Early stages of chronic kidney disease (CKD) are characterized by development of progressive anemia1 as well as concurrent marked elevation of the phosphaturic hormone fibroblast growth factor 23 (FGF23).2 As kidney function declines, FGF23 further increases and anemia worsens, due to either inadequate production of renal erythropoietin (EPO) or incidence of hypoferremia. Moreover, in CKD, anemia and elevated FGF23 levels are associated with left ventricular hypertrophy (LVH),3 CKD progression,4 and mortality.2 Treatment of CKD-related anemia involves iron repletion and erythropoietin (EPO) administration. EPO is one of the most extensively used medications in CKD, but its administration is associated with increased risks of cardiovascular disease and mortality.5 Although FGF23 levels increase early in CKD, the pathophysiological regulation of FGF23 is still not completely understood. Phosphate, 1,25-dihydroxyvitamin D (1,25D), parathyroid hormone, and calcium affect FGF23 production; however, these factors are still within normal ranges when bone and circulating FGF23 increase. Recent studies demonstrate intriguing associations between hypoxia, iron deficiency, and FGF23 upregulation. Indeed, in the settings of normal and impaired6 kidney function, iron deficiency potently increases bone Fgf23 expression. However, other anemia-related factors, including EPO, could potentially contribute to elevated FGF23 production. As both EPO therapy and FGF23 are associated with adverse outcomes in CKD, we explored the hypothesis that EPO is a previously unrecognized regulator of this phosphaturic hormone. Collectively, our pre-clinical findings suggest that modulating EPO exposure in CKD patients may lower FGF23 and thereby decrease its adverse effects.
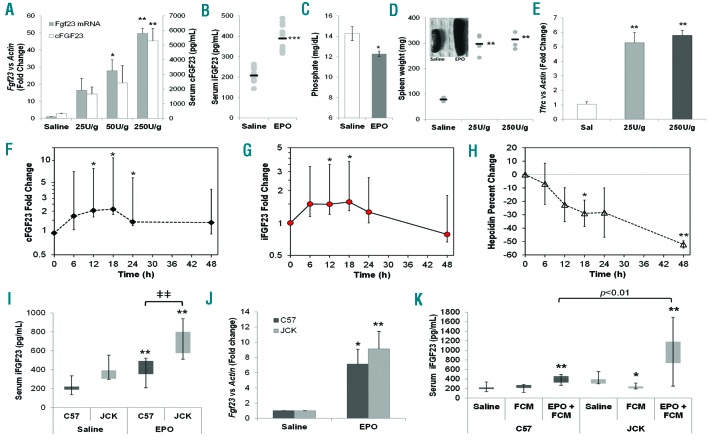
To examine whether exogenous EPO stimulates FGF23 in vivo, wild-type C57BL/6 mice at 6–8 weeks of age were injected with increasing doses of recombinant human EPO (25–250 U/g of body weight). A 3-day regimen induced a dose-dependent, 40-fold maximal increase in whole bone Fgf23 mRNA expression (Figure 1A), paralleled by increased serum total FGF23 as measured with an ELISA that detects both C-terminal FGF23 fragments (‘cFGF23’) and bioactive intact FGF23 (‘iFGF23’) (Figure 1A). Compared to controls, three days of an intermediate dose of 125 U/g/day EPO doubled iFGF23 concentrations (Figure 1B), with a parallel reduction in serum phosphate (Figure 1C). EPO stimulated erythropoiesis, as indicated by increased spleen weight (Figure 1D) and whole bone transferrin receptor-1 (TfRc) mRNA expression (Figure 1E).
Figure 1.
Erythropoietin (EPO) is a stimulator of FGF23. (A) Injections of EPO (50–250 U/g/d of body weight) over a 3-day time course dose-dependently increased serum cFGF23 as well as whole intact femur Fgf23 mRNA (*P<0.05, **P<0.01 vs. saline controls). (B) Serum iFGF23, measured with the Quidel rodent specific ELISA, was significantly elevated following EPO delivery (125 U/g/d body weight; ***P<0.001), (C) which correlated with a significant decrease in serum phosphate (*P<0.05). Markers of EPO activity, including (D) spleen weight and (E) whole bone TfRc mRNA, were increased in EPO-injected mice (n=3–8 mice at each time point; **P<0.01). (F) Serum cFGF23 levels progressively increased in 4 anemic patients treated with EPO, with a significant elevation over baseline levels observed at the 12–24 hour (h) time points (P<0.05). (G) Serum iFGF23, measured with the Quidel human-specific ELISA, mirrored cFGF23, with significant increases observed 12 and 18 h post injection. (H) After receiving EPO, serum hepcidin was significantly decreased in these subjects, indicative of normal EPO function. (I) Jck and C57 mice were injected with either saline or 125 U/g EPO. EPO treatment increased iFGF23 in C57 and Jck mice, with Jck iFGF23 concentrations significantly higher versus C57 mice; n=6 mice per group; **P<0.01 versus saline treatment of the same genotype, ǂǂP<0.01 versus C57 same treatment. (J) EPO treatment increased whole bone Fgf23 mRNA in C57 and in Jck mice (*P<0.02 and **P<0.01 compared to saline treatment of the same genotype). (I) Jck and C57 mice were injected with either ferric carboxymaltose (FCM) alone or in combination with EPO (EPO+FCM) during the 3-day regimen. FCM alone reduced serum iFGF23 in the Jck mice, whereas FCM+EPO increased iFGF23 in both genotypes, with higher iFGF23 concentrations observed in the Jck mice compared to C57 (n=6 mice per group; *P<0.05 and **P<0.01 vs. saline treatment of the same genotype).
We next performed a study in which 4 human subjects with anemia of unclear etiology but normal renal function received a subcutaneous dose of EPO. Serum was sampled pre-injection and every six hours post-injection for 48 hours (h) and then analyzed for cFGF23, iFGF23, iron, hepcidin, and phosphate (Online Supplementary Table S1). Despite variations in baseline ferritin levels among the 4 patients, indicative of different iron and inflammatory status, all subjects responded similarly to EPO administration by increasing FGF23. Indeed, 12 h post-EPO administration, cFGF23 was significantly increased (P<0.05) (Figure 1F and Online Supplementary Figure S1); iFGF23 paralleled this response (Figure 1G and Online Supplementary Figure S1). There was little change in serum phosphate over this short time course (Online Supplementary Figure S2). As expected, EPO significantly decreased hepcidin, which helped maintain serum iron (Figure 1H and Online Supplementary Figure S3).
Erythropoietin effects on FGF23 were also examined in the juvenile cystic kidney (Jck) mouse, a model characterized by increased iFGF23 (Online Supplementary Figure S4A) and progressive CKD.7 Reductions in red blood cell count, hemoglobin and hematocrit were observed as early as four weeks of age in Jck mice compared to normal C57BL/6 (C57) mice (Online Supplementary Table S2), along with reduced serum iron and inappropriately low EPO (Online Supplementary Figure S4B and C). In line with the studies above, 6-week old mice were administered EPO (125 U/g) which increased iFGF23 in both C57 and Jck mice; however, Jck mice had higher iFGF23 (Figure 1I), which paralleled whole bone Fgf23 mRNA expression (Figure 1J). To rule out EPO-induced functional iron deficiency, ferric carboxymaltose (FCM; 80 mg/kg) was administered through tail vein injection either alone or with EPO. While there was no measured change in serum iron, whole bone Fgf23 mRNA and the EPO-responsive erythroblast-derived hormone erythroferrone (Erfe) mRNA were reduced with FCM treatment, in contrast to increases with EPO+FCM. As controls, FCM and EPO increased and reduced, respectively, liver hepcidin levels, demonstrating appropriate changes in iron metabolism with treatment (Online Supplementary Figure S5). Whereas FCM alone reduced serum iFGF23 in the Jck mice, EPO+FCM increased serum iFGF23 in both genotypes (and again was significantly higher in the Jck mice compared to C57), confirming iron-independent EPO stimulation of FGF23 (Figure 1K).
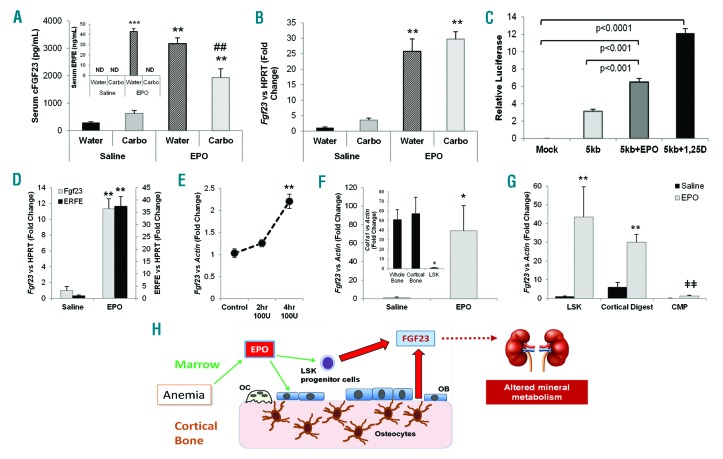
Erythropoietin is known to target marrow erythropoietic precursors, so to assess the EPO-dependent contribution of these cells to FGF23 production, wild-type mice were given a single dose of marrow ablative carboplatin (2.5 mg/mouse)8 one day prior to 125 U/g/d EPO injections administered for three days, using saline as control. Compared to mock ablation controls, in carboplatin pretreated mice, ERFE was undetectable (Figure 2A, inset) confirming ablation, and serum cFGF23 showed around 40% reduced response (Figure 2A). However, there was no difference in Fgf23 mRNA expression within the cortical bone flushed of marrow (Figure 2B), supporting the hypothesis that a lack of marrow FGF23 production decreased circulating levels, and that EPO had direct effects on cortical FGF23-producing bone cells. These data also support no direct role for ERFE during EPO stimulation of FGF23. Interestingly, we found that iron chelation with deferoxamine (DFO) increased EPO receptor (EpoR) mRNA expression in the osteoblast-like cell line UMR-106 (Online Supplementary Figure S6A), possibly providing a mechanism for enhancing EPO-responsiveness in bone. Since we had previously found that DFO independently stimulates Fgf23 transcription,9 ROS osteoblast-like cells were co-transfected with a -5kb mouse Fgf23 promoter-luciferase construct and the human EPOR to mimic EpoR upregulation. Luciferase activity was significantly enhanced after 24 h of treatment with both 100 U/mL EPO and the positive control 1,25D (10-8M), compared to control treated cells (Figure 2C). Furthermore, EPO treatment of UMR-106 cells overexpressing the EPOR elicited a significant increase in Fgf23 mRNA expression, as well as increased phosphorylation of STAT5 and ERK1/2 (Online Supplementary Figure S6B and C).
Figure 2.
Marrow-derived FGF23 contributes to the response to erythropoietin (EPO). (A) Marrow ablation in wild-type mice using carboplatin (‘Carbo’) completely blocked the induction of ERFE in response to EPO (inset; ND: not detected). Although serum cFGF23 was induced in all groups treated with EPO, marrow ablation reduced these levels by approximately 40%. (B) Fgf23 mRNA, normalized to Hprt, was induced in cortical bone flushed of marrow in all groups treated with EPO (n=5–6; **P<0.01 vs. same pre-treatment + Saline; #P<0.05 and ##P<0.01 vs. water ablation control+same post-treatment). (C) A -5kb Fgf23 promoter fragment responded positively to EPO treatment versus the promoter fragment alone or mock transfected ROS17/2.8 osteoblastic cells. 1,25D treatment served as the positive control (n=3 replicates per treatment). (D) Flushed bone marrow RNA from mice treated with EPO showed a significant increase in Fgf23 and Erfe mRNA levels (normalized to Hprt) compared to flushed bone marrow from saline-injected mice (n=4 mice per group; **P<0.001). (E) Isolated bone marrow cells were cultured ex vivo and treated with 100 U/mL of EPO for 4 h, showing a significant increase in Fgf23 mRNA expression (**P<0.01). (F) An enriched population of Lineage− cells was isolated from flushed bone marrow using a hematopoietic progenitor separation kit. Treatment of wild-type C57 mice with EPO resulted in a significant increase of Fgf23 mRNA expression compared to that of saline-treated mice (n=4 replicates, pools from at least 2 mice; P<0.05). Col1a1 mRNA, a marker of osteoblasts, was significantly reduced in this enriched population compared to wild-type whole bone and wild-type cortical bone flushed of marrow to show a lack of osteoblasts within this preparation (inset; *P<0.05 vs. whole bone and cortical bone). (G) Marrow from C57 control mice injected with saline or EPO were stained and co-sorted with flow cytometry for LSK and CMP cell populations while the cortical bone was collagenase digested. The sorted LSK cells significantly induced Fgf23 mRNA in response to EPO (n=4 pools from 2–3 mice; **P<0.01). Cortical digested cells also showed a significant Fgf23 mRNA induction with EPO treatment (**P<0.01), whereas the CMP cells were at the limit of detection and remained significantly lower compared to the LSK and cortical digested cells (ǂǂP<0.01 vs. LSK and cortical digests treated with EPO). Data represented as mean+standard error of mean (SEM). (H) Our data demonstrate that EPO can induce FGF23 production in osteoblasts/osteocytes and hematopoietic progenitors, with excess FGF23 potentially leading to altered renal mineral metabolism and other pathogenic actions.
The NCBI GEO gene array data set GSE7874 demonstrated that human peripheral blood CD34+ hematopoietic stem cells cultured with EPO have an 8-fold increase in FGF23 mRNA expression. Accordingly, in mice treated with EPO, Fgf23 mRNA expression was increased 11-fold in marrow flushed from femurs (Figure 2D). As expected, EPO also increased marrow Erfe expression (Figure 2D). In addition, isolated bone marrow cells treated ex vivo with 100 U/mL EPO increased Fgf23 mRNA after 4 h (Figure 2E). To determine if other EPO-responsive tissues could express Fgf23, spleen RNA was isolated in parallel with bone and flushed marrow. Fgf23 mRNA expression was significantly elevated in spleen, further supporting an effect of EPO on FGF23 in erythroid precursor cells (Online Supplementary Figure S7). To begin to identify the marrow cell populations capable of expressing FGF23, hematopoietic progenitor cells were collected from the marrow of EPO- and saline-injected mice using negative selection to enrich Lineage− cells,10 as Fgf23 mRNA was previously found in Ter119+ erythrocytes.11 Fgf23 mRNA was detectable in control cells, but was potently induced more than 70-fold with EPO treatment (Figure 2F). Flow cytometry sorting of Lineage− c-kit+ Sca1+ (LSK) cells from EPO-injected mice showed a significant induction of Fgf23 mRNA (>40-fold vs. saline-injected controls) (Figure 2G). Collagenase digested bone showed 5-fold higher levels of Fgf23 mRNA compared to LSK at baseline, which significantly increased in response to EPO (Figure 2G). The common myeloid progenitor (CMP) cell population was negative for Fgf23 mRNA and rose only to the limit of detection with EPO treatment, thus remaining far lower than both LSK and the cortical digests from EPO-treated mice (Figure 2G). In summary, our results strongly support a new functional model whereby EPO directly affects FGF23 production in hematopoietic progenitor cell subsets and cortical bone, revealing novel roles for these sites in controlling crossover iron and phosphate homeostasis (Figure 2H).
FGF23 regulation is determined by an interplay of systemic and local factors that now extends beyond the ‘typical’ feedback loops associated with phosphate and 1,25D homeostasis. Notably, iron deficiency stimulates FGF23 production,6,9,12 and most CKD patients develop anemia with iron deficiency or iron restriction.1 However, studies of the effects of various intravenous iron formulations on cFGF23 and/or iFGF23 in CKD patients have been inconclusive. In the light of our results demonstrating EPO-mediated stimulation of FGF23, these previous studies may have been confounded by the interactive effects of endogenous or exogenous EPO on FGF23 production and stabilization. In addition to its well-established role in erythropoiesis, EPO can directly activate transcription of osteogenic genes in human cell lines;13 thus, EPO may influence FGF23 expression in osteoblastic cells as well as in hematopoietic cells. Clinical trials with CKD patients have shown that higher EPO doses are associated with adverse cardiovascular effects,5 although the mechanism is not well understood. Elevated FGF23 levels are also associated with poor patient outcomes,2–4 with a plausible mechanism suggested by animal models linking high FGF23 levels to cardiomyopathy.3 Expanded studies in patient cohorts will be necessary to derive the full overlap of specific interactions between EPO and FGF23. Therefore, our collective findings may have important clinical implications for patients, supporting an exploration of current CKD-associated anemia and CKD-mineral bone disorder treatment paradigms to optimize the therapeutic use of EPO.
Supplementary Material
Acknowledgments
The authors would like to thank the Indiana University Melvin and Bren Simon Cancer Center Flow Cytometry Resource Facility for their outstanding technical help. This core facility is partially funded by National Cancer Institute grant P30 CA082709 and National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK090948. The authors would also like to acknowledge the important advice from Susan C. Schiavi, PhD.
Footnotes
Funding: the authors would like to acknowledge support by NIH grants DK063934, DK095784, and AR059278 (KEW); F32-AR065389 (ELC); T32-HL007910 (JMH); an AHA postdoctoral fellowship 16POST27260108 (JMH); NIH-K12-HD034610 (MRH); UCLA CTSI Team Science Award UL1-TR000124 (MRH and IS); DK101008 (EN); Ralph W. and Grace M. Showalter Trust Fund (MAK and KEW).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13(2):504–510. [DOI] [PubMed] [Google Scholar]
- 2.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–2608. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Barnhart HX, Inrig JK, et al. Cardiovascular toxicity of epoetinalfa in patients with chronic kidney disease. Am J Nephrol. 2013;37(6):549–558. [DOI] [PubMed] [Google Scholar]
- 6.Hanudel MR, Chua K, Rappaport M, et al. Effects of Dietary Iron Intake and Chronic Kidney Disease on Fibroblast Growth Factor 23 Metabolism in Wild Type and Hepcidin Knockout Mice. Am J Physiol Renal Physiol. 2016:ajprenal 00281 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbagh Y, Graciolli FG, O’Brien S, et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27(8):1757–1772. [DOI] [PubMed] [Google Scholar]
- 8.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108(46):E1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitteti BR, Cheng YH, Poteat B, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115(16):3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289(14):9795–9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinkenbeard EL, Farrow EG, Summers LJ, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29(2):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L, Luo T, Fang Y, et al. Effects of erythropoietin on osteoblast proliferation and function. Clin Exp Med. 2014;14(1):69–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.