GA induces PCD in maize aleurone layers through down-regulating the HAT activity and thereby indirectly increasing the relative HDAC activity, followed by the ROS-mediated signaling pathway.
Abstract
Recent discoveries have shown that epigenetic regulation is an integral part of phytohormone-mediated processes. The phytohormone gibberellin (GA) triggers a series of events in cereal aleurone cells that lead to programmed cell death (PCD), but the signaling cascade mediating GA-induced PCD in cereal aleurone layers remains largely unknown. Here, we showed that histone deacetylase (HDAC) activity gradually increased relative to histone acetyltransferase (HAT) activity, leading to a global decrease in histone H3 and H4 acetylation levels during PCD of maize (Zea mays) embryoless aleurone layers after 3 d of treatment with GA. HDAC inhibition prevented GA-induced PCD in embryoless aleurone cells, whereas HAT inhibition resulted in PCD even in the absence of GA. Hydrogen peroxide concentrations increased in GA- or HAT inhibitor-treated aleurone cells due to reduced levels of reactive oxygen species scavengers. Hydrogen peroxide-treated aleurone cells showed no changes in the activity or expression of HATs and HDACs. We show that it is possible to predict whether epigenetic modification enzymes serve as a regulator of the GA-triggered PCD signaling pathway in maize aleurone layers. Taken together, these findings reveal that HDAC activity is required for GA-induced PCD in maize aleurone layers and regulates PCD via the reactive oxygen species-mediated signal transduction pathway.
Programmed cell death (PCD) is a physiological cell death process that plays an important role in plant growth and development and in the response to environmental stress. The cereal aleurone layer is the outermost living cell layer of the endosperm, and the metabolism and gene expression of cereal aleurone layers are controlled by the plant hormones gibberellin (GA) and abscisic acid (ABA) (Mozer, 1980; Kuo et al., 1996; Bethke et al., 1999). During seed germination, aleurone cells hydrolyze carbohydrate reserves and secrete hydrolases to the inner starchy endosperm, which is immediately followed by PCD (Fath et al., 2000). The PCD of aleurone cells is elicited by GA that was secreted by the embryo of the germinated seed so that nutrients in the aleurone layers can finally be completely utilized by the developing embryo (Fath et al., 2001a; Domínguez and Cejudo, 2014). Some putative GA-triggered PCD regulators have been identified in cereal aleurone cells (Fath et al., 2001b). The concentration of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anion, and hydroxyl radicals, was increased rapidly in GA-triggered PCD in the cereal aleurone, implicating ROS in the GA-mediated developmental program that culminates in PCD (Schopfer et al., 2001; Fath et al., 2002; Bissenbaev et al., 2007). Cell death has been demonstrated to result from increased H2O2 stimulated by GA in barley (Hordeum vulgare) aleurone layers and protoplasts (Fath et al., 2002). Ca2+ also plays a vital role in regulating the PCD of aleurone layers (Jones, 2001). The finding that treatment with the phosphatase inhibitor okadaic acid prevents the PCD of aleurone cells suggests that protein phosphorylation is involved in aleurone cell death (Lovegrove and Hooley, 2000). However, the signaling pathway controlling GA-induced aleurone PCD remains largely undefined.
Eukaryotic chromatin consists of DNA and histones. The histone tails are subject to various modifications, including methylation, acetylation, phosphorylation, ubiquitination, and ADP-ribosylation (Peterson and Laniel, 2004). Among these chromatin-associated marks, the balance between histone acetylation and deacetylation has been shown to play an important role in regulating gene expression and is involved in many biological processes (Berger, 2002, 2007; Karlić et al., 2010). Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are two key enzymes that are responsible for establishing and maintaining the state of histone acetylation across the genome (Kuo and Allis, 1998; Strahl and Allis, 2000; Yang and Seto, 2007). Histone acetylation often is associated with gene transcription, whereas histone hypoacetylation typically marks silenced genes (Grunstein, 1997; Turner, 2000). Altered expression of HAT or HDAC genes has been linked to many regulatory processes, such as rapid responses to internal or external signals, changes in cell proliferation, the switch from vegetative to reproductive growth, and PCD (Kouzarides, 1999; Pawlak and Deckert, 2007).
Aleurone cell death is a typical PCD process in plants, which is thought to be an excellent model system in which to explore the molecular mechanisms involved in PCD (Buckner et al., 1998). In this study, we showed that the relative activity of HDACs to HATs was increased in maize (Zea mays) embryoless aleurone layers after treatment with GA for 3 d. HDAC inhibition blocked GA-triggered PCD, whereas HAT inhibition induced the developmental program of embryoless aleurones, leading to PCD in the absence of GA, which suggests that HDACs are regulated by GA during aleurone PCD and are a necessary regulator in the signaling cascade of GA-initiated PCD of the maize aleurone layer. Furthermore, we found that HAT inhibition induced an increase in H2O2 content, leading to the PCD of aleurone cells. In contrast, HDAC inhibition did not cause the accumulation of H2O2, even in the presence of GA, and no PCD occurred. We concluded that GA induces PCD in maize aleurone layers through the regulation of HDACs, followed by the ROS-mediated signaling pathway.
RESULTS
GA Induces PCD in the Maize Embryoless Aleurone Layers
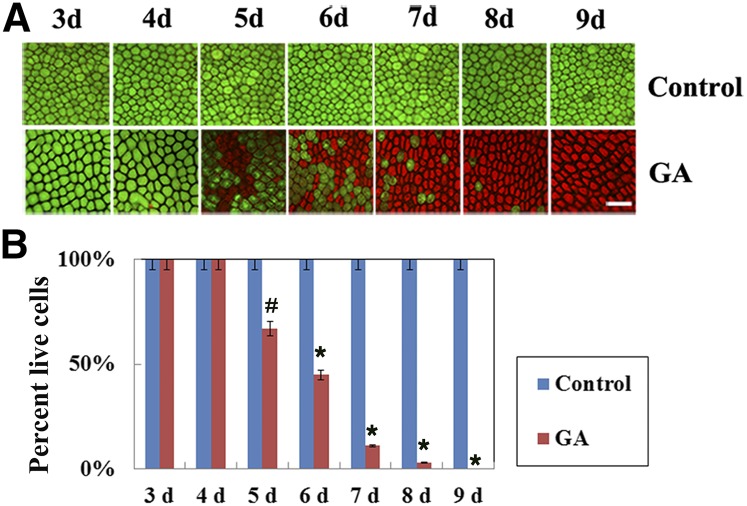
Cereal aleurone cells have been shown to undergo PCD regulated by plant hormones (Bethke et al., 1999). During seed germination, plant aleurone layers undergo PCD in response to GA produced by the seed embryo, and this effect is prevented by ABA (Fath et al., 2000). In this study, we first investigated PCD in maize embryoless aleurone layers after GA or ABA treatment. Fluorescein diacetate (FDA) is a live-cell stain that is taken up by living aleurone cells and then hydrolyzed to yield green fluorescein (Schwille et al., 1999). Propidium iodide (PI) is a red fluorescent dye that accumulates rapidly in dead cells and often is applied to identify dead cells in a population (Ergen et al., 2013). Thus, we monitored the viability and death of aleurone cells in an intact, dissected maize aleurone layer by FDA/PI staining. Embryoless aleurone layers were isolated from maize seeds as described by Hou et al. (2015). The prepared aleurone layers were treated with or without different reagents and cultured for the indicated times. FDA/PI staining showed that the number of green (e.g. living) cells decreased gradually, whereas the number of red (e.g. dead) cells increased gradually in aleurone layers, and half of the cells were dead by 6 d after GA treatment (Fig. 1A). When aleurone layers were cultured for 9 d, almost no living cells remained in the treated aleurones, whereas the untreated aleurone cells were all alive (Fig. 1A). In contrast, no dead cells were observed when aleurone layers were treated with ABA for up to 9 d (Supplemental Fig. S1). The number of living and dead cells at the indicated time points was counted and statistically analyzed (Fig. 1B). These results were similar to the PCD process of barley aleurone protoplasts treated with the phytohormone GA (Bethke et al., 1999) and the time course of GA-induced PCD in barley aleurone layers (Fath et al., 2001a).
Figure 1.
Analysis of the PCD time course in maize aleurone layers treated with GA by staining with FDA in combination with PI. A, Representative images from aleurone layers incubated with GA from 3 to 9 d and visualized by epifluorescence microscopy. Live cells appear green, and dead cells appear red. Bar = 100 μm. B, Quantification of viability and death in GA-treated maize aleurone layers. Control, Distilled, deionized water. Each assay was repeated three times for every sample in three independent experiments. Error bars indicate the se in three independent experiments (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
After aleurone layer cell PCD was characterized by FAD/PI staining, next, we wanted to identify the PCD at the molecular level. Hexokinase inhibits PCD, and caspase-like proteins are vital marks of PCD (Kim et al., 2006). To investigate how the transcription of these genes changes during phytohormone-induced PCD, two caspase-like protein genes, metacaspase type II (gene identifier 100285605) and putative metacaspase (gene identifier 100191541) (Ahmad et al., 2012), and three PCD inhibition genes, hexokinase (gene identifier 100192075), fdad1 (gene identifier 541686), and ABP9 (GenBank accession no. GU237073) (Zhang et al., 2011; Repka et al., 2015), were selected for analysis in this study. Quantitative real-time PCR analysis revealed that the expression of two metacaspase genes was increased at the late stage of GA-induced PCD, and the expression of three PCD inhibition genes increased initially, which then decreased at the stage of GA-induced PCD (Supplemental Fig. S2), indicating that these genes also may regulate aleurone cell death.
Histones Are Deacetylated during GA-Induced PCD in Maize Aleurone Layers
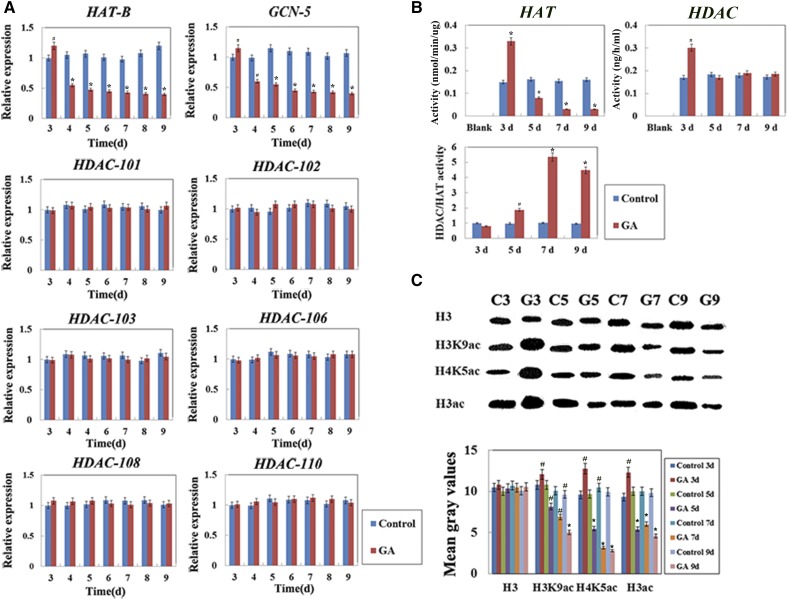
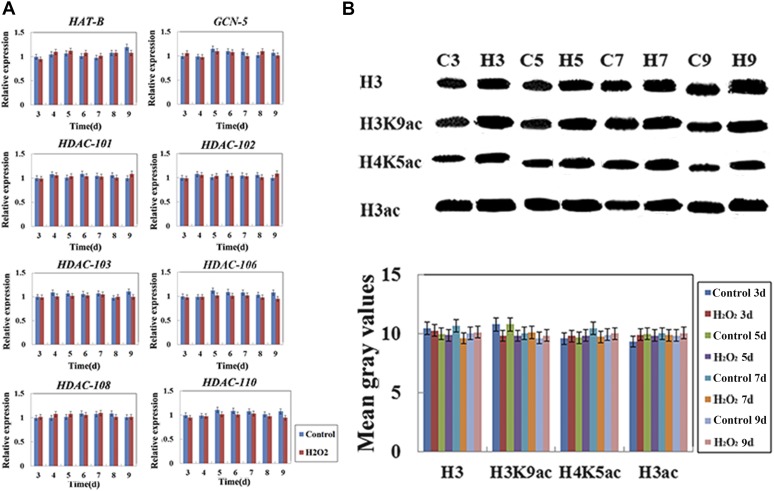
Previous studies have shown that epigenomes are involved in the PCD induced by heat stress in maize seedling leaves (Wang et al., 2015). To analyze whether and how histone modification enzymes are involved in the GA-induced PCD process of maize aleurone cells, we examined the expression and activity of histone modification enzymes. Histone acetylation is regulated by HATs and HDACs. Fifteen kinds of deacetylases have been identified in maize, including 10 RPD3/HDA1 proteins, one SIR2 protein, and four HD2 proteins (Demetriou et al., 2009). RPD3/HDA1 family proteins are closely related to plant development, and HD2 family proteins are shown to be associated with plant tolerance to environmental stress in barley (Zhou et al., 2005; Demetriou et al., 2009). To determine the expression levels of HATs and HDACs, two HAT genes (GCN5 and HAT-B) and six HDAC genes (HDAC101, HDAC102, HDAC103, HDAC106, HDAC108, and HDAC110) were analyzed in this study. GCN5 and HAT-B genes were selected from two types of HATs (HAT-A and HAT-B), and six HDAC genes were chosen from two types of HDACs (RPD3-like and HD2-like). The results showed that histone acetylation-related gene expression decreased significantly, while histone deacetylation-related gene expression remained stable, in aleurone layers after treatment for 3 d with GA (Fig. 2A; Supplemental Fig. S3). HAT activity also decreased, whereas HDAC activity remained unchanged after GA treatment for 3 d, leading to an increase in the relative HDAC activity within the cell (Fig. 2B). Furthermore, to examine the relative activity of histone modification enzymes, the histone modification state of chromatin was detected by western blotting using antibodies against H3K9ac, H4K5ac, and H3ac, which are three euchromatin markers. The results showed that the total levels of histone H3K9, H4K5, and H3 acetylation decreased significantly during the PCD process after GA treatment for 3 d (Fig. 2C). These results suggested that HDAC or histone deacetylation was involved in the GA-induced PCD process of maize aleurones. Histone acetylation-related gene expression remained stable, while histone deacetylation-related gene expression decreased significantly, in ABA-treated aleurone layers (Supplemental Fig. S4A). After ABA treatment, the relative HAT activity was increased, accompanied by elevated total levels of histone H3K9, H4K5, and H3 acetylation (Supplemental Fig. S4, B and C).
Figure 2.
GA affected H3K9ac, H4K5ac, and H3ac protein levels in the aleurone layers. A, Transcript levels of histone acetylation-related genes. The transcript levels of all genes were measured by quantitative real-time PCR using mRNA prepared from normal and treated maize aleurone layers. The x axis indicates time, and the y axis indicates relative expression values. The relative expression value of the control group (3 d) was defined as 1. The ubiquitin gene was used as an internal control. B, HAT and HDAC enzyme activities after histone modification enzyme inhibitor treatment in aleurone layers. C, Antibodies specific to H3K9ac, H4K5ac, and H3ac were employed to analyze histone extracts from aleurone layers treated with or without GA for 3, 5, 7, and 9 d. Control, Distilled, deionized water; C, control; G, GA. Quantitative analysis of western blotting was performed using ImageJ. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
HDACs Regulate the PCD Process of Maize Aleurone Layers
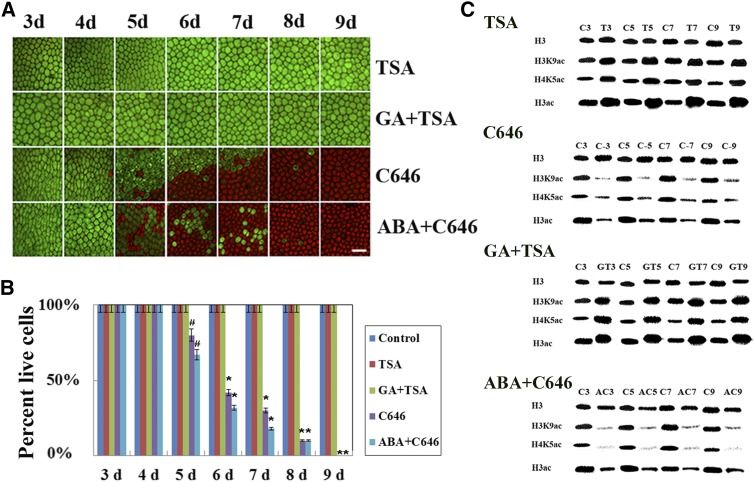
Histone acetylation/deacetylation is important for both animal and plant physiology and development (Marks et al., 2001; Shahbazian and Grunstein, 2007). Trichostatin A (TSA), CUDC-101, C646, and anacardic acid (AA) are four widely used epigenetic enzyme inhibitors with clear functional mechanisms (Wang et al., 2016). To investigate how these epigenetic enzymes affected the hormone-induced PCD process in maize aleurone layers, we further treated maize aleurone layers with the HDAC inhibitor TSA and the HAT inhibitor C646. Almost all cells were still alive after treatment with TSA for up to 9 d, even in the presence of GA (Fig. 3, A and B). In contrast, the cells in C646-treated aleurone layers started to die after 5 d, and all cells died after 8 d of incubation. Even ABA could not prevent PCD induced by C646 (Fig. 3, A and B). We also performed western blotting using antibodies against H3K9ac, H4K5ac, and H3ac. As expected, the total levels of histone H3K9, H4K5, and H3 acetylation increased significantly after treatment with TSA or TSA plus GA (Fig. 3C). However, the total levels of histone H3K9, H4K5, and H3 acetylation remained lower in live cells treated with C646 or C646 plus ABA (Fig. 3C). These results indicated that histone deacetylase is necessary for the GA-mediated PCD process of maize aleurone layers. To exclude the possibility that drug treatment nonspecifically induced PCD, we treated maize seedlings with another histone deacetylase inhibitor, CUDC-101, and another HAT inhibitor, AA, and observed the same effects (Supplemental Fig. S5). C646 or AA treatment decreased HAT activity but had no effect on HDAC activity, similar to GA treatment (Supplemental Fig. S6). In contrast, HDAC activity was decreased, whereas HAT activity remained unchanged, after TSA, CUDC-101, or ABA treatment (Supplemental Fig. S6). We also found that treatment with DMSO or water showed no difference in gene expression levels and enzyme activity (Supplemental Fig. S7).
Figure 3.
Time course of PCD in maize aleurone layers determined by staining with FDA and PI. A, Representative images from aleurone layers incubated in histone deacetylation inhibitors (TSA), HAT inhibitors (C646), TSA + GA, and C646 + ABA from 3 to 9 d and visualized by epifluorescence microscopy. Live cells appear green, and dead cells appear red. Bar = 100 μm. B, Quantification of viability and death in different treated maize aleurone layers. C, Histone acetylation inhibitors affect H3K9ac, H4K5ac, and H3ac protein levels in the aleurone layers. Antibodies specific to H3K9ac, H4K5ac, and H3ac were employed to analyze histone extracts from aleurone layers treated with or without histone acetylation inhibitors for 3, 5, 7, and 9 d. Control, 1 μm dimethyl sulfoxide (DMSO); C, control; T, TSA; GT, GA + TSA; C-, C648; AC, ABA + C656. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
HDAC Is Located Upstream of the ROS Signaling in the GA-Mediated PCD Pathway
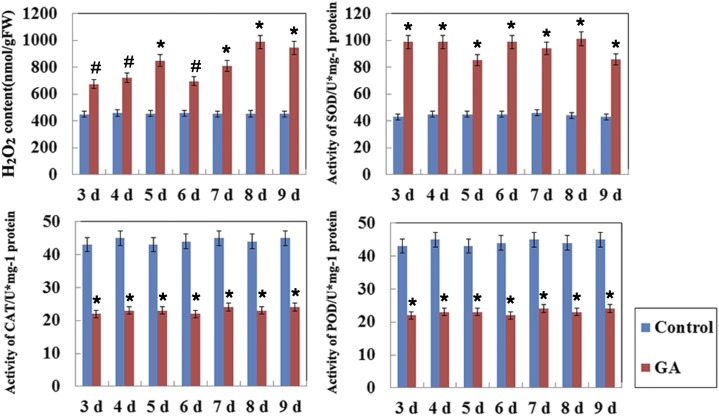
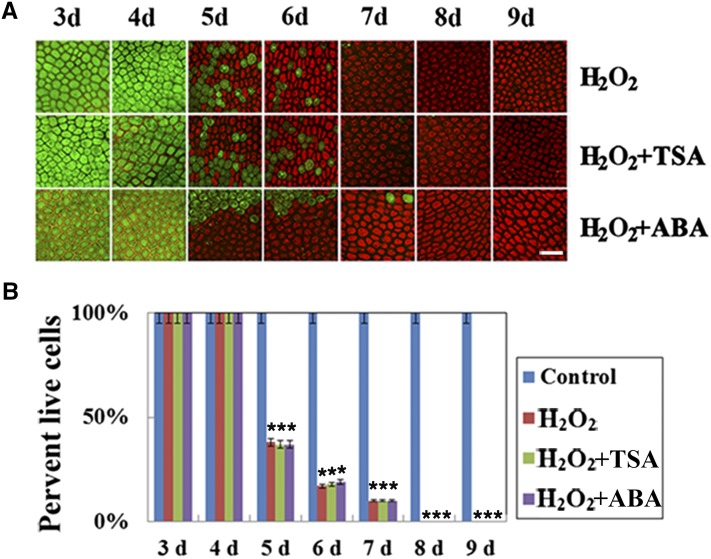
ROS such as H2O2 have been demonstrated to be key players in stress and PCD signaling pathways (Wang et al., 2015). Thus, we measured the concentrations of H2O2 and the activities of ROS-related enzymes, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), in maize aleurone layers during the GA-induced PCD process. The results showed that H2O2 accumulated and remained at a high level during the GA-induced PCD process, whereas H2O2 levels remained relatively low in the live cells of the untreated maize aleurone layers (Fig. 4). GA-treated maize aleurone layers showed increased SOD activity and decreased CAT and POD activity during PCD (Fig. 4). Because SOD is responsible for producing H2O2, while POD and CAT catalyze the decomposition of H2O2, our results indicated that GA initiated the PCD process in maize aleurone layers by modulating ROS-related enzymes to produce H2O2. We also examined cell death and viability in H2O2-treated maize aleurone layers. H2O2-treated aleurone cells showed a decrease in the number of living cells and an increase in the number of dead cells (Fig. 5A). The extent of cell death at several time points was quantified by counting the number of living and dead cells. Virtually all cells in the H2O2-treated aleurone layers died after 8 d of incubation (Fig. 5B). Treatment with ROS scavengers (thiourea or butyl hydroxyanisole) plus GA can delay the PCD process in aleurone layers, showing that H2O2 also is necessary for aleurone PCD (Supplemental Fig. S8). These results indicated that ROS and ROS-related enzymes regulated the PCD process of maize aleurone layers.
Figure 4.
Concentration of ROS-related molecules and enzyme activities after GA treatment in aleurone layers. Each assay was repeated three times for every sample in three independent experiments. Control, Distilled, deionized water; FW, fresh weight. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
Figure 5.
Time course of PCD in maize aleurone layers treated with H2O2 or H2O2 plus TSA or ABA determined by staining with FDA and PI. A, Representative images from aleurone layers incubated with H2O2, H2O2 + TSA, and H2O2 + ABA from 3 to 9 d and visualized by epifluorescence microscopy. Live cells appear green, and dead cells appear red. Bar = 100 μm. B, Quantification of viability and death in different treated maize aleurone layers. Control, 1 μm DMSO. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). *, P < 0.01 versus the control group according to Student’s t test.
As described above, histone modification enzyme inhibitors and H2O2 both affected the GA-mediated PCD process of maize aleurone layers; hence, we decided to explore their effects on a series of events triggered by GA. Interestingly, when aleurone layers were treated simultaneously with H2O2 and epigenetic enzyme inhibitors, the number of living cells decreased and the number of dead cells increased, as with treatment with H2O2 alone, regardless of HDAC or HAT inhibition (Fig. 5). We further measured the concentrations of ROS and the activities of the aforementioned ROS-related enzymes in maize aleurone layers treated with histone modification enzyme inhibitors during the PCD process. Our results showed an increase in SOD activity and a decrease in CAT and POD activity in dissected aleurone layers treated with HAT inhibitors (C646 or AA; Supplemental Fig. S9). H2O2 also accumulated and remained elevated as a result of the high level of histone acetylation after HAT inhibitor (C646 or AA) treatment during the PCD process, whereas H2O2 levels remained relatively low in the live aleurone layers after HDAC inhibitor (TSA or CUDC-101) treatment (Supplemental Fig. S9). Meanwhile, we monitored cell death and viability in aleurone cells in maize aleurone layers treated with H2O2 plus HDAC inhibitor (TSA or CUDC-101) or ABA. Aleurone cells showed a decrease in the number of living cells and an increase in the number of dead cells, just as with H2O2 alone, although HDAC inhibition and ABA each inhibited cell death in maize aleurone layers (Fig. 5; Supplemental Fig. S10, A and B). Quantitative real-time PCR analysis revealed that the expression of eight histone-modifying enzyme genes was not changed after H2O2 treatment (Fig. 6A). Western blotting showed that the total acetylation levels of histones H3K9, H4K5, and H3 were unchanged under H2O2 treatment (Fig. 6B). These data showed that histone deacetylase regulated the GA-mediated PCD by modulating ROS production.
Figure 6.
A, Transcript levels of histone modification-related enzyme genes after treatment with H2O2. The transcript levels of all genes were measured by quantitative real-time PCR using mRNA prepared from normal and H2O2-treated maize aleurone layers. The x axis indicates time, and the y axis indicates relative expression values. The relative expression value of the control group (3 d) was defined as 1. The ubiquitin gene was used as an internal control. B, H2O2 did not affect H3K9ac, H4K5ac, and H3ac protein levels in the aleurone layers. Antibodies specific to H3K9ac, H4K5ac, and H3ac were employed to analyze histone extracts from aleurone layers treated with or without H2O2 for 3, 5, 7, and 9 d. Control, Distilled, deionized water; C, control; H, H2O2. Quantitative analysis of western blotting was performed using ImageJ. Error bars represent se (n = 3). No significant differences was observed, as compared with the Control group according to Student’s t test.
GA Regulates the PCD Process in Maize Aleurone Layers via HDAC-Mediated Changes in ROS-Related Gene Expression
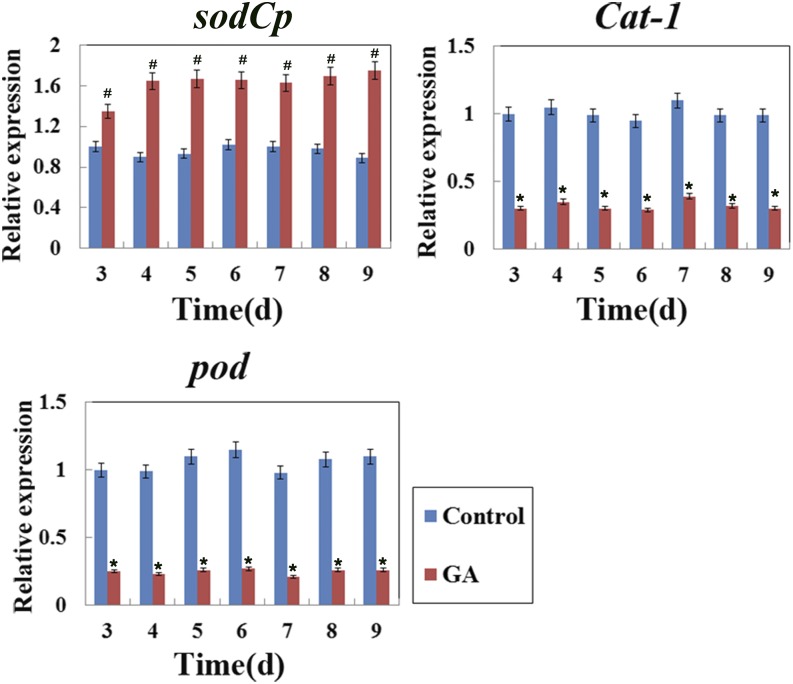
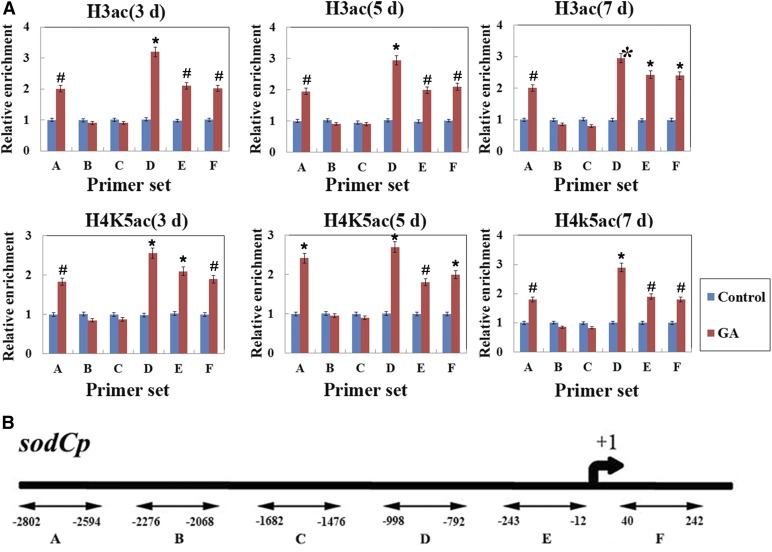
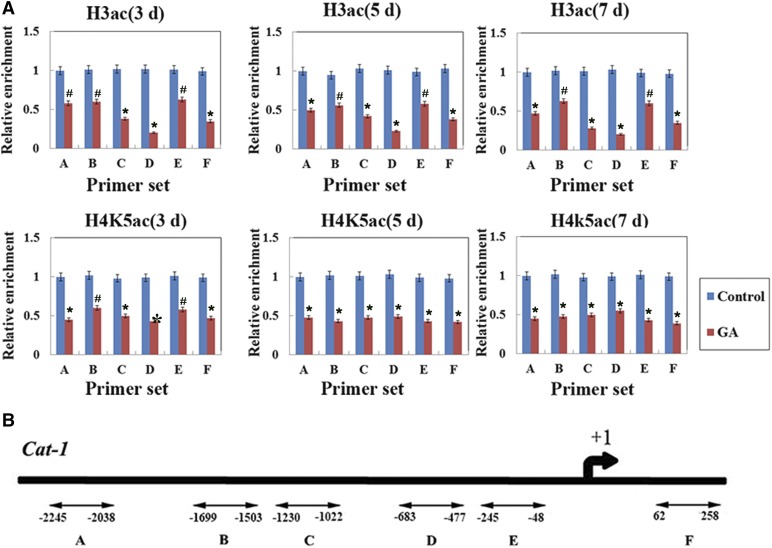
HATs/HDACs have been shown to regulate gene expression by being recruited to target genes and altering histone acetylation (Kurdistani and Grunstein, 2003; Minucci and Pelicci, 2006). In plants, a number of ROS-related genes were found to be involved in the regulation of PCD (Wang et al., 2009), and our previous results showed that histone acetylation was involved in GA-regulated sodCp gene expression in maize aleurone layers during 3 d of germination (Hou et al., 2015). First, we analyzed the expression of these genes during the GA-regulated PCD in maize aleurones. Dissected aleurone layers were cultured with or without GA, and RNA was then prepared at each time point. Quantitative real-time PCR results indicated that sodCp gene expression was up-regulated, whereas the Cat-1 and Pod genes were down-regulated, in GA-treated maize aleurone layers from 3 to 9 d (Fig. 7). Next, we performed chromatin immunoprecipitation to compare H4K5 and H3 acetylation levels at the promoter regions of these ROS-related genes in aleurone layers with or without GA treatment. Our results showed that hyperacetylation occurred in the promoter regions (regions A, D, and E) and the first exon (region F) of the sodCp gene in GA-treated aleurone layers. However, there was no significant decrease in regions B and C after GA treatment compared with untreated aleurone layers (Fig. 8A). GA-treated aleurone layers showed relatively low acetylation in the promoter regions and an exon region of the Cat-1 gene, corresponding to the reduced expression of this gene (Fig. 9A).
Figure 7.
Quantitative real-time PCR analysis of ROS-related genes at different time points in maize aleurone layers during the PCD process with or without GA. The relative expression value of the control group (3 d) was defined as 1. The ubiquitin gene was used as an internal control. Control, Distilled, deionized water. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
Figure 8.
Chromatin immunoprecipitation (ChIP) results of sodCp. A, H3ac and H4K5ac levels in the promoter regions (sets A–E) and the exon (set F) of the sodCp gene after GA treatment in maize aleurone layers during the PCD process. The x axis indicates time, and the y axis indicates relative expression values. The relative expression value of the control group (set A) was defined as 1. The ubiquitin gene was used as an internal control. B, Schematic representation of the sodCp gene from −2,802 to +242 bp. Control, Distilled, deionized water. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
Figure 9.
ChIP results of Cat-1. A, H3ac and H4K5ac levels in the promoter regions (sets A–E) and the exon (set F) of the Cat-1 gene after GA treatment in maize aleurone layers during the PCD process. The x axis indicates time, and the y axis indicates relative expression values. The relative expression value of the control group (set A) was defined as 1. The ubiquitin gene was used as an internal control. B, Schematic representation of the Cat-1 gene from −2,245 to +258 bp. Control, Distilled, deionized water. Each assay was repeated three times for every sample in three independent experiments. Error bars represent se (n = 3). #, P < 0.05 and *, P < 0.01 versus the control group according to Student’s t test.
DISCUSSION
HDACs Are Necessary for Inducing the GA-Initiated PCD of Maize Aleurone Layers
Cereal aleurone cells are known to undergo PCD initiated by the phytohormone GA during seed germination. The embryos of germinated grains produce GA, and GA is transferred to aleurone cells, which do not synthesize GA themselves. GA has been reported to promote cell death in barley aleurone protoplasts and aleurone layers (Bethke et al., 1999). Phytohormones have been shown to regulate plant growth and development, as well as responses to exogenous stress, by mediating epigenetic processes (Chinnusamy et al., 2008). Many studies have indicated that the epigenome is involved in many cellular functions. HDAC inhibitors induce growth arrest, cell differentiation, and cell apoptosis, while HAT inhibitors also have been shown to inhibit proliferation and induce apoptosis in cancer cells (Marks et al., 2000, 2001; Landreville et al., 2012). Simultaneous FDA and PI staining showed that maize embryoless aleurone cells underwent PCD induced by GA, which was characterized by the up-regulation of PCD-related gene expression. Following GA treatment, a rapid increase in the relative activity of HDACs occurs during the developmental stage at which cells show typical, apoptosis-like morphological features, suggesting that GA elevated the relative activity of HDACs by inhibiting HATs, leading to global deacetylation of histones H3 and H4, and that HDACs were involved in the GA-triggered PCD of aleurone layers. In the presence of HDAC inhibitors, aleurone cell death was blocked, and the lifespan of the cells was extended.
The ability of HAT inhibition to induce aleurone cell death in the absence of GA was due to a change in the HDAC/HAT balance, which led to higher HDAC activity relative to HAT activity, as confirmed by global deacetylation of the genome. Collectively, these data indicated that HDACs were required for GA to induce PCD in aleurone layers. Maize HDACs included 10 maize RPD3/HDA1 genes, one SIR2 gene, and four HD2-like genes (Demetriou et al., 2009). RPD3-type HDAC expression is required for plant development, and HD2 genes were found to be associated with plant resistance to plant biotic and abiotic stress (Zhou et al., 2005). Thus, precisely which of the multiple HDACs is involved needs to be investigated in the future. Similarly, HDACs have been shown to act as a positive signaling molecule that selectively mediates cold-responsive gene expression in plants (Miura and Furumoto, 2013). In fact, the balance of histone acetylation and deacetylation plays a critical role in regulating gene expression, and the altered expression of HDAC genes has been linked to tumor development (Ropero and Esteller, 2007). Global histone deacetylation is always associated with the condensed chromatin that is a cytological feature of PCD. Thus, reduced acetylation might be implicated in the cytological changes of aleurone cell death. DNA methylation and histone monoubiquitination have been shown to play an important role in regulating PCD in tapetum (Solís et al., 2014; Cao et al., 2015).
HDACs Cause PCD of Maize Aleurone Layers by Increasing the Content of H2O2 in Aleurone Cells
H2O2 has been suggested to act as a signal molecule in regulating PCD in aleurone cells (Gechev and Hille, 2005). Epigenetic modifications occur in response to exogenous oxidative stress, and thus, it is also likely that ROS, as signaling molecules, are implicated in epigenetic modulation (Rahman and Adcock, 2006; Franco et al., 2008). However, ABA and HDAC inhibition prevented an increase in H2O2 concentrations, whereas GA and HAT inhibition provoked epigenetic modification changes and elevated H2O2, suggesting that histone acetylation/deacetylation regulated the expression of ROS-related genes, resulting in changes in H2O2 levels. Several enzymatic systems may contribute to this overproduction of H2O2. In contrast, the addition of H2O2 induced PCD in aleurone layers in the absence of GA and regardless of HDAC or HAT inhibition, which indicated that HDACs are located upstream of H2O2 in the GA-triggered signaling cascade. The epigenome also has been shown to play a role in heat stress-induced apoptosis by inducing ROS production (Brunet et al., 2004). Cat-1 gene transcription was down-regulated because of hypoacetylation of the promoters. Our previous ChIP data showed a higher acetylation level of H3K9 and H4K5 at the promoter regions of the sodCp gene in aleurone layers treated with GA for 24 and 48 h, which is associated with the high transcription level of the sodCp gene in aleurone layers during seed germination or in GA-treated aleurone layers (Hou et al., 2015). Histone acetylation levels started to decrease after treatment with GA for 3 d due to a gradual increase in relative HDAC/HAT activity, but the acetylation level of the sodCp gene decreased slightly after GA treatment, and the sodCp gene expression level still was higher.
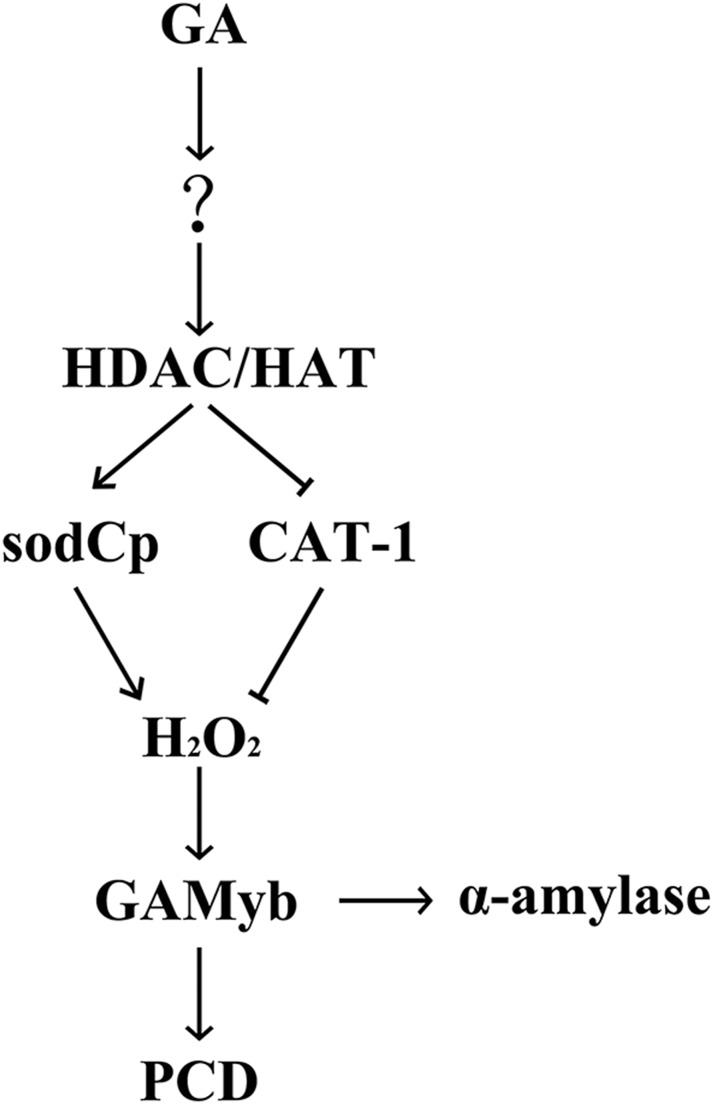
Actually, dual functions of HDACs have been reported in plants and animals (Robert et al., 2004; Tanaka et al., 2008). HDACs have been demonstrated to repress gene expression by the removal of acetyl groups from histones (Peterson and Laniel, 2004). However, recent studies have identified genes that also need HDACs for efficient activation via different mechanisms. For example, HDACs may induce proinflammatory gene expression by enabling transcription factor recruitment (Bode et al., 2007). HDACs regulate cold-tolerance gene expression by preventing the binding of transcriptional repressors (Zhu et al., 2008). Rpd3p up-regulates telomeric genes in yeast through the deacetylation of histones that inhibited the binding of SIR repressors (Robert et al., 2004). Thus, based on these data and previous results, we propose a model of HDAC function as shown in Figure 10. HDACs are integral regulators of GA-induced PCD in maize aleurone layers, wherein GA first induces the relative activity of HDACs/HATs through an unknown mechanism, which then activates or suppresses the expression of downstream ROS-related genes that, in turn, regulate H2O2 production (Hou et al., 2015). H2O2 might regulate the induction of the transcription factor GAMyb and then promote the expression of the α-amylase gene (Ishibashi et al., 2012) and PCD of aleurone cells (Guo and David, 2008). There exists another GA signaling pathway that activates GAMyb through the degradation of a repressor SLN1 (Guo and David, 2008; Aoki et al., 2014).
Figure 10.
Model for the role of local histone acetylation in ROS signaling in maize aleurone cells during PCD.
CONCLUSION
The main goal of this work was to analyze the possible relationship between the epigenome and PCD in aleurone layers. For this purpose, the dynamics of the epigenome during the PCD of aleurone layers was characterized by a multidisciplinary approach. The results showed that the relative activity of HDACs was increased in GA-treated aleurone cells, leading to PCD. HDAC inhibition suppressed the induction of PCD in the presence of GA, whereas embryoless aleurone layers underwent PCD following HAT inhibition, which resulted in the deacetylation of chromatin. Furthermore, treating dissected aleurones with GA or HAT inhibitors increased H2O2 levels, whereas exogenous H2O2 did not affect HAT or HDAC expression. These data identified HDACs as key players that lie between GA and H2O2 in the GA-mediated signal transduction cascade leading to PCD in maize aleurone layers (Fig. 10).
MATERIALS AND METHODS
Plant Materials and Chemicals
Seedlings of the maize (Zea mays) hybrid line Huayu5 were grown on Murashige and Skoog medium under controlled conditions: 25°C, dark regime, and 70% humidity. Embryoless aleurone layers were isolated from intact maize seeds as described by Hou et al. (2015). TSA, CUDC-101, C646, and AA are four widely used epigenetic enzyme inhibitors with clear functional mechanisms, which were produced by Selleck Chemicals. TSA is an HDAC inhibitor (purity, 99.03%). CUDC-101 is a specific inhibitor of the type I and type II HDAC family that cannot inhibit the third class of HDACs. CUDC-101 also has a weak inhibitory activity against other protein kinases, including KDR/VEGFR2, Lyn, Lck, Abl-1, FGFR-2, Flt-3, and Ret (purity, 99.36%; Wang et al., 2016). C646 is a HAT inhibitor that can lower histone H3 and H4 acetylation levels in cells and also can abolish TSA-induced acetylation (purity, 99.66%; Kurita et al., 2017). AA is found to be a HAT inhibitor (purity, 99.36%; Sukumari-Ramesh et al., 2011). These inhibitors were dissolved in DMSO to a concentration of 1 μm. Thiourea and butyl hydroxyanisole are two ROS scavengers. Isolated aleurone layers were collected and processed in various assays. Aleurone layers were treated with distilled water or DMSO and different reagents: 100 μm GA, 10 μm ABA, 10 μm TSA, 10 μm CUDC-101, 10 μm C646, 10 μm AA, and 10 μm H2O2. Since H2O2 is not stable and will decompose instantly, the separated aleurone layers were treated in gauze soaked with 10 μm H2O2 and the aleurone layers were transferred to a new gauze with 10 μm H2O2 every day.
FDA/PI Staining
Aleurone layers were washed with phosphate buffer (0.04575 mol L−1 Na2HPO4 and 0.00425 mol L−1 NaH2PO4, pH 7.8) and mounted on microscope slides. Cell death and viability in maize aleurone layers were determined by simultaneous staining with FDA (100 μg mL−1) and PI (60 μg mL−1) for 15 min. Aleurone cells were observed on an Olympus BX-60 fluorescence microscope, and fluorescence images were captured with a Sensys 1401E monochrome CCD camera using MetaMorph 4.6.3 software (Universal Imaging). Randomly selected fields from at least three different aleurone layers were counted per treatment to determine the percentage of live cells.
Antibodies
A variety of antibodies were used for western blotting. Anti-H3K9ac (07-352), anti-H4K5ac (07-327), and anti-H3ac (06-599) antibodies were produced by Millipore. Anti-H3 (ab1791) and AP-conjugated goat anti-rabbit IgG (A4187) antibodies were produced by Abcam. Anti-H3ac antibody recognizes histone H3 acetylation at the N terminus, anti-H3K9ac antibody recognizes histone H3 acetylated on Lys-9, and anti-H4K5ac antibody recognizes histone H4 acetylated on Lys-5. These antibodies have been shown to work for ChIP using chromatin from different cells.
Western Blotting
Proteins were isolated by grinding maize aleurone layers in liquid nitrogen and resuspended in extraction buffer (100 mm Tris-HCl, pH 7.4, 50 mm NaCl, 5 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride). The supernatant after centrifugation was collected for the experiment. Western blotting was carried out as described previously (Nieswandt et al., 2000). Histone H3 was used as a loading control. Three independent experiments were performed for each sample.
Quantitative Real-Time PCR
According to the reported amplification condition and method (Hou et al., 2015), quantitative real-time PCR of total RNA isolated from aleurone layer samples was carried out using the SYBR Green Real-Time PCR Master Mix (Toyobo) on a StepOne Plus real-time PCR system (Applied Biosystems). Relative expression levels were normalized to the maize ubiquitin gene. Quantitative real-time PCR primers that were designed to amplify fragments of ∼200 bp are listed in Table I. Real-time PCR was repeated three times for each sample in three independent experiments.
Table I. Primers used for quantitative real-time PCR.
| Primer | Sequence (5′–3′) | Efficiency |
|---|---|---|
| ZmUbiquitin | ATCTTTGTGAAGACCCTCAC | 98% |
| CCTAAGGCGCAGCACCAAGT | ||
| Hexokinase | AATGCCGAGGACCGTAGTTG | 101% |
| GTCATCAACTACCTGGGCAC | ||
| Metacaspase typeII | GGCATCAACTACCTGGGCAC | 98% |
| GCCAACATCCGGCTGGAGCT | ||
| Putative metacaspase | CTCCTCTTCTTCCACTACAG | 96% |
| GGCTGTCTCTTTACCATAGT | ||
| GCN5 | GGACGGCTGAAGTTTCTCTG | 97% |
| GCTTGCATAAGGGCGATAAG | ||
| HAT-B | CAGCTGACCTGATGGAGACT | 96% |
| TTGGCATCTGCAACAGACGC | ||
| HDAC101 | GCCATACTCGAGCTGCTCAA | 102% |
| TAGAGGGACATTCAGGGAGT | ||
| HDAC102 | TCATTGGCGAGGGATCGTTT | 99% |
| TCTCATTTGGGAGTTCTGTG | ||
| HDAC106 | AGATAGTTCCGATGAAGAGC | 104% |
| GAGTCATCTTCCTCAGACAT | ||
| HDAC108 | GCTTTAACGTCGGTGAGGAC | 96% |
| GGCGAGGACGATGTCGTTGA | ||
| ZmsodCp | ATAACAACGCAGCACAGGTG | 101% |
| CGTGCTCCCACAAGTCTAGG | ||
| ZmCat-1 | GAACAACTTCAAGCAGCCCG | 98% |
| CGTTCAGTTCAGCGAGCAAA |
HAT and HDAC Activity Assay
The nuclei were isolated using a nuclear extraction kit (Epigenetic; base catalog no. 00021). HAT activity was determined using the HAT Activity Colorimetric Assay Kit (BioVision; catalog no. K332-100). The nuclear extracts isolated from aleurone layers were assayed on a 96-well plate. For background reading, add 10 μL of water into the sample instead of HAT substrates I and II; for a positive control, add 10 μL of the cell nuclear extract and 30 μL of water. Add assay mix to each well to start the reaction. Plates were incubated at 37°C for 1 to 4 h depending on the color development, and the absorbance was read on a microplate reader at 440 nm. HDAC activity was determined using the EpiQuik HDAC Activity/Inhibition Assay Kit (Epigenetic; base catalog no. P-4002). Nuclear extracts were added to each strip well, except the wells for the control and blank. Strip wells were mixed, covered, and incubated at 37°C for 45 to 60 min. For the control and standard curves, add HDAC assay buffer instead of nuclear extract. For HDAC inhibition, add different amounts of inhibitors. For the blank, add HDAC assay buffer into the blank wells. The capture antibody was added to each strip well and incubated at room temperature for 60 min. Then the detection antibody was added to each strip well and incubated at room temperature for 25 to 30 min. The absorbance could be read on a microplate reader at 450 nm within 2 to 15 min.
H2O2 Measurements
H2O2 concentrations were measured according to the method described by Schumb et al. (1955). Briefly, crude extracts from aleurone layers were extracted with acetone and reacted with 5% (w/v) titanium sulfate to form a yellow precipitate that then was dissolved in H2SO4. Finally, extracts were analyzed at 415 nm.
ROS-Related Enzyme Assays
Aleurone layers were mixed with quartz sand and precooled phosphate buffer (0.04575 mol L−1 Na2HPO4 and 0.00425 mol L−1 NaH2PO4, pH 7.8) and then homogenized on ice and centrifuged at 4°C and 12,000g for 20 min. The supernatant enzyme solution was used for the following experiments.
SOD was assayed according to the method reported by Beauchamp and Fridovich (1971). POD was measured using a published procedure (Gajewska and Skłodowska, 2007). CAT assays were carried out following the reported method (Chance and Maehly, 1955).
ChIP-PCR
ChIP-PCR of the promoter region (regions A–E) and the exon region of the sodCp gene and the Cat-1 gene was carried out using H3ac and H4K5ac antibodies following the procedure reported by Zhang et al. (2011) and Haring et al. (2007). The primer sets used in this test are listed in Table II. A negative control was performed using rabbit serum for mock immunoprecipitation. Briefly, aleurone layers were ground into powder in liquid nitrogen. Chromatin after digestion with 50 units of micrococcal nuclease was combined with salmon sperm DNA/protein A agarose (Upstate) and then incubated with H3ac and H4K5ac antibodies overnight at 4°C. Chromatin-antibody complexes were collected on protein A agarose beads (Upstate), and then chromatin was eluted and reverse cross-linked. DNA was recovered by phenol/chloroform extraction and ethanol precipitation. Purified DNA was used for quantitative real-time PCR as described above.
Table II. Primers used for ChIP.
| Primer | Sequence (5′–3′) | Efficiency |
|---|---|---|
| ZmsodCp Set A | AGCCCCTCTCATGGATCTGT | 101% |
| ACCCAGAGGGGAATGCCATA | ||
| ZmsodCp Set B | TCCATGCATGCAGCTAACCT | 99% |
| CTTGGATCCACCGACACCTC | ||
| ZmsodCp Set C | TCGAGAGCTAGCGGGTGATA | 102% |
| AGTTTTTCGACGGCGTTTGG | ||
| ZmsodCp Set D | CCTAGAAACCAAACACCCCCT | 97% |
| AGGACAGGTACAACCGTACA | ||
| ZmsodCp Set E | CCTGCAATAATCAGTGTCGCC | 96% |
| CGAGCTACATGCTTGGTCGT | ||
| ZmsodCp Set F | ATAACAACGCAGCACAGGTG | 101% |
| CGTGCTCCCACAAGTCTAGG | ||
| ZmCat-1 Set A | CGAGATCAGGACCATCTGGC | 98% |
| TCCGGCCATTGCACATGTAT | ||
| ZmCat-1 Set B | ATACATGTGCAATGGCCGGA | 97% |
| CGCCATTTTGATGGCCCAAA | ||
| ZmCat-1 Set C | CTGAACGAGCTGCACTCTGA | 96% |
| AGAGCTTGTCCGGCCATTG | ||
| ZmCat-1 Set D | CCAATACATGTGCAATGGCCG | 101% |
| ACAGATGCGCTGCTGATGTA | ||
| ZmCat-1 Set E | CTGTCTGCTTTCGCTCATGC | 99% |
| CAGAGCTTGTCCGGCCATTG | ||
| ZmCat-1 Set F | GGACCATCTGGCTCTCCAAC | 97% |
| TCCGGCCATTGCACATGTATT | ||
| ZmUbiquitin | ATCTTTGTGAAGACCCTCAC | 98% |
| CCTAAGGCGCAGCACCAAGT |
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers metacaspase type II (Gene identifier 100285605); putative metacaspase (Gene identifier 100191541); hexokinase (Gene identifier 100192075); fdad1 (Gene identifier 541686); ABP9 (Genebank assession no. GU237073).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Analysis of the PCD time course in maize aleurone layers treated with ABA.
Supplemental Figure S2. Transcript levels of PCD-related genes.
Supplemental Figure S3. Transcript levels of histone acetylation-related genes at 5 d.
Supplemental Figure S4. H3K9ac, H4K5ac, and H3ac protein levels in the aleurone layers after ABA treatment.
Supplemental Figure S5. Time course of PCD in maize aleurone layers treated with CUDC-101 or AA plus GA or ABA determined by staining with FDA and PI.
Supplemental Figure S6. HAT and HDAC enzyme activities after treatment in aleurone layers.
Supplemental Figure S7. Gene expression levels and enzyme activity after treatment with DMSO or ddH2O.
Supplemental Figure S8. Time course of PCD in maize aleurone layers incubated with GA or ROS scavengers plus GA determined by staining with FDA and PI.
Supplemental Figure S9. Concentration of ROS-related molecules and enzyme activities after histone modification enzyme inhibitor treatment in aleurone layers.
Supplemental Figure S10. Time course of PCD in maize aleurone layers incubated with CUDC-101 or CUDC-101 plus H2O2 determined by staining with FDA and PI.
Acknowledgments
We thank Huan Wen, Fei Gao, and Ningjie Ma for helpful discussions; we also thank the reviewers for their suggestions and constructive remarks.
Footnotes
This work was supported by the National Natural Science Foundation of China (no. 31571265).
Articles can be viewed without a subscription.
References
- Ahmad R, Zuily-Fodil Y, Passaquet C, Bethenod O, Roche R, Repellin A (2012) Ozone and aging up-regulate type II metacaspase gene expression and global metacaspase activity in the leaves of field-grown maize (Zea mays L.) Chemosphere 87: 789–795. [DOI] [PubMed] [Google Scholar]
- Aoki N, Ishibashi Y, Kai K, Tomokiyo R, Yuasa T, Iwaya-Inoue M (2014) Programmed cell death in barley aleurone cells is not directly stimulated by reactive oxygen species produced in response to gibberellin. J Plant Physiol 171: 615–618 [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287 [DOI] [PubMed] [Google Scholar]
- Berger SL. (2002) Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12: 142–148 [DOI] [PubMed] [Google Scholar]
- Berger SL. (2007) The complex language of chromatin regulation during transcription. Nature 447: 407–412 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Lonsdale JE, Fath A, Jones RL (1999) Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 11: 1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissenbaev AK, Altybaeva NA, Kolbaeva GA (2007) Role of reactive oxygen species and antioxidant enzymes in hormone regulating programmed cell death of wheat aleurone layer. J Cell Mol Biol 6: 41–48 [Google Scholar]
- Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ, Dalpke AH (2007) Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 122: 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Buckner B, Janick-Buckner D, Gray J, Johal GS (1998) Cell-death mechanisms in maize. Trends Plant Sci 3: 218–223 [Google Scholar]
- Cao H, Li X, Wang Z, Ding M, Sun Y, Dong F, Chen F, Liu L, Doughty J, Li Y, et al. (2015) Histone H2B monoubiquitination mediated by HISTONE MONOUBIQUITINATION1 and HISTONE MONOUBIQUITINATION2 is involved in anther development by regulating tapetum degradation-related genes in rice. Plant Physiol 168: 1389–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2: 764–775 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Gong Z, Zhu JK (2008) Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integr Plant Biol 50: 1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou K, Kapazoglou A, Tondelli A, Francia E, Stanca MA, Bladenopoulos K, Tsaftaris AS (2009) Epigenetic chromatin modifiers in barley: I. Cloning, mapping and expression analysis of the plant specific HD2 family of histone deacetylases from barley, during seed development and after hormonal treatment. Physiol Plantarum 136: 358–368 [DOI] [PubMed] [Google Scholar]
- Domínguez F, Cejudo FJ (2014) Programmed cell death (PCD): an essential process of cereal seed development and germination. Front Plant Sci 5: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen AV, Jeong M, Lin KK, Challen GA, Goodell MA (2013) Isolation and characterization of mouse side population cells. Methods Mol Biol 946: 151–162 [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53: 1273–1282 [PubMed] [Google Scholar]
- Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R (2000) Programmed cell death in cereal aleurone. Plant Mol Biol 44: 255–266 [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke PC, Belligni MV, Spiegel YN, Jones RL (2001a) Signalling in the cereal aleurone: hormones, reactive oxygen and cell death. New Phytol 151: 99–107 [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke PC, Jones RL (2001b) Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol 126: 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI (2008) Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett 266: 6–11 [DOI] [PubMed] [Google Scholar]
- Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20: 27–36 [DOI] [PubMed] [Google Scholar]
- Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168: 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. (1997) Histone acetylation in chromatin structure and transcription. Nature 389: 349–352 [DOI] [PubMed] [Google Scholar]
- Guo WJ, Ho T-HD (2008) An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant physiol 147: 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Offermann S, Danker T, Horst I, Peterhansel C, Stam M (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Wang P, Zhang H, Wen H, Gao F, Ma N, Wang Q, Li L (2015) Histone acetylation is involved in gibberellin-regulated sodCp gene expression in maize aleurone layers. Plant Cell Physiol 56: 2139–2149 [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, Zheng SH, Yuasa T, Iwaya-Inoue M (2012) Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol 158: 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. (2001) Programmed cell death in development and defense. Plant Physiol 125: 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlić R, Chung HR, Lasserre J, Vlahoviček K, Vingron M (2010) Histone modification levels are predictive for gene expression. Proc Natl Acad Sci USA 107: 2926–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Lim JH, Ahn CS, Park K, Kim GT, Kim WT, Pai HS (2006) Mitochondria-associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana. Plant Cell 18: 2341–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. (1999) Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev 9: 40–48 [DOI] [PubMed] [Google Scholar]
- Kuo A, Cappelluti S, Cervantes-Cervantes M, Rodriguez M, Bush DS (1996) Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 8: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Allis CD (1998) Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 20: 615–626 [DOI] [PubMed] [Google Scholar]
- Kurdistani SK, Grunstein M (2003) Histone acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol 4: 276–284 [DOI] [PubMed] [Google Scholar]
- Kurita K, Sakamoto T, Yagi N, Sakamoto Y, Ito A, Nishino N, Sako K, Yoshida M, Kimura H, Seki M, et al. (2017) Live imaging of H3K9 acetylation in plant cells. Sci Rep 7: 45894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, Bowcock AM, Harbour JW (2012) Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 18: 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove A, Hooley R (2000) Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci 5: 102–110 [DOI] [PubMed] [Google Scholar]
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1: 194–202 [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA (2000) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92: 1210–1216 [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51 [DOI] [PubMed] [Google Scholar]
- Miura K, Furumoto T (2013) Cold signaling and cold response in plants. Int J Mol Sci 14: 5312–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer TJ. (1980) Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell 20: 479–485 [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H (2000) Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem 275: 23998–24002 [DOI] [PubMed] [Google Scholar]
- Pawlak S, Deckert J (2007) Histone modifications under environmental stress. Biol Lett 44: 65–73 [Google Scholar]
- Peterson CL, Laniel MA (2004) Histones and histone modifications. Curr Biol 14: R546–R551 [DOI] [PubMed] [Google Scholar]
- Rahman I, Adcock IM (2006) Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28: 219–242 [DOI] [PubMed] [Google Scholar]
- Repka V, Fiala R, Čiamporová M, Martinka M, Pavlovkin J (2015) Antibody microarray expression profiling of maize roots treated with cadmium and nickel. Agriculture (Polnohospodárstvo) 61: 41–49 [Google Scholar]
- Robert F, Pokholok DK, Hannett NM, Rinaldi NJ, Chandy M, Rolfe A, Workman JL, Gifford DK, Young RA (2004) Global position and recruitment of HATs and HDACs in the yeast genome. Mol Cell 16: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropero S, Esteller M (2007) The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 1: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125: 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumb WC, Satterfield CN, Wentworth RL (1955) Hydrogen Peroxide. ACS Monograph No. 128. Reinhold Publishing, New York [Google Scholar]
- Schwille P, Haupts U, Maiti S, Webb WW (1999) Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys J 77: 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76: 75–100 [DOI] [PubMed] [Google Scholar]
- Solís MT, Chakrabarti N, Corredor E, Cortés-Eslava J, Rodríguez-Serrano M, Biggiogera M, Risueño MC, Testillano PS (2014) Epigenetic changes accompany developmental programmed cell death in tapetum cells. Plant Cell Physiol 55: 16–29 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Sukumari-Ramesh S, Singh N, Jensen MA, Dhandapani KM, Vender JR (2011) Anacardic acid induces caspase-independent apoptosis and radiosensitizes pituitary adenoma cells. J Neurosurg 114: 1681–1690 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kikuchi A, Kamada H (2008) The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol 146: 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM. (2000) Histone acetylation and an epigenetic code. BioEssays 22: 836–845 [DOI] [PubMed] [Google Scholar]
- Wang P, Zhang H, Hou H, Wang Q, Li Y, Huang Y, Xie L, Gao F, He S, Li L (2016) Cell cycle arrest induced by inhibitors of epigenetic modifications in maize (Zea mays) seedling leaves: characterization of the process and possible mechanisms involved. New Phytol 211: 646–657 [DOI] [PubMed] [Google Scholar]
- Wang P, Zhao L, Hou H, Zhang H, Huang Y, Wang Y, Li H, Gao F, Yan S, Li L (2015) Epigenetic changes are associated with programmed cell death induced by heat stress in seedling leaves of Zea mays. Plant Cell Physiol 56: 965–976 [DOI] [PubMed] [Google Scholar]
- Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, Chen JG (2009) Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radical Bio Med 47: 229–240 [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zhang L, Qiu Z, Hu Y, Yang F, Yan S, Zhao L, Li B, He S, Huang M, Li J, et al. (2011) ABA treatment of germinating maize seeds induces VP1 gene expression and selective promoter-associated histone acetylation. Physiol Plant 143: 287–296 [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhang L, Duan J, Miki B, Wu K (2005) HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 17: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu JK, Hasegawa PM, Bohnert HJ, Shi H, Yun DJ, et al. (2008) Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc Natl Acad Sci USA 105: 4945–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]