Abstract
IT-901 is a novel and selective NF-κB inhibitor with promising activity in pre-clinical models. Here we show that treatment of chronic lymphocytic leukemia cells (CLL) with IT-901 effectively interrupts NF-κB transcriptional activity. CLL cells exposed to the drug display elevated mitochondrial reactive oxygen species, which damage mitochondria, limit oxidative phosphorylation and ATP production, and activate intrinsic apoptosis. Inhibition of NF-κB signaling in stromal and myeloid cells, both tumor-supportive elements, fails to induce apoptosis, but impairs NF-κB-driven expression of molecules involved in cell-cell contacts and immune responses, essential elements in creating a pro-leukemic niche. The consequence is that accessory cells do not protect CLL cells from IT-901-induced apoptosis. In this context, IT-901 shows synergistic activity with ibrutinib, arguing in favor of combination strategies. IT-901 is also effective in primary cells from patients with Richter syndrome (RS). Its anti-tumor properties are confirmed in xenograft models of CLL and in RS patient-derived xenografts, with documented NF-κB inhibition and significant reduction of tumor burden. Together, these results provide pre-clinical proof of principle for IT-901 as a potential new drug in CLL and RS.
Introduction
Nuclear factor-kappa B (NF-κB) is a ubiquitous transcription factor, composed of a family of five structurally related proteins, including p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB and c-Rel, which can form homo- and hetero-dimers. While NF-κB is normally kept inactivated through binding to the inhibitory subunit (IκB), IκB phosphorylation and degradation releases the dimer that translocates to the nucleus and binds to target sequences on DNA.1–3
NF-κB signaling plays essential roles in inflammation, immune responses, proliferation, and cell survival.4–6 In cancer cells, NF-κB promotes tumor growth by contributing to maintenance/expansion of tumor-initiating cells and by shaping the tumor microenvironment.7 Deregulated NF-κB signaling is a common finding in most, if not all, B-lymphoid malignancies.8
Chronic lymphocytic leukemia cells (CLL) exhibit high constitutive NF-κB activation compared to normal B lymphocytes, with the p65 subunit being the most active and relevant for transcription.9–12 Moreover, p65 levels correlate with leukemic cell survival in vitro, as well as with tumor burden and shorter lymphocyte doubling time.13,14 NF-κB is activated by B-cell receptor (BCR) signaling, the driving force behind CLL pathogenesis and progression.15 However, additional signals from the micro-environment increase NF-κB activity and enhance CLL cell survival, with members of the Bcl-2 family among the most important transcriptional targets.16–19
Approximately 5–10% of CLL patients undergo transformation of the leukemia into an aggressive diffuse large B-cell lymphoma (DLBCL), a complication known as Richter syndrome (RS).20 The clinical outcome of RS is generally poor, with a median survival of a few months, mainly because of limited therapeutic options and responses.21 In a significant proportion of cases, RS patients carry activating mutations in genes belonging to the NF-κB pathway.22
Given the central role of the NF-κB signals in CLL and in RS transformation, and specifically of the p65 subunit, this complex represents an important therapeutic target, even if so far none of the studied inhibitors have entered clinical trials. IT-901 was recently reported as an NF-κB inhibitor (acting through c-Rel and p65) and redox modulator, showing significant activity in mouse models of graft-versus-host disease (GvDH) and graft-versus-lymphoma (GvL), as well as in a xenograft model of human-B-cell lymphoma, mediating promising anti-lymphoma effects.23,24
The aim of this work is to determine the in vitro and in vivo effects of IT-901 in CLL and RS primary cells and derived line models.
Methods
Cell lines and primary samples
Leukemic cells were purified using Ficoll-Hypaque (Sigma-Aldrich, Milan, Italy) from peripheral blood (PB) of CLL patients or lymph node (LN) of RS patients presenting with typical morphology and immunophenotype.21 Samples were obtained at Weill Cornell Medicine after written informed consent in accordance with institutional guidelines and the Declaration of Helsinki. The referring physician provided molecular and genetic characterization of patients’ samples. Normal circulating B cells were purified from healthy donors. Mec-1 and OSU-CLL CLL cell lines were obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures and Ohio State University, respectively, and cultured in RPMI+10% fetal bovine serum (FBS). HS-5 stromal cells were obtained from ATCC and cultured in DMEM+10% FCS.
Metabolic assays
Chronic lymphocytic leukemia cells were exposed to vehicle (0.02% DMSO in RPMI-1640, indicated as NT) or IT-901 (10 μM in the same solution as vehicle) for 6 hours (h), before dynamically measuring the metabolic profile using the XF96e Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). Cells (5×105 for primary cells and 105 for cell lines) were seeded in specialized tissue culture plates, coated with CellTak (BD Biosciences). An hour before measurement, cells were incubated at 37°C in a CO2-free atmosphere. Oxygen consumption rate (OCR), an indicator of mitochondrial respiration, was measured in basal conditions and following addition of specific drugs, oligomycin (1 μM), carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP, 1 μM) and Rotenone/Antimycin A (0.5 μM) able to interfere with different steps of the oxidative phosphorylation (OXPHOS) process (XF Cell Mito Stress test kit, Seahorse Bioscience). Maximal OCR and ATP production were measured. In all experiments, measurements were performed in quadruplicates.
In vivo experiments and treatments
Mec-1 (5×105) cells were intravenously injected (i.v; tail vein) in 8-week old NOD/SCID/gamma chain−/− (NSG) mice and left to engraft for ten days before starting treatment. Mice received intra-peritoneal (i.p.) injection of IT-901 (15 mg/kg) or vehicle (Polyethene glycol-12 Glycerol-Dimyristate, GDM 4% in PBS). At the end of treatment, mice were euthanized, organs collected and partially dismantled to obtain single cell suspension or formalin-fixed for immunohistochemistry analyses. Mec-1 cell distribution in the different organs was analyzed by flow cytometry, after staining single cell suspensions with anti-human-CD19FITC and -CD45PerCP antibodies to identify leukemic cells. A different set of mice was monitored for survival.
Richter syndrome model
Primary RS cells were obtained from PB or LN biopsies of clinically diagnosed RS patients. Purified cells (20×106) or LN fragments were injected sub-cutaneously (s.c., double flank) in 6-week old NSG mice and left to engraft. Tumor masses were then collected, partially dismantled and re-implanted in new animals for several passages to obtain a stable model of RS. Genetic stability and relationship to the original tumor was confirmed by exome sequencing (T Vaisitti and JN Allan, 2017, manuscript in preparation).
After several passages in vivo, RS cells obtained from the tumor mass were put in culture over a stromal layer of HS-5 cells and were able to grow in vitro, maintaining the original genetic and phenotypic characteristics. RS-PDX models were established while TV and SD were Visiting Scientists at Weill Cornell Medicine. The Institutional Animal Care and Use Committee approved all the experiments involving mice.
Additional details are provided in the Online Supplementary Appendix.
Results
IT-901 blocks NF-κB activity in primary CLL cells and derived cell lines
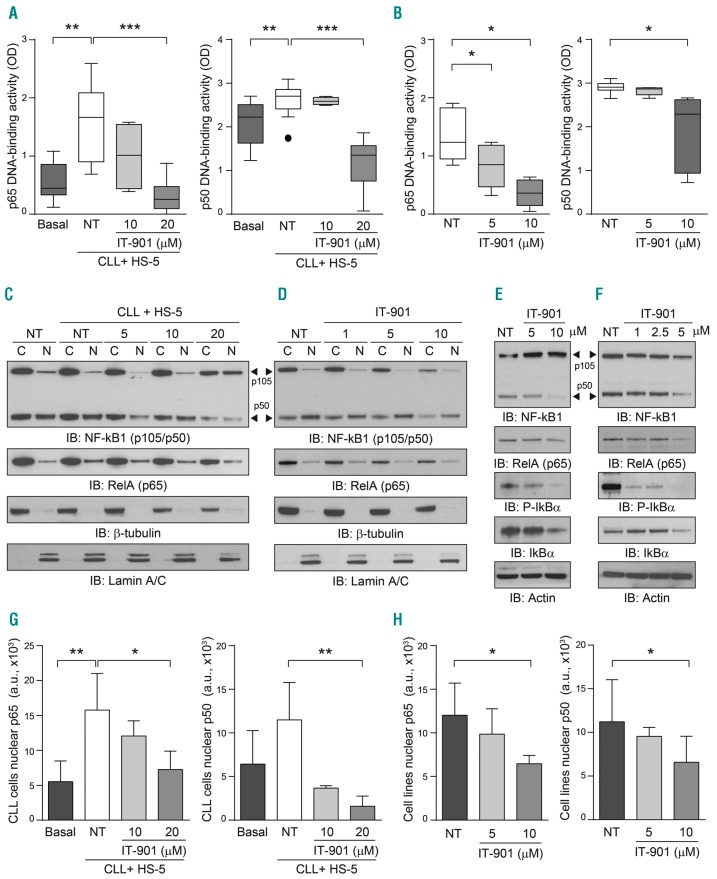
IT-901 is a recently described NF-κB inhibitor, with promising activity in mouse models of GvHD and lymphoma.23,24 In consideration of the high levels of constitutive NF-κB activation in CLL and in RS cells, the aim of this work is to provide pre-clinical evidence of the therapeutic effects of IT-901. In the experimental setting adopted, maximal NF-κB activation was obtained by culturing primary CLL cells for 6 h over a layer of HS-5, a stromal cell line with documented nurturing properties for CLL cells.25 By using an ELISA assay that measures NF-κB interactions with its DNA consensus sequences, we confirmed significant upregulation of p65 and p50 DNA-binding activities compared to the basal condition (Figure 1A). Conversely, addition of IT-901 during the co-culture period significantly decreased DNA binding of both subunits, with p65 being most sensitive (Figure 1A). In line with previous reports, IT-901 limited the DNA binding ability of c-Rel (Online Supplementary Figure S1A); however, in CLL cells this subunit is expressed and active at very low levels, suggesting that the main effects of the drug are through p65. Inhibition of NF-κB transcriptional activity was confirmed using nuclear extracts of Mec-1 and OSU-CLL, two CLL cell lines, where NF-κB activity is independent of micro-environmental conditions (Figure 1B and Online Supplementary Figure S1B).
Figure 1.
IT-901 blocks nuclear factor-kappa B (NF-κB) activity in primary chronic lymphocytic leukemia (CLL) cells and derived cell line. (A) DNA binding activity of the p65 and p50 subunits in primary CLL cells (n=13) was analyzed using an ELISA kit, applying the same amount of nuclear extracts. Leukemic cells were co-cultured on a stromal layer (HS-5) to maximize the activation of the pathway, in the presence of increasing doses of drug or vehicle (NT) for 6 hours (h). (B) Cumulative DNA binding activity of NF-κB (p65 and p50) in 2 different cell line models of CLL, Mec-1 (n=5), and OSU-CLL (n=3). (C, D, G and H) Cytoplasmic (C) and nuclear (N) fractions obtained from primary CLL cells (C) or cell lines (D) cultured as indicated above were resolved by SDS-PAGE and blotted with specific antibodies to detect the expression of NF-κB1 subunits p105/p50 (arrow head) and RelA (p65). Lamin A/C and β-tubulin were used as nuclear and cytoplasmic markers, respectively. Nuclear p65 and p50 band intensities in primary CLL samples and CLL cell lines are reported in (G) and (H), respectively. (E and F) Total lysates from primary CLL cells (E) or CLL cell lines (F) cultured alone and exposed to different doses of IT-901 or vehicle (NT) for 6 h were resolved by SDS-PAGE and expression of the NF-κB complex analyzed using specific antibodies. Actin was used as a loading control. OD: optical density.
Diminished expression of p65 and p50 in nuclear and cytosolic fractions following IT-901 exposure was documented in primary cells and cell lines, suggesting that the drug induces degradation of the complex (Figure 1C, G and D, H, respectively). The same treatment also decreased the expression of the inhibitory subunit IκBα both in the phosphorylated and non-phosphorylated forms, in primary CLL cells (Figure 1E) and cell lines (Figure 1F), in line with the hypothesis that IT-901 interacts with the NF-κB-IκB cytoplasmic complex triggering its degradation.
IT-901 induces mitochondrial reactive oxygen species accumulation
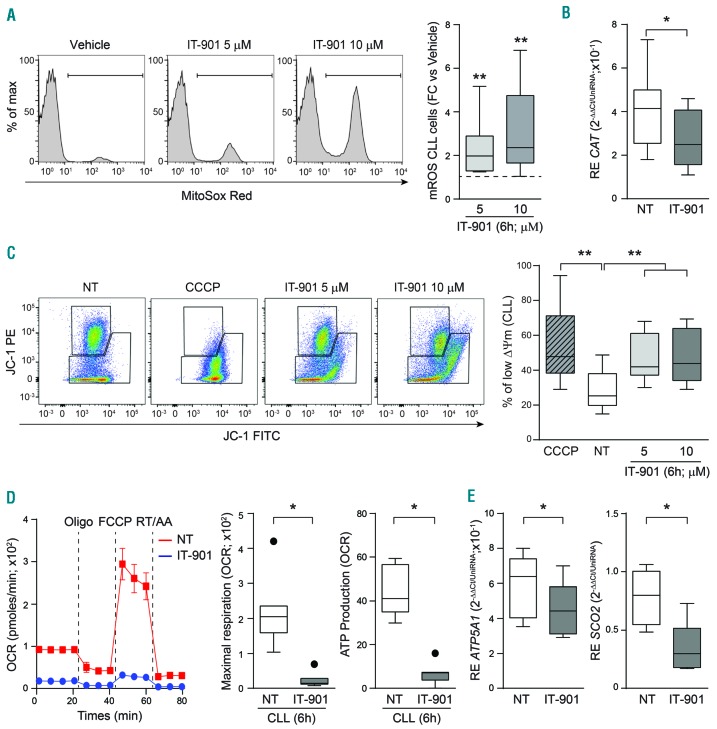
Consistent with the documented cross-talk between NF-κB and ROS,26,27 a prominent and dose-dependent increase in mitochondrial reactive oxygen species (mROS) was measured starting 6 h after treatment with IT-901, both in primary cells and in cell lines (Figure 2A and Online Supplementary Figure S2A, respectively).
Figure 2.
IT-901 induces mitochondrial damage and compromises mitochondrial respiration. (A) Representative plots and cumulative data of mitochondrial reactive oxygen species (mROS) concentration in chronic lymphocytic leukemia (CLL) cells. Data are represented as fold change (FC) over the vehicle (n=10). (B) Box plot reporting the catalase (CAT) mRNA expression levels in vehicle (NT)- or IT-901-treated primary cells (n=10). (C) Representative plots and cumulative data of inner mitochondrial membrane potential (ΔΦm) in primary CLL cells (n=10) exposed to vehicle (NT) or increasing doses of IT-901 for 6 hours (h). CCCP was used as positive control. (D) Dynamic mitochondrial metabolic profile (OCR; pmoles/min) of a representative CLL patient treated with vehicle (red line) or IT-901 10 μM (blue line) for 6 h. Maximal respiration (calculated as: OCR after FCCP injection-late OCR measurement after RT/AA addition) and ATP production (calculated as last rate measurement of OCR before Oligo injection-minimum rate measurement after Oligo injection) in primary CLL patients (n=7). (E) Box plots reporting ATP-synthase (ATP5A1) and Cytochrome C Oxidase Assembly Protein (SCO2) mRNA expression levels in vehicle- or IT-901-treated primary cells (n=7). CCCP: carbonyl cyanide m-chlorophenyl hydrazone; Oligo: oligomycin; FCCP: carbonyl cyanide p-trifluoromethoxyphenylhydrazone; RT/AA: rotenone + antimycin A; OCR: oxygen consumption rate.
In principle, progressive mROS accumulation could be caused by increased production or impaired scavenging, due, at least in part, to the interruption of NF-κB-dependent transcription of genes coding for scavenging enzymes.26,27 The finding of a significantly decreased expression of the NF-κB-regulated catalase enzyme favored the hypothesis of a contribution of impaired scavenging to increased mROS (Figure 2B and Online Supplementary Figure S2B).
Elevated mROS levels decreased mitochondrial membrane potential (Δϕm), as shown by using the potentiometric probe JC-1, whose decrease in red fluorescence depends solely on mitochondrial depolarization (Figure 2C). Similar results were obtained in CLL cell lines, with a dose-dependent loss of (Δϕm), starting at the 2.5 μM dose (Online Supplementary Figure S2C). These results were confirmed using the cationic dye TMRM that binds to the inner membrane of intact mitochondria (Online Supplementary Figure S2D).
Mitochondrial respiration is severely compromised following IT-901 treatment
Consistent with the finding of high levels of mROS in IT-901-treated CLL cells, mitochondrial energetic processes were severely compromised. By using the Seahorse metabolic analyzer, and in keeping with previous data,28 we observed that leukemic cells have a distinct preference for oxidative phosphorylation (OXPHOS), with limited glycolytic capacity. After 6 h of culture with 10 μM IT-901, a dramatic drop in respiration was highlighted, leading to a marked decrease in ATP production (Figure 2D). In line with the hypothesis that these metabolic alterations are due to the brusque interruption of NF-κB transcriptional circuits, as documented in other models,29 we found significant downmodulation of ATP5A1, the enzyme responsible for ATP synthesis, and of SCO2, a mitochondrial chaperone molecule that controls assembly of the cytochrome c oxidase subunit II of the electron transfer chain (Figure 2E). A similar behavior was measured in the cell lines, with a clear dose-dependent response to IT-901 (Online Supplementary Figure S2E and F).
IT-901 rapidly induces apoptosis in primary CLL cells, but not in normal B and T lymphocytes
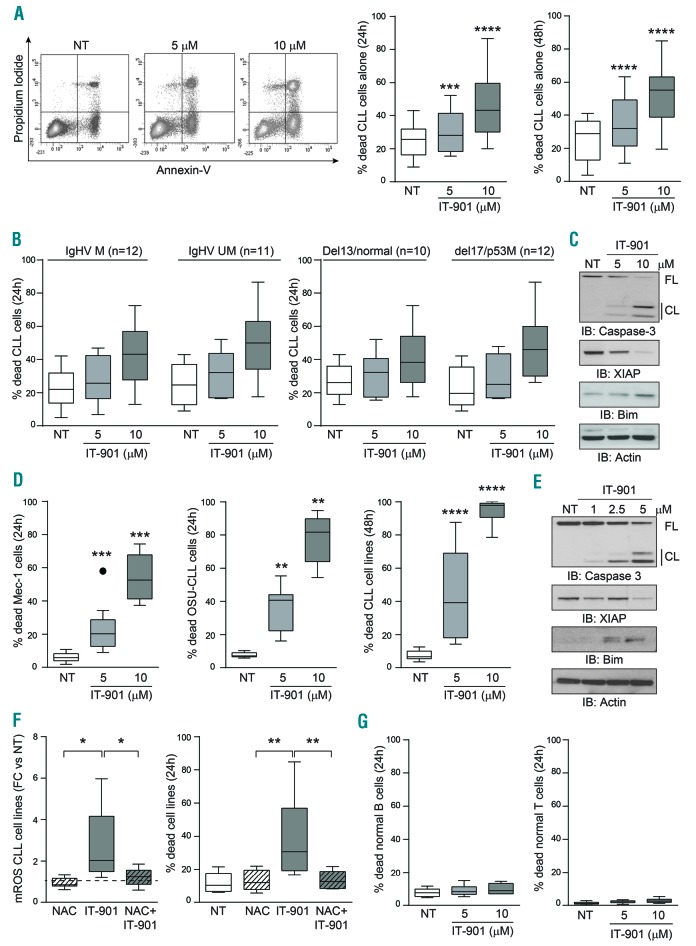
Induction of mROS with subsequent mitochondrial damage activated the apoptotic cascade in primary CLL cells from 25 different patients (Figure 3A). Apoptosis was time- and dose-dependent, starting at 5 μM after 24-h culture with IT-901 (Figure 3A). In the CLL cohort examined, the apoptotic response to IT-901 was apparently independent of the mutational status of the IgHV genes and of the presence of mutations or deletions in TP53, classically considered as markers of a more aggressive form of the disease (Figure 3B). Activation of the apoptotic cascade was confirmed by highlighting caspase 3 cleavage, downregulation of anti-apoptotic XIAP and upregulation of pro-apoptotic BIM proteins, evident at the 5 μM dose and peaking at 10 μM for primary cells (Figure 3C).
Figure 3.
IT-901 rapidly induces apoptosis selectively in primary chronic lymphocytic leukemia (CLL) cells. (A) Representative plots and cumulative data of apoptotic staining with Annexin-V and propidium iodide (PI) of CLL patient cells (n=25) after 24–48 hours (h) of exposure to increasing doses of IT-901 or vehicle (NT). (B) Apoptotic data were plotted according to the mutational status of IgHV genes [mutated (M) vs. unmutated (UM)] and to the presence of specific genetic abnormalities (deletion 13/normal vs. deletion 17/mutation in TP53). (C) Total lysates from primary CLL cells cultured alone and exposed to different doses of IT-901 or vehicle (NT) for 6 h were resolved by SDS-PAGE and expression of pro- and anti-apoptotic proteins and activation of the caspase pathway analyzed using specific antibodies. Actin was used as a loading control. (D) Cumulative data of apoptosis obtained from Mec-1 (n=12) and OSU-CLL cell lines (n=9) at 24 h and both cell lines (n=21) at 48 h, exposed to increasing doses of IT-901. (E) Total lysates of CLL cell lines, cultured alone and exposed to different doses of IT-901 or vehicle (NT) for 6 h were resolved by SDS-PAGE and expression of pro- and anti-apoptotic proteins and activation of the caspase pathway analyzed using specific antibodies. Actin was used as a loading control. (F) Cumulative data of mROS and apoptosis of CLL cell lines (n=6) measured in the presence of N-acetyl-cysteine (NAC) and/or IT-901. (G) Cumulative data of apoptosis in normal B and T lymphocytes purified from 6 different healthy donors and exposed to increasing doses of IT-901 for the indicated time points. FL: full-length; CL: cleaved.
These results were then confirmed in the two CLL cell line models, which were more sensitive to IT-901, in keeping with higher constitutive NF-κB activation (Figure 3D and E). Treatment of cell lines with the N-acetyl cysteine (NAC), a ROS scavenger, completely abrogated mROS accumulation and prevented apoptosis, clearly indicating that mROS are responsible for leukemic cell death (Figure 3F).
In line with a markedly lower NF-κB expression and constitutive activation,10 PB, and B and T lymphocytes purified from healthy donors were less sensitive to apoptosis by IT-901 (Figure 3G). After 24h cultures at the 10 μM concentration, less than 10% (9.8±3.2) of normal B lymphocytes were dead, as opposed to 44% (44.7±17.7) of primary CLL cells, indicating the presence of a therapeutic window.
NF-κB silencing recapitulates IT-901 functional effects
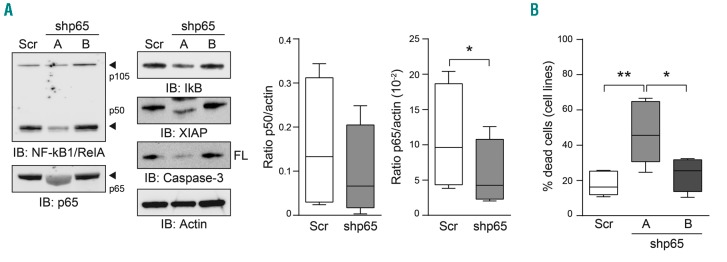
To confirm that IT-901 effects on CLL cells were due to specific NF-κB inhibition, we silenced the p65 subunit of this complex. This choice was based on evidence of its high expression and critical role in CLL,3,13,14 also confirmed by our results. Lentiviral particles containing a control GFP-shRNA [Scramble (Scr)], as well as 4 different GFP-shRNA sequences targeting p65 were used to infect CLL cell lines. One of the shRNA p65 sequences (shp65A) significantly inhibited expression of p65 compared to the Scr sequence, as shown by western blot (Figure 4A). A second shRNA p65 sequence (shp65B) was used as an additional control because of its limited inhibition of expression of the target protein (Figure 4A). Infected cells analyzed for viability by flow cytometry at day 6 post infection, after staining for AnnexinV and PI, showed that silencing by shp65A significantly enhanced apoptosis of leukemic cells compared to cells infected with the Scr or shp65B sequences (Figure 4B). Consistently, expression of proteins related to the apoptotic pathway, such as XIAP and Caspase-3, was influenced by p65 silencing, confirming the results obtained in IT-901 treated primary CLL cells (Figure 4A).
Figure 4.
Silencing of the p65 subunit confirms results obtained with IT-901. (A) chronic lymphocytic leukemia (CLL) cell lines were infected with lentiviral particles containing plasmids coding for an shRNA scramble (Scr) or shRNA p65 (A and B). Expression of the NF-κB complex was checked by western blot and p50 and p65 expression quantified over actin, showing a significant reduction for p65, while for p50 statistical significance was not reached. Silencing of p65 diminished also the expression of the anti-apoptotic protein XIAP and of the full-length (FL) Caspase3 protein. Actin was used as a loading control. (B) Apoptosis analysis by flow cytometry of cell lines infected with shRNA lentiviral particles (n=5).
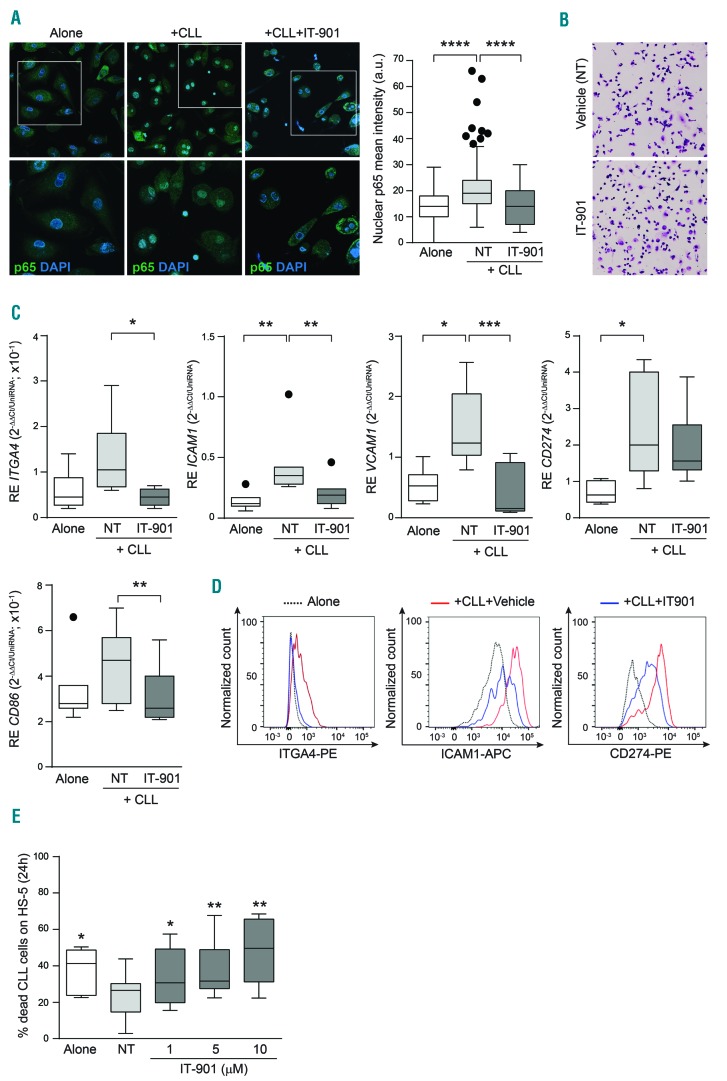
IT-901 inhibits the nurturing properties of nurse-like cells (NLC)
Given: i) the critical role played by the leukemic microenvironment in conditioning CLL cell survival and resistance to therapy; and ii) the central role of NF-κB in the interactions between CLL cells and stromal/myeloid components,30 we explored the effects of IT-901 on the stromal cell line HS-5 and on nurse-like cells (NLC), a population of alternatively activated macrophages typical of CLL patients.31
Exposure of both cell types to lipopolysaccharides (LPS) strongly activated NF-κB, as shown by nuclear translocation of the p65 subunit, which was almost completely abrogated by IT-901 treatment (Online Supplementary Figure S3A), confirming that the drug is effective. We then co-cultured CLL cells on a layer of autologous NLC, documenting NF-κB activation in both cell types, which was prevented by IT-901 (Figure 5A). However, exposure of HS-5 or NLC to IT-901 did not significantly alter their viability (Figure 5B and Online Supplementary Figure S3B). Consistently, no IT-901-induced modulation of metabolic genes, mROS and ROS scavengers could be highlighted, suggesting that CLL cells are uniquely sensitive to the drug, likely due to their basal metabolic conditions (Online Supplementary Figure S3C).
Figure 5.
IT-901 inhibits the nurturing properties of nurse-like cells (NLC). (A) Confocal microscopy analysis of nuclear factor-kappa B (NF-κB) activation in NLC alone or co-cultured with autologous CLL cells in the presence of vehicle (+CLL) or IT-901 (+CLL+IT-901). Cumulative results of the nuclear p65 fluorescence intensity, in NLC, are reported in the graph. (B) Giemsa staining of vehicle- or IT-901-treated NLC. (C and D) mRNA expression level (C; n=8) and phenotypic analysis by flow cytometry (D) of molecules involved in cell-cell interactions (ITGA4, ICAM1 and VCAM1) and immune-modulatory pathway (CD274 and CD86) in NLC cultured as indicated above. (E) Cumulative data of apoptosis of primary leukemic cells co-cultured over HS-5 cells in the presence of increasing doses of IT-901 for 24h. a.u.: arbitrary units.
NF-κB signaling in NLC induced transcription of genes encoding integrins, including ITGA4, ICAM1 and VCAM-1, and immune-modulatory molecules, such as CD274 and CD86.30 Consistent with the inhibitory effects of IT-901, expression of these target genes (Figure 5C) and derived proteins (Figure 5D) was inhibited at the 10 μM dose, indicating that IT-901 prevents the tumor-supportive phenotype of stromal or NLC cells.
In line with this hypothesis, IT-901 was equally effective in inducing apoptosis in CLL cells cultured alone or over a layer of HS-5 or NLC, without measurable differences in degradation of the NF-κB complex, caspase 3 activation and modulation of apoptosis-related proteins (Figure 5E and Online Supplementary Figure S3D and E).
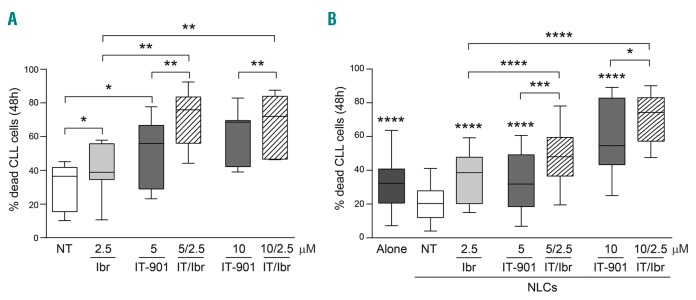
IT-901 co-operates with ibrutinib in inducing apoptosis of CLL cells
We then asked whether IT-901 potentiated the effects of the btk inhibitor ibrutinib, selected because of its current use in the treatment of CLL patients, including the high-risk subgroups32 and considering that both drugs target the same signaling pathway.33 The combination of IT-901 with ibrutinib markedly enhanced the effects of both drugs used alone, as determined both by treating CLL cells alone or after co-culturing leukemic cells on NLC with consequent protection from spontaneous apoptosis (Figure 6A and B). As an example, when cultured over NLC, treatment with ibrutinib 2.5 μM + IT-901 5 μM induced 48% (48±16.1) of the leukemic cells to undergo apoptosis after 48 h, compared to 36% (36±14.5) and 33% (33±17) when treating cells with ibrutinib or IT-901 alone, respectively (Figure 6B).34
Figure 6.
IT-901 co-operates with ibrutinib in inducing apoptosis of chronic lymphocytic leukemia (CLL) cells. Cumulative results of apoptosis of primary CLL cells (n=16) cultured alone (A) or over autologous nurse-like cells (NLC) (B) for 48 hours (h) in the presence of ibrutinib (2.5 μM) and IT-901 (5 and 10 μM) alone or in combination. Statistical significance of the combined effect of IT-901 and ibrutinib compared to single drugs alone was calculated according to the effect-based strategy, using the Highest Single Agent approach, as described by Foucquier and Guedj.34 *Over single box plot indicates statistical significance compared to untreated (NT).
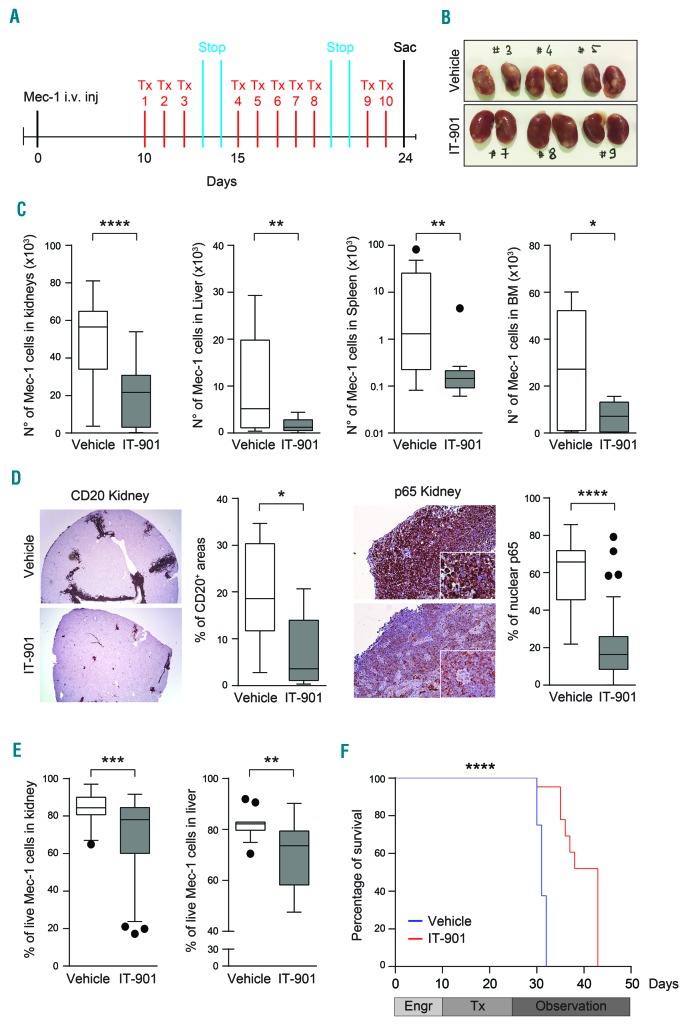
IT-901 limits in vivo growth and spread of CLL cells
We then tested whether treatment with IT-901 of mice xenografted with CLL cell lines induced an anti-tumor effect. To address this point, Mec-1 cells were injected in NSG mice,35 a model considered to be reproducible and instructive for therapeutic testing.36 Cells were injected in the tail vein of 8-week old mice, left to engraft for ten days, before beginning treatment with IT-901 (15 mg/kg, every day in PBS+GDM) or vehicle only. (A scheme of the in vivo experiment is reported in Figure 7A). This experiment had two distinct end points: i) at the end of the treatment schedule, vehicle- and IT-901-treated animals were analyzed for tumor growth and leukemic cell infiltration of different target organs; ii) the remaining mice were analyzed for survival.
Figure 7.
IT-901 limits in vivo growth and spread of chronic lymphocytic leukemia (CLL) cells. (A) Representative scheme of the in vivo model. Mec-1 cells were intravenously injected in tail vein of NSG mice, left to engraft for ten days before starting the treatment with IT-901 or vehicle. (B) Images of kidneys obtained from vehicle- or IT-901-treated mice. (C) Mec-1 engraftment in different organs evaluated by flow cytometry after labeling of leukemic cells with anti-human-CD45 and -CD19 antibodies. Cumulative data of engraftment in kidneys, liver, spleen and bone marrow (BM) (n=8 different mice/group). (D) Immunohistochemical analyses and quantification of CD20 and nuclear p65 staining (reported as percentage of positive cells) in kidneys of vehicle- or IT-901-treated mice. (E) Viability of Mec-1 cells, purified from kidneys and liver of vehicle- or IT-901-treated mice, analyzed by flow cytometry (69% vs. 85% of viable cells in kidneys and 70% vs. 82% of viable cells in liver, respectively). (F) Kaplan-Meier curves showing survival of mice treated with IT-901 (n=8; red line; 43 days) compared to vehicle (n=8; blue line; 31 days). Engr: engraftment; Tx: treatment; Stop: drug holidays; Sac: euthanasia.
Examination of the mice immediately after the last treatment revealed significant differences in tumor growth and localization. In agreement with previous data,35 vehicle-treated mice showed extensive Mec-1 colonization of the kidneys, significantly reduced in the IT-901-treated group (Figure 7B). Moreover, flow cytometry (Figure 7C) and immunohistochemistry (IHC) analyses, using an anti-human CD20 antibody (Figure 7D and Online Supplementary Figure S4A), indicated that IT-901 treatment significantly reduced the number of Mec-1 cells in liver, spleen and bone marrow (BM). In line with the specificity of action of IT-901, a significant decrease in total expression and nuclear localization of the p65 subunit was apparent in IT-901-treated mice (Figure 7D and Online Supplementary Figure S4B). Accordingly, Mec-1 cells obtained from this group of mice were characterized by diminished viability compared to cells from vehicle-treated mice (Figure 7E).
Kaplan-Meier curves indicated that vehicle-treated mice were characterized by a significantly shorter survival (median: 31 days) when compared with those injected with IT-901 (median: 43 days; P<0.0001) (Figure 7F).
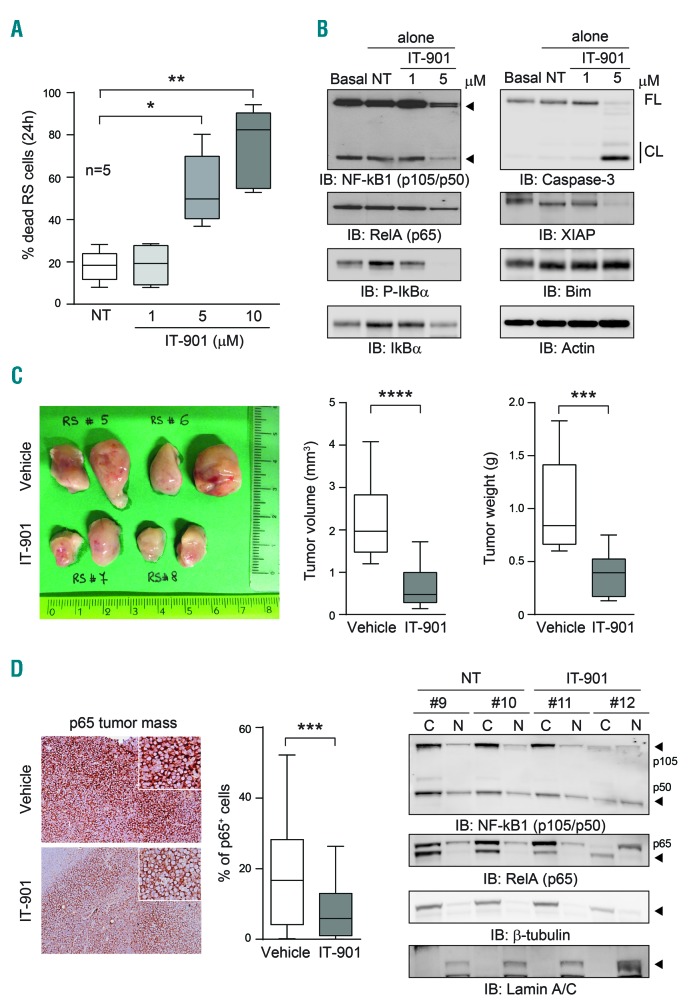
IT-901 is active on primary RS cells and in patient-derived xenograft
We then focused on RS, typically a DLBCL developing in a minority of patients with a previous or concomitant diagnosis of CLL.37
Exposure of RS cells to increasing concentrations of IT-901 induced apoptosis in a dose-dependent way. These cells were clearly more sensitive to the NF-κB inhibitor than CLL cells, independently of the presence of a protective stromal layer (Figure 8A). The mechanism behind increased apoptotic responses following IT-901 treatment was similar to the one highlighted for CLL cells, with decreased expression of the NF-κB complex, including the inhibitory subunit. Concomitantly, there was the activation of the caspase pathway, with modulation of the expression of pro- and anti-apoptotic proteins (Figure 8B and Online Supplementary Figure S4C). To prove the efficacy of IT-901 in vivo, we then exploited 2 different patient-derived xenograft (PDX) models, recently established in the lab. RS cells were s.c. injected in NSG mice and left to engraft, until tumors became palpable. Mice were then randomized to receive vehicle or IT-901 treatment, with the same schedule adopted for the CLL xenografts. Results indicate that IT-901 significantly reduced tumor growth, as highlighted by size and weight of tumor masses (Figure 8C). Moreover, a significant reduction in p65 mRNA and protein levels were evident in IT-901 treated mice (Figure 8D and Online Supplementary Figure S4C). This diminished expression and activity was also confirmed by checking localization of the NF-κB complex in cytoplasmic and nuclear extracts (Figure 8E).
Figure 8.
IT-901 is active on primary Richter syndrome (RS) cells and in patient-derived xenograft (PDX). (A) Cumulative data of apoptosis of primary or PDX-tumor-derived RS cells. (B) Western blot analysis of the expression of the nuclear factor-kappa B (NF-κB) complex, pro- and anti-apoptotic proteins and caspase-3 in RS cells exposed to the indicated doses of IT-901 for 6 hours (h). Actin was used as loading control. (C) Tumor masses from vehicle- or IT-901-treated RS-PDX mice compared for tumor volume (cm3) and weight (g) (6 mice/group; double-flank injected). (D) Immunohistochemistry analysis of p65 expression within the tumor mass. Staining was reported as percentage of positive cells. (E) Cytoplasmic (C) and nuclear (N) fractions obtained from RS cells purified from the tumor mass were resolved by SDS-PAGE and expression of the NF-κB complex analyzed. β-tubulin and Lamin A/C were used as cytoplasmic and nuclear controls, respectively. FL: full-length; CL: cleaved.
Together, these results provide a proof-of-principle that IT-901 is efficacious in CLL and RS cells.
Discussion
This work studies the effects of IT-901, a novel inhibitor of the c-Rel/p65 NF-kB subunits in CLL and in RS. Our findings suggest that IT-901 induces cell death in both cell types, with tumor cells being significantly more sensitive to the drug than normal circulating B cells.
Even if the mechanism of action of this novel inhibitor is still partially unclear, some conclusions can now be drawn. Firstly, our data indicate that treatment with IT-901 reduces NF-κB binding to its consensus DNA sequences. This effect is particularly evident when considering p65, which is the most active subunit in CLL cells. Biochemical analysis of the complex, however, reveals global degradation of all the subunits, including p50 and the inhibitory subunit IκB, suggesting that IT-901 binds to the complex in the cytosol, causing its degradation. Secondly, IT-901 exposure is followed by an increase of mROS, which become toxic after a few hours. In principle, increased mROS could be due to active production or impaired degradation. On the basis of RT-PCR data indicating that IT-901 decreases expression of genes favoring ROS scavenging, including catalase, impaired ROS degradation seems to be the main mechanism. According to our working model, IT-901 would bind the NF-κB complex in the cytosol, interrupting its cycle, which is constitutively active in neoplastic B cells, and hence blocking transcription of NF-κB-controlled genes. This would limit the cells’ ability to scavenge mROS causing their accumulation over time, eventually leading over 6 h to mitochondrial damage, highlighted by the finding of altered membrane polarity and of severely impaired mitochondrial respiration which in turn decrease ATP levels. The final result for the leukemic cell is the cleavage of caspase-3, resulting in induction of intrinsic apoptosis. The observation that the ROS scavenger N-acetyl cysteine fully prevents the apoptotic effects of IT-901 in cell lines confirms the central role of ROS in the process.
This behavior in the presence of IT-901 seems to be specific for CLL cells as normal B lymphocytes need significantly higher doses to undergo apoptosis, while NLC, as well as stromal cells do not go into apoptosis with IT-901. The reason behind this different sensitivity to IT-901 remains unclear, even if it is tempting to speculate a connection to the basal metabolic state of the cell and to basal levels of mROS. According to this hypothesis, apoptotic responses to IT-901 would be specific to those cells that have constitutively high levels of mROS, with rapid toxicity following the interruption of genetic circuits regulating expression of the scavengers. On the contrary, NLC, which are essentially tumor-associated macrophages, possess a radically different basal metabolism, with low levels of mROS. In this context, the genes most affected by NF-κB inhibition are those coding for surface molecules important for the crosstalk with tumor cells.
These findings suggest that IT-901 treatment could be beneficial by acting through different mechanisms. On the one hand, IT-901 targets leukemic cells directly, leading to rapid toxicity and death by apoptosis. On the other hand, it perturbs molecular circuits driven by the leukemic cells and aimed at creating favorable growth conditions. The finding that PD-L1 expression is modulated when leukemic cells interact with NLC further suggests that IT-901 helps restore an immunocompetent host.
The third finding favoring further investigation of the therapeutic properties of IT-901 derives from the observation of a combined effect when administered together with the btk inhibitor ibrutinib. While this observation can be explained on the basis of the convergence of the two drugs on the same signaling pathway, it is interesting to note that addition of ibrutinib renders CLL cells sensitive to lower doses of IT-901, broadening its therapeutic window.
Using an established xenograft model based on i.v. injection of Mec-1 cells into NSG mice, we showed that IT-901 administered as a single agent for two weeks after documented disease engraftment significantly reduces tumor burden in all the organs examined. Importantly, a marked reduction in the nuclear expression of p65 was observed, confirming NF-κB targeting in vivo.
Lastly, we investigated whether IT-901 is effective also in experimental models of RS. This is an important issue as RS remains a disease for which there is an urgent need for active drugs. One of the difficulties in studying RS is the lack of experimental models as there are no available cell lines. For this reason, we exploited two different patient-derived xenografts established from patients with RS (T Vaisitti and JN Allan, 2017, manuscript in preparation). Subsequent adoptive transfer of the lymphoma, which remained genetically similar to patients’ cells, confirmed successful disease engraftment. By exploiting these models, we first demonstrated inhibition of NF-κB activation after IT-901 treatment, with rapid mROS accumulation, mitochondrial damage and death by apoptosis. Furthermore, the drug was used in monotherapy to treat xenografts obtained from RS patients. We found that IT-901 markedly reduced tumor weight and volume and inhibited NF-kB expression, suggesting a similar mechanism of action.
In conclusion, this work offers pre-clinical evidence supporting the use of IT-901 in the treatment of CLL and RS patients.
Supplementary Material
Acknowledgments
We thank K. Gizzi (Italian Institute for Genomic Medicine) for excellent technical support.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1878
Funding
This work is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG-17314 to SD), by the Italian Ministry of Health (Bando Giovani Ricercatori GR-2011-02349282 to TV and GR-2011-02346826 to SD), by the Leukemia and Lymphoma Society (6465-15 to JLZ), and by ImmuneTarget Inc.. IT-901 was provided by ImmuneTarget Inc.
References
- 1.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–227. [DOI] [PubMed] [Google Scholar]
- 2.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13(5):759–772. [DOI] [PubMed] [Google Scholar]
- 3.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. [DOI] [PubMed] [Google Scholar]
- 5.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. [DOI] [PubMed] [Google Scholar]
- 6.Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14(2):64–69. [DOI] [PubMed] [Google Scholar]
- 7.Bradford JW, Baldwin AS. IKK/nuclear factor-kappaB and oncogenesis: roles in tumor-initiating cells and in the tumor microenvironment. Adv Cancer Res. 2014;121:125–145. [DOI] [PubMed] [Google Scholar]
- 8.Gasparini C, Celeghini C, Monasta L, Zauli G. NF-kappaB pathways in hematological malignancies. Cell Mol Life Sci. 2014;71(11):2083–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18(8):1391–1400. [DOI] [PubMed] [Google Scholar]
- 10.Furman RR, Asgary Z, Mascarenhas JO, Liou HC, Schattner EJ. Modulation of NF-kappa B activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol. 2000;164(4):2200–2206. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tracey L, Perez-Rosado A, Artiga MJ, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206(2):123–134. [DOI] [PubMed] [Google Scholar]
- 13.Hewamana S, Alghazal S, Lin TT, et al. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111(9):4681–4689. [DOI] [PubMed] [Google Scholar]
- 14.Hewamana S, Lin TT, Rowntree C, et al. Rel a is an independent biomarker of clinical outcome in chronic lymphocytic leukemia. J Clin Oncol. 2009;27(5):763–769. [DOI] [PubMed] [Google Scholar]
- 15.Sutton LA, Rosenquist R. The complex interplay between cell-intrinsic and cell-extrinsic factors driving the evolution of chronic lymphocytic leukemia. Semin Cancer Biol. 2015;34:22–35. [DOI] [PubMed] [Google Scholar]
- 16.Bernal A, Pastore RD, Asgary Z, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98(10):3050–3057. [DOI] [PubMed] [Google Scholar]
- 17.Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99(8):2969–2976. [DOI] [PubMed] [Google Scholar]
- 18.Zaninoni A, Imperiali FG, Pasquini C, Zanella A, Barcellini W. Cytokine modulation of nuclear factor-kappaB activity in B-chronic lymphocytic leukemia. Exp Hematol. 2003;31(3):185–190. [DOI] [PubMed] [Google Scholar]
- 19.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):4820–4827. [DOI] [PubMed] [Google Scholar]
- 20.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103(2):216–228. [DOI] [PubMed] [Google Scholar]
- 21.Rossi D, Gaidano G. Richter syndrome: pathogenesis and management. Semin Oncol. 2016;43(2):311–319. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri G, Khiabanian H, Holmes AB, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210(11):2273–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shono Y, Tuckett AZ, Ouk S, et al. A small-molecule c-Rel inhibitor reduces alloactivation of T cells without compromising anti-tumor activity. Cancer Discov. 2014;4(5):578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shono Y, Tuckett AZ, Liou HC, et al. Characterization of a c-Rel Inhibitor That Mediates Anticancer Properties in Hematologic Malignancies by Blocking NF-kappaB-Controlled Oxidative Stress Responses. Cancer Res. 2016;76(2):377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seiffert M, Stilgenbauer S, Dohner H, Lichter P. Efficient nucleofection of primary human B cells and B-CLL cells induces apoptosis, which depends on the microenvironment and on the structure of transfected nucleic acids. Leukemia. 2007;21(9):1977–1983. [DOI] [PubMed] [Google Scholar]
- 26.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13(5):730–737. [DOI] [PubMed] [Google Scholar]
- 28.Jitschin R, Hofmann AD, Bruns H, et al. Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia. Blood. 2014;123(17):2663–2672. [DOI] [PubMed] [Google Scholar]
- 29.Mauro C, Leow SC, Anso E, et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13(10):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutzny G, Kocher T, Schmidt-Supprian M, et al. Protein kinase c-beta-dependent activation of NF-kappaB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell. 2013;23(1):77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 32.Jain P, Keating MJ, Wierda W, et al. Long term follow up of treatment with ibrutinib and rituximab (IR) in patients with high-risk Chronic Lymphocytic Leukemia (CLL). Clin Cancer Res. 2017;23(9):2154–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiestner A. BCR pathway inhibition as therapy for chronic lymphocytic leukemia and lymphoplasmacytic lymphoma. Hematology Am Soc Hematol Educ Program. 2014;2014(1):125–134. [DOI] [PubMed] [Google Scholar]
- 34.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3(3):e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaisitti T, Audrito V, Serra S, et al. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia. 2015;29(2):356–368. [DOI] [PubMed] [Google Scholar]
- 36.Bertilaccio MT, Scielzo C, Simonetti G, et al. Xenograft models of chronic lymphocytic leukemia: problems, pitfalls and future directions. Leukemia. 2013;27(3):534–540. [DOI] [PubMed] [Google Scholar]
- 37.Rossi D. Richter’s syndrome: Novel and promising therapeutic alternatives. Best Pract Res Clin Haematol. 2016;29(1):30–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.