancer (CCIRGE-BFC 2016).
References
- 1.Garnache-Ottou F, Feuillard J, Ferrand C, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145(5):624–636. [DOI] [PubMed] [Google Scholar]
- 2.Feuillard J, Jacob MC, Valensi F, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556–1563. [DOI] [PubMed] [Google Scholar]
- 3.Julia F, Dalle S, Duru G, et al. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014;38(5):673–680. [DOI] [PubMed] [Google Scholar]
- 4.Petrella T, Bagot M, Willemze R, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662–675. [PubMed] [Google Scholar]
- 5.Dalle S, Beylot-Barry M, Bagot M, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2009;162(1):74–79. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich S, Andrulis M, Hegenbart U, et al. Blastic plasmacytoid dendritic cell neoplasia (BPDC) in elderly patients: results of a treatment algorithm employing allogeneic stem cell transplantation with moderately reduced conditioning intensity. Biol Blood Marrow Transplant. 2011;17(8):1250–1254. [DOI] [PubMed] [Google Scholar]
- 7.Gilis L, Lebras L, Bouafia-Sauvy F, et al. Sequential combination of high dose methotrexate and L-asparaginase followed by allogeneic transplant: a first-line strategy for CD4+/CD56+ hematodermic neoplasm. Leuk Lymphoma. 2012;53(8):1633–1637. [DOI] [PubMed] [Google Scholar]
- 8.Kharfan-Dabaja MA, Lazarus HM, Nishihori T, Mahfouz RA, Hamadani M. Diagnostic and therapeutic advances in blastic plasmacytoid dendritic cell neoplasm: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(7): 1006–1012. [DOI] [PubMed] [Google Scholar]
- 9.Pagano L, Valentini CG, Pulsoni A, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multi-center study. Haematologica. 2013;98(2): 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos-Weil D, Dietrich S, Boumendil A, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121(3):440–446. [DOI] [PubMed] [Google Scholar]
- 11.Sapienza MR, Fuligni F, Agostinelli C, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014;28(8):1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120(5):947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceroi A, Masson D, Roggy A, et al. LXR agonist treatment of blastic plasmacytoid dendritic cell neoplasm restores cholesterol efflux and triggers apoptosis. Blood. 2016;128(23):2694–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaperot L, Perrot I, Jacob MC, et al. Leukemic plasmacytoid dendritic cells share phenotypic and functional features with their normal counterparts. Eur J Immunol. 2004;34(2):418–426. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Murata K, Fukushima T, et al. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81(2):148–154. [DOI] [PubMed] [Google Scholar]
- 16.Angelot-Delettre F, Biichle S, Ferrand C, et al. Intracytoplasmic detection of TCL1–but not ILT7-by flow cytometry is useful for blastic plasmacytoid dendritic cell leukemia diagnosis. Cytometry. 2012;81(8):718–724. [DOI] [PubMed] [Google Scholar]
- 17.Garnache-Ottou F, Chaperot L, Biichle S, et al. Expression of the myeloid-associated marker CD33 is not an exclusive factor for leukemic plasmacytoid dendritic cells. Blood. 2005;105(3):1256–1264. [DOI] [PubMed] [Google Scholar]
- 18.Garnache-Ottou F, Feuillard J, Saas P. Plasmacytoid dendritic cell leukaemia/lymphoma: towards a well defined entity? Br J Haematol. 2007;136(4):539–548. [DOI] [PubMed] [Google Scholar]
- 19.Angelot-Delettre F, Roggy A, Frankel AE, et al. In vivo and in vitro sensitivity of blastic plasmacytoid dendritic cell neoplasm to SL-401, an interleukin-3 receptor targeted biologic agent. Haematologica. 2015;100(2): 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agliano A, Martin-Padura I, Marighetti P, et al. Therapeutic effect of lenalidomide in a novel xenograft mouse model of human blastic NK cell lymphoma/blastic plasmacytoid dendritic cell neoplasm. Clin Cancer Res. 2011;17(19):6163–6173. [DOI] [PubMed] [Google Scholar]
- 21.Angelot-Delettre F, Garnache-Ottou F. Blastic plasmacytoid dendritic cell neoplasm. Blood. 2012;120(14):2784. [DOI] [PubMed] [Google Scholar]
- 22.Piccaluga PP, Paolini S, Sapienza MR, Pileri SA. Blastic plasmacytoid dendritic cell neoplasm: is it time to redefine the standard of care? Expert Rev Hematol. 2012;5(4):353–355. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Suzuki R, Kuwatsuka Y, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125(23):3559–3562. [DOI] [PubMed] [Google Scholar]
- 24.Dalle S, Beylot-Barry M, Bagot M, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2010;162(1):74–79. [DOI] [PubMed] [Google Scholar]
- 25.Gruson B, Vaida I, Merlusca L, et al. L-asparaginase with methotrexate and dexamethasone is an effective treatment combination in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol. 2013;163(4):543–545. [DOI] [PubMed] [Google Scholar]
- 26.Roy SS, Kirma NB, Santhamma B, Tekmal RR, Agyin JK. Effects of a novel proteasome inhibitor BU-32 on multiple myeloma cells. Cancer Chemother Pharmacol. 2014;73(6): 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirai M, Kadowaki N, Kitawaki T, et al. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 2011;117(2):500–509. [DOI] [PubMed] [Google Scholar]
- 28.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–2622. [PubMed] [Google Scholar]
- 29.Piperdi B, Ling YH, Liebes L, Muggia F, Perez-Soler R. Bortezomib: understanding the mechanism of action. Mol Cancer Ther. 2011;10(11):2029–2030. [DOI] [PubMed] [Google Scholar]
- 30.Koyama D, Kikuchi J, Hiraoka N, et al. Proteasome inhibitors exert cytotoxicity and increase chemosensitivity via transcriptional repression of Notch1 in T-cell acute lymphoblastic leukemia. Leukemia. 2014;28(6): 1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastian L, Hof J, Pfau M, et al. Synergistic activity of bortezomib and HDACi in preclinical models of B-cell precursor acute lymphoblastic leukemia via modulation of p53, PI3K/AKT, and NF-kappaB. Clin Cancer Res. 2013;19(6):1445–1457. [DOI] [PubMed] [Google Scholar]
- 32.Zhang QL, Wang L, Zhang YW, et al. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia. 2009;23(8):1507–1514. [DOI] [PubMed] [Google Scholar]
- 33.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. [DOI] [PubMed] [Google Scholar]
- 34.Kim SM, Lee EJ, Lee JH, et al. Simvastatin in combination with bergamottin potentiates TNF-induced apoptosis through modulation of NF-kappaB signalling pathway in human chronic myelogenous leukaemia. Pharm Biol. 2016;54(10):2050–2060. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 36.Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503–1512. [DOI] [PubMed] [Google Scholar]
- 37.Walker AR, Klisovic RB, Garzon R, et al. Phase I study of azacitidine and bortezomib in adults with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2014;55(6):1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.