MiR408 positively regulates rice grain yield by targeting the uclacyanin gene OsUCL8 and, in turn, affects photosynthesis.
Abstract
Increasing grain yield is the most important object of crop breeding. Here, we report that the elevated expression of a conserved microRNA, OsmiR408, could positively regulate grain yield in rice (Oryza sativa) by increasing panicle branches and grain number. We further showed that OsmiR408 regulates grain yield by down-regulating its downstream target, OsUCL8, which is an uclacyanin (UCL) gene of the phytocyanin family. The knock down or knock out of OsUCL8 also increases grain yield, while the overexpression of OsUCL8 results in an opposite phenotype. Spatial and temporal expression analyses showed that OsUCL8 was highly expressed in pistils, young panicles, developing seeds, and inflorescence meristem and was nearly complementary to that of OsmiR408. Interestingly, the OsUCL8 protein was localized to the cytoplasm, distinct from a majority of phytocyanins, which localize to the plasma membrane. Further studies revealed that the cleavage of OsUCL8 by miR408 affects copper homeostasis in the plant cell, which, in turn, affects the abundance of plastocyanin proteins and photosynthesis in rice. To our knowledge, this is the first report of the effects of miR408-OsUCL8 in regulating rice photosynthesis and grain yield. Our study further broadens the perspective of microRNAs and UCLs and provides important information for breeding high-yielding crops through genetic engineering.
Increasing grain yield is the most important object of crop breeding. To date, a couple of genes related to grain yield have been identified and functionally characterized. For example, PTB1 regulates rice (Oryza sativa) seed setting rate by affecting pollen tube growth (Li et al., 2013). Ghd7 and Ghd8 regulate the number of rice grains per panicle (Xue et al., 2008; Yan et al., 2011). DEP1 controls panicle architecture and grain number by promoting cell division (Huang et al., 2009). GW2 and GS3 are the two major quantitative trait loci that regulate grain size (Fan et al., 2006; Song et al., 2007; Mao et al., 2010). Notably, most of the reported genes encode proteins and are identified in quantitative trait loci. Despite the knowledge of these protein-coding genes, our understanding of the regulating networks that control rice grain yield remains limited, especially that noncoding RNAs modulated agronomic traits.
MicroRNAs (miRNAs), a class of abundant small noncoding RNAs, have been identified as important regulators of gene expression in plants, affecting many aspects of plant development (Bartel, 2004). Recently, several miRNAs have been reported to regulate rice grain yield. The perturbed OsmiR156-mediated regulation of a transcription factor gene, OsSPL14, reduces the tiller number and increases the number of panicle branches, resulting in an increase of grain yield (Jiao et al., 2010; Miura et al., 2010). We demonstrated previously that miR397 can improve rice yield by increasing grain size and promoting panicle branching (Zhang et al., 2013). More recently, a rare mutation in GS2 was reported to affect the binding of OsmiR396c, which causes elevated expression of OsGRF4, the gene that encodes GROWTH-REGULATING FACTOR4, leading to an increase in grain weight and yield (Li et al., 2016). These results illustrate the potential roles of miRNAs in controlling rice yield-related traits.
Given the importance of crop yield improvement, we asked whether other miRNAs in the rice genome could positively regulate rice grain size or other yield-related traits. Our previous study revealed that miR397 regulates rice grain yield by affecting a blue copper protein, laccase. This mechanism is conserved between monocots and dicots (Zhang et al., 2013; Wang et al., 2014), suggesting that miRNA mediation of blue copper protein could be a novel method for improving rice yield. More interestingly, we performed a genome-wide screening of miRNA expression during embryogenesis and postembryogenesis and found that, among all the known miRNAs, only one conserved miRNA, miR408, showed the same expression pattern as that of miR397 during embryogenesis. Coincidently, miR408 also targets the blue copper protein (Luo et al., 2006; Zhu et al., 2008; Xue et al., 2009; Chen et al., 2011). Thus, we asked whether miR408 could regulate rice grain yield or other agronomic traits.
MiR408 has been reported to target a variety of blue copper protein members, including those in the phytocyanin family (Jones-Rhoades and Bartel, 2004; Sunkar et al., 2005). Both phytocyanin and laccase are blue copper proteins, but they belong to different groups (Rydén and Hunt, 1993). Blue copper proteins could be divided into three groups: small blue proteins, blue oxidases, and coagulation factors. Laccase belongs to blue oxidases and combines four coppers. Phytocyanin and plastocyanin (PC) both belong to small blue proteins and combine only one copper. However, the functions of phytocyanin members in plant developmental processes, especially in rice, are not fully understood. Notably, it has been revealed that a member of the phytocyanin family is the target of miR408 in Arabidopsis (Arabidopsis thaliana) and responds to copper deficiency and light (Abdel-Ghany and Pilon, 2008). Another member of the phytocyanin family also was reported as a target in wheat (Triticum aestivum; Zhao et al., 2016), suggesting that miR408 is a conserved miRNA that regulates pathways that are involved in phytocyanin.
In this study, we analyzed the functions of OsmiR408 and its target gene in rice. We revealed that a high expression level of OsmiR408 or a knockout of its target gene, OsUCL8, an uclacyanin (UCL) gene in the phytocyanin family, could promote panicle branches and increase grain number, leading to higher grain productivity. We further elucidated the molecular mechanisms of OsmiR408 activity, finding that the cleavage of OsUCL8 by miR408 affects copper homeostasis in plant cells, which, in turn, affects the abundance of PC proteins and photosynthesis in rice. Our study provides, to our knowledge, the first functional evidence of rice UCLs in regulating crop agronomic traits and further broadens the function of miRNAs in breeding high-yielding crops through genetic engineering.
RESULTS
OsmiR408 Promotes Panicle Branching and Increases Grain Weight
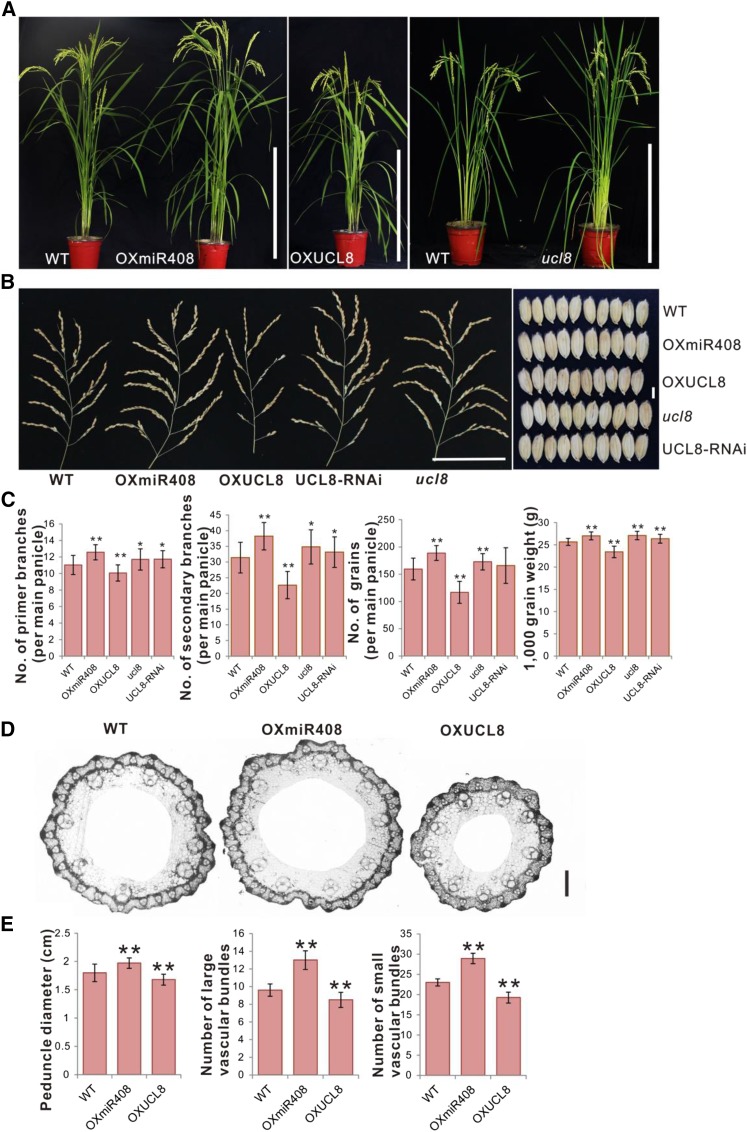
To investigate the function of OsmiR408, we generated OsmiR408-overexpressing (OXmiR408) lines (Fig. 1A) to evaluate the effect of OsmiR408 on rice development. RNA-blot hybridization was performed to analyze the expression levels of OsmiR408 in the OxmiR408 lines (Supplemental Fig. S1A). The OXmiR408 transgenic plants grew slightly faster than the wild-type plants, and the heights of OXmiR408 plants (plant height, ∼112.3 cm; P < 0.001, n = 100) were slightly taller than those of wild-type plants (plant height, ∼109 cm; n = 80) at the mature stage (Fig. 1A), indicating that miR408 might positively regulate plant growth.
Figure 1.
Phenotypes of OXmiR408, OXUCL8, ucl8, and UCL8-RNAi transgenic rice plants. A, Gross morphology of wild-type (WT), OXmiR408, OXUCL8, ucl8, and UCL8-RNAi transgenic plants. Bars = 40 cm. B, Panicle and grain morphologies of the wild-type and transgenic plants. Bars = 10 cm for the panicles and 3 mm for the grains. C, Number of primer branches per main panicle, number of secondary branches per main panicle, number of grains per main panicle, and 1,000-grain weight of the wild-type plants and the transgenic plants. Values are expressed as means ± sd (n = 30 plants). Significant differences were identified at the 5% (*) and 1% (**) probability levels using Student’s t test. D, Cross sections of peduncles in wild-type, OXmiR408, and OXUCL8 plants. Bar = 500 µm. E, Comparison of the peduncle diameters, number of large vascular bundles, and small vascular bundles in wild-type, OXmiR408, and OXUCL8 plants. Values are expressed as means ± sd (n = 15 plants). Significant differences were identified at the 1% (**) probability level using Student’s t test.
To further determine whether OsmiR408 positively regulates grain yield, we measured yield-related traits in the OXmiR408 plants. Intriguingly, compared with the wild-type plants, the OXmiR408 transgenic lines showed a significant increase in the number of panicle branches and had slightly longer grains (Fig. 1B). The numbers of primary and secondary branches in the OXmiR408 lines were approximately 14% and 22% higher than those in the wild-type plants, respectively, and the effective grains per main panicle in the OXmiR408 plants were approximately 18% higher than those in the wild-type plants (Fig. 1C). In addition, the 1,000-grain weight of OXmiR408 also was increased significantly (Fig. 1C). It has been reported that the number of vascular bundles in the peduncle corresponds to the panicle branches and the grain number; thus, we analyzed the peduncle vascular bundles. Consistently, the peduncle diameter and the number of peduncle vascular bundles also were increased in the OXmiR408 main panicles (Fig. 1, D and E). These results demonstrate the positive regulation of OsmiR408 on increasing panicle branches and grain weight.
MiR408 Exerts Its Functions through Its Downstream Target, UCLACYANIN-LIKE PROTEIN8
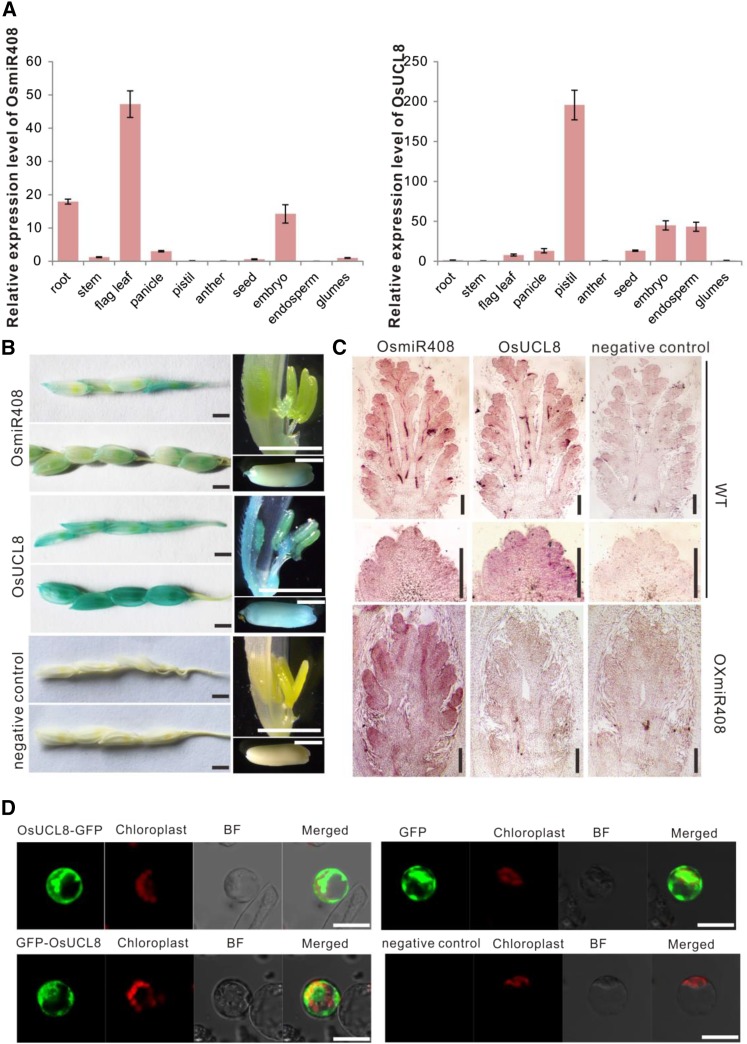
It is well known that miRNAs exert their functions on the development of organisms through their downstream targets (Bartel, 2004; Jones-Rhoades et al., 2006). To determine the molecular mechanism by which OsmiR408 regulates rice development in vivo, we further investigated its downstream targets. In Arabidopsis, three laccase genes, LAC3, LAC12, and LAC13, and one plantacyanin gene have been validated to be the targets of miR408 and to respond to light and copper conditions (Abdel-Ghany and Pilon, 2008). In rice, two members of the UCL family, LOC_Os03g50140 (UCL8) and LOC_Os08g37670 (UCL30), were identified as targets of OsmiR408 (Zhou et al., 2010). However, a recent study on the variety-specific regulatory schema for OsmiR408 expression in rice showed that the transcript accumulation of OsUCL30 does not correspond to the variation of miR408 expression levels (Mutum et al., 2013). Further analysis of the expression pattern of OsUCL30 showed that it was relatively abundantly expressed in most of the examined tissues (Ma et al., 2011). In contrast, the spatial expression pattern of OsmiR408 and OsUCL8 by quantitative reverse transcription (qRT)-PCR detection showed that OsmiR408 is highly expressed in flag leaf, root, and embryo, whereas the expression pattern of OsULC8 is approximately complementary with that of OsmiR408 (Fig. 2A). Thus, in this study, we focus mainly on the regulatory schema of OsmiR408 and OsUCL8.
Figure 2.
Expression patterns of OsmiR408 and OsUCL8, and subcellular localization of the OsUCL8 protein. A, Relative expression patterns of OsmiR408 and OsUCL8 in different tissues. Values are expressed as means ± sd. B, Spatial expression pattern analysis of OsmiR408 and OsUCL8 in young panicles and grains by GUS staining. Bars = 2 mm. C, In situ hybridization of OsmiR408 and OsUCL8 during panicle development in wild-type (WT) plants and in OXmiR408 plants. Bars = 100 µm. D, Subcellular localization of OsUCL8. The empty vector with or without ATG before the GFP gene was used as a positive or negative control, respectively. BF, Bright field. Bars = 10 µm.
To further confirm the expression of OsmiR408 and OsUCL8, we analyzed their spatial expression patterns by GUS activity analysis and in situ hybridization. Both OsmiR408 and OsULC8 are expressed in inflorescence meristem, young panicle, and seeds 5 d after pollination, but the expression levels of OsUCL8 in young panicle and pistil are higher than in OsmiR408 (Fig. 2, B and C). Moreover, the mRNA abundance of OsUCL8 is reduced in the OXmiR408 inflorescence meristem (Fig. 2C). We further validated the cleavage of OsUCL8 mRNA by OsmiR408 using RNA ligase-mediated RACE and found that the cleavage was between the 11th and 12th bases of the OsmiR408 target site (Supplemental Fig. S1B). These results implied that OsmiR408 might play a role in regulating panicle and seed development through cleaving OsUCL8 mRNA.
To identify the effect of OsUCL8 on grain yield in rice, we generated transgenic plants that ectopically expressed OsUCL8 (OXUCL8) and investigated their phenotype (Fig. 1A). We used qRT-PCR to analyze the expression levels of OsUCL8 mRNA in these OXUCL8 transgenic lines (Supplemental Fig. S1C). Because OsmiR408 was found to promote panicle branching, to increase grain number per panicle and grain weight, we then evaluated the effects of OsUCL8 on the traits of panicles and grains. We hypothesized that the overexpression of OsUCL8 might have a negative phenotype compared with that of the overexpressing OsmiR408 plants. Notably, OXUCL8 plants showed semidwarf phenotypes (plant height, ∼98.3 cm; P < 0.001, n = 90), the number of panicle branches and effective grains per panicles decreased dramatically (Fig. 1, C–F), and the 1,000-grain weight of the OXUCL8 plants also was decreased significantly compared with the wild-type plants (Fig. 1, B and C). The peduncle diameter and the number of peduncle vascular bundles also were decreased in the OXUCL8 main panicles (Fig. 1, D and E). These results indicated that the phenotypes observed in the plants with high expression levels of OXUCL8 were opposite to those observed in the OXmiR408 plants, which expressed lower levels of OsUCL8 than the wild-type plants.
To further validate the function of OsUCL8, we constructed transgenic plants that knock down OsUCL8 by RNA interference (UCL8-RNAi) and plants that knock out OsUCL8 by CRISPR-Cas9 (ucl8; Fig. 1A; Supplemental Fig. S1, C and D). Consistently, the plants that knock down or knock out OsUCL8 had more panicle branches and effective grains than the wild-type plants, and their 1,000-grain weight and grain yield per plant were higher than those of the wild-type plants (Fig. 1, B and C). To observe the actual grain yield in the OXmiR408, OXUCL8, ucl8, and UCL8-RNAi plants, we conducted a plot field experiment of different transgenic lines with different OsmiR408 and OsUCL8 expression levels. Overall, compared with the wild-type plot yield, overexpression of OsmiR408 increased plot yield by ∼16.2% and knock out of OsUCL8 increased plot yield by ∼21.4% (Table I). Moreover, the expression level of OsmiR408 was positively correlated with grain yield and the level of OsUCL8 was negatively correlated with grain yield (Table I; Supplemental Fig. S1C). These observations suggest that miR408 regulates rice panicle branching and grain weight via OsUCL8.
Table I. Yield test in a paddy between wild-type, OXmiR408, OXUCL8, ucl8, and UCL8-RNAi plants.
Values shown are means ± sd (n = 8 plots). Significant differences were identified using Student’s t test.
| Plants | Yield per Plant | Yield per Plot |
|---|---|---|
| g | ||
| Wild type | 20.1 ± 2.3 | 1,256.0 ± 96.2 |
| OXmiR408 18-3 | 23.0 ± 1.8 (P < 0.001) | 1,443.9 ± 70.2 (P < 0.001) |
| OXmiR408 50-1 | 23.2 ± 2.5 (P = 0.001) | 1,462.0 ± 83.1 (P < 0.001) |
| OXmiR408 56-7 | 23.5 ± 2.7 (P < 0.001) | 1,500.0 ± 97.7 (P < 0.001) |
| OXmiR408 61-1 | 22.9 ± 2.3 (P = 0.002) | 1,432.6 ± 92.8 (P = 0.002) |
| OXUCL8 19-8 | 15.8 ± 2.8 (P < 0.001) | 972.1 ± 82.2 (P < 0.001) |
| OXUCL8 21-2 | 15.6 ± 2.6 (P < 0.001) | 955.7 ± 86.8 (P < 0.001) |
| OXUCL8 33-4 | 16.7 ± 3.7 (P = 0.005) | 986.2 ± 70.6 (P < 0.001) |
| ucl8 | 23.8 ± 2.3 (P < 0.001) | 1,525.0 ± 84.6 (P < 0.001) |
| UCL8-RNAi | 22.4 ± 2.2 (P = 0.02) | 1,473.6 ± 108.1 (P < 0.001) |
Subcellular Localization of OsUCL8 Protein and Its Effects on Plastocyanin Abundance and Photosynthesis
We then asked about the mechanism by which OsUCL8 exerts its effect. UCLs are members of the ancient and plant-specific phytocyanin family, which is a subfamily of blue copper proteins (Nersissian et al., 1998; Ma et al., 2011). Thus far, most of the research on phytocyanins has been on their biochemical characteristics and redox properties (Sato et al., 2007; Guzzi et al., 2008; Ma et al., 2011; Cao et al., 2015). Recent studies showed that phytocyanin is a pollen tube growth-controlling protein (Dong et al., 2005). However, the subcellular location and function of UCL8 remain largely unknown.
We first evaluated the subcellular location of OsUCL8. Bioinformatic analysis suggested that lots of phytocyanins are predicted to have glycosylphosphatidylinositol-anchor signals responsible for plasma membrane localization, and several members have been identified to be plasma membrane localized (Ma et al., 2011). To determine the subcellular localization of OsUCL8, we fused GFP to the C terminus and N terminus of OsUCL8. These plasmids were then transiently transformed into rice protoplasts. The fluorescence signal in the protoplasts showed that the OsUCL8 protein localized in the cytoplasm but not the plasma membrane (Fig. 2D), indicating that the subcellular localization of OsUCL8 is different from that of other phytocyanins reported.
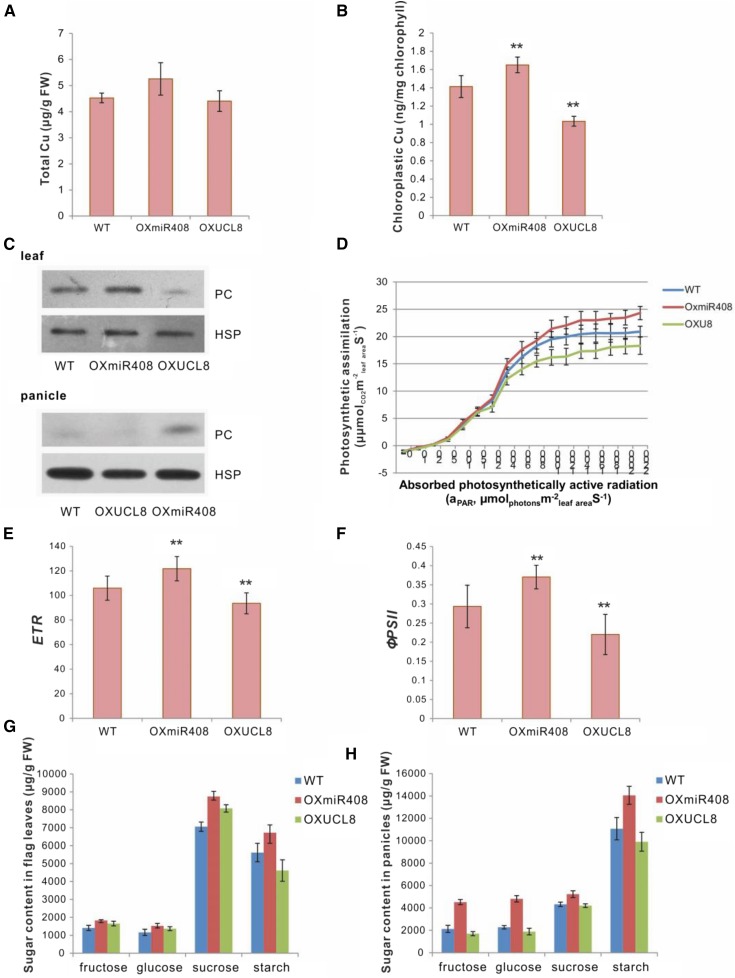
The observations above indicated that OsUCL8 is located specifically in the cytoplasm, suggesting that it might have a different function from UCLs located in the plasma membrane. Although miR408 has different target genes in different species, its targets all encode copper proteins. Moreover, miR408 responds to copper deficiency and is involved in copper dynamics through its target genes. Copper is an essential micronutrient in plants, and copper proteins, including UCLs and PC, competitively bind copper ions. Previous studies showed that, under conditions of copper deficiency, copper is preferentially supplied to PC, which is one of the most abundant copper proteins and is essential to photosynthetic electron transport. We speculated that OsmiR408 might regulate copper homeostasis through its target, OsUCL8. We then determined the total copper content and the chloroplastic copper content in OXmiR408, OXUCL8, and wild-type plants. We found that copper uptake in chloroplast is affected when OsUCL8 levels are altered (Fig. 3, A and B).
Figure 3.
Mechanistic analysis of OsmiR408-mediated OsUCL8 silencing with respect to increasing grain yield. A, Total copper content in wild-type (WT), OXmiR408, and OXUCL8 leaves. B, Chloroplastic copper content in wild-type, OXmiR408, and OXUCL8 leaves. C, PC content in the leaves and panicles of wild-type, OXmiR408, and OXUCL8 plants as determined by western blot. OsHSP90 was used as a control. D, Photosynthetic light-response curves of wild-type, OXmiR408, and OXUCL8 flag leaves. E, Electron transport rate (ETR) in wild-type, OXmiR408, and OXUCL8 flag leaves. F, PSII quantum yield (ϕPSII) in wild-type, OXmiR408, and OXUCL8 flag leaves. G and H, Sugar content in the flag leaves (G) and panicles (H) of wild-type, OXmiR408, and OXUCL8 plants. Values in A, B, and D to H are expressed as means ± sd (for A, B, G, and H, n = 3 replicates; for D–F, n = 25 plants). Significant differences were identified at the 1% (**) probability level using Student’s t test. FW, Fresh weight.
In Arabidopsis, increased levels of miR408 under copper deficiency could repress its target laccase to maintain PC abundance (Zhang et al., 2014). Thus, we asked if OsUCL8 affected the function of PC in rice. To address this question, we collected proteins from the panicles and flag leaves of the OXmiR408, OXUCL8, and wild-type plants for western-blot analysis of PC. In Arabidopsis, there are two PC proteins (AtPETE1 and AtPETE2), and they have only one ortholog in rice (LOC_Os06g01210), which is 55.4% identical to AtPETE1 and 61.8% identical to AtPETE2. We transiently transformed OsPC-Flag into rice protoplasts and detected OsPC by both anti-Flag antibody and anti-PC antibody, and the result showed that the PC antibody is specific for OsPC protein (Supplemental Fig. S1E). We then used PC antibody to detect rice endogenous PC protein. As expected, high expression levels of OsUCL8 decreased PC abundance, while overexpression of OsmiR408 caused a slight increase in the amount of PC, both in the panicles and in the flag leaves (Fig. 3C).
PC is an electron carrier for the photosynthetic machinery and is located in the thylakoid lumen of the chloroplast. Up-regulation of PC proteins in the OXmiR408 plants suggested that miR408 might affect photosynthesis. We next measured the light-responsive curves and chlorophyll florescence of the flag leaves in transgenic plants. The results showed that the photosynthetic rate and the photosynthetic electron transport rate were indeed up-regulated in the OXmiR408 plants and down-regulated in the OXUCL8 plants (Fig. 3, D–F). We further analyzed the sugar (Fru, Glc, Suc, and starch) content of the transgenic plants because the photosynthetic rate directly regulates carbon assimilation. Consistently, the OXmiR408 plants accumulate more sugars in the leaves and panicles than the wild-type plants, while the sugar content in the OXUCL8 plants was less than that of the wild-type plants (Fig. 3, G and H). Consistently, OsmiR408 was highly expressed in the flag leaf (Fig. 2C), which suggests that the expression of OsmiR408 in the flag leaf might be necessary for the abundance of PC and photosynthesis. The results show that the OXmiR408 plants were more efficient at saving and converting light energy into sugars, suggesting that OsmiR408 might promote grain yield through regulating the PC content and photosynthesis by down-regulating OsUCL8.
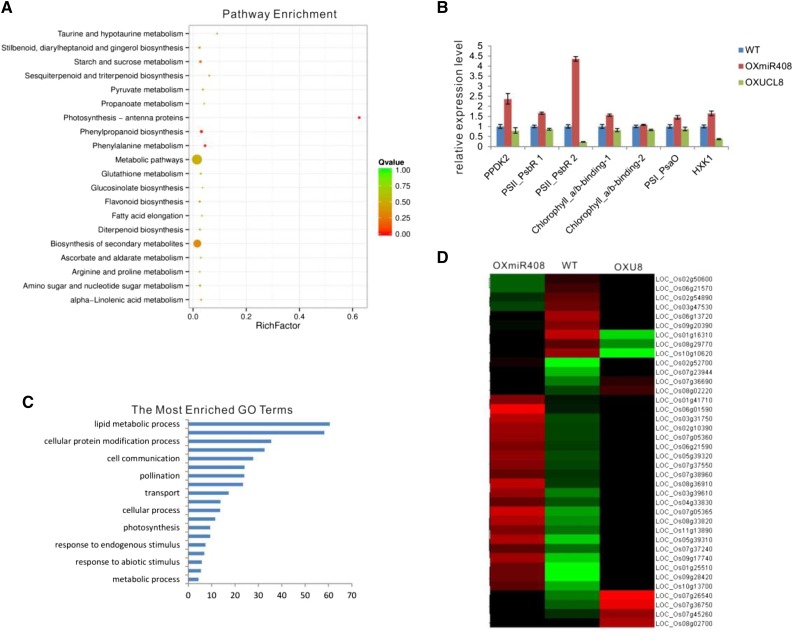
Transcriptome Analysis Further Showed That OsmiR408-Mediated OsUCL8 Acts through the Photosynthesis Pathway
The results indicated that miR408 regulates rice grain yield by down-regulating OsUCL8 and controlling photosynthesis. To further reveal the biological pathways that UCL members might regulate to control rice grain yield-related traits, we performed RNA sequencing (RNA-seq) on the seedlings and young panicles of wild-type, OXmiR408, and OXUCL8 plants to screen for differentially expressed genes. We generated an average of 3.1 × 107 reads per sample, and the gene expression profiles derived from RNA-seq were estimated using fragments per kilobase of transcript per million reads mapped. To investigate the functions of the differentially expressed genes, we performed Gene Ontology (GO) analyses and Kyoto Encyclopedia of Genes and Genomes pathway analysis. The differentially expressed genes could be divided into several groups, including the photosynthesis group and two photosynthesis-related pathways (starch and Suc metabolism and photosynthesis-antenna proteins; Fig. 4A). These were included in the top 10 enriched pathways, and the photosynthesis-antenna protein pathway was the most significantly enriched pathway (Fig. 4B). We further confirmed the expression of the photosynthesis-related genes by qRT-PCR to validate the RNA-seq data (Fig. 4C). Moreover, the genes that were differentially expressed between the OXmiR408 and wild-type plants were significantly enriched in photosynthesis and sugar metabolism GO terms, including photosynthesis light harvesting, starch catabolic process, and Suc catabolic process (Fig. 4D; Supplemental Fig. S2). These results are consistent with the effects of miR408 and OsUCL8 on photosynthesis, sugar content, and grain yield.
Figure 4.
Transcriptome analysis of wild-type (WT), OXmiR408, and OXUCL8 seedlings. A, Pathway enrichment of differentially expressed genes between wild-type and OXmiR408 plants. B, Most enriched GO terms of differentially expressed genes between wild-type and OXmiR408 plants. C, Relative expression levels of photosynthesis-related genes in wild-type, OXmiR408, and OXUCL8 seedlings analyzed by qRT-PCR. D, Hierarchical ontology tree of the differentially expressed genes in wild-type, OXmiR408, and OXUCL8 seedlings.
DISCUSSION
Novel Function of Phytocyanin Proteins
Plant phytocyanins are blue copper proteins and function as electron transporters. Most of the phytocyanins are expected to have glycosylphosphatidylinositol-anchor signals (Ma et al., 2011). Phytocyanins can be categorized into three families: UCLs, stellacyanins, and nodulin-like proteins (Ma et al., 2011). Previous studies have indicated that phytocyanin proteins are involved in various plant activities, including pollen tube germination and anther pollination (Kim et al., 2003; Dong et al., 2005), reproductive potential determination (Khan et al., 2007), and apical bud organ development (Yoshizaki et al., 2000). However, the role of UCLs in plant development is poorly identified.
In this study, we revealed the functions of OsUCL8, which belongs to the UCL family. OsUCL8 is subcellularly located in the cytoplasm, not in the plasma membrane or chloroplast, like most other phytocyanin family members. We demonstrated that OsUCL8 is regulated by OsmiR408 and is associated with panicle branching and grain weight by regulating PC content and photosynthesis. To our knowledge, this is the first report on the effects of UCLs on photosynthesis and grain yield regulation.
miR408 and miR397 Are Two miRNAs That Positively Regulate Rice Grain Yield
Identifying new genes that modulate agronomic traits, especially grain yield, would facilitate the breeding of new varieties with increased yields. MiRNAs have been identified as important regulators of gene expression and are involved in many aspects of plant development. For example, OsmiR156 negatively controls the number of panicle branches and grain yield. Down-regulation of OsmiR396c causes elevated expression of OsGRF4, leading to increased grain weight and yield (Li et al., 2016). However, miRNAs that positively regulate grain size and grain yield are very few, and currently, only one such miRNA, miR397, was reported in both rice and Arabidopsis (Zhang et al., 2013; Wang et al., 2014).
In this study, we found another conserved miRNA, miR408, that positively regulates grain yield. Intriguingly, miR408 shows the same expression pattern as miR397 (Luo et al., 2006; Zhu et al., 2008; Xue et al., 2009; Chen et al., 2011) and also targets a blue copper protein, as does miR397. We reported previously that OsmiR397 increases grain yield by down-regulating its target, OsLAC, a gene encoding a laccase-like protein (Zhang et al., 2013). OsLAC does not affect PC protein content; instead, it was found to be involved in the sensitivity of plants to brassinosteroids (Zhang et al., 2013). In contrast, miR408 regulates rice grain yield by regulating a UCL protein and affecting PC abundance, which, in turn, affects photosynthesis, suggesting that the regulatory networks of blue copper proteins involved are complicated. Altogether, our results indicate that the miRNA regulation of blue copper proteins may be a novel method for improving rice yield.
Multiple Methods for Controlling Copper Ion Homeostasis in Plant Cells
The homeostasis of copper ions is essential for plant development and physiology (Bashir et al., 2016). Copper is a cofactor for various enzymes and plays roles in photosynthetic and respiratory electron transport, oxidative stress protection, ethylene perception, and cell wall metabolism. Additionally, copper deficiency leads to abnormal root, stem, and leaf development. Thus, it is necessary to control copper ion homeostasis in plant cells.
The plant utilizes chelating agents to solubilize metals, and transporters actively participate in the regulation of cellular metal homeostasis. However, compared with other metals, such as Fe, Zn, and Mn, Cu homeostasis is poorly understood. Copper enters the plant cell via a family of high-affinity copper transporter proteins (Grotz and Guerinot, 2006). Copper is used as a cofactor by plant proteins involved in neutralizing reactive oxygen species, lignification of the cell wall, ethylene perception, and the formation of phenolic compounds (Burkhead et al., 2009). Plants can maintain copper homeostasis and prioritize the use of cellular copper by regulating the abundance of these proteins.
Interestingly, some of these copper proteins are targeted by several miRNAs, including miR397, miR398, miR408, miR528, and miR857, and the pathways mediated by these miRNAs are important in controlling copper ion homeostasis (Abdel-Ghany and Pilon, 2008). It has been shown that miR408 targets laccases and regulates copper homeostasis in Arabidopsis (Zhang et al., 2014). In this study, we found that, in rice, miR408 targets another copper protein, OsUCL8, and affects copper homeostasis, suggesting that miR408 is important in regulating blue copper protein pathways and copper homeostasis in both monocots and dicots.
Specific Regulatory Schema of UCL Regulation by miR408
The miR408-mediated regulation of specific plant blue copper proteins has been reported as widely conserved across distant plant species. In Arabidopsis, miR408 targets the laccase genes LAC3, LAC12, LAC13, and PLANTACYANIN1 (Abdel-Ghany and Pilon, 2008). In rice, two OsUCLs have been predicted to be the targets of miR408 (OsUCL8 and OsUCL30), which have been validated by 5′ RACE (Zhou et al., 2010). However, a previous study made the interesting observation that miR408 levels are not always inversely related to that of its target genes, although the target mRNAs were cleaved by miR408 experimentally. For example, OsUCL30 was confirmed to be the target of miR408 (Zhou et al., 2010). However, its transcript accumulation is stable in most tissues and does not match the expression pattern of miR408, indicating the complex nature of the relationship between miR408 and its target genes. Thus, it is necessary to examine the expression patterns of miRNAs and the target genes before functional analysis.
CONCLUSION
Increasing grain yield is the most important aspect of crop breeding. In this study, we report a conserved miRNA, OsmiR408, that is a positive regulator of grain yield in rice. OsmiR408 regulates grain yield by down-regulating its downstream target, OsUCL8, which is a UCL gene of the phytocyanin family. We further elucidated the molecular mechanisms of OsmiR408 activity, finding that the abundance of OsUCL8 affects copper homeostasis in the plant cell, which, in turn, affects the abundance of PC proteins and photosynthesis in rice. To our knowledge, this is the first report of the effects of miR408-UCLs on photosynthesis and grain yield regulation. Our study provides important information for breeding high-yielding crops through genetic engineering.
MATERIALS AND METHODS
Plant Materials and Plant Growth Conditions
The ‘Zhonghua 11’ (Oryza sativa subspecies japonica) rice cultivar was used in the experiments. Rice seeds were germinated in one-half-strength Murashige and Skoog medium in a 28°C incubator for 20 d and then grown in the field in Guangzhou, China (23°08′N, 113°18′E). The growing season begins in April and extends to October, with the average temperature ranging from 29.7°C to 32.9°C.
Construction of Plasmids and Plant Transformation
A binary vector, pHQSN, which has been described by Zhang et al. (2013), was used in this work to generate the overexpressing transgenic lines. The genomic DNA sequences of the premiR408 and OsUCL8 genes were cloned into the EcoRI, BamHI, and SpeI sites of pHQSN between the cauliflower mosaic virus 35S promoter and the Nos terminator. The primers used to amplify the inserts were as follows: miR408, 5′-CGGAATTCTTGGGACAGGTCAGCATC-3′ and 5′-GAAGATCTTGCAACAGCCCTTGAAGTG-3′; OsUCL8, 5′-CGGAATTCATGGCTCGGGGAAGAGGCA-3′ and 5′-GACTAGTTCACACGGCGGTGACGACCA-3′. Another binary vector, the pRNAi-35S vector, which has been described by Zhang et al. (2013), was used to generate the OsUCL8-RNAi lines. The primers used to amplify the insert for the UCL8-RNAi vector were 5′-CACCCTGACGCGTGGTGTTACTTCTGAAGAGG-3′ and 5′-ACTAGAACTGCAGCCTCAGATCTACCATGGTCG-3′. The UCL8 knockout mutants were constructed using CRISPR-Cas9-based genome-editing technology as described previously (Ma et al., 2015). The primers were as follows: target site 1, 5′-GCCAGCATCGATGGCTCGGGGAAG-3′ and 5′-AAACCTTCCCCGAGCCATCGATGC-3′; target site 2, 5′-GCCGAGTACTCCTATGATTTACCG-3′ and 5′-AAACCGGTAAATCATAGGAGTACT-3′.
Northern-Blot Analysis
Total RNAs were isolated from seedlings of rice with TRIzol reagent (Invitrogen). Northern-blot analysis was performed as described with some modifications (Zhang et al., 2013). A 100-µg aliquot of total RNA from different transgenic plants was separated on a denaturing 10% polyacrylamide gel (w/v) at 300 V for 3.5 h, then RNA was transferred to Hybond-N+ membranes (Amersham, GE Life Sciences) using a semidry blotting apparatus (Bio-Rad). After electroblotting, the RNAs were fixed to the membrane by UV cross-linking for 4 min and then baked at 80°C for 50 min. DNA oligonucleotides complementary to the miRNA sequence were synthesized (Sangon). The 5′ ends of the DNA probes were labeled with [γ-32P]ATP (Yahui) using T4 polynucleotide kinase (Takara). The membrane was prehybridized in hybridization buffer (5× SSC, 20 mm NaH2PO4, pH 7.2, 7% SDS (w/v), and 2× Denhardt’s solution) for at least 30 min and then was hybridized overnight at 42°C. After washing three times with 2× SSPE/0.1% SDS (w/v) at room temperature, the membrane was exposed to a phosphor screen and visualized using a Typhoon variable mode imager (Amersham Biosciences). The probe used for the northern-blot analysis was as follows: miR408, 5′-GCCAGGGAAGAGGCAGTGCAG-3′.
qRT-PCR Analysis
Total RNA from rice was reverse transcribed using the PrimeScript RT Reagent Kit (Takara). Real-time PCR was carried out using SYBR Premix Ex Taq (Takara) to detect PCR products. ACTIN2 and U6 were chosen as the reference genes. Real-time PCR was performed according to the manufacturer’s instructions (Takara). Relative expression levels were analyzed using the 2−∆∆Ct method. The data represent means ± sd of three independent experiments. The primers were as follows: for ACTIN2, 5′-TCTTACGGAGGCTCCACTTAAC-3′ and 5′-TCCACTAGCATAGAGGGAAAGC-3′; for U6, 5′-TACAGATAAGATTAGCATGGCCCC-3′ and 5′-GGACCATTTCTCGATTTGTACGTG-3′; for OsUCL8, 5′-TCGTCGTGAGCTGCATCTTC-3′ and 5′-ACCTGAACACGAGGATGTCG-3′; for miR408, 5′-TGCAGCTGCACTGCCTCTTC-3′ and 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCCAGG-3′; for PPDK2, 5′-TGCGGCTGCTGGAATTCTTA-3′ and 5′-TGACCTCTCCAGTCGAACCA-3′; for PSII-PsbR1, 5′-GGTAGCCAAGGGAAAGGGTC-3′ and 5′-AATAGCGCCGCCAACTAGAA-3′; for PSII-PsbR2, 5′-GATTGGCCATGCTGGCTTTT-3′ and 5′-CTCGGGATGATGATGTCGCC-3′; for chlorophyll a/b-binding1, 5′-TGGTGGTCTGTGGTTTGACC-3′ and 5′-TGGAACCAAGCTCCCATGAC-3′; for chlorophyll a/b-binding2, 5′-TTTCCGCAGGACTGGCTTAG-3′ and 5′-GAGAAGAAGAGCCCCGTGAG-3′; for PSI-PsaO, 5′-GTCATGGCCTCTTGTGCTCT-3′ and 5′-ACCGTCTTTCAGGTTCGGTC-3′; and for HXK1, 5′-ATCGCCAAGCTACACCCATC-3′ and 5′-TCGTCGAACTCCGTCAATGG-3′.
Plot Field Experiment
Plants of the wild type, OXmiR408, OXUCL8, ucl8, and UCL8-RNAi were grown under natural conditions in Guangzhou and Zhuhai in China. The planting density was 15 cm × 25 cm, with one plant per hill. Each plot contained 8 × 8 plants. We chose four OXmiR408 lines and four OXUCL8 lines with different levels of OsmiR408 and OsUCL8, respectively, and each line was divided into eight plots. Wild-type, ucl8, and UCL8-RNAi plants also were divided into eight plots. The grain yield per plant and grain yield per plot were determined when the seeds were harvested. Data are from a randomized complete block design with eight replications. Values are means ± sd.
Histochemical GUS Staining
A binary vector, pCAMBIA1303, was used to generate the GUS-OsmiR408 and GUS-OsUCL8 transgenic lines. The ∼2,000-bp region upstream of OsmiR408 or OsUCL8 was used as the promoter region to control the expression of the GUS gene. GUS activity in the transgenic plants was localized by histochemical staining with 5-bromo-4-chloro-3-indolyl-β-d-GlcA. Transgenic plants were cut and incubated overnight at 37°C in staining buffer [1 mm 5-bromo-4-chloro-3-indolyl-β-d-GlcA, 100 mm sodium phosphate (pH 7), 10 mm EDTA, 0.5 mm K4Fe(CN)6, 0.5 mm K3Fe(CN)6, and 0.1% (v/v) Triton X-100] and then destained in 70% ethanol before photographing.
In Situ Hybridization
RNA in situ hybridization was performed as described previously with minor modifications (Kouchi and Hata, 1993). Briefly, plant materials were fixed in FAA (formaldehyde – acetic acid – ethanol) fixative for 8 h at 4°C after vacuum infiltration, dehydrated using a graded ethanol series followed by a xylene series, and embedded in Paraplast Plus (Sigma-Aldrich). Microtome sections (8 μm) were mounted on Probe-On Plus microscope slides (Fisher). The 214-bp region of OsUCL8 was amplified with the primers 5′-TGTTCAGGTACTTGCCGTGG-3′ and 5′-TGTTTCTCCCTCAGTCACACG-3′, subcloned into the pEASY-T3 (TransGen Biotech) vector, and used as the template to generate sense and antisense RNA probes. The antisense probe was transcribed using T7 RNA polymerase, and the sense probe was synthesized using SP6 RNA polymerase. Digoxigenin-labeled RNA probes were prepared using the DIG RNA Labeling Kit (SP6/T7; Roche) according to the manufacturer’s instructions. The custom 5′ and 3′ double-labeled LNA (locked nucleic acid)-modified oligonucleotides (EXIQON; 5′-CUGCACUGCCUCUUCCCUGGC-3′) were used to detect OsmiR408-3p produced from miRNA precursors. A scrambled miRNA control probe was used as a negative control. Photomicrographs were taken using a bright-field microscope (Leica DM5000B).
Subcellular Localization
Two-week-old rice shoots were used to isolate protoplast. A bundle of rice plants (approximately 30 seedlings) was cut together into approximately 0.5-mm strips with propulsive force using sharp razors. The strips were incubated in an enzyme solution (1.5% cellulose RS, 0.75% macerozyme R-10, 0.6 m mannitol, 10 mm MES at pH 5.7, 10 mm CaCl2, and 0.1% BSA) for 4 to 5 h in the dark with gentle shaking (40–50 rpm). After the enzymatic digestion, an equal volume of W5 solution (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, and 2 mm MES at pH 5.7) was added, followed by shaking (60–80 rpm) for 30 min. Protoplasts were released by filtering through 40-μm nylon mesh into round-bottom tubes and washing the strips with W5 solution three to five times. The pellets were collected by centrifugation at 800 rpm for 3 min in a swinging bucket. After washing once with W5 solution, the pellets were then resuspended in MMG solution (0.4 m mannitol, 15 mm MgCl2, and 4 mm MES at pH 5.7) at a concentration of 2 × 106 cells mL−1. Polyethylene glycol-mediated transfections were carried out as described previously (Zhang et al., 2011). Protoplasts were observed using a confocal laser scanning microscope (Zeiss 7 DUO NLO) at 488- and 561-nm excitation. All manipulations described above were performed at room temperature.
Measurement of Chloroplast Copper Content
Leaves from plants of different genotypes were harvested and weighed, then washed twice with double-distilled water. The plant material was then digested in 1% nitric acid (Sigma-Aldrich) and used directly for copper analysis with inductively coupled plasma-atomic emission spectroscopy as described previously (Zhang et al., 2014).
Chloroplasts were isolated from rice leaves as described previously (Kubis et al., 2008). Plant tissues were homogenized in chloroplast isolation buffer (0.3 m sorbitol, 5 mm MgCl2, 5 mm EGTA, 5 mm EDTA, 20 mm HEPES/KOH, pH 8, and 10 mm NaHCO3). The extract was filtered through two layers of Miracloth to remove cell debris. Then, 6 mL of the filtrate was layered on a discontinuous Percoll density gradient solution (top layer, 3 mL of 40%; bottom layer, 1 mL of 80%) containing isolation buffer and centrifuged at 8,000g for 10 min at 4°C. A band at the 40%-80% interface, which contains intact chloroplasts, was collected. Afterward, intact chloroplasts were measured using 40 mg of chlorophyll. The intact chloroplasts were digested in 1% nitric acid and used directly for copper analysis with inductively coupled plasma mass spectrometry.
Photosynthesis
Photosynthesis rates of flag leaves were measured using an infrared gas analyzer (Li-6400XT; LI-COR) with a 2-cm2 leaf chamber fluorescent light head (6400-40; LI-COR). Light-response curves were constructed at 28°C at relative humidity greater than 60% in a saturating chamber with 400 µL L−1 CO2 under light densities of 2,200, 2,000, 1,800, 1,600, 1,400, 1,200, 1,000, 800, 600, 400, 200, 150, 100, 50, 20, 10, and 0 µmol photons m−2 s−1. The electron transport rate and PSII quantum yield were measured in a fluorescence chamber with CO2 of 400 µL L−1 under the light density of 600 µmol photons m−2 s−1. The data were collected with 30 individuals for each genotype.
Measurement of Glc, Fru, and Starch Content
The Glc and Fru content was measured by gas chromatography-mass spectrometry as described previously (Lisec et al., 2006). Generally, 100 mg (fresh weight) of flag leaves and spikelets was harvested and ground into powder in liquid nitrogen. Then, 700 µL of methanol and 200 µL of 0.2 mg mL−1 ribitol (Sigma-Aldrich) were added to the powder. The mixture was shaken at 950 rpm at 70°C for 10 min. After centrifugation at 11,000g for 10 min, the supernatant was transferred to a new tube and dried. Forty microliters of methoxyamination reagent was added at 37°C and shaken for 2 h. Afterward, 40 µL of N-methyl-N-(trimethylsilyl)trifluoroacetamide was added, and the mixture was incubated at 37°C for 30 min. Gas chromatography-mass spectrometry analysis was performed using an Agilent 7890A series device. The gas chromatograph was combined with a quadrupole mass selective detector (Agilent). Starch was measured using a starch assay kit (product no. SA20-1KT; Sigma-Aldrich).
Accession Numbers
Sequence data from this article can be found in the NCBI SRA data libraries under accession number: BioProject PRJNA412295 (including six biosamples: SAMN07709121, SAMN07709122, SAMN07709123, SAMN07709124, SAMN07709125, SAMN07709126).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The cleavage site of OsmiR408 on the OsUCL8 mRNA, the expression levels of OsmiR408 and OsUCL8 in different transgenic lines, and the validation of the PC antibody.
Supplemental Figure S2. Hierarchical ontology tree of the differentially expressed genes in wild-type, OXmiR408, and OXUCL8 seedlings.
Footnotes
This research was supported by the National Natural Science Foundation of China (grant nos. 91640202 and 91335104), the Science and Technology Project (grant no. 2014ZX0800934B), and grants from Guangdong Province (grant nos. 2014T70833 and 2016A030308015) and Guangzhou Science and Technology Project (grant no. 201707020018).
References
- Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283: 15932–15945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Bashir K, Rasheed S, Kobayashi T, Seki M, Nishizawa NK (2016) Regulating subcellular metal homeostasis: the key to crop improvement. Front Plant Sci 7: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182: 799–816 [DOI] [PubMed] [Google Scholar]
- Cao J, Li X, Lv Y, Ding L (2015) Comparative analysis of the phytocyanin gene family in 10 plant species: a focus on Zea mays. Front Plant Sci 6: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Liu Q, Zhang YC, Qu LH, Chen YQ, Gautheret D (2011) Genome-wide discovery and analysis of microRNAs and other small RNAs from rice embryogenic callus. RNA Biol 8: 538–547 [DOI] [PubMed] [Google Scholar]
- Dong J, Kim ST, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112: 1164–1171 [DOI] [PubMed] [Google Scholar]
- Grotz N, Guerinot ML (2006) Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta 1763: 595–608 [DOI] [PubMed] [Google Scholar]
- Guzzi R, Sportelli L, Sato K, Cannistraro S, Dennison C (2008) Thermal unfolding studies of a phytocyanin. Biochim Biophys Acta 1784: 1997–2003 [DOI] [PubMed] [Google Scholar]
- Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41: 494–497 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, et al. (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet 42: 541–544 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14: 787–799 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57: 19–53 [DOI] [PubMed] [Google Scholar]
- Khan JA, Wang Q, Sjölund RD, Schulz A, Thompson GA (2007) An early nodulin-like protein accumulates in the sieve element plasma membrane of Arabidopsis. Plant Physiol 143: 1576–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100: 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Hata S (1993) Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet 238: 106–119 [DOI] [PubMed] [Google Scholar]
- Kubis SE, Lilley KS, Jarvis P (2008) Isolation and preparation of chloroplasts from Arabidopsis thaliana plants. Methods Mol Biol 425: 171–186 [DOI] [PubMed] [Google Scholar]
- Li S, Gao F, Xie K, Zeng X, Cao Y, Zeng J, He Z, Ren Y, Li W, Deng Q, et al. (2016) The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol J 14: 2134–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li W, Huang B, Cao X, Zhou X, Ye S, Li C, Gao F, Zou T, Xie K, et al. (2013) Natural variation in PTB1 regulates rice seed setting rate by controlling pollen tube growth. Nat Commun 4: 2793. [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Luo YC, Zhou H, Li Y, Chen JY, Yang JH, Chen YQ, Qu LH (2006) Rice embryogenic calli express a unique set of microRNAs, suggesting regulatory roles of microRNAs in plant post-embryogenic development. FEBS Lett 580: 5111–5116 [DOI] [PubMed] [Google Scholar]
- Ma H, Zhao H, Liu Z, Zhao J (2011) The phytocyanin gene family in rice (Oryza sativa L.): genome-wide identification, classification and transcriptional analysis. PLoS ONE 6: e25184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, et al. (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8: 1274–1284 [DOI] [PubMed] [Google Scholar]
- Mao H, Sun S, Yao J, Wang C, Yu S, Xu C, Li X, Zhang Q (2010) Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc Natl Acad Sci USA 107: 19579–19584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M (2010) OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42: 545–549 [DOI] [PubMed] [Google Scholar]
- Mutum RD, Balyan SC, Kansal S, Agarwal P, Kumar S, Kumar M, Raghuvanshi S (2013) Evolution of variety-specific regulatory schema for expression of osa-miR408 in indica rice varieties under drought stress. FEBS J 280: 1717–1730 [DOI] [PubMed] [Google Scholar]
- Nersissian AM, Immoos C, Hill MG, Hart PJ, Williams G, Herrmann RG, Valentine JS (1998) Uclacyanins, stellacyanins, and plantacyanins are distinct subfamilies of phytocyanins: plant-specific mononuclear blue copper proteins. Protein Sci 7: 1915–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén LG, Hunt LT (1993) Evolution of protein complexity: the blue copper-containing oxidases and related proteins. J Mol Evol 36: 41–66 [DOI] [PubMed] [Google Scholar]
- Sato K, Crowley PB, Dennison C (2007) Transient protein interactions between cytochrome f and the phytocyanin umecyanin. ChemBioChem 8: 732–735 [DOI] [PubMed] [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39: 623–630 [DOI] [PubMed] [Google Scholar]
- Sunkar R, Girke T, Jain PK, Zhu JK (2005) Cloning and characterization of microRNAs from rice. Plant Cell 17: 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Zhang S, Yu Y, Luo YC, Liu Q, Ju C, Zhang YC, Qu LH, Lucas WJ, Wang X, et al. (2014) MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol J 12: 1132–1142 [DOI] [PubMed] [Google Scholar]
- Xue LJ, Zhang JJ, Xue HW (2009) Characterization and expression profiles of miRNAs in rice seeds. Nucleic Acids Res 37: 916–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR, Ding ZH, Zhang YS, Yu SB, Xing YZ, et al. (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4: 319–330 [DOI] [PubMed] [Google Scholar]
- Yoshizaki M, Furumoto T, Hata S, Shinozaki M, Izui K (2000) Characterization of a novel gene encoding a phytocyanin-related protein in morning glory (Pharbitis nil). Biochem Biophys Res Commun 268: 466–470 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao X, Li J, Cai H, Deng XW, Li L (2014) MicroRNA408 is critical for the HY5-SPL7 gene network that mediates the coordinated response to light and copper. Plant Cell 26: 4933–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, et al. (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YC, Yu Y, Wang CY, Li ZY, Liu Q, Xu J, Liao JY, Wang XJ, Qu LH, Chen F, et al. (2013) Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat Biotechnol 31: 848–852 [DOI] [PubMed] [Google Scholar]
- Zhao XY, Hong P, Wu JY, Chen XB, Ye XG, Pan YY, Wang J, Zhang XS (2016) The tae-miR408-mediated control of TaTOC1 gene transcription is required for the regulation of heading time in wheat. Plant Physiol 170: 1578–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MG, Gu LF, Li PC, Song XW, Wei LY, Chen ZY, Cao XF (2010) Degradome sequencing reveals endogenous small RNA targets in rice (Oryza sativa L. ssp. indica). Front Biol 5: 67–90 [Google Scholar]
- Zhu QH, Spriggs A, Matthew L, Fan L, Kennedy G, Gubler F, Helliwell C (2008) A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res 18: 1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]