Cytokinin promotes auxin biosynthesis in and efflux from the medial gynoecial domain in Arabidopsis, whereas auxin blocks cytokinin from apical and lateral domains to regulate patterning.
Abstract
The Arabidopsis (Arabidopsis thaliana) gynoecium consists of two congenitally fused carpels made up of two lateral valve domains and two medial domains, which retain meristematic properties and later fuse to produce the female reproductive structures vital for fertilization. Polar auxin transport (PAT) is important for setting up distinct apical auxin signaling domains in the early floral meristem remnants allowing for lateral domain identity and outgrowth. Crosstalk between auxin and cytokinin plays an important role in the development of other meristematic tissues, but hormone interaction studies to date have focused on more accessible later-stage gynoecia and the spatiotemporal interactions pivotal for patterning of early gynoecium primordia remain unknown. Focusing on the earliest stages, we propose a cytokinin-auxin feedback model during early gynoecium patterning and hormone homeostasis. Our results suggest that cytokinin positively regulates auxin signaling in the incipient gynoecial primordium and strengthen the concept that cytokinin regulates auxin homeostasis during gynoecium development. Specifically, medial cytokinin promotes auxin biosynthesis components [YUCCA1/4 (YUC1/4)] in, and PINFORMED7 (PIN7)-mediated auxin efflux from, the medial domain. The resulting laterally focused auxin signaling triggers ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6), which then represses cytokinin signaling in a PAT-dependent feedback. Cytokinin also down-regulates PIN3, promoting auxin accumulation in the apex. The yuc1, yuc4, and ahp6 mutants are hypersensitive to exogenous cytokinin and 1-napthylphthalamic acid (NPA), highlighting their role in mediolateral gynoecium patterning. In summary, these mechanisms self-regulate cytokinin and auxin signaling domains, ensuring correct domain specification and gynoecium development.
The Arabidopsis (Arabidopsis thaliana) female reproductive organ, the gynoecium, is initiated from the terminating remains of the floral meristem after the initiation of organ primordia in the outer three floral whorls. At floral stage 5 (stages according to Smyth et al., 1990), in the mound of meristematic cells that give rise to the gynoecial primordium, genes marking the medial and lateral gynoecial domains are already differentially expressed (Sessions et al., 1997; Bowman et al., 1999; Ferrándiz et al., 1999; Siegfried et al., 1999; Larsson et al., 2014). The specified lateral domains will subsequently produce the valves, which will enclose the reproductive tissues produced by the medial domain. What regulates the specification of the two types of domains has long been an open question. It is well known that crosstalk between auxin and cytokinin signaling has a major influence on meristematic maintenance and differentiation in many tissues [see review by Schaller et al. (2015)]. In the shoot apical meristem (SAM), cytokinin signaling plays a major role maintaining meristem size, opposite to its role in the root apical meristem, where it instead promotes differentiation (Werner et al., 2003; Dello Ioio et al., 2008; Bartrina et al., 2011; Chickarmane et al., 2012). On the other hand, auxin signaling and polar auxin transport regulate lateral organ initiation, outgrowth, and phyllotaxis (Reinhardt et al., 2003; Kierzkowski et al., 2013).
Recently, we could show that already at stage 5, in the floral meristem remnants, PAT mediated by the PINFORMED1 (PIN1) auxin efflux carrier creates focused auxin response peaks in the center of the two lateral domains, and this is required to repress medial expressed genes in the lateral domain and for valve outgrowth (Larsson et al., 2014). Blocking PAT at this stage results in unfocused and ectopic auxin signaling throughout both medial and lateral domains, reduction of lateral and promotion of medial expressed genes, and consequently valveless gynoecia develop (Nemhauser et al., 2000; Larsson et al., 2014). In contrast to the lateral domains, the medial domains retain meristematic activity, and at floral stage 7, the adaxial side of the two medial domains forms what are called the “carpel margin meristems” (CMMs). At floral stage 8, the CMMs fuse in the center of the gynoecial cylinder and during the subsequent stages 9 through 12, the CMM forms the placenta, ovules, septum, and transmitting tract, all of which are vital for successful reproduction (Smyth et al., 1990; Sessions, 1997; Bowman et al., 1999). Cytokinin is important for the initiation and/or development of these tissues arising from the CMM. Gynoecia of the triple cytokinin response mutant arr1 arr10 arr12 produce less ovules, show septum fusion defects, and reduced transmitting tract proliferation (Reyes-Olalde et al., 2017). In addition, mutations in SPATULA (SPT), which encodes a bHLH transcription factor suggested to activate cytokinin response, show similar phenotypes (Reyes-Olalde et al., 2017), whereas mutations in CYTOKININ OXIDASE/DEHYDROGENASE3 (CKX3) and CKX5, which encode cytokinin catabolic enzymes, result in increased ovule formation and thus seed set (Bartrina et al., 2011).
Interestingly, cytokinin promotes auxin biosynthesis genes in various organs (Jones et al., 2010; Di et al., 2016). The main auxin biosynthesis pathway in plants is a simple two-step pathway regulated by the TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA1)/TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS RELATED enzymes followed by the rate-limiting YUCCA (YUC) enzymes [see review by Zhao (2012)]. These enzymes are crucial throughout plant development, including the reproductive phase, and multiple mutants of genes in each family make gynoecia that have severely reduced valves or are completely valveless (Stepanova et al., 2008, 2011; Mashiguchi et al., 2011; Won et al., 2011). Recently, Reyes-Olalde et al. (2017) could show that exogenous cytokinin and ectopic activation of cytokinin signaling increase TAA1 expression in developing medial tissues of stage-9 gynoecia. In addition, cytokinin regulates the activity of PINs, either on a transcriptional or posttranscriptional level in many organs or tissue types, including in the developing ovules at later stages (Laplaze et al., 2007; Dello Ioio et al., 2008; Pernisová et al., 2009; Ruzicka et al., 2009; Bishopp et al., 2011; Zhang et al., 2011; Galbiati et al., 2013; Marhavý et al., 2014). During gynoecium development, PIN3 is activated by cytokinin in the medial domain of stage-9 gynoecia, where it appears to be important for proper transmitting tract development (Reyes-Olalde et al., 2017).
Auxin, on the other hand, activates genes encoding cytokinin-signaling repressors such as ARABIDOPSIS RESPONSE REGULATOR (ARR) type-A genes and ARABIDOPSIS HISTIDINE PHOSPHOTRANSPHER6 (AHP6) in tissue types requiring high auxin output (Müller and Sheen, 2008; Zhao et al., 2010; Bishopp et al., 2011; Besnard et al., 2014a, 2014b). AHP6 in particular establishes domains of reduced cytokinin signaling to ensure clearly defined auxin peaks for robustness during phyllotaxy regulation (Besnard et al., 2014a, 2014b). Reyes-Olalde et al. (2017) also studied AHP6 expression in differentiating gynoecia (stage 9) and suggested a model of cytokinin-auxin crosstalk during placenta and ovule development including cytokinin-directed activation of auxin biosynthesis (TAA1) and efflux (PIN3) genes in the medial reproductive tissues, and auxin-induced repression of cytokinin signaling in the lateral valves via AHP6.
Given the multiple levels of interaction, it is plausible that an auxin-cytokinin interaction would contribute to medial-lateral boundary development already in the floral meristem remnants giving rise to the gynoecial primordia. So far, no studies of the activity or importance of cytokinin and its crosstalk with auxin in the early stages of gynoecial primordium patterning have been reported.
Here we focus on identifying mechanisms of auxin-cytokinin interactions in the medial-lateral patterning of the floral meristem remnants. This is the developmental time point when the patterning decision is established, which lays the foundation for the further development of the gynoecium. We show that auxin and cytokinin signaling peak in mutually exclusive domains, and while cytokinin promotes auxin signaling, cytokinin signaling remains repressed from peak auxin signaling domains through, in part, AHP6. Cytokinin promotes some medial but not lateral identity genes in early stage gynoecia. In addition, both YUC1 and YUC4 are promoted by cytokinin in the medial domain, and their expression is important for hormone homeostasis during later processes such as valve outgrowth and ovule, style, and stigma development. Cytokinin also targets PAT by promoting medial auxin efflux via the up-regulation of PIN7 and apical auxin accumulation via PIN3 repression. Together, our results both strengthen preexisting models of auxin-cytokinin interactions and provide, to our knowledge, new insights on a crosstalk network in which cytokinin regulates auxin biosynthesis and transport in the incipient gynoecial primordium to ensure auxin maxima are established, whereas auxin and PAT restrict cytokinin signaling to the medial tissue, so that correct gynoecium patterning ensues.
RESULTS
Cytokinin and Auxin Signaling Act in Mutually Exclusive Domains in the Youngest Gynoecial Primordium
To correlate changes in auxin and cytokinin signaling peaks, we analyzed plants containing reporters for both auxin and cytokinin signaling, DR5::RFP (Marin et al., 2010) and TCSn::GFP (Zürcher et al., 2013), respectively. At stage 5 (Fig. 1A), DR5::RFP marks the two lateral foci and is strongest in the apical-most cells (until about 10 μm below the apex; Fig. 1, B, and D to G), as previously shown (Larsson et al., 2014). In the same tissues, TCSn::GFP is expressed in both apical and subapical medial cells, peaking in expression between 10 μm and 15 μm below the apex (Fig. 1, B, and D to G). TCSn::GFP also forms two peaks in the basal lateral domain around 20 μm below the apex (Fig. 1G). Taken together, these results indicate that the two hormones act in complementary primordial domains, in line with what has been suggested for later stage gynoecia (Marsch-Martínez et al., 2012). To understand how this early tissue responds to exogenous cytokinin, we assessed the expression of these markers after treatment with the synthetic cytokinin 6-benzylaminopurine (BAP). A strong up-regulation of TCSn 24 hours (h) after treatment suggests that nearly all stage-5 cells are BAP sensitive (Fig. 1, C, and H to K). To examine if exogenous cytokinin could affect auxin signaling, we assessed the expression of DR5::RFP in these same BAP-treated tissues. Indeed, BAP treatment induces a broader, less-focused apical DR5::RFP response (Fig. 1, C, H, and I), indicating that exogenous cytokinin promotes increased auxin signaling in the apical domain. Interestingly, in these DR5 expressing cells, TCSn::GFP is generally not detectable or drastically weaker than neighboring nonDR5-expressing cells (Supplemental Fig. S1, A, B, and D), suggesting that auxin signaling feeds-back to repress cytokinin signaling in its peak domains. Alternatively, cytokinin response components may simply not express in these cells.
Figure 1.
Coexpression of cytokinin and auxin signaling reporters reveals mutually exclusive response domains in early stage gynoecial primordia. A, Transmitted light images of a stage-5 gynoecial primordia from an apical and medial perspective with domains and scales indicated. B to K and M to S, Confocal microscopy images of wild type plants containing both TCSn::GFP (green) and DR5::RFP (magenta) reporters were used to identify coexpression in early stage gynoecial primordia (A and L) at stage 5 (B to K), stage 6 (M and Q), stage 7 (N, O, R, and S), and stage 8 (P) after 24 h mock (B, D to G, and M to P) or 24-h BAP treatment (C, H to K, and Q to S). L, Transmitted light images indicate the domains for each stage and have artificial coloring for medial (beige) or lateral (blue) domains. Transmitted light images were overlaid for cell clarity (D to K and P). Schematic drawings in each subfigure show the image perspective (red line) and denote either the position from the apex (snap) or that the image is a maximum intensity projection of serial images (stack). Gynoecium periphery (solid white line), stamen (dotted line), sepal (asterisk), and medial domain invagination (arrowhead). Scale bar = 10 µm.
Stage-6 gynoecia had comparable expression patterns to stage-5 gynoecia (Fig. 1, L, M, and Q). In later stage 7 and stage 8, TCSn::GFP becomes restricted to the basal part of the gynoecium, predominantly in the adaxial medial domain, and DR5::RFP expands to the lateral and medial preprocambial domains (Fig. 1, N to P) as previously described for DR5rev::3xVENUS (Larsson et al., 2014), remaining complementary to the observed cytokinin signaling peaks. After 24 h BAP treatment, cytokinin signaling expands to almost all cells of the stage-7 gynoecium except those in the gynoecium apex and the preprocambial domains, where instead enlarged DR5::RFP expression is detected (Fig. 1, R and S; Supplemental Fig. S1, C to E). Together, these results show that cytokinin-auxin crosstalk dynamics act on mutual regulation to ensure complementary signaling domains in early stage gynoecia.
Ectopic Cytokinin Affects Select Medial, but Not Lateral, Reporters
We have previously shown that blocking PAT in early stage gynoecia with 1-napthylphthalamic acid (NPA) results in an expansion of medially expressed reporters (pSHP2::YFP and TAA1::TAA1::GFP), and a reduced expression of the abaxial lateral reporter pFIL::GFP, indicating that PAT is important in early stages for correct tissue identity (Larsson et al., 2014). It has been suggested that cytokinin may affect mediolateral patterning via auxin transport, since long-term treatment with both NPA and BAP results in valveless gynoecia (Zúñiga-Mayo et al., 2014). To test whether cytokinin may affect early domain specification in a similar way to NPA, we assessed the expression of the same reporters after transient BAP treatment. After 24 h BAP treatment, pSHP2::YFP showed a weak expansion into the lateral domain (Fig. 2, A and B), although this appears less pronounced compared to previously published effects on pSHP2::YFP by NPA (Larsson et al., 2014). In contrast to NPA, laterally expressed pFIL::GFP was unaltered after 24h BAP (Fig. 2, D and E). Although it has recently been reported that medial expressed pTAA1::TAA1::GFP can be up-regulated by cytokinin in later-stage gynoecia (Reyes-Olalde et al., 2017), we detected no deviations in TAA1 expression 24 h, 48 h, or 72 h after BAP in the earliest stage-5 to stage-8 gynoecia (Supplemental Fig. S2). Together, these results suggest that cytokinin treatment of early stage gynoecia can partially promote ectopic medial identity via SHP2, but that lateral identity appears unaffected after short treatments. The gynoecial primordia likely robustly buffers ectopic and transient presence of BAP.
Figure 2.
Cytokinin signaling regulates medial boundaries and directs local auxin response peaks required for maintaining correct cytokinin signaling via AHP6. A to L, Confocal microscopy images of pSHP2::YFP (A to C), pFIL::GFP (D to F), and DR5::3xVENUS (G-L) in early stage gynoecial primordia of 24 h mock-treated wild type Col-0 (A, D, G, and J), 24-h BAP-treated Col-0 (B, E, H, and K), and the ahp6-1 mutant (C, F, I, and L). M to O, Confocal microscopy images of pAHP6::GFP expression in gynoecial primordia at stage 5 (M), stage 7 (N), and in stage 5 after a 24-h NPA treatment (O). P, Heat map of relative signal intensity in arbitrary units applied to (Q to V). Q to V, Confocal microscopy images of stage-5 gynoecial primordia expressing TCSn::GFP in mock-treated Col-0 (Q) and ahp6-1 (T), 24-h NPA-treated Col-0 (R), and ahp6-1 (U), and 72 h after NPA treatment in Col-0 (S) and ahp6-1 (V). Arrows indicate AHP6 expression in medial preprocambium (N), TCSn repression (R), or TCSn peaks (T) compared to controls. Magenta indicates chloroplast autofluorescence (A to F, M, and O). Each image is a maximum intensity projection of serial images (stack) and the schematic drawings in each subfigure indicate the image perspective (red line). Gynoecial primordium periphery (white circle). Scale bar = 10 µm.
Auxin-directed AHP6 Represses Lateral Cytokinin Signaling to Establish Hormone Homeostasis and Promote Domain Specification in the Gynoecial Primordium
In both roots and the SAM, auxin activates AHP6, which in turn represses cytokinin signaling (Mähönen et al., 2006; Bishopp et al., 2011; Besnard et al., 2014a, 2014b). We therefore asked whether AHP6 is important also during early gynoecial primordium formation. In the earliest stage-5 gynoecial primordia, pAHP6::GFP is distinctly expressed in the lateral domains, and by late stage 7, expression can also be detected in a file of medial cells presumed to be preprocambium (Fig. 2, M and N), overlapping with auxin response peaks (Fig. 1, D and O). In later stages, AHP6 expression weakens drastically and becomes vascular specific (Supplemental Fig. S3A) as previously described (Reyes-Olalde et al., 2017). Although AHP6 is repressed by BAP in roots (Mähönen et al., 2006), it remains laterally expressed after BAP treatment in gynoecial primordia (Supplemental Fig. S3, B and C), in line with its behavior in the SAM (Besnard et al., 2014a, 2014b). Next, we assessed the effects of the ahp6-1 mutation on the expression of the domain markers pSHP2::YFP and pFIL::GFP. Similar to short-term BAP effects, the medial marker pSHP2::YFP is expanded into the lateral domains of ahp6-1 (Fig. 2C), whereas the lateral marker pFIL::GFP is expressed as in wild type (Fig. 2F). Together, these results suggest that AHP6-mediated repression of cytokinin signaling in the auxin response domains may confine medial fate to nonlateral positions for correct domain boundary specification in early stage gynoecia.
If AHP6 is important for restricting cytokinin signaling from the medial domains, and BAP treatment broadens the auxin response domain (Fig. 1, B to K), then we expect that auxin response may also be affected in the ahp6-1 mutant as a result of increased cytokinin signaling. Indeed, the ahp6-1 mutant exhibited broader, less-focused auxin maxima and significantly more cells expressing DR5 (Fig. 2, G, I, J, and L), similar to what is observed after BAP treatment (Fig. 2, H and K; Supplemental Fig. S3F). This data suggests that cytokinin signaling is regulated by AHP6 in the early stage primordium, and that this in turn acts to balance auxin-signaling domains.
Next, we assessed the role of PAT in the regulation of AHP6. We have previously shown that NPA treatment of early stage gynoecial primordia results in strong, ectopic auxin response as seen by DR5 (Larsson et al., 2014); similarly, a 24 h NPA treatment causes expanded pAHP6::GFP in stage-5 gynoecial primordia, resulting in a ring of AHP6 expression at the apex (Fig. 2O). Therefore, the PAT essential for forming focused auxin peaks in the young primordium apex also ensures correct activation of AHP6. To understand if blocking auxin transport affects cytokinin signaling, we assessed TCSn::GFP after NPA treatment in wild type and ahp6-1. Indeed, a 24 h NPA treatment caused a clear reduction of cytokinin signaling in wild type gynoecial primordia, as seen by reduced TCSn::GFP in a ring around the adaxial, medial domain (Fig. 2, P, Q and R), complementary to the observed expansion of AHP6 (Fig. 2O). Longer NPA treatment (72 h) causes a drastic reduction in cytokinin signaling, where TCSn::GFP is most prominently reduced in the medial domain but also clearly reduced in the lateral domain (Fig. 2S). If AHP6 is important for hormone homeostasis in the early stage primordium, we expect to see evidence of this in the ahp6-1 mutant. Indeed, stage-5 ahp6-1 gynoecial primordia have increased TCSn expression in the lateral domains (Fig. 2T), indicating that AHP6 is important for dampening cytokinin signaling in the lateral domain. At stage 8, we observed that TCSn::GFP was frequently expressed ectopically in the apical, lateral domain in ahp6-1 mutants, which was not observed in wild type (Supplemental Fig. S3D), which is in accordance with Reyes-Olalde et al. (2017), who recently showed ectopic TCS::GFP activity in the lateral valves of stage-9 and mature ahp6-1 gynoecia. In contrast to wild type gynoecial primordia, NPA treatment of ahp6-1 did not reduce the cytokinin signaling reporter activity as readily after 24 h or 72 h (Fig. 2, R, S, U, and V). Taken together, these results suggest that auxin-mediated AHP6 regulation is a key component in auxin-dependent repression of cytokinin signaling in early stage gynoecial primordia. AHP6 is a clear player in ensuring a balanced relationship between auxin and cytokinin signaling domains in the earliest stages of gynoecium development.
Cytokinin Promotes Auxin Biosynthesis Gene Activity and Regulates Auxin Transport Activities in the Gynoecial Primordium
In roots, cytokinin signaling can regulate the expression of several YUC auxin biosynthesis genes (Jones et al., 2010; Di et al., 2016), and PIN1, PIN3, and PIN7 auxin efflux carriers (Dello Ioio et al., 2008; Ruzicka et al., 2009; Bishopp et al., 2011; Šimášková et al., 2015). We and others have previously shown that PIN1, PIN3, and PIN7 translational reporters are expressed in tissue-specific patterns in early stage gynoecial primordia (Larsson et al., 2014; Moubayidin and Ostergaard, 2014). In searching the Arabidopsis eFP browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) for auxin biosynthesis components that are transcriptionally affected by cytokinin, we noted that YUC1 and YUC4 responded to cytokinin in seedlings. In addition, the yuc1 yuc4 double mutant has a severe gynoecial phenotype (Cheng et al., 2006), reminiscent of NPA or BAP treatment of wild type gynoecia. However, while YUC1 and YUC4 are expressed in inflorescence meristems and later stage gynoecia (Cheng et al., 2006), their activity in the gynoecial primordium has not been investigated. Therefore, we looked further into a possible interaction between cytokinin and components regulating auxin biosynthesis and transport in the gynoecial primordium.
In the stage-5 gynoecial primordium, both pYUC1::n3GFP and pPIN7::PIN7::GFP are expressed in a few subapical medial cells, peaking at 10 μm to 20 μm below the apex (Fig. 3, A, A′, K, and K′), overlapping with the cytokinin signaling peak (Fig. 1B). BAP treatment (24 h) results in elevated and ectopic expression of both genes. YUC1 expression is up-regulated and ectopic throughout the medial domain and occasionally also in the lateral domain (Fig. 3, D, D′, and E), whereas longer (48 h) BAP treatment consistently leads to ectopic YUC1 expression in the lateral domains (Supplemental Fig. S4A). By stage 7, YUC1 is expressed only in the adaxial-most part of the medial domains that will later fuse to form the placenta and transmitting tract (Fig. 3, B and C), whereas BAP treatment strongly activates YUC1 throughout the medial domain (Fig. 3, F and G).
Figure 3.
Cytokinin up-regulates YUC1, YUC4, and PIN7 and represses PIN3 in the gynoecial primordium. Confocal microscopy images of YUC1, YUC4, PIN7, and PIN3 reporter lines. A to J, pYUC1::n3GFP expression after 24-h mock treatment in stage 5 (A, A′; n = 15/21) and stage 7 (B, C; n = 8/11), after 24-h BAP treatment in stage 5 (D, D′; n = 4/5 and E; n = 1/5), in stage-7 gynoecial primordia after 24 h (F; n = 6) and 48 h (G) BAP treatment, and after 24 h PI-55 treatment in stage 5 (H, H′; n = 14/16) and stage 7 (I, J; n = 12/14). A, D, and H shown from the medial perspective in (A′), (D′), and (H′), respectively. Magenta indicates chloroplast autofluorescence. K to N′, pPIN7::PIN7::GFP expression in stage 5 gynoecial primordia after 24-h mock (K, K′; n = 25/27), 24-h BAP (L, L′; n = 10/11 and M; n = 1/11), and 24-h PI-55 treatment (N, N′; n = 10/16). O, Chart of pPIN7::PIN7::GFP expression patterns observed in stage-5 gynoecial primordia 24 h after mock, BAP or PI-55 treatment (n = 54). K, L, and N shown from the medial perspective in (K′), (L′), and (N′), respectively. P-U, pYUC4::n3GFP expression in stage-5 (P, R, T) and stage-7 (Q, S, U) gynoecial primordia after 24-h mock (P and Q; n = 14 and 8), 24-h BAP (R and S; n = 10 and 11), and 24-h PI-55 treatment (T and U; n = 10 and 8). V, Heat map of relative signal intensity in arbitrary units to visualize semiquantitative expression levels (in P to U and W to DD). W to DD, pPIN3::PIN3::GFP expression in stage-5 (W and AA), stage-6 (X and BB), stage-7 (Y and CC), and stage-8 (Z and DD) gynoecial primordia after 24-h mock (W to Z; n = 28) and 24-h BAP treatment (AA to DD; n = 35). Each image is a maximum intensity projection of serial images (stack) and the schematic drawings in each subfigure indicate the image perspective (red line). Gynoecial primordium periphery (white circle), border in medial perspective (dashed white line) and sepal (asterisk) are indicated. Scale bar = 10 μm.
Although the stage 5 PIN7-expression is evident in most gynoecia, it is often weak (Fig. 3O); however, 24 h BAP treatment results in stronger, clearly detectable PIN7 activity in the corresponding subapical medial cells of all stage-5 gynoecia (Fig. 3, L and L′). In addition, BAP treatment also induces ectopic PIN7 expression in a wider range of subapical medial cells and occasionally also in the lateral domains (Fig. 3M). The activation of PIN7 by cytokinin in gynoecial primordia is in line with the reported cytokinin-induced PIN7 expression in roots (Ruzicka et al., 2009; Bishopp et al., 2011; Šimášková et al., 2015). Together our data suggest that YUC1 and PIN7 expression overlap with peak cytokinin signaling in the subapical medial tissue of the gynoecial primordium, and that cytokinin can promote medial auxin biosynthesis in, and PIN7-mediated auxin efflux from, this domain.
pYUC4::n3GFP is expressed in all apical, epidermal cells of stage-5 and stage-7 gynoecial primordia; the signal is slightly stronger in the medial domain at stage 5 and in a few adaxial-most cells of the medial domain at stage 7 (Fig. 3, P and Q). A 24 h BAP treatment up-regulates YUC4 expression predominantly in the medial epidermis, and at stage 7, the strongest YUC4 activation occurs in the adaxial-most medial cells (Fig. 3, R and S). Interestingly, pPIN3::PIN3::GFP is expressed in a few adaxial, medial cells at stage 5, partly overlapping with YUC4 and apical TCSn (Fig. 3W), and at stage 6, PIN3 expression is also detected in one or two apical, lateral cells and a few basal, medial cells (Fig. 3X). By stage 7 and stage 8, PIN3 becomes stronger and broadens in the medial apex, and apparently marks preprocambial positions in a few apical, lateral cells (Fig. 3, Y and Z). Twenty-four hours after BAP treatment, PIN3 is significantly reduced in all early gynoecial primordia stages in the medial domain, and also reduced in the lateral domain of stage 7 and stage 8 (Fig. 3AA-DD and Supplemental Fig. S4D). Together, these data suggest that cytokinin in the apical medial domain can activate YUC4 and repress PIN3 expression, and this may serve to promote auxin accumulation in the apex and to delay auxin drainage from the apical medial domain. These data are in accordance with the broadened, less-focused auxin response observed after cytokinin treatment (Figs. 1 and 2), suggesting that cytokinin may spatiotemporally control the timing of medial auxin drainage and consequently, medial preprocambium formation. Accordingly, the provascular marker pIAA2::GUS (Bishopp et al., 2011) exhibits reduced provascular expression in later stage gynoecia both 72 h and 9 d after BAP treatment (Supplemental Fig. S5).
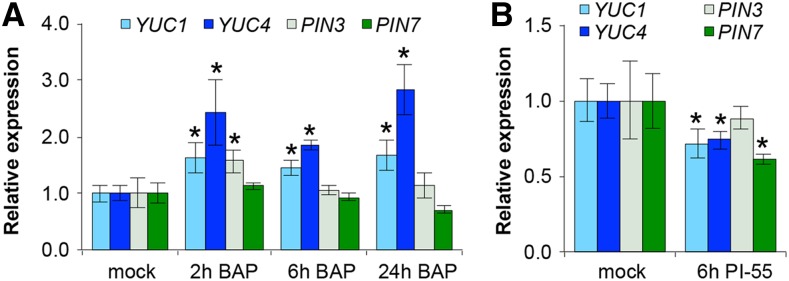
To confirm and better characterize the timing of the observed expression changes after treatment with BAP, we performed quantitative real-time PCR. Because it is not feasible to hand-extract such early stage gynoecial primordia without neighboring tissue contamination, we instead extracted RNA from primary inflorescences with stage 9 and older flowers removed. As expected, both YUC1 and YUC4 are significantly up-regulated at 2 h, 6 h, and 24 h after BAP treatment (Fig. 4A), suggesting that BAP has an enduring influence on the expression of both of these auxin biosynthesis genes. In contrast and unexpectedly, the PIN3 transcript level was elevated instead of repressed shortly after BAP treatment (2 h), although no change compared to mock was detected after 6 h and 24 h BAP treatment (Fig. 4A). We therefore examined the PIN3 reporter in gynoecia after short-term BAP treatment (2 h), and detected a small but significant reduction in PIN3 activity compared to mock (Supplemental Fig. S4, B to D), in support of the confocal results after 24h BAP (Fig. 3W-DD). No significant change in transcript abundance of PIN7 in young floral buds was detected at any time point after BAP treatment (Fig. 4A). The discrepancies between BAP effects on PIN3 and PIN7 expressions obtained by qPCR and confocal experiments could reflect a differential response to BAP in the additional floral tissues included only in the qPCR experiment. Alternatively, cytokinin’s effects on the PIN3 and PIN7 translational reporters may indicate a role of posttranscriptional regulation by cytokinin on PINs, as has been previously demonstrated in roots (Zhang et al., 2011).
Figure 4.
Quantitative expression of YUC1, YUC4, PIN3, and PIN7 in response to increased or decreased cytokinin signaling. A and B, qPCR analyses of YUC1, YUC4, PIN3, and PIN7 expression in early floral buds after mock versus 2-h, 6-h, and 24-h BAP treatment (A) and mock versus 6-h PI-55 treatment (B). Error bars indicate SD from three biological replicates; *, P < 0.05, Student’s t test.
In contrast to other organs (Dello Ioio et al., 2008; Ruzicka et al., 2009), no perceptible deviation in pPIN1::PIN1::GFP signal intensity or localization was detected in early stage BAP-treated gynoecia compared to mock treatment (Supplemental Fig. S4, E and F), suggesting that PIN1 is not an early cytokinin target in gynoecia.
As auxin can negatively feed-back on YUC expression (Suzuki et al., 2015), we treated both YUC and PIN reporters with two different synthetic auxins, 1-naphthaleneacetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D), but we observed no alterations of YUC1, YUC4, or PIN7 activity after a 24 h treatment (Supplemental Fig. S6, A to C; compare to Fig. 3, A to C, K, P, and Q). This clearly indicates that the observed BAP-induced expression changes are not explained by cytokinin’s influence on auxin homeostasis alone. In contrast, both BAP and auxin treatments exerted similar repressive effects on apical PIN3 expression (Supplemental Fig. S6D, compare to Fig. 3, W to DD), hinting that cytokinin’s repressive effect on PIN3 may be the result of up-regulated auxin signaling.
Endogenous Cytokinin Signaling is Required for YUC1, YUC4, and PIN7 Expression in the Gynoecial Primordium
We next asked whether endogenous cytokinin signaling is important for activation of YUC1 and YUC4. Because of gene redundancy in the cytokinin signaling pathway, multiple mutants are often necessary to observe any phenotypic changes to gynoecium development; however, these mutants exhibit severe growth defects already in the vegetative state that may carry over secondary defects to the development of the gynoecium, as the gynoecium is the last organ to develop (Higuchi et al., 2004; Argyros et al., 2008; Ishida et al., 2008; Kinoshita-Tsujimura and Kakimoto, 2011; Cheng and Kieber, 2013; Cheng et al., 2013). We instead chose to reduce cytokinin signaling transiently in wild type using the competitive cytokinin antagonist PI-55 (Spíchal et al., 2009). In our hands, higher concentrations of PI-55 (5 μM to 10 μM) halted meristematic growth, whereas 1 μM PI-55 allowed floral development to continue; therefore, we used 1 μM PI-55 to study the effect of this compound on gene expression in early gynoecial primordia. First, we tested the response of ARR5, an A-type ARR that is rapidly induced by BAP treatment (D’Agostino et al., 2000) in floral buds (Supplemental Fig. S7A). Indeed, a 6 h treatment of 1 μM PI-55 was sufficient to significantly reduce ARR5 expression (Supplemental Fig. S7B), signifying the efficacy of PI-55 to transiently reduce cytokinin signaling in floral tissue. In these PI-55 treated floral buds, YUC1, YUC4, and PIN7 transcript levels were also significantly reduced, whereas PIN3 expression was unaffected (Fig. 4B). We corroborated that these changes also occur in the gynoecium in a spatiotemporal manner by analyzing the effects of reporter gene expression after short-term PI-55 treatment. Indeed, 24 h after PI-55 treatment, the activity of pYUC1::n3GFP, pYUC4::n3GFP, and pPIN7::PIN7::GFP reporters were drastically reduced in early stage gynoecia (Fig. 3, H to J, N to O, T, and U) in addition to the TCSn::GFP reporter also being down-regulated (Supplemental Fig. S7C). These data clearly suggest that YUC1, YUC4, and PIN7 are targets of endogenous cytokinin signaling in the medial domain of the early gynoecial primordium.
Whereas double yuc1 yuc4 mutants are severely affected in gynoecium development, as evidenced by the large frequency of valveless gynoecia, the single yuc1 and yuc4 mutants alone have no observable lateral-medial developmental defects (Cheng et al., 2006). Similarly, ahp6-1 single mutants have no observable gynoecium phenotype (Supplemental Fig. S3E). To better understand the developmental roles of YUC1, YUC4 and AHP6 in gynoecial development, we performed long-term BAP and NPA treatments on the yuc1, yuc4 and ahp6-1 single mutants. In wild type, long-term BAP treatment causes pleiotropic effects in the gynoecium, most notably: outgrowths from the replum, increased ovule number, and occasionally reduced valve length or even valveless gynoecia (Bartrina et al., 2011; Marsch-Martínez et al., 2012; Zúñiga-Mayo et al., 2014). After BAP treatment, yuc1, yuc4 and ahp6 mutants formed a considerably higher frequency of valveless gynoecia compared to wild type (6.7%, 67.8% and 50% of gynoecia respectively, versus 2.3% of wild type gynoecia) (Fig. 5A); similarly, blocking auxin transport in these single mutants with long-term NPA treatment also resulted in a higher frequency of reduced valve and valveless gynoecia compared to wild type (Fig. 5A). Together, these results suggest that each of these three genes are individually required for stabilizing auxin response domains for lateral domain outgrowth (Fig. 5A). In contrast, other developmental defects induced by long-term BAP treatment in wild type, such as replum outgrowths, were less pronounced in the yuc4 mutant (Fig. 5B), indicating that cytokinin-induced YUC4 may contribute to the formation of these outgrowths. In accordance, hemizygous yuc1/+ yuc4/+ double mutants have been shown to also produce fewer outgrowths upon BAP treatment compared to wild type (Lucero et al., 2015). Taken together, these results suggest that cytokinin-induced YUC1 and YUC4 are both required for the stability of the auxin response domains contributing to valve outgrowth.
Figure 5.
YUC1, YUC4 and AHP6 are required for valve outgrowth and hormone homeostasis. A, Quantification of valve phenotypes in stage-12 gynoecia from yuc1, yuc4, and ahp6 mutants analyzed 14 d after 1 d transient treatment with mock, BAP, or NPA. B, Quantification of outgrowth severity in Col-0, yuc1, and yuc4 mutants after five consecutive days of mock or BAP treatment, analyzed 14 d after the first spray.
DISCUSSION
Distinct Signaling Domains for Auxin and Cytokinin in the Youngest Gynoecial Primordium Resembles that of the SAM and Floral Meristem
We present new insights on cytokinin and auxin crosstalk in the earliest stages of gynoecial primordium establishment. First, we show that TCSn, AHP6, and DR5 expression in stage-5 gynoecial primordia are spatially comparable to that in the SAM and floral meristem (FM) (Yoshida et al., 2011; Murray et al., 2012; Zürcher et al., 2013), suggesting that the FM organization partially remains during gynoecium initiation (Figs. 1 and 2). DR5 and AHP6 are expressed mainly in lateral domains, while TCSn is active in the medial domain, peaking a few cell layers below the apex. This implies that the lateral domains of the incipient gynoecial primordia experience a similar hormonal regulation used for lateral organ initiation by the SAM and FM, while the medial domains retain a regulation common for the meristematic region. In the respective domains, cytokinin promotes medial markers, such as SHP2, whereas auxin peaks promote lateral identity (e.g. FIL). This may not be surprising, considering the suggestion that the carpels evolutionarily represent ancestral leaflike structures carrying spores along their edges [for review, see Hawkins and Liu (2014)].
We show that through stage 6 and stage 7, the TCSn signal becomes restricted to the adaxial, basal part of the medial domain (Fig. 1), and in subsequent stages, when the medial part grows out to form the CMM, from which the reproductive tissues develop, the complementary hormonal regulation largely remains (Marsch-Martínez et al., 2012). At these later stages, the TCS::GFP reporter is active in the CMM, septum, and replum initials; however, the responsiveness to BAP appears to be restricted to the innermost medial domain (Marsch-Martínez et al., 2012). In contrast, we show that most of the tissues in young gynoecial primordia (stage 5 to stage 8) can respond to BAP treatment (Fig. 1), suggesting that after complete lateral domain specification around stage 8, cytokinin responsiveness is efficiently blocked from this domain. This may be due to the repressive effects of auxin signaling perhaps in part through promotion of AHP6, which remains in the lateral domains of later-stage gynoecial primordia (Reyes-Olalde et al., 2017).
Interestingly, apart from an elevated TCSn expression in stage-5 ahp6-1 lateral domains, which is in accordance with published data on later-stage gynoecia (Reyes-Olalde et al., 2017), TCSn expression was weaker in the medial domains of stage-5 ahp6-1 gynoecia (Fig. 2T), suggesting that increased cytokinin signaling in auxin response domains may autonomously feed-back on cytokinin signaling in domains normally without strong auxin response. Because BAP treatment resulted in broader, less-focused auxin response, we hypothesized that this feedback likely occurs via auxin signaling. Indeed, auxin response, as visualized by the DR5::3xVENUS reporter (Vernoux et al., 2011; Besnard et al., 2014a, 2014b), was expanded and less focused in ahp6-1 gynoecial primordia, and more cells expressed DR5, similarly to DR5 in wild type after 24 h BAP treatment (Fig. 2, G to L; Supplemental Fig. S3F). Although our data show that AHP6 is an important component during gynoecial primordium development to secure correct positioning of not only cytokinin but also auxin signaling maxima, the ahp6-1 mutants develop only slightly fewer stamens and exhibit no discernable gynoecial patterning defects under normal growth conditions (Supplemental Fig. S3E). However, long-term BAP or NPA treatment indicated the importance of AHP6 during development, as the ahp6-1 mutant is hypersensitive to both treatments and forms more reduced valve and valveless gynoecia than BAP or NPA-treated wild type. These findings advocate for a resilient developmental system with multiple and partially redundant layers of cytokinin-auxin feedback, which includes but is not limited to AHP6, to ensure balanced gynoecium development.
Cytokinin Signaling Spatiotemporally Regulates Auxin Homeostasis Genes in Cytokinin Response Domains during Gynoecial Primordium Establishment
Although cytokinin responsiveness of YUC1 and YUC4 was not previously established in wild type gynoecia, the laterally expressed TEOSINTE BRANCHED1-CYCLOIDEA-PCF (TCP) transcription factor TCP15 has been suggested to repress YUC1 and YUC4 activity in developing valves, and to participate in a feedback loop adjusting the auxin-cytokinin balance (Lucero et al., 2015). Here, we reveal expressional colocalization of YUC1 with cytokinin signaling peaks in the subapical medial domain, demonstrate BAP-mediated expressional activation and show the inhibition of YUC1 activity upon cytokinin signaling repression, which together give strong support for a connection between YUC1 expression and cytokinin signaling in wild type. YUC4, which is expressed in the apical epidermal cells of the newly initiated gynoecial primordium, responds similarly to changes in cytokinin signaling. It is activated by BAP predominantly in the medial epidermal cells, and drastically repressed in these cells upon reduced cytokinin signaling. Together, our data support a role for cytokinin to activate auxin biosynthesis in the medial domain during gynoecial primordium initiation and development.
Although a connection between YUC1 and cytokinin signaling at later developmental stages has not been clarified (Lucero et al., 2015), attention has been given to TAA1, which is BAP-induced in the medial domains of stage-9 gynoecia (Reyes-Olalde et al., 2017). At this stage, when the CMM initiates internal tissue differentiation, TAA1 transcription appears to be directly regulated by the cytokinin response activator ARR1 (Reyes-Olalde et al., 2017). ARR1 in turn is directly activated by SPT, a bHLH transcription factor important for correct differentiation of septum, transmitting tract and style tissues from the medial domain (Heisler et al., 2001; Groszmann et al., 2010; Reyes-Olalde et al., 2017). In addition, the HECATE (HEC) proteins, closely related to SPT, appear important to support apical YUC4 activity in older gynoecia (stage 10; Schuster et al., 2015). Interestingly, at stage 5, when TAA1 is also expressed in the medial domain, it does not respond to BAP treatment (Supplemental Fig. S2), suggesting that at gynoecial initiation, this first step in the Trp-dependent auxin biosynthetic pathway is not rate limiting, whereas cytokinin promotion instead acts at the rate-limiting step mediated by YUC enzymes. This also suggests that TAA1 is differentially controlled by cytokinin at different stages of development.
Cytokinin Signaling Differentially Regulates PIN Expression in the Early Stage Gynoecial Primordium
Interestingly, the medial expression of YUC1 and YUC4 does not overlap with peak auxin signaling, which instead peaks in the lateral apical domains. This is in line with observations made from various auxin biosynthetic components in other tissues (Stepanova et al., 2008; Ursache et al., 2014), where researchers have postulated that the discrepancy between auxin biosynthesis and signaling peaks likely underscores the necessity of a functional auxin transport system to correctly position auxin maxima for various developmental processes.
Indeed, using short-term BAP and PI-55 treatments, we could show that cytokinin regulates auxin transport by promoting PIN7 expression in the basal, medial domain of stage-5 gynoecial primordia, where cytokinin signaling and YUC1 expression peak (Fig. 3); however, in later stages, when the lateral domains have already expanded, no PIN7 activity is detectable in the medial domain (Reyes-Olalde et al., 2017). In contrast, cytokinin represses PIN3 expression in apical tissues, where cytokinin-regulated YUC4 is most strongly expressed. These results suggest that cytokinin effectively encourages auxin transport out of the basal medial domain and reduces auxin efflux from the apical tissues of early stage gynoecial primordia. In line with these findings, PIN7 is up-regulated while PIN3 is repressed in cytokinin-treated roots (Ruzicka et al., 2009; Bishopp et al., 2011). As PIN3, in contrast to YUC1, YUC4, and PIN7, is also responding to auxin, the cytokinin-induced repression of the PIN3 translational reporter may be mediated by cytokinin’s effects on auxin in young gynoecial primordia. At later stages, PIN3 appears to be activated in medial tissues via the same regulatory route as TAA1 (SPT and ARR1) and possibly also directly by HEC proteins (Schuster et al., 2015; Reyes-Olalde et al., 2017).
In line with these observed differences between early and later stage gynoecia, cytokinin modulates auxin flow in ovules by promoting PIN1, leading to balanced auxin response domains, and BAP treatment leads to increases in PIN1 and exogenous DR5 signal in the developing ovule (Bencivenga et al., 2012). However, we did not observe any obvious changes to PIN1 after BAP treatment in the gynoecial primordium, suggesting that cytokinin-regulated PIN1 instead has a later role during gynoecium development, at the level of ovule differentiation.
Recently, cytokinin has been shown to directly regulate PIN1 and PIN7 transcription in roots via CYTOKININ RESPONSE FACTOR (CRF) transcription factors that bind to specific PIN CYTOKININ RESPONSE ELEMENT (PCRE) domains found in PIN1 and PIN7 promoters (Šimášková et al., 2015). In addition, cytokinin’s activation of PIN1 in ovules has been linked to the PCRE domain, and multiple CRF mutants have reduced seed-set (Rashotte et al., 2006; Bencivenga et al., 2012; Cucinotta et al., 2016). Therefore, it is plausible that cytokinin could regulate PIN7 and possibly PIN3 by a similar mechanism via CRF transcription factors during gynoecial primordium initiation.
Blocking auxin transport with NPA leads to increased as well as broadened auxin response domains (Larsson et al., 2014), which causes valveless and reduced-valve gynoecia (Nemhauser et al., 2000; Larsson et al., 2014). In line with our findings, loss-of-function pin3 and pin7 mutants are hypersensitive to BAP treatment and form more valveless gynoecia after BAP treatment than BAP-treated wild type (Züñiga-Mayo et al., 2014), suggesting PIN3 and PIN7 each have a role in promoting correct auxin maxima drainage to ensure lateral tissue development. Taken together with our findings, we suggest that cytokinin regulates auxin transport thus stabilizing auxin maxima formation, ultimately ensuring the correct formation of the lateral domains.
We hereby propose a model elucidating a sophisticated cytokinin-auxin feedback mechanism in the earliest stage of gynoecial primordium initiation (Fig. 6). This intricate network creates a homeostatic hormone balance vital for correct mediolateral patterning and tissue specification in the early stage gynoecium. The expression of the identified molecular components, as well as their homologs, in various plant tissues hints that this feedback mechanism may be conserved in other meristematic systems. In addition, the model contains both similarities and differences compared to the recently presented model for cytokinin-auxin interactions in differentiating stage-9 to stage-12 gynoecia (Reyes-Olalde et al., 2017). Common to both models is the restriction of cytokinin signaling to the medial domain through the action of auxin-induced AHP6 in the lateral domains. Additionally, in medial tissues, cytokinin promotes the expression of multiple auxin biosynthesis genes as well as the flow and accumulation of auxin by regulating the expression of auxin efflux carriers. However, our models differ in the specificity of the target genes, the way in which these are regulated, and in the direction of auxin flow. We show evidence of YUC1 and YUC4 regulation at early stages, whereas Reyes-Olalde et al. (2017) show support for TAA1 activation at later stages. In addition, we propose that auxin flows outward from the medial domain (via PIN7), then apically where it accumulates (through repression of PIN3) and promotes the formation of auxin signaling maxima and valve outgrowth as auxin is subsequently drained. In later stages, Reyes-Olalde et al. (2017) propose that cytokinin-induced PIN3 directs auxin flow from central to peripheral medial tissues and subsequently to lateral domains.
Figure 6.
Model of cytokinin-auxin feedback mechanisms in the earliest stage gynoecial primordium. A stage-5 gynoecial primordium depicted from the medial domain showing regions of cytokinin signaling (green), auxin signaling (blue), auxin transport (arrowheads), and interactions between components (inhibiting lines and activating arrows). We propose a model in which cytokinin in the central medial domain is important to activate auxin biosynthesis via YUC1 and transport via PIN7 in the basal medial domain. PAT is important for setting up auxin peaks in the apical, lateral domains, ensuring the activation of AHP6, which contributes to the inhibition of cytokinin signaling from the lateral domains. Additionally, cytokinin promotes auxin signaling in the apex by promoting biosynthesis through YUC4 and blocking efflux via repression of PIN3, which together likely contribute to the apical accumulation of auxin. Together, these components are important for ensuring lateral and apical auxin accumulation and valve outgrowth.
The differences observed among the regulation of PIN3, PIN7, and TAA1, as well as the proposed destination of auxin produced in the medial domain of the incipient gynoecial primordium compared to the following developmental stages, highlight the dynamic regulation occurring in such complex tissues, and emphasizes the necessity for more stage-focused, spatiotemporal hormone interaction studies. It also clearly reveals that it is not possible to superimpose data found in one stage of development upon other stages.
CONCLUSION
We have presented a network of cytokinin-auxin interactions in the incipient gynoecial primordium in which cytokinin up-regulates both the auxin biosynthesis genes YUC1 and YUC4 and the auxin transport gene PIN7 in the medial domain, whereas auxin blocks cytokinin signaling in the lateral domains via promotion of AHP6. In addition, cytokinin down-regulates apically expressed PIN3 to ensure auxin accumulation at the apex. Together, this network promotes hormone homeostasis, correct domain specification, and valve outgrowth. As this network is far from complete, future work is needed to identify additional players and enrich our understanding of those components involved in hormone crosstalk during the development of the gynoecial primordium.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All lines used in this study are in the Arabidopsis (Arabidopsis thaliana) Columbia ecotype background. The following lines have been described previously: DR5::RFP (Marin et al., 2010), TCSn::GFP (Zürcher et al., 2013), DR5::GFP (Friml et al., 2003); pSHP2::YFP (Larsson et al., 2014; Villarino et al., 2016); pFIL::GFP (Watanabe and Okada, 2003); pTAA1::TAA1::GFP (Stepanova et al., 2008); ahp6-1 and pAHP6::GFP (Mähönen et al., 2006); pYUC1::n3GFP and pYUC4::n3GFP (Robert et al., 2013); pPIN1::PIN1::GFP (Benková et al., 2003); pPIN3::PIN3::GFP (Zádniková et al., 2010); pPIN7::PIN7::GFP (Blilou et al., 2005); DR5::3xVENUS in Col-0 and ahp6- 1 (Besnard et al., 2014a, 2014b); and pIAA2::GUS (Bishopp et al., 2011).
All mutant alleles were genotyped by PCR using gene- and T-DNA-specific primers except for ahp6-1, whose PCR product from the mutant but not the wild type was cleaved enzymatically with PvuII and separated by gel electrophoresis. All primers are listed in Supplemental Table S1. Seeds were surface-sterilized using 10% (v/v) commercial bleach containing 0.15% (v/v) SDS for 15 min, then 70% (v/v) ethanol for 1 min, and germinated on plates containing 0.5× Murashige & Skoog medium with 1% (w/v) Suc and 1% (w/v) plant agar. One- to two-week-old seedlings were transplanted to soil and grown under controlled long-day conditions (16-h light/8-h dark photoperiod at 110 μmol m−2 s−1 and 22°C).
For imaging of 24 h or longer hormone treatments, plants were sprayed once in the morning (0 h) and once in the afternoon (8 h) as described previously (Nemhauser et al., 2000; Larsson et al., 2014) with 100 μM BAP, 1 μM PI-55, 100 μM NPA, 100 μM NAA, or 10 μM 2,4-D containing 0.01% Silwet L-77; working concentrations for NPA, 2,4D, and BAP were based on previous reports of efficacy (Marsch-Martínez et al., 2012; Yamaguchi et al., 2013; Larsson et al., 2014). BAP and NAA were dissolved in 1 M NaOH to a stock concentration of 100 mM; PI-55, 2,4-D, and NPA were dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 10 mm PI-55, 10 mM 2,4-D, and 100 mM NPA. Mock treatments contained 0.1% (v/v) NaOH or DMSO and 0.01% Silwet L-77 only. Plants were washed with a distilled water spray 24 h after the first hormone spray. For hormone treatments less than 24 h and treatments for RNA extraction, plants were sprayed just once and analyzed or collected at the specified hour after treatment. For valve and outgrowth quantification of yuc and ahp6 mutants, plants were sprayed with 100 μM BAP once in the morning and once in the afternoon and sprayed with water on d 2 (valve quantification) or sprayed 1± for 5 d and then sprayed with water on d 6. Inflorescences were fixed and cleared with chloral hydrate (see below) on d 14.
Sample Preparation
Gynoecia were individually hand-dissected out from the inflorescences and mounted on 1% agarose (w/v) for confocal microscopy in water or 30% glycerol for fluorescence microscopy, or in chloral hydrate (2.5 g chloral hydrate in 30% [v/v] glycerol) for GUS-stained tissues. A minimum of 10 gynoecia of each stage was analyzed from at least two (Supplemental Data) or three independent experiments. For pPIN1::PIN1::GFP fixation and localization and GUS staining, samples were prepared as previously described (Larsson et al., 2014). All GUS-staining times were equal for all treatments within one experiment.
Microscopy, Imaging, and Analysis
Confocal laser-scanning microscopy was performed using a model no. 780 inverted AxioObserver (Zeiss) with a supersensitive GaASp detector. An argon laser was used at 488 nm for GFP, 514 nm for YFP, and 633 nm for RFP and chlorophyll a excitation. Emissions were detected between 491 nm and 535 nm for GFP, 517 nm and 606 nm for YFP, 624 nm and 642 nm for RFP, and 648 nm and 721 nm for chlorophyll a. All images were taken using a C-Apochromat 63× water immersion objective with the pinhole set to 1 Airy unit. The images presented are either a snapshot of a single focal plane (snap) or a maximum intensity projection of multiple individual images taken as a z-series and assembled as a three-dimensional reconstruction (stack) using the ZEN Black software (v. 2011; Zeiss). Fluorescence microscopy of whole-mounted older stages was performed with a model no. DMI 4000 inverted microscope (10×, 20×, or 40× objectives; Leica) with differential interference contrast (DIC) optics, a DFC360FX camera (Leica), and LAS AF filters (Leica Microsystems) software. GFP/YFP (L5 GFP band-pass) and chlorophyll-a autofluorescence (rhodamine/red fluorescent protein long-pass) filters were used. GUS-stained tissues were analyzed using an AxioScope A1 microscope (10×, 20×, or 40× objectives; Zeiss) with DIC optics. All images were processed using Photoshop CS5 (Adobe), including image merging for some late-stage gynoecia and brightness and contrast adjustments, which were done equally to the whole image as needed for clarification. Figures were compiled in Illustrator CS6 (Adobe).
Quantification of signal intensity in confocal images was performed using the ZEN Black software (v. 2011; Zeiss). For quantification of DR5::RFP and TCSn::GFP expressing cells, single confocal snap images from 24 h BAP-treated gynoecia expressing both DR5::RFP and TCSn::GFP reporters were used. The mean signal intensity (in arbitrary units) of RFP and GFP was calculated for a square area (5 µm2, roughly the size of a single cell) in two places on the image. First, the mean intensity was calculated for a strong DR5::RFP-expressing cell, and then for a neighboring cell that appeared to express strong TCSn::GFP.
For pPIN3::PIN3::GFP, a maximum intensity projection image was created from the z-stacks, and from this image, the mean signal intensity was calculated (in arbitrary units) from a 10 µm2 square area in the medial or lateral domains as specified. For consistency, the square was placed over the area in the medial or later domain giving the highest mean intensity for each image. Between 5 and 10 gynoecia were analyzed for each stage and treatment. Only those gynoecia imaged in a clear orientation to visualize both medial and lateral domains apically were used.
For pPIN7::PIN7::GFP quantification, confocal image stacks were merged to create a maximum intensity projection. These images were compared and fell into visually distinct categories of none (no distinct expression), weak (barely perceptible expression), strong (clear and distinct expression observed in about half of wild type gynoecia), and strong + ectopic (expanded and sometimes very strong signal).
For DR5::3xVENUS, confocal images of stage-7 gynoecia viewed apically were used to quantify cell number. The number of cells expressing DR5 was counted by hand while scanning through each individual slice from a multislice scan encompassing 1 µm to 20 µm of the gynoecium apex. For consistency, out of the two medial and lateral domains present in each sample imaged, only the medial and lateral domain with the most DR5-expressing cells was used for quantification of each sample.
Gene Expression Analysis
For qPCR analyses, inflorescences with floral buds ≥ stage 9 removed were snap-frozen, homogenized in a Fast-prep TissueLyser (Savant) with glass beads, and total RNA extracted using the RNeasy Plant mini kit (Qiagen) with on-column DNase digestion (Qiagen) according to the manufacturer’s instructions. Ten inflorescences were used per biological replicate, and all comparable treatments were collected at the same time of day to reduce potential circadian rhythm effects. RNA quantity was calculated using the BR RNA Assay Kit with Qubit 2.0 (Life Technologies). cDNA was synthesized from 500 ng total RNA using the Maxima First-Strand cDNA Synthesis kit (Thermo Fisher Scientific). qPCR was carried out using Maxima SYBR Green/ROX master mix (Thermo Fisher Scientific) and gene-specific primers in an iCycler iQ Real-Time PCR machine (Bio-Rad). Primer efficiencies and gene normalization were calculated according to Pfaffl (2001). Two previously described normalization genes, ADENINE PHOSPHORIBOSYL TRANSFERASE1 and PROTEIN PHOSPHATASE2A SUBUNIT A3 (Czechowski et al., 2005; Gutierrez et al., 2008), have been used as normalization controls. A minimum of three biological and two technical replicates were performed for each assay. All primers can be found in Supplemental Table S1.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Primers used in this study
Supplemental Figure S1. Peak cytokinin and auxin signaling domains are exclusive after BAP treatment.
Supplemental Figure S2. pTAA1::TAA1::GFP expression is unaltered in gynoecial primordia after BAP treatment.
Supplemental Figure S3. AHP6 expression and ahp6-1 mutant phenotype and auxin response.
Supplemental Figure S4. Expression of auxin biosynthesis and transport components after BAP treatment.
Supplemental Figure S5. pIAA2::GUS expression in gynoecia after BAP treatment.
Supplemental Figure S6. Cytokinin-responsive YUC1, YUC4 and PIN7 reporters are not changed by exogenous auxin, while the PIN3 reporter is repressed by exogenous auxin.
Supplemental Figure S7. BAP upregulates ARR5 while both ARR5 and the reporter TCSn::GFP are reduced after PI-55 treatment.
Acknowledgments
We thank Robert G. Franks and Katarina Landberg for thoughtful comments on this article, Patrick Martin for helpful assistance, and Hélène Robert-Boisivon (Central-European Technology Institute), Teva Vernoux (INRA, University of Lyon), Bruno Müller (University of Zürich), and Annelie Carlsbecker (Uppsala University) for kindly providing seeds.
Footnotes
Articles can be viewed without a subscription.
References
- Argyros RD, Mathews DE, Chiang Y-H, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE (2008) Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20: 2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencivenga S, Simonini S, Benková E, Colombo L (2012) The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis. Plant Cell 24: 2886–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Lainé S, Thévenon E, Farcot E, et al. (2014a) Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 505: 417–421 [DOI] [PubMed] [Google Scholar]
- Besnard F, Rozier F, Vernoux T (2014b) The AHP6 cytokinin signaling inhibitor mediates an auxin-cytokinin crosstalk that regulates the timing of organ initiation at the shoot apical meristem. Plant Signal Behav 9: e28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, Helariutta Y (2011) A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol 21: 917–926 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Baum SF, Eshed Y, Putterill J, Alvarez J (1999) Molecular genetics of gynoecium development in Arabidopsis. Curr Top Dev Biol 45: 155–205 [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Kieber JJ (2013) The role of cytokinin in ovule development in Arabidopsis. Plant Signal Behav 8: e23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-Y, Mathews DE, Schaller GE, Kieber JJ (2013) Cytokinin-dependent specification of the functional megaspore in the Arabidopsis female gametophyte. Plant J 73: 929–940 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20: 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickarmane VS, Gordon SP, Tarr PT, Heisler MG, Meyerowitz EM (2012) Cytokinin signaling as a positional cue for patterning the apical-basal axis of the growing Arabidopsis shoot meristem. Proc Natl Acad Sci USA 109: 4002–4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta M, Manrique S, Guazzotti A, Quadrelli NE, Mendes MA, Benkova E, Colombo L (2016) Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 143: 4419–4424 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino IB, Deruère J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124: 1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- Di D-W, Wu L, Zhang L, An C-W, Zhang T-Z, Luo P, Gao H-H, Kriechbaumer V, Guo G-Q (2016) Functional roles of Arabidopsis CKRC2/YUCCA8 gene and the involvement of PIF4 in the regulation of auxin biosynthesis by cytokinin. Sci Rep 6: 36866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C, Pelaz S, Yanofsky MF (1999) Control of carpel and fruit development in Arabidopsis. Annu Rev Biochem 68: 321–354 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Galbiati F, Sinha Roy D, Simonini S, Cucinotta M, Ceccato L, Cuesta C, Šimášková M, Benkova E, Kamiuchi Y, Aida M, Weijers D, Simon R, et al. (2013) An integrative model of the control of ovule primordia formation. Plant J 76: 446–455 [DOI] [PubMed] [Google Scholar]
- Groszmann M, Bylstra Y, Lampugnani ER, Smyth DR (2010) Regulation of tissue-specific expression of SPATULA, a bHLH gene involved in carpel development, seedling germination, and lateral organ growth in Arabidopsis. J Exp Bot 61: 1495–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Guénin S, Pelloux J, Lefebvre J-F, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, van Wuytswinkel O (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J 6: 609–618 [DOI] [PubMed] [Google Scholar]
- Hawkins C, Liu Z (2014) A model for an early role of auxin in Arabidopsis gynoecium morphogenesis. Front Plant Sci 5: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Atkinson A, Bylstra YH, Walsh R, Smyth DR (2001) SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development 128: 1089–1098 [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, Helariutta Y, Sussman MR, et al. (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 101: 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Yamashino T, Yokoyama A, Mizuno T (2008) Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol 49: 47–57 [DOI] [PubMed] [Google Scholar]
- Jones B, Gunnerås SA, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K (2010) Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22: 2956–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzkowski D, Lenhard M, Smith R, Kuhlemeier C (2013) Interaction between meristem tissue layers controls phyllotaxis. Dev Cell 26: 616–628 [DOI] [PubMed] [Google Scholar]
- Kinoshita-Tsujimura K, Kakimoto T (2011) Cytokinin receptors in sporophytes are essential for male and female functions in Arabidopsis thaliana. Plant Signal Behav 6: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, Offringa R, Graham N, et al. (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E, Roberts CJ, Claes AR, Franks RG, Sundberg E (2014) Polar auxin transport is essential for medial versus lateral tissue specification and vascular-mediated valve outgrowth in Arabidopsis gynoecia. Plant Physiol 166: 1998–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero LE, Uberti-Manassero NG, Arce AL, Colombatti F, Alemano SG, Gonzalez DH (2015) TCP15 modulates cytokinin and auxin responses during gynoecium development in Arabidopsis. Plant J 84: 267–282 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y (2006) Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311: 94–98 [DOI] [PubMed] [Google Scholar]
- Marhavý P, Duclercq J, Weller B, Feraru E, Bielach A, Offringa R, Friml J, Schwechheimer C, Murphy A, Benková E (2014) Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr Biol 24: 1031–1037 [DOI] [PubMed] [Google Scholar]
- Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A (2010) miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22: 1104–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch-Martínez N, Ramos-Cruz D, Irepan Reyes-Olalde J, Lozano-Sotomayor P, Zúñiga-Mayo VM, de Folter S (2012) The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning. Plant J 72: 222–234 [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L, Ostergaard L (2014) Dynamic control of auxin distribution imposes a bilateral-to-radial symmetry switch during gynoecium development. Curr Biol 24: 2743–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JA, Jones A, Godin C, Traas J (2012) Systems analysis of shoot apical meristem growth and development: integrating hormonal and mechanical signaling. Plant Cell 24: 3907–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Feldman LJ, Zambryski PC (2000) Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127: 3877–3888 [DOI] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Soucek P, Reichman P, Hoyerová K, Dubová J, Friml J, Zazímalová E, Hejátko J (2009) Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci USA 106: 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ (2006) A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc Natl Acad Sci USA 103: 11081–11085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Reyes-Olalde JI, Zúñiga-Mayo VM, Serwatowska J, Chavez Montes RA, Lozano-Sotomayor P, Herrera-Ubaldo H, Gonzalez-Aguilera KL, Ballester P, Ripoll JJ, Ezquer I, Paolo D, Heyl A, et al. (2017) The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium. PLoS Genet 13: e1006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J (2013) Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr Biol 23: 2506–2512 [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Šimášková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, Friml J, Van Montagu MC, Benková E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 106: 4284–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27: 44–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C, Gaillochet C, Lohmann JU (2015) Arabidopsis HECATE genes function in phytohormone control during gynoecium development. Development 142: 3343–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC (1997) ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124: 4481–4491 [DOI] [PubMed] [Google Scholar]
- Sessions R. (1997) Arabidopsis (Brassicaceae) flower development and gynoecium patterning in wild type and Ettin mutants. Am J Bot 84: 1179. [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126: 4117–4128 [DOI] [PubMed] [Google Scholar]
- Šimášková M, O’Brien JA, Khan M, van Noorden G, Ötvös K, Vieten A, De Clercq I, van Haperen JM, Cuesta C, Hoyerová K, Vanneste S, Marhavý P, et al. (2015) Cytokinin response factors regulate PIN-FORMED auxin transporters. Nat Commun 6: 8717. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spíchal L, Werner T, Popa I, Riefler M, Schmülling T, Strnad M (2009) The purine derivative PI-55 blocks cytokinin action via receptor inhibition. FEBS J 276: 244–253 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23: 3961–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Yamazaki C, Mitsui M, Kakei Y, Mitani Y, Nakamura A, Ishii T, Soeno K, Shimada Y (2015) Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep 34: 1343–1352 [DOI] [PubMed] [Google Scholar]
- Ursache R, Miyashima S, Chen Q, Vatén A, Nakajima K, Carlsbecker A, Zhao Y, Helariutta Y, Dettmer J (2014) Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development 141: 1250–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Brunoud G, Farcot E, Morin V, van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, Guédon Y, Armitage L, et al. (2011) The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino GH, Hu Q, Manrique S, Flores-Vergara M, Sehra B, Robles L, Brumos J, Stepanova AN, Colombo L, Sundberg E, Heber S, Franks RG (2016) Transcriptomic signature of the SHATTERPROOF2 expression domain reveals the meristematic nature of Arabidopsis gynoecia medial domain. Plant Physiol 171: 42–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Okada K (2003) Two discrete cis elements control the Abaxial side-specific expression of the FILAMENTOUS FLOWER gene in Arabidopsis. Plant Cell 15: 2592–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 108: 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Wu MF, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA, Wagner D (2013) A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell 24: 271–282 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádníková P, Petrásek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, van der Straeten D, Friml J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Zhang W, To JPC, Cheng C-Y, Schaller GE, Kieber JJ (2011) Type-A response regulators are required for proper root apical meristem function through post-transcriptional regulation of PIN auxin efflux carriers. Plant J 68: 1–10 [DOI] [PubMed] [Google Scholar]
- Zhao Y. (2012) Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant 5: 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU (2010) Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092 [DOI] [PubMed] [Google Scholar]
- Zúñiga-Mayo VM, Reyes-Olalde JI, Marsch-Martínez N, de Folter S (2014) Cytokinin treatments affect the apical-basal patterning of the Arabidopsis gynoecium and resemble the effects of polar auxin transport inhibition. Front Plant Sci 5: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher E, Tavor-Deslex D, Lituiev D, Enkerli K, Tarr PT, Müller B (2013) A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol 161: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]