Rosemary leaves contain two phenolic diterpenes, carnosic acid and carnosol, which provide protection against oxidative stress by distinct mechanisms involving ROS scavenging or inhibition of lipid oxidation.
Abstract
Carnosic acid, a phenolic diterpene specific to the Lamiaceae family, is highly abundant in rosemary (Rosmarinus officinalis). Despite numerous industrial and medicinal/pharmaceutical applications of its antioxidative features, this compound in planta and its antioxidant mechanism have received little attention, except a few studies of rosemary plants under natural conditions. In vitro analyses, using high-performance liquid chromatography-ultraviolet and luminescence imaging, revealed that carnosic acid and its major oxidized derivative, carnosol, protect lipids from oxidation. Both compounds preserved linolenic acid and monogalactosyldiacylglycerol from singlet oxygen and from hydroxyl radical. When applied exogenously, they were both able to protect thylakoid membranes prepared from Arabidopsis (Arabidopsis thaliana) leaves against lipid peroxidation. Different levels of carnosic acid and carnosol in two contrasting rosemary varieties correlated with tolerance to lipid peroxidation. Upon reactive oxygen species (ROS) oxidation of lipids, carnosic acid was consumed and oxidized into various derivatives, including into carnosol, while carnosol resisted, suggesting that carnosic acid is a chemical quencher of ROS. The antioxidative function of carnosol relies on another mechanism, occurring directly in the lipid oxidation process. Under oxidative conditions that did not involve ROS generation, carnosol inhibited lipid peroxidation, contrary to carnosic acid. Using spin probes and electron paramagnetic resonance detection, we confirmed that carnosic acid, rather than carnosol, is a ROS quencher. Various oxidized derivatives of carnosic acid were detected in rosemary leaves in low light, indicating chronic oxidation of this compound, and accumulated in plants exposed to stress conditions, in parallel with a loss of carnosic acid, confirming that chemical quenching of ROS by carnosic acid takes place in planta.
Carnosic acid is a labdane-type diterpene present in plant species of the Lamiaceae family, such as rosemary (Rosmarinus officinalis) and common salvia (Salvia officinalis; Hossain et al., 2010; Birtić et al., 2015). This lipid-soluble compound is recognized for its high antioxidative capacities, which have led to many industrial applications in the fields of foods and beverages, personal care, nutrition, and health (Birtić et al., 2015). The antioxidant properties of carnosic acid, presumably due to the presence of a catechol moiety (Supplemental Fig. S1), were evaluated mainly in vitro in a large variety of artificial and/or model systems. For instance, when tested in bulk and emulsified lipid systems, carnosic acid was found to protect fatty acids and triglycerides against oxidation (Hopia et al., 1996; Cuvelier et al., 1996). Carnosic acid also was observed to prevent low-density lipoprotein oxidation in human aortic endothelial cells (Pearson et al., 1997) and lipid hydroperoxide-mediated oxidative stress in Caco-2 cells (Wijeratne and Cuppett, 2007). Inhibition of lipid peroxidation by carnosic acid was reported in rat liver microsomes and ox brain phospholipid liposomes (Aruoma et al., 1992; Romo Vaquero et al., 2013). Food material such as oil, raw and cooked meat, and cooked meat patties were protected from oxidation by carnosic acid, in most cases with a higher efficiency than synthetic antioxidants (Erkan et al., 2008; Zhang et al., 2010; Naveena et al., 2013; Jordán et al., 2014). Carnosic acid also was described as a scavenger of hydroxyl and 2,2-diphenyl-1-picrylhydrazyl radicals (Aruoma et al., 1992; Luis and Johnson, 2005). While the potential antioxidative activity of carnosic acid is well documented, its exact mechanism of action has not been studied extensively. In particular, little is known of the interactions of carnosic acid with distinct reactive oxygen species (ROS) or lipid radicals. Moreover, in most studies, in vitro oxidation was generated by prolonged and artificial heating treatments, so that it is difficult to extrapolate the results to the in vivo situation in plants. Surprisingly, the role of carnosic acid in plant leaves has received little attention, and the biological role of this compound in plants is not firmly established.
Carnosic acid is present at very high concentrations, up to several percent of dry weight, in leaves of the Mediterranean half-shrub rosemary (Munné-Bosch and Alegre, 2001; del Baño et al., 2003; Luis and Johnson, 2005). Carnosic acid biosynthesis and accumulation take place exclusively in young rosemary leaves at the branch apices, with the diterpene molecule being partially consumed during leaf development and aging (Hidalgo et al., 1998; Brückner et al., 2014; Božić et al., 2015). Beside carnosic acid, less abundant phenolic diterpenes can be measured in rosemary leaves, including carnosol (Supplemental Fig. S1), the major oxidation product of carnosic acid. The antioxidative activity of the latter compound, produced spontaneously from carnosic acid by nonenzymatic reaction, has been seldom investigated (Aruoma et al., 1992; Zeng et al., 2001). Diterpene levels in field-grown rosemary plants displayed seasonal changes, with a tendency for carnosic acid losses in response to environmental stress conditions (Luis and Johnson, 2005). In particular, carnosic acid concentrations in rosemary leaves under natural conditions were found to decrease at high temperatures and low precipitation rates in summer with concomitant increases in oxidized derivatives, suggesting that cellular oxidative stress is accompanied by the consumption of carnosic acid (Munné-Bosch et al., 1999; Munné-Bosch and Alegre, 2003). Both carnosic acid and carnosol accumulate in photosynthetic green tissues only (leaves, sepals, and petals) and have be localized in the chloroplasts (Munné-Bosch and Alegre, 2001), although the synthesis of carnosic acid also has been reported in glandular trichomes (Brückner et al., 2014).
Because the functions of the major rosemary diterpenes in plant leaves are poorly understood, we performed a comprehensive study of the antioxidant activity of carnosic acid and its oxidized derivative carnosol, both in vitro and in rosemary plants. This study reveals different modes of action for carnosic acid and carnosol against ROS and lipid radicals, which make this diterpenoid tandem a peculiar and efficient antioxidant system in planta. It is likely that this carnosic acid-based protection mechanism is an important component in the ability of rosemary to withstand harsh climatic conditions that can prevail in its natural Mediterranean habitat.
RESULTS
Lipid Protection by Carnosic Acid and Carnosol in Vitro
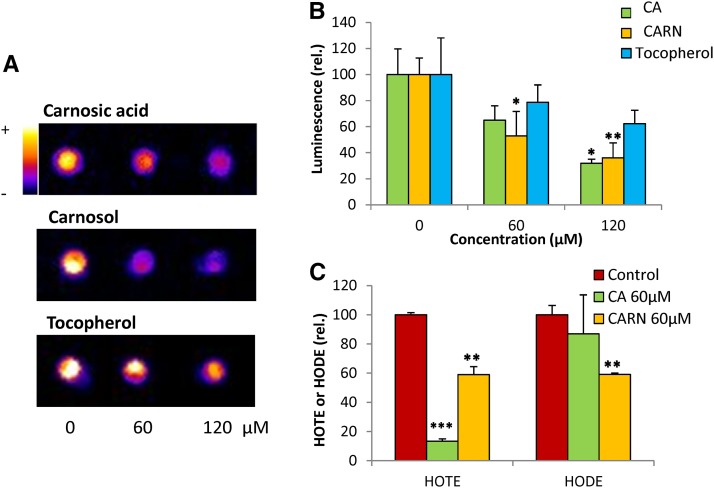
The lipid monogalactosyldiacylglycerol (MGDG), solubilized in methanol/chloroform, was oxidized with singlet oxygen (1O2) generated by illuminating the photosensitizing agent Methylene Blue. As expected (Birtić et al., 2011), the MGDG solution became luminescent after this oxidation treatment as imaged with a high-sensitivity cooled CCD camera (Fig. 1A). This photon emission originates from lipid peroxides whose slow decomposition produces light-emitting species such as triplet carbonyls and singlet oxygen, with the intensity of this signal being correlated with the extent of lipid peroxidation in the sample (Birtić et al., 2011; Cifra and Pospisil, 2014). When MGDG was supplemented with carnosic acid during 1O2 oxidation, the luminescence signal intensity was reduced noticeably (Fig. 1, A and B), indicating lower levels of MGDG oxidation and of lipid peroxides. Protection of MGDG against oxidation also was observed with carnosol and tocopherol, with the protective effect of the latter compound, however, appearing to be slightly lower than the protection provided by carnosol and carnosic acid. Lipid protection by carnosic acid and carnosol also was obtained when the experiments were done with linolenic acid (C18:3) instead of MGDG (Supplemental Fig. S2). Those observations show that, similar to tocopherols (Liebler et al., 1986), both carnosic acid and carnosol are lipid protectors against attack by 1O2. These effects were confirmed by analyzing hydroxyoctadecatrienoic acid (HOTE), an oxidation product of the main fatty acid in leaves, linolenic acid, and hydroxyoctadecadienoic acid (HODE), the oxidation product of linoleic acid, in the MGDG solution after 1O2 oxidation (Fig. 1C). Both HOTEs and HODEs were reduced substantially by carnosic acid and carnosol.
Figure 1.
Effects of carnosic acid, carnosol, and α-tocopherol on in vitro oxidation of lipids by 1O2. 1O2 was produced by a 30-min illumination of the lipid solution (MGDG) in the presence of Methylene Blue. 1O2 oxidation of MGDG was performed in the presence of 60 or 120 μm carnosic acid, carnosol, or α-tocopherol. A, Luminescence images of the oxidized solutions. The color palette indicates signal intensity from black (0) to white (highest values). B, Quantification of the luminescence signals. Data are normalized to the control signal values measured in the absence of antioxidant. C, Hydroxy fatty acid quantification (HOTE and HODE). Data are normalized to the control HOTE values measured in the absence of antioxidant. CA, Carnosic acid; CARN, carnosol. Asterisks indicate significant differences from control (0 μm) at P < 0.05 (*), P < 0.01 (**), and P < 0.005 (***) by Student’s t test.
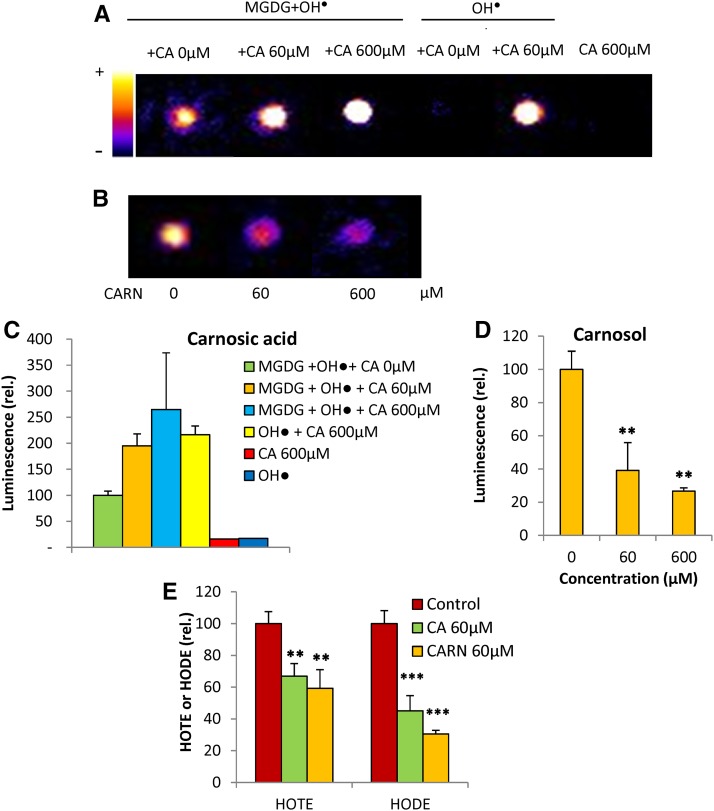
MGDG also was oxidized by hydroxyl radicals produced by hydrogen peroxide (H2O2) and iron (Fenton reaction), leading to luminescence emission (Fig. 2, A and B). The addition of carnosol to MGDG protects the galactolipid solution against oxidation, as shown by the marked decrease in luminescence (Fig. 2, B and D). Surprisingly, the addition of carnosic acid did not reduce MGDG luminescence after oxidation by hydroxyl radicals (Fig. 2, A and C). On the contrary, carnosic acid strongly increased luminescence, and this phenomenon was still observed when carnosic acid concentrations were increased up to 600 μm. Actually, this luminescence enhancement was observed to be due to carnosic acid itself, which became highly luminescent when incubated in the presence of free radicals (without lipid). We checked that the mixture H2O2 + iron or a solution of carnosic acid in the absence of any ROS was not luminescent. The data of Figure 2A thus suggest that carnosic acid reacts with free radicals, leading to its oxidation and to the formation of light-emitting derivatives. This is confirmed in Supplemental Figure S2: a drastic loss of carnosic acid occurred when lipids were oxidized by 1O2 or free radicals, whereas carnosol levels were less affected. As a consequence, the lipid protective action of carnosic acid cannot be assessed through autoluminescence measurements. HPLC analyses of HOTE and HODE levels can overcome this problem. The data shown in Figure 2 revealed that carnosic acid, similar to carnosol, does protect MGDG from oxidation by free radicals: the HOTE and HODE levels were reduced noticeably in the presence of carnosic acid or carnosol.
Figure 2.
Effects of carnosic acid and carnosol on in vitro oxidation of lipids by free radicals. Hydroxyl radicals were produced by the Fenton reaction using H2O2 + Fe2+ in the presence of 60 or 600 μm carnosic acid (CA) or carnosol (CARN). A, Luminescence imaging of MGDG oxidized by the hydroxyl radical in the presence or absence of carnosic acid (60 and 600 μm). The luminescence signals of the mixture H2O2 + iron (hydroxyl radicals) and of carnosol in the presence or absence of hydroxyl radicals also were measured as controls. B, Luminescence imaging of MGDG oxidized by hydroxyl radical in the presence of carnosol (60 and 600 μm). C, Quantification of the luminescence signals shown in A. Data are normalized to the signal values measured from oxidized MGDG in the absence of antioxidant. D, Quantification of the luminescence signals shown in B. Data are normalized to the signal values measured from oxidized MGDG in the absence of antioxidant. E, Hydroxy fatty acid quantification (HOTE and HODE). Data are normalized to the HOTE or HODE values measured in the absence of antioxidant. Asterisks indicate significant differences from control at P < 0.01 (**) and P < 0.005 (***) by Student’s t test.
Interactions of Carnosol and Carnosic Acid with ROS
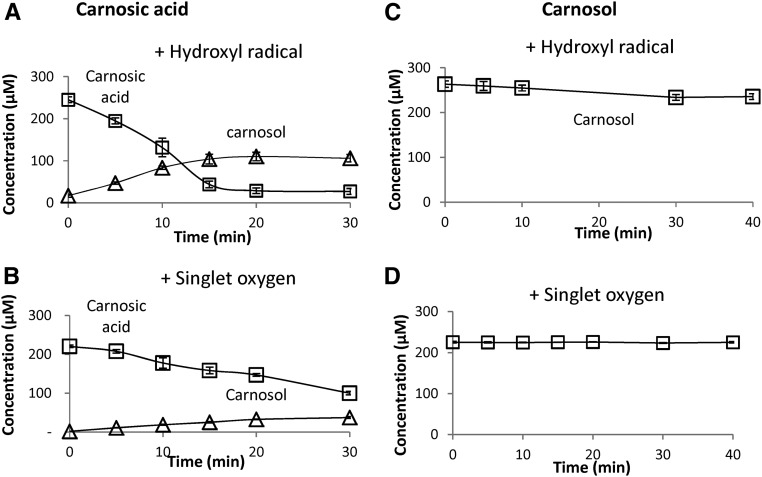
The oxidative degradation of carnosic acid by the hydroxyl radical is confirmed in Figure 3. When H2O2 and iron were added to a solution of carnosic acid, the diterpene concentration fell rapidly, and an accumulation of carnosol was observed in parallel (Fig. 3A). The same phenomena were found with 1O2, although the rates of carnosic acid disappearance and carnosol accumulation were slower compared with the effect of hydroxyl radicals. In striking contrast, carnosol was resistant to this oxidation: the carnosol concentration remained stable in the presence of hydroxyl radical or 1O2 (Fig. 3B). These findings indicate that carnosic acid has a high reactivity toward ROS and is easily oxidizable. Therefore, it is likely that the antioxidant activity of carnosic acid relies on chemical quenching of ROS.
Figure 3.
Time course of the changes in carnosic acid and carnosol concentrations upon exposure to 1O2 or hydroxyl radicals. 1O2 was produced by illumination of Methylene Blue, and hydroxyl radicals were produced by the Fenton reaction using H2O2 + Fe2+. A and B, Carnosic acid. C and D, carnosol.
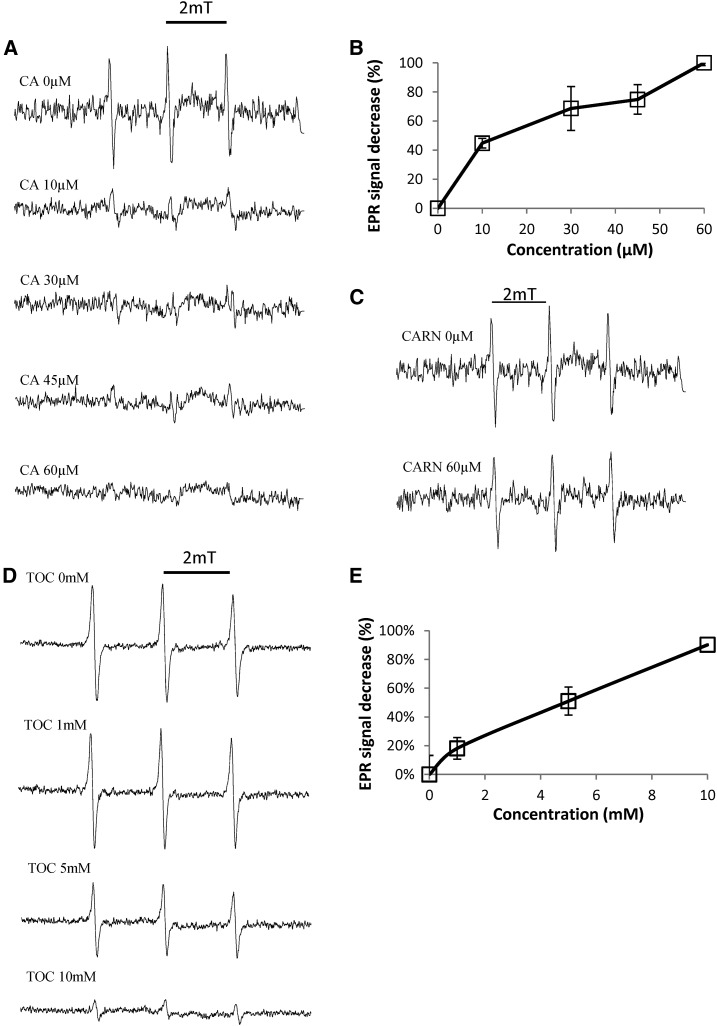
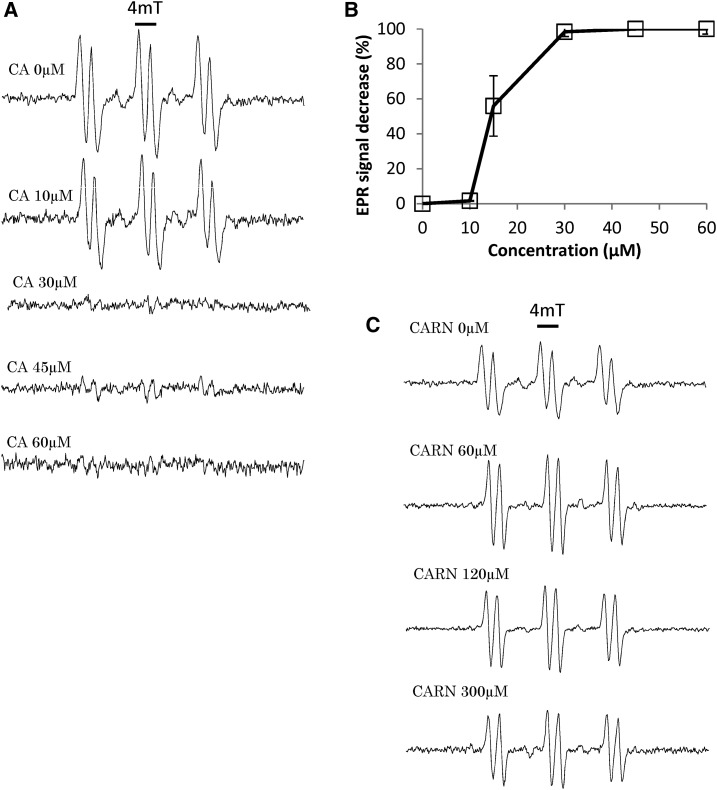
In Figure 4, 1O2 was produced from Rose Bengal in the light and was quantified using 2,2,6,6-tetramethyl-4-piperidone hydrochloride (TEMPD), a 1O2-specific spin probe (Hideg et al., 2011). The amplitude of the electron paramagnetic resonance (EPR) spectra of TEMPD was strongly reduced by carnosic acid, and this effect was visible even at the low concentration of 10 μm (Fig. 4, A and B). As expected from the data of Figure 3, EPR analyses showed that carnosol does not quench 1O2: 60 μm carnosol had very little effect on the amplitude of the 1O2 EPR spectrum (Fig. 4C). This result confirms that carnosol is not able to eliminate 1O2 in the micromolar concentration range, although it protects lipids against oxidation in this concentration range. We also examined the effects of α-tocopherol, a known quencher of 1O2 (Foote et al., 1974; Di Mascio et al., 1990). The quenching effect of tocopherol was visible at concentrations in the millimolar range only (Fig. 4D), indicating that tocopherol is a less efficient 1O2 quencher than carnosic acid.
Figure 4.
1O2 quenching capacity of carnosic acid and carnosol. 1O2 was generated by a 5-min illumination of 100 µm Rose Bengal in the presence of the spin probe TEMPD. A, Effects of different concentrations of carnosic acid (CA) on the EPR spectra of TEMPD. B, Quantification of the decrease in the EPR signal amplitude induced by increasing concentrations of carnosic acid. C, Effects of carnosol (CARN) on the EPR spectra of TEMPD. D, Effects of a-tocopherol (TOC) on the EPR spectra of TEMPD. E, Quantification of the decrease in the EPR spectra by increasing concentrations of α-tocopherol.
The spin probe 4-pyridyl-1-oxide-N-tert-butylnitrone (POBN) was used to measure the hydroxyl radical by EPR spectroscopy (Hideg et al., 2011). Carnosic acid was able to quench this ROS (Fig. 5, A and B), while carnosol had virtually no effect on ROS concentration (Fig. 5C). Taken together, the data of Figures 4 and 5 confirm that carnosic acid and carnosol differ in their reaction with ROS, although both can protect lipids against ROS-induced lipid peroxidation (Fig. 1).
Figure 5.
Quenching of hydroxyl radicals by carnosic acid and carnosol. Hydroxyl radicals were generated by the Fenton reaction using H2O2 + Fe2+ in the presence of the spin probe POBN. A, Effects of different concentrations of carnosic acid (CA) on the EPR spectra of POBN. B, Quantification of the decrease in the EPR signal amplitude induced by increasing concentrations of carnosic acid. C, Effects of carnosol (CARN) on the EPR spectra of POBN.
Using liquid chromatography coupled with mass spectrometry (LC-HRMS), we characterized oxidized derivatives (besides carnosol) generated during the in vitro oxidation of carnosic acid by 1O2 or hydroxyl radical in solution (Supplemental Fig. S3). Authentic standards were used to determine the retention times, mass-to-charge ratios (m/z), full mass spectra, and tandem mass spectrometry (MS/MS) spectra of carnosic acid and carnosol (Supplemental Figs. S4 and S5), allowing unambiguous identification of those compounds in oxidized solutions and in leaf extracts. Oxidation of carnosic acid was confirmed by a decrease in the carnosic acid peak and a concomitant production of carnosol. A variety of compounds obtained by the oxidation of carnosic acid by 1O2 and by hydroxyl radical was detected. There was a strong overlap between the oxidation profiles of carnosic acid induced by 1O2 and by the hydroxyl radical. Structures of rosmanol, isorosmanol, and 12-o-methyl carnosic acid were confirmed by matching their retention times and MS/MS spectra with those of the reference compounds (Supplemental Figs. S6–S8), while rosmaridiphenol, 11′12-o-methylrosmanol, 7-methylisorosmanol, isorosmanol, rosmadial isomers, and 5,6,7,10-tetrahydroxyrosmariquinone were putatively identified by matching bibliography data with the retention times and MS/MS spectra obtained experimentally with oxidized carnosic acid solutions and with rosemary leaf extracts (Supplemental Figs. S9–S13).
Carnosic Acid and Carnosol Levels in Rosemary Leaves
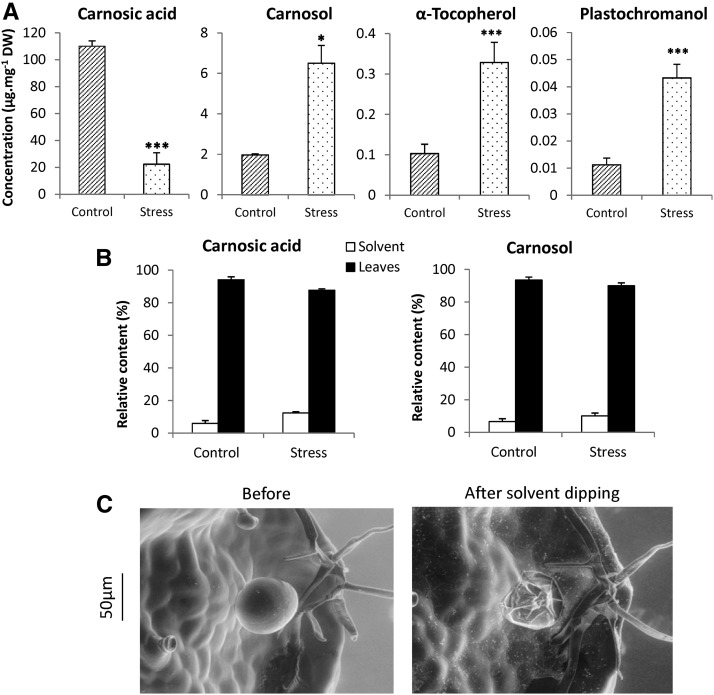
Rosemary leaves are known to accumulate high amounts of carnosic acid (Birtić et al., 2015), as confirmed in Figure 6A for young leaves of the Sudbury Blue variety, in which carnosic acid represented up to 10% of leaf dry weight under control growth conditions (250 μmol photons m−2 s−1 and 25°C). The major oxidized derivative of carnosic acid, carnosol, was less abundant (∼2 μg mg−1 dry leaf weight), nevertheless representing about 0.2% of dry weight. We also analyzed the prenyl lipids, tocopherols and plastochromanol, which are both ubiquitous plastid antioxidants (Kruk et al., 2014, 2016). Both compounds were found in rosemary leaves at concentrations noticeably lower than carnosol: approximately 0.1 and 0.01 μg mg−1, respectively.
Figure 6.
Carnosic acid and carnosol in leaves of rosemary plants (Sudbury Blue variety) grown under two different conditions of light and temperature (250 μmol photons m−2 s−1 at 25°C/15°C day/night [control] or 1,200 μmol photons m−2 s−1 at 35°C/5°C [stress]). A, Carnosic acid, carnosol, α-tocopherol, and plastochromanol-8 concentrations in leaves. DW, Dry weight. Asterisks indicate significant differences from control at P < 0.05 (*) and P < 0.005 (***) by Student’s t test. B, Carnosic acid and carnosol were measured in leaves after organic solvent dipping and in the organic solvent after leaf dipping (representing the compounds stored in the trichomes). C, Glandular trichomes before and after solvent dipping.
Growing rosemary plants for 4 weeks under harsh conditions of light and temperature (1,200 μmol photons m−2 s−1 and 35°C/5°C [day/night]) led to a strong decrease in carnosic acid compared with control conditions (Fig. 6A). Concomitantly, the loss of carnosic acid was associated with a marked increase (about 3×) in carnosol levels, suggesting consumption of the former compound during its antioxidant activity under stress conditions with partial conversion to its oxidized metabolite carnosol. A strong accumulation of tocopherols and plastochromanol also was observed after exposure of rosemary plants to high light and heat, thus exhibiting a behavior that contrasts with that of carnosic acid. This contrasting response was observed previously for carnosic acid and α-tocopherol in sage (Salvia officinalis) and rosemary exposed to natural drought conditions (Munné-Bosch and Alegre, 2003).
Carnosic acid and carnosol are present in photosynthesizing green tissues only (Munné-Bosch and Alegre, 2001; Luis and Johnson, 2005) and, in leaves, they have been found in the chloroplasts (Munné-Bosch and Alegre, 2001). However, carnosic acid and carnosol also have been reported to partition between trichomes at the leaf surface and internal leaf tissues (Božić et al., 2015). This partitioning was estimated by briefly washing rosemary leaves (for 30 s) with dichloromethane in order to extract hydrophobic compounds from the trichomes. As shown in Figure 6C, this treatment caused a complete emptying of the glandular trichomes while epidermal cells remained unaltered. The solvent after leaf dipping was found to contain both carnosic acid and carnosol (Fig. 6B), indicating storage of those compounds in the trichomes. However, the amounts of diterpenes present in this fraction were relatively small, representing less than 10% of total amounts. Thus, in the Sudbury Blue variety investigated here, carnosic acid and carnosol are stored mainly within the leaves. This partitioning of carnosic acid and carnosol between trichomes and internal leaf tissues was not modified significantly by growth in high light at high temperature (Fig. 6B).
Oxidized Derivatives of Carnosic Acid in Planta
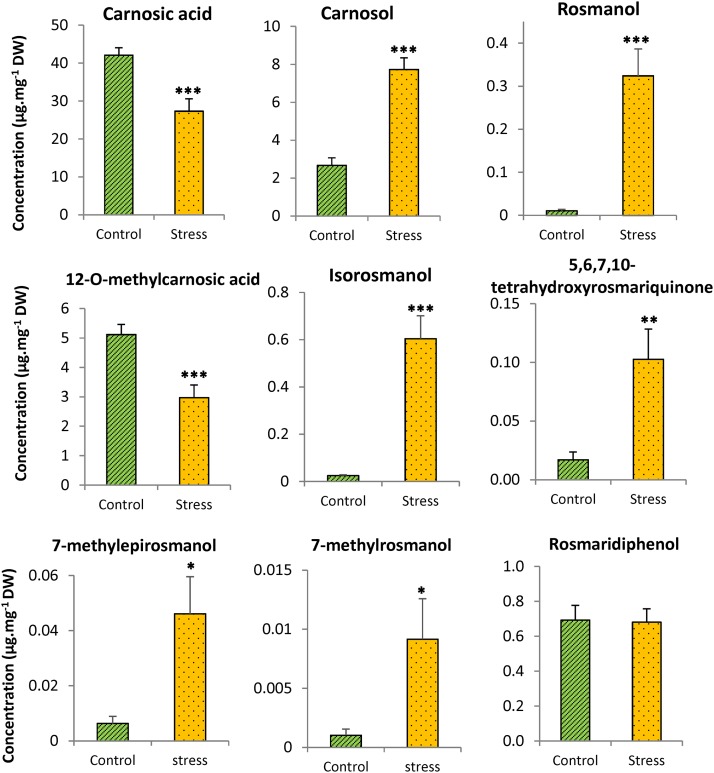
Some of the compounds detected in vitro after ROS oxidation of carnosic acid (Supplemental Fig. S3) also were found in rosemary leaves grown under control conditions: rosmanol, isorosmanol, rosmaridiphenol, 7-methyl-epirosmanol, 7-methyl-rosmanol, 12-o-methylcarnosic acid, and 5,6,7,10-tetrahydroxyrosmariquinone (Fig. 7; Supplemental Figs. S4–S12), indicating chronic oxidation of carnosic acid by ROS in leaves. In line with this conclusion, a 30-h adaptation of rosemary plants to darkness brought about a strong decrease in those compounds (Supplemental Fig. S14), confirming the link with light and the associated ROS production in the chloroplasts.
Figure 7.
Oxidation products of carnosic acid in planta. Rosemary plants were grown under two different conditions (control and stress, described in the legend of Fig. 6). The carnosic acid metabolites were measured in rosemary leaves by ultra-performance liquid chromatography-MS. Quantification of the metabolites for which no standard is available was done using the conversion factor of carnosic acid. DW, Dry weight. Asterisks indicate significant differences at P < 0.05 (*), P < 0.01 (**), and P < 0.005 (***) by Student’s t test.
Under stress conditions that caused a strong decrease in carnosic acid and a concomitant accumulation of carnosol (Fig. 6), the levels of several oxidation products of carnosic acid, including rosmanol, isorosmanol, 5,6,7,10-tetrahydroxyrosmariquinone, 7-methyl-epirosmanol, and 7-methyl-rosmanol, increased strongly in rosemary leaves (Fig. 7). The concentration of other oxidized metabolites of carnosic acid, such as rosmaridiphenol and 12-o-methylcarnosic acid, did not increase with the stress conditions. The accumulation of rosmanol and isorosmanol, as well as of methylated isorosmanol, was reported previously in rosemary plants exposed to drought stress in the field (Munné-Bosch et al., 1999). The accumulation of oxidized derivatives in rosemary leaves exposed to high light and high temperature supports the idea that the loss of carnosic acid observed under those conditions (Fig. 6) resulted from its oxidative degradation by ROS.
Exogenous Carnosic Acid and Carnosol Protect Thylakoid Membranes
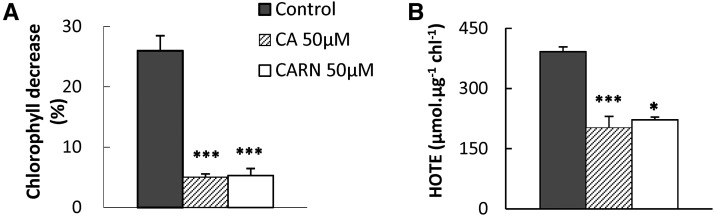
Supplementing chloroplast membranes with carnosic acid was shown previously to preserve α-tocopherol and to reduce oxidative damage in high light (Munné-Bosch and Alegre, 2003). Moreover, a marked consumption of the exogenously applied carnosic acid was observed during the high-light treatment. We performed a similar experiment with thylakoid membranes prepared from leaves of Arabidopsis (Arabidopsis thaliana), a species that does not contain carnosic acid, supplemented with carnosol or carnosic acid. Thylakoid suspensions were exposed for 2 h to high light (3,000 μmol photons m−2 s−1), causing photooxidative damage, including the loss of chlorophyll and the accumulation of lipid peroxidation products (HOTE; Fig. 8). Adding carnosic acid or carnosol to the membrane suspensions noticeably reduced photooxidative damage: loss of chlorophyll was reduced to 5% (versus 30% in control samples) by carnosic acid and carnosol, and lipid peroxidation was very low. These results confirm that both carnosic acid and carnosol can protect biomembranes and function as membrane lipid protectors in vivo.
Figure 8.
Effects of carnosic acid or carnosol on Arabidopsis thylakoid membranes exposed to high light. Thylakoid suspensions were exposed to white light of photon flux density (PFD) 1,500 μmol photons m−2 s−1 for 2 h. A, Decrease in chlorophyll content after light treatment. B, HOTE with or without the addition of 50 μm carnosic acid (CA) or carnosol (CARN) to the membrane suspensions. Asterisks indicate significant differences from control at P < 0.05 (*) and P < 0.005 (***) by Student’s t test.
Direct Interaction of Carnosol with the Lipid Peroxidation Process
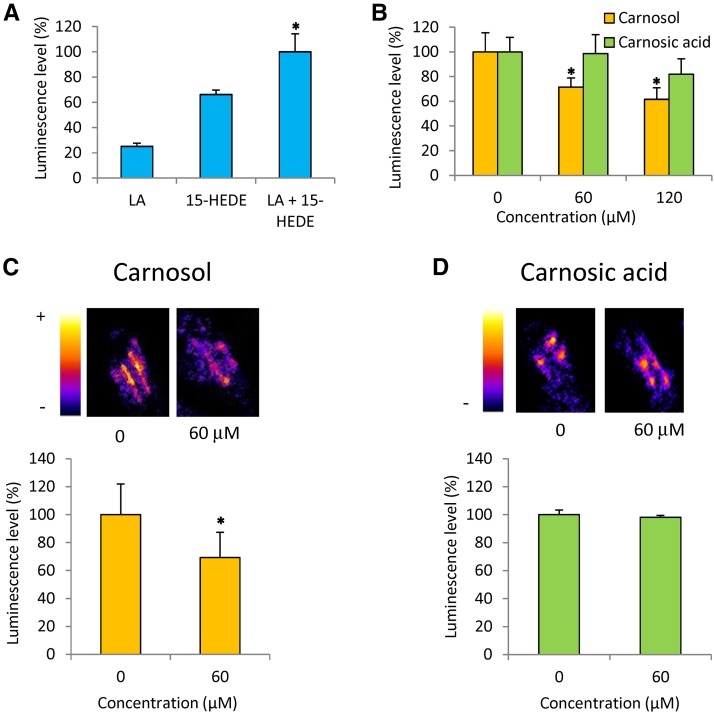
As shown above (Fig. 3), carnosol is resistant to direct oxidation by ROS. However, when exposure to ROS took place in a lipid environment (linolenic acid; Supplemental Fig. S2), some loss of carnosol was observed. This could suggest that carnosol has the capacity to interact directly with the lipid peroxidation mechanism itself and can be degraded by reactions with some lipid oxidation-derived products. It has been shown that lipid hydroperoxides are capable of inducing membrane damage and lipid peroxidation in cell cultures (Wijeratne and Cuppett, 2006). Based on this observation, in vitro oxidation of linolenic acid was triggered with a hydroxy fatty acid, 15-hydroxyeicosadienoic acid (15-HEDE), in darkness and in the absence of ROS or ROS generator. Luminescence from linolenic acid was noticeably increased by adding 15-HEDE (Fig. 9A), indicating oxidation of the fatty acid molecule. The luminescence of HEDE was found to be higher than that of linolenic acid (but lower than the linolenic acid + 15-HEDE combination), probably due to the spontaneous decomposition of the hydroxy fatty acid and the generation of light-emitting species. The addition of 60 or 120 μm carnosol to the mixture of linolenic acid + 15-HEDE decreased luminescence significantly (by 30% or 40%, respectively; Fig. 9B). This indicates that carnosol has a direct inhibitory effect on the lipid peroxidation process. We checked that carnosol had no effect of the 15-HEDE intrinsic luminescence (data not shown), excluding an action of the diterpene on 15-HEDE decomposition products. In contrast with carnosol, carnosic acid had no significant effect on the HEDE-induced oxidation of linolenic acid (Fig. 9B).
Figure 9.
Inhibition of ROS-independent lipid peroxidation by carnosol. A, Induction of linolenic acid (LA) oxidation by addition of the hydroxy fatty acid 15-HEDE in the dark, as measured by luminescence emission. B, Effects of 60 and 120 μm carnosol or carnosic acid on in vitro oxidation of linolenic acid induced by the addition of 15-HEDE. C and D, Effects of leaf infiltration with carnosol or carnosic acid on wounding-induced lipid peroxidation in Arabidopsis leaves in the dark, as measured by autoluminescence. Top images show autoluminescence emission of Arabidopsis leaves wounded with a scalpel. Leaves were preinfiltrated with a buffer (10 mm MES, pH 5.6, 10 mm MgSO4, and 1% dimethyl sulfoxide) containing 0 or 60 μm carnosic acid or carnosol. Bottom graphs show autoluminescence intensity of the wounds in leaves infiltrated with 0 or 60 μm carnosic acid or carnosol. Asterisks indicate significant differences from control (0 μm) at P < 0.05 by Student’s t test.
The effect of carnosic acid in ROS-independent lipid oxidation also was tested in vivo. Wounding is known to trigger lipoxygenase activity in leaves, causing enzymatic lipid peroxidation (Chauvin et al., 2013) and inducing the associated generation of photon emission (Birtić et al., 2011). In Figure 9C, leaves were injured with a scalpel in darkness. As shown previously (Birtić et al., 2011), the wounds can be visualized by the lipid oxidation-related luminescence emission. The intensity of this luminescence signal was decreased significantly in leaves preinfiltrated with carnosol compared with leaves preinfiltrated with a buffer that did not contain carnosol. Then, in line with the in vitro data shown in Figure 9B, carnosol can reduce lipid peroxidation in planta through a mechanism different from ROS scavenging. Similar to what we observed with linolenic acid oxidized by 15-HEDE, carnosic acid was unable to inhibit lipid peroxidation in wounded Arabidopsis leaves. Thus, taken together, our results show that the antioxidant activities of carnosic acid and carnosol rely on distinct mechanisms, involving direct interactions with ROS or with the lipid oxidation process, respectively.
Comparison of Two Rosemary Varieties Containing Different Concentrations of Carnosic Acid and Carnosol
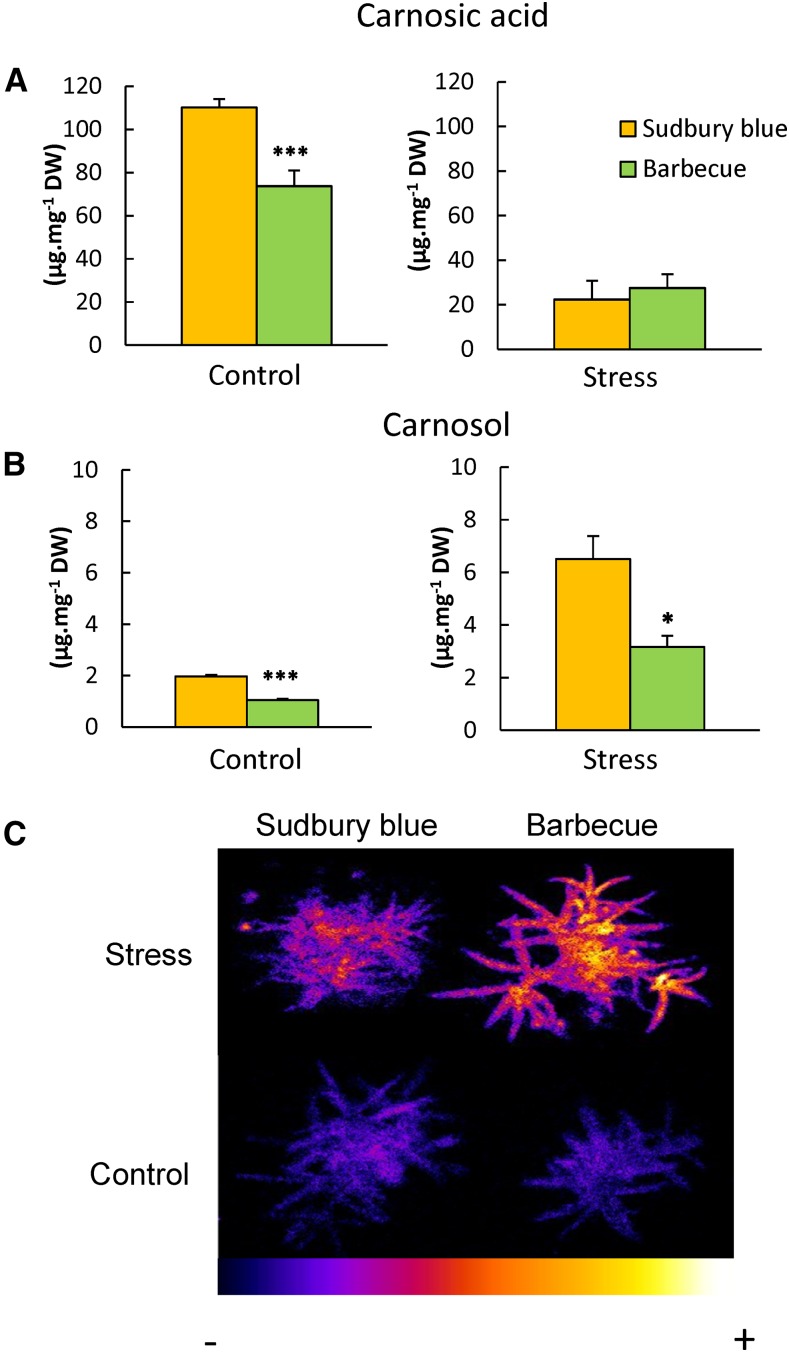
As shown in Figure 10A, leaves of the Barbecue variety contain substantially less carnosic acid and carnosol than Sudbury Blue. As expected from the data of Figure 6, exposure to high light at high-temperature conditions caused a drastic loss of carnosic acid in both varieties (Fig. 10A), which was accompanied by increased levels of carnosol (Fig. 10B). However, the latter effect was less pronounced in the Barbecue variety relative to Sudbury Blue. Photooxidative damage to lipids in plants grown in high light or in control conditions was visualized in both rosemary varieties by autoluminescence imaging (Fig. 10C). Interestingly, Barbecue plants exposed to stress conditions were noticeably more luminescent than Sudbury Blue plants, indicating more oxidative stress and lipid peroxidation in the former variety. The correlation found in the experiment of Figure 10 between the leaf content of carnosic acid and carnosol and the tolerance of rosemary to photooxidative stress is consistent with the lipid-protective functions of those diterpenes observed in vitro (Figs. 1 and 8). However, the differential tolerance of Sudbury Blue and Barbecue to photooxidative stress must be interpreted with caution, because the involvement of other factors in the responses of the two rosemary varieties cannot be excluded.
Figure 10.
Comparison of two rosemary varieties (Sudbury Blue and Barbecue) containing different amounts of carnosic acid. Control, Plants grown in low light; Stress, plants grown in high light at high temperature (see legends of Fig. 6). A, Carnosic acid. B, Carnosol. C, Autoluminescence imaging of lipid peroxidation in rosemary plants. DW, Dry weight. Asterisks indicate significant differences at P < 0.05 (*) and P < 0.005 (***) by Student’s t test.
DISCUSSION
This study has confirmed that the phenolic diterpene carnosic acid is a potent antioxidant and has shown that this compound can efficiently protect lipids from oxidation, both in vitro (lipid solutions) and in vivo (biomembranes). This study also provides some insights into the mechanism underlying the antioxidative activity of carnosic acid. This compound was found to have a very high reactivity toward ROS, being readily oxidized and converted into a variety of metabolites in this process. Thus, carnosic acid acts as a ROS scavenger that can eliminate toxic ROS through its oxidation. Both singlet oxygen, an excited form of oxygen, and free radicals can be scavenged by carnosic acid, giving rise to overlapping profiles of oxidized molecules. Oxidized derivatives of carnosic acid were observed in rosemary leaves, both under control and stress conditions, and prolonged adaptation of rosemary plants to darkness brought about a marked decrease in their concentrations. This indicates chronic oxidation of carnosic acid in plants in the light and suggests that carnosic acid plays a protective role, not only under excess light energy, when ROS production is expected to be elevated, but also in low light. This is in agreement with previous observations showing the presence of 1O2-specific degradation products of polyunsaturated fatty acids in plant leaves in low light, reflecting continuous generation of 1O2 in illuminated chloroplasts (Triantaphylidès et al., 2008). This phenomenon has led to the concept of lipid membranes acting as supramolecular antioxidants that capture ROS (Schmid-Siegert et al., 2016). This concept could be extended to the carnosic acid pool in rosemary leaves.
Interestingly, carnosol, the major oxidized metabolite of carnosic acid, was found to be an antioxidant and lipid protector as efficient as carnosic acid. This result is in line with early works that showed a protective effect of carnosol against lipid peroxidation in microsomal and liposomal systems (Aruoma et al., 1992). In previous studies, other carnosic acid-derived metabolites, such as rosmanol, epirosmanol, or rosmaridiphenol, also were found to possess some antioxidative capacities. For instance, carnosol, rosmanol, and epirosmanol were able to inhibit the oxidation of lipoproteins in vitro (Zeng et al., 2001). Methyl carnosate was reported to be even more active than carnosic acid in the protection of triglyceride emulsions at 60°C (Huang et al., 1996). Rosmanol and epirosmanol were reported to inhibit mitochondrial and microsomal lipid peroxidation (Haraguchi et al., 1995), and the antioxidative activity of rosmanol and 20-deoxocarnosol was observed using the 2,2-diphenyl-1-picrylhydrazyl antioxidant assay (Escuder et al., 2002). The in vitro antioxidant activity of rosmanol, epirosmanol, and isorosmanol was found to be higher than that of α-tocopherol (Nakatani and Inatani, 1984). Thus, when scavenging ROS, carnosic acid can generate a variety of secondary antioxidants. This cascade-type process is likely to amplify the antioxidative power of carnosic acid and to constitute an effective defense mechanism. Moreover, ROS scavenging by carnosic acid can be fueled by the very large pools of this compound (representing several percentages of leaf dry weight) that rosemary plants are able to accumulate in their leaves.
Carnosol was much more resistant to oxidation by ROS than carnosic acid, although it protected lipids from oxidation as efficiently as carnosic acid. Contrary to carnosic acid, carnosol could not lower the concentration of singlet oxygen or hydroxyl radical in solution. The chemical quenching capacities of carnosol thus appear to be weak compared with those of carnosic acid; therefore, the antioxidative activity of carnosol relies on a different mechanism that does not involve its direct oxidation by ROS. A possibility is that carnosol reacts directly with lipid radicals and, hence, blocks the lipid peroxidation chain process. This idea was supported by the inhibitory effect of carnosol on lipid peroxidation induced in vitro by a lipid hydroperoxide or in vivo by lipoxygenase. Since this effect was observed in darkness under conditions where ROS production was not induced, carnosol can act by interfering with the lipid peroxidation process, playing a lipid oxidation-blocking role like tocopherols (Tavadyan et al., 2007). This phenomenon was not observed with carnosic acid. In the case of tocopherol, the tocopheroxyl radical and tocopherol quinone formed in this process are recycled either by reductants such as ascorbate (Liebler et al., 1986; Szarka et al., 2012) or by enzymatically catalyzed reactions (Eugeni Piller et al., 2014). A similar recycling mechanism could take place for carnosol. Interestingly, it has been shown that carnosol quinone, an oxidized form of carnosol, is converted into carnosol in water-containing solvent (Masuda et al., 2005). Similarly, thermal treatments of carnosol quinone in lipids can reform carnosol (Masuda et al., 2004). These results suggest the possibility of a recycling mechanism for carnosol that promotes the recovery of its antioxidant activity under oxidative conditions. Also, it has been shown that the antioxidative efficiency of carnosol surpasses that of carnosic acid when assayed in model membranes (Pérez-Fons et al., 2006, 2010). This effect was attributed to the enhanced lipid order by carnosol at the hydrophobic core of the membrane, presumably contributing to membrane stabilization and the hindrance of radical propagation. Independently of the exact mechanism underlying the antioxidative function of carnosol, the fact that the modes of action of carnosic acid and carnosol differ widens the action spectrum of rosemary diterpenes in the defense of plants against oxidative stress.
Carnosic acid is present exclusively in some species of the Lamiaceae family, such as rosemary, sage, and oregano (Origanum vulgare; Hossain et al., 2010; Birtić et al., 2015). However, some Lamiaceae species, such as basil (Ocimum basilicum) and thyme (Thymus vulgaris), accumulate carnosol rather than carnosic acid. Most carnosic acid/carnosol-containing species are Mediterranean plants that can adapt to harsh climatic conditions and, therefore, need to protect themselves from oxidative stress. Although the in vitro antioxidant properties of carnosic acid have led to numerous applications in food science and medicine (Birtić et al., 2015), evidence for an antioxidative role in plants is missing. The main source of information on this aspect is the pioneering work by Munné-Bosch and coworkers, who showed the interdependence between the concentrations of carnosic acid and other low-molecular antioxidant molecules in rosemary leaves (Munné-Bosch and Alegre, 2003) as well as the presence of oxidized abietane diterpenes in field-grown rosemary plants in the summer (Munné-Bosch et al., 1999). Our work extends those previous studies and provides several arguments supporting that carnosic acid does fulfill an antioxidant function in planta. First, both carnosic acid and carnosol can protect chloroplast membranes against high light-induced oxidation. Because biomembranes are targets of high light, drought, and high temperatures (Schwab and Heber, 1984; Conde et al., 2011), the accumulation of those antioxidants is beneficial in Mediterranean climatic conditions. In rosemary, there is a wide diversity of carnosic acid accumulation levels in leaves (Wellwood and Cole, 2004). In a preliminary experiment, we analyzed the carnosic acid concentrations in leaves of a large range of rosemary varieties from various geographic origins (data not shown). The Barbecue variety contained low levels of carnosic acid. When grown under control conditions in a phytotron, the leaf concentration in carnosic acid was lower by 40% in Barbecue compared with the Sudbury Blue variety. Also, under stress conditions, Barbecue was found to contain less carnosol than Sudbury Blue. Interestingly, these lower concentrations of carnosic acid and carnosol were correlated with a lower resistance to photooxidative stress, in line with a role for those diterpenes in the resistance of rosemary plants to photooxidative stress. Moreover, considering that carnosic acid functions as a chemical quencher of ROS, the light-dependent presence of oxidized carnosic acid derivatives in rosemary leaves and their marked accumulation in plants exposed to stress conditions indicate that the ROS-scavenging antioxidative action of carnosic acid does operate in vivo.
The biosynthesis pathway of carnosic acid is currently being elucidated. In particular, the enzymatic activities responsible for the first three steps in the pathway have been identified, and the synthesis of the carnosic acid precursor ferruginol was achieved using yeast and Nicotiana benthamiana expression systems (Božić et al., 2015). Subsequently, four P450 cytochromes have been identified, the combined activities of which account for all of the oxidation events leading to the biosynthesis of carnosic acid when expressed in yeast (Ignea et al., 2016). As a perspective, it could be envisaged from those results to introduce the whole carnosic acid biosynthetic pathway in model plants that are naturally deficient in carnosic acid, such as tobacco or Arabidopsis. It is clear that a successful transformation of a vascular plant to express the newly elucidated steps and, hence, to induce carnosic acid accumulation would provide a useful tool to confirm the antioxidative and lipid-protective activities of carnosic acid and carnosol described here.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rosemary cuttings (Rosmarinus officinalis varieties Sudbury Blue and Barbecue) were obtained from the plant nursery SARL du Tilleul at Chateaurenard, France. Plants were grown on a soil:sand mixture (70:30) in a phytotron under a PFD of 250 μmol photons m−2 s−1, a photoperiod of 12 h, and day/night temperatures of 25°C/19°C. Stress conditions were imposed by transferring plants to a high PFD of 1,200 μmol photons m−2 s−1 (photoperiod, 12 h) at a high day temperature of 35°C combined with a low night temperature of 5°C for 4 weeks. Young leaves at the top of plants aged 2 months were collected, weighed, frozen in liquid nitrogen, and stored at −80°C before analyses.
In Vitro Oxidation of Biological Molecules
Linolenic acid (1–3 mg mL−1; obtained from Fluka), MGDG (1–2 mg mL−1; from Larodan), α-tocopherol (Naturex), carnosic acid (Extrasynthèse), and carnosol (Sigma-Aldrich) were supplemented with Methylene Blue (final concentration, 0.1 mm). Oxidation of these molecules by 1O2 was induced by exposing the mixture to white light produced by HQI metal halide lamps (Osram; PFD of 750 µmol photons m−2 s−1) at 7°C (except when specified otherwise). For the oxidation of linolenic acid (5–8 mg mL−1 methanol), MGDG (5 mg mL−1 methanol/CHCl3), carnosic acid, and carnosol by hydroxyl radicals, H2O2 and iron chloride (Fenton reaction) were added to the solutions and left to react for 20 s. 15-HEDE also was used to oxidize linolenic acid in vitro: 15-HEDE in methanol was incubated at 60°C for 10 s and then mixed with linolenic acid (5 mg mL−1 in methanol) at a final concentration of 10 μm. 15-HEDE was prepared from eicosadienoic acid and soybean (Glycine max) lipoxygenase according to the procedure described by Martini et al. (1994).
Preparation of Thylakoid Membranes
Seven grams of leaves (fresh weight) was ground for 2 s in 50 mL of extraction buffer (330 mm sorbitol, 50 mm Tricine, 2 mm EDTA-Na2, 1 mm MgCl2, and 2 mm ascorbate, pH 7.7) with 5 mm DTT in a Waring Blendor at low speed. The liquid phase was removed and set aside, and 50 mL of extraction buffer was added for a second extraction. The extracts were filtered onto four Miracloth layers, and the filtrate was centrifuged for 4 min at 1,500g at 4°C. The pellet was washed twice with the extraction buffer and centrifuged for 4 min at 1,500g at 4°C. The washed pellet was resuspended in 21 mL of lysis buffer, pH 7.8 (10 mm Tricine, 10 mm NaCl, and 10 mm MgCl2), with 1 mm phenylmethylsulfonyl fluoride with occasional stirring for 15 min. The sample was centrifuged at 48,400g for 15 min. The pellet was resuspended in 1.75 mL of storage buffer (100 mm Tricine, 10 mm NaCl, 10 mm MgCl2, and 400 mm Suc, pH 7.8) and stored at −80°C before analyses.
HPLC-UV Determination of Carnosic Acid and Carnosol
A total of 5 mL of methanol:H3PO4 (99.5:0.5, v/v) was added to 25 mg of leaves (fresh weight). The mix was ground for 1 min with an Ultra-Turrax T25 (IKA-Werke) at 24,000 rpm. After centrifugation at 4,500g for 10 min at 4°C, the pellet was resuspended in 2.5 mL of methanol:H3PO4 for a second extraction. After filtration through a 0.45-µm polytetrafluoroethylene Costar filter, the extract was analyzed by HPLC-UV with a reverse-phase column (Waters NovaPak; 4 μm, 39 × 300 mm), isocratic elution with 65:34.8:0.2 (v/v/v) acetonitrile:water:H3PO4 at a flow rate of 1 mL min−1, and UV detection at 230 nm. Quantification was done using authentic standards of carnosic acid and carnosol.
Diterpene Extraction from Trichomes by Leaf Dipping in Solvent
Carnosic acid and carnosol extraction from leaf trichomes was performed by dipping detached rosemary leaves for 30 s in 1 mL of dichloromethane. The solvent was then evaporated under nitrogen. A total of 250 µL of methanol with 0.5% H3PO4 (v/v) was then added, and the solution was analyzed subsequently by HPLC-UV, as described above. Diterpenes were extracted from the solvent-dipped leaves as described above.
Prenyl Lipid Determinations
A total of 60 mg of leaves was ground for 1 min in 2 mL of 100% ethyl acetate with an Ultra-Turrax at 24,000 rpm. After centrifugation for 3 min at 16,900g, 600 µL of extract was filtered with a 0.2-µm polytetrafluoroethylene filter. The extract was evaporated under a stream of nitrogen, and 1 mL of methanol:hexane (17:1, v/v) was added to the tubes before analysis by HPLC-UV fluorescence. The samples were submitted to reverse-phase HPLC using a Phenomenex Kinetex 2.6-µm column (100 × 4.6 mm) operating in the isocratic mode with methanol:hexane (17:1, v/v) as a solvent system at a flow rate of 0.8 mL min−1, as described previously (Ksas et al., 2015). Tocopherols and prenyl lipids, except oxidized plastoquinone-9, were detected by their fluorescence at 330 nm with excitation at 290 nm. Plastoquinone-9 in the oxidized state was measured by its A255.
Chlorophyll Fluorometry
Chlorophyll fluorescence emission from leaves attached to the plant was measured with a PAM-2000 modulated fluorometer (Walz), as described previously (Havaux et al., 2003). The maximal quantum yield of PSII photochemistry was measured in dark-adapted samples by (Fm − Fo)/Fm = Fv/Fm, where Fo is the initial fluorescence level and Fm is the maximal fluorescence level and Fv is the difference between Fm and Fo. Fm was measured with an 800-ms pulse of intense white light, and Fo was measured with a 1-s pulse of far-red light.
Lipid Peroxidation Imaging
Lipid peroxides were visualized by autoluminescence imaging (Havaux et al., 2006). Imaged autoluminescence signals are attributed to the spontaneous decomposition of lipid peroxides (Birtić et al., 2011). Spontaneous photon emission from whole rosemary plants was measured after 2.5 h of dark adaptation using a liquid N2-cooled CCD camera, as detailed previously (Birtić et al., 2011). Acquisition time was 20 min, and pixel binning was 2 × 2. In vitro oxidation of lipid solutions (MGDG) also was measured by this method without dark preadaptation and with a pixel binning of 5 × 5. The luminescence signals were analyzed and quantified with ImageJ software.
Biochemical Analysis of Lipid Peroxidation
In vitro oxidation solution with 30% (w/v) MGDG was ground with Ultraturax T25 (IKA-Werk) in CHCl3:methanol (50:50, v/v) containing 5 mm triphenyl phosphine, 1 mm butylated hydroxytoluene, and 1 m citric acid. 15-Hydroxy-11,13(Z,E)-eicosadienoic acid was added as an internal standard. After centrifugation at 700g for 5 min at 4°C, the organic phase (CHCl3) was evaporated under a stream of N2 at 40°C for 30 min. Then, the organic phase was resolubilized in ethanol and NaOH (3.5 m). The sample was hydrolyzed at 80°C for 30 min. pH was adjusted between 4 and 5 by the addition of citric acid (1 m), and hydroxy fatty acids were then extracted with hexane:ether (50:50, v:v). HOTE isomers (produced by the oxidation of linolenic acid) and HODE isomers (produced by the oxidation of linoleic acid) were separated and quantified by straight-phase HPLC-UV analysis, as described previously (Montillet et al., 2004).
1O2 and Hydroxyl Radical Detection by EPR Spin Trapping
Spin-trapping assays with POBN to detect the formation of hydroxyl radicals were carried out using 50 µm H2O2 solution, 50 mm POBN, and 50 mm Fe-EDTA in the presence of carnosic acid, carnosol, or α-tocopherol. To detect singlet oxygen, the spin probe TEMPD (100 mm) was illuminated for 2 min with red light (RG 630; 1,000 μmol photons m−2 s −1) with Rose Bengal (100 µm) in the presence of carnosic acid, carnosol, or α-tocopherol. EPR spectra were recorded at room temperature in a standard quartz flat cell using an ESP-300 X-band spectrometer (Bruker). The following parameters were used: microwave frequency, 9.73 GHz; modulation frequency, 100 kHz; modulation amplitude, 1 G; microwave power, 63 mW in TEMPD assays and 6.3 mW in POBN assays; receiver gain, 2 × 104; time constant, 40.96 ms; number of scans, 16.
Mass Spectrometric Analysis of Metabolites
Mass spectrometry analyses were performed at the Criblage Biologique Marseille (CRIBIOM) platform (Centre Hospitalier Universitaire Timone). The LC-MS method was developed from Zhang et al. (2012) and Song et al. (2014). Samples were diluted in acetonitrile:water (65:35 v:v) and then analyzed by ultra-performance liquid chromatography-HRMS and MS/MS.
The chromatographic separation was carried out on a Dionex Ultimate 3000 (Thermo Fisher Scientific) consisting of a rapid separation pump (LGP-3400 RS), an autosampler (WPS-3000 TRS), and a column compartment (TCC-3000 RS), all operated by Chromeleon 6.8 software. A Hypersil Gold reverse-phase column (100 nm × 2.1 mm × 1.9 µm; Thermo Scientific) was used for the compound separation. Accurate mass measurements were performed on the Q-Exactive Plus mass spectrometer (Thermo Fisher Scientific) with a heated electrospray ionization probe. Thermo Xcalibur 3.0.63 software was used for the instrument setup, control of the LC-MS system during acquisition, and data treatment. The Tune Q Exactive Plus 2.5 application was used for the direct control of the mass spectrometer. The column oven was maintained at 40°C, while the sample chamber temperature was set at 4°C. The mobile phase was 0.1% formic acid aqueous solution (v/v) (A) and acetonitrile containing 0.1% formic acid (B), eluting according to the following program: 0 to 10 min, 40% to 80% B, 10 to 12 min, 80% B, 12 to 12.1 min, 80% to 40% B, 12.1 to 18 min, 40% B. The flow rate was set at 0.4 mL min−1, and the injection volume was 5 µL.
LC-HRMS analyses were performed with external calibration in positive and negative ionization modes, providing a mass precision lower than 3 ppm. The heated electrospray ionization probe and the transfer capillary temperatures were kept at 310°C and 320°C, respectively. The spray voltage was set at 3,500 V, and the S-lens RF (radio frequency) level was 55. Sheath and auxiliary gas were maintained at 30 and 8 arbitrary units. Mass resolving power was set to 70,000 full width at half maximum for m/z 200, the maximum injection time was set to 250 ms, and auto gain control was set to 10e6. LC-MS spectra were acquired in the mass range from m/z 80 to 700.
MS/MS analyses were performed on the Q-Exactive Plus mass spectrometer (Thermo Fisher Scientific) using parallel reaction monitoring (HCD, higher energy collision-induced dissociation) experiments. For this purpose, resolving power was set to 70,000 for m/z 200, auto gain control target was set to 1e6, and maximum injection time was set to 250 ms. Precursor ions were isolated in the 2 m/z isolation window in the quadrupole and then fragmented in the higher collision energy (HCD) cell under normalized collision energies determined previously. Thermo Xcalibur 3.0.63 software was used for the instrument setup and control of the LC-MS system during acquisition as well as for data treatment. Carnosic acid and isorosmanol were obtained from Sigma-Aldrich, carnosol was purchased from Extrasynthèse, and rosmanol and 12-o-methyl carnosic acid were obtained from Phytolab. Carnosic acid-derived metabolites for which standards are not available were quantified using the conversion factor of carnosic acid.
Environmental Scanning Electron Microscopy
Rosemary leaves were dipped in dichloromethane for 30 s, and the leaf surface was examined with an FEI QUANTA 200 FEG environmental scanning electron microscope operating at 30 kV. The sample was placed in a 5-mm-diameter platinum crucible inside the microscope analysis chamber at a water vapor pressure of 600 Pa at 2°C.
Statistical Analyses
All experiments were done at least in triplicate. Statistical differences between measurements on different treatments were analyzed following Student’s t test. Differences were considered significant at P < 0.05. One, two, or three asterisks was assigned to 0.01 < P < 0.05, 0.005 < P < 0.01, and P < 0.005, respectively.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Chemical structures of carnosic acid, carnosol, rosmanol, isorosmanol, 7-methyl-epirosmanol, 7-methyl-rosmanol, 12-o-methylcarnosic acid, 5,6,7,10-tetrahydroxyrosmariquinone, 11-12-di-o-methylisorosmanol, rosmadial, and rosmaridiphenol.
Supplemental Figure S2. In vitro oxidation of linolenic acid with 1O2 or with hydroxyl radicals in the presence of carnosic acid or carnosol.
Supplemental Figure S3. List of carnosic acid derivatives measured after in vitro oxidation of carnosic acid by singlet oxygen or hydroxyl radical.
Supplemental Figure S4. LC-HRMS analysis of carnosic acid in a standard product solution, a solution of oxidized carnosic acid, and a rosemary leaf extract.
Supplemental Figure S5. LC-HRMS analysis of carnosol in a standard product solution, a solution of oxidized carnosic acid, and a rosemary leaf extract.
Supplemental Figure S6. LC-HRMS analysis of 12-o-methylcarnosic acid in a standard product solution, a solution of oxidized carnosic acid, and a rosemary leaf extract.
Supplemental Figure S7. LC-HRMS analysis of isorosmanol in a standard product solution and a solution of oxidized carnosic acid.
Supplemental Figure S8. LC-HRMS analysis of rosmanol in a standard product solution and a solution of oxidized carnosic acid.
Supplemental Figure S9. LC-HRMS analysis of probable 7-methyl-rosmanol and 7-methyl-epirosmanol in a rosemary leaf extract and in a solution of oxidized carnosic acid.
Supplemental Figure S10. LC-HRMS analysis of probable rosmadial isomers in a rosemary leaf extract and in a solution of oxidized carnosic acid.
Supplemental Figure S11. LC-HRMS analysis of probable isomers of 5,6,7,10-tetrahydroxyrosmariquinone in a rosemary leaf extract and in a solution of oxidized carnosic acid.
Supplemental Figure S12. LC-HRMS analysis of probable rosmaridiphenol in a rosemary leaf extract and in a solution of oxidized carnosic acid.
Supplemental Figure S13. LC-HRMS analysis of probable 11,12-o-dimethylrosmanol in a rosemary leaf extract and in a solution of oxidized carnosic acid.
Supplemental Figure S14. Effects of growth under dark adaptation (30 h) on the levels of several oxidized metabolites of carnosic acid in rosemary leaves.
Acknowledgments
We are grateful to the members of the Phytotec platform (Commissariat à l’Energie Atomique et aux Energies Alternatives Cadarache) for help in growing plants under control and stress conditions, to Bernard Genty (Commissariat à l’Energie Atomique et aux Energies Alternatives Cadarache) for help with the autoluminescence imaging technique, to Brigitte Ksas (Commissariat à l’Energie Atomique et aux Energies Alternatives Cadarache) for advices in HPLC analyses, to Jerzy Kruk (Jagiellonian University, Krakow) for the gift of plastochromanol standard, and to Renaud Podor (Commissariat à l’Energie Atomique et aux Energies Alternatives Marcoule) for help with environmental scanning electron microscopy analyses. We also thank Alain Tissier (Leibniz Institute of Plant Biochemistry, Halle) and Jean-Luc Montillet (Commissariat à l’Energie Atomique et aux Energies Alternatives Cadarache) for useful discussions.
Footnotes
M.L. was supported by a CIFRE studentship from the French National Association for Research and Technology (ANRT).
Articles can be viewed without a subscription.
References
- Aruoma OI, Halliwell B, Aeschbach R, Löligers J (1992) Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica 22: 257–268 [DOI] [PubMed] [Google Scholar]
- Birtić S, Dussort P, Pierre FX, Bily AC, Roller M (2015) Carnosic acid. Phytochemistry 115: 9–19 [DOI] [PubMed] [Google Scholar]
- Birtić S, Ksas B, Genty B, Mueller MJ, Triantaphylidès C, Havaux M (2011) Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J 67: 1103–1115 [DOI] [PubMed] [Google Scholar]
- Božić D, Papaefthimiou D, Brückner K, de Vos RC, Tsoleridis CA, Katsarou D, Papanikolaou A, Pateraki I, Chatzopoulou FM, Dimitriadou E, et al. (2015) Towards elucidating carnosic acid biosynthesis in Lamiaceae: functional characterization of the three first steps of the pathway in Salvia fructicosa and Rosmarinus officinalis. PLoS ONE 10: e0124106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner K, Božić D, Manzano D, Papaefthimiou D, Pateraki I, Scheler U, Ferrer A, de Vos RCH, Kanellis AK, Tissier A (2014) Characterization of two genes for the biosynthesis of abietane-type diterpenes in rosemary (Rosmarinus officinalis) glandular trichomes. Phytochemistry 101: 52–64 [DOI] [PubMed] [Google Scholar]
- Chauvin A, Caldelari D, Wolfender JL, Farmer EE (2013) Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 197: 566–575 [DOI] [PubMed] [Google Scholar]
- Cifra M, Pospisil P (2014) Ultra-weak photon emission from biological samples: definitions, mechanisms, properties, detection and applications. J Photochem Photobiol B Biol 139: 2–10 [DOI] [PubMed] [Google Scholar]
- Conde A, Chaves MM, Gerós H (2011) Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol 52: 1583–1602 [DOI] [PubMed] [Google Scholar]
- Cuvelier ME, Richard H, Berset C (1996) Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chem Soc 73: 645–652 [Google Scholar]
- del Baño MJ, Lorente J, Castillo J, Benavente-García O, del Río JA, Ortuño A, Quirin KW, Gerard D (2003) Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis: antioxidant activity. J Agric Food Chem 51: 4247–4253 [DOI] [PubMed] [Google Scholar]
- Di Mascio P, Devasagayam TP, Kaiser S, Sies H (1990) Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans 18: 1054–1056 [DOI] [PubMed] [Google Scholar]
- Erkan N, Ayranci G, Ayranci E (2008) Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110: 76–82 [DOI] [PubMed] [Google Scholar]
- Escuder B, Torres R, Lissi E, Labbé C, Faini F (2002) Antioxidant capacity of abietanes from Sphacele salviae. Nat Prod Lett 16: 277–281 [DOI] [PubMed] [Google Scholar]
- Eugeni Piller L, Glauser G, Kessler F, Besagni C (2014) Role of plastoglobules in metabolite repair in the tocopherol redox cycle. Front Plant Sci 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote CS, Ching TY, Geller GG (1974) Chemistry of singlet oxygen. XVIII. Rates of reaction and quenching of α-tocopherol and singlet oxygen. Photochem Photobiol 20: 511–513 [DOI] [PubMed] [Google Scholar]
- Haraguchi H, Saito T, Okamura N, Yagi A (1995) Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med 61: 333–336 [DOI] [PubMed] [Google Scholar]
- Havaux M, Lütz C, Grimm B (2003) Chloroplast membrane photostability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol 132: 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Triantaphylidès C, Genty B (2006) Autoluminescence imaging: a non-invasive tool for mapping oxidative stress. Trends Plant Sci 11: 480–484 [DOI] [PubMed] [Google Scholar]
- Hidalgo PJ, Ubera JL, Tena MT, Valcarcel M (1998) Determination of the carnosic acid content in wild and cultivated Rosmarinus officinalis. J Agric Food Chem 46: 2624–2627 [Google Scholar]
- Hideg E, Kálai T, Hideg K (2011) Direct detection of free radicals and reactive oxygen species in thylakoids. Methods Mol Biol 684: 187–200 [DOI] [PubMed] [Google Scholar]
- Hopia AI, Huang SW, Schwarz K, German JB, Frankel EN (1996) Effect of different lipid systems on antioxidant activity of rosemary constituents. J Agric Food Chem 44: 2030–2036 [Google Scholar]
- Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C (2010) Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J Agric Food Chem 58: 10576–10581 [DOI] [PubMed] [Google Scholar]
- Huang SW, Frankel EN, Schwarz K, Aeschbach R, German JB (1996) Antioxidant activity of carnosic acid and methyl carnosate in bulk oils and oil-in-water emulsions. J Agric Food Chem 44: 2951–2956 [Google Scholar]
- Ignea C, Athanasakoglou A, Ioannou E, Georgantea P, Trikka FA, Loupassaki S, Roussis V, Makris AM, Kampranis SC (2016) Carnosic acid biosynthesis elucidated by a synthetic biology platform. Proc Natl Acad Sci USA 113: 3681–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordán MJ, Castillo J, Bañón S, Martínez-Conesa C, Sotomayor JA (2014) Relevance of the carnosic acid/carnosol ratio for the level of rosemary diterpene transfer and for improving lamb meat antioxidant status. Food Chem 151: 212–218 [DOI] [PubMed] [Google Scholar]
- Kruk J, Szymańska R, Cela J, Munné-Bosch S (2014) Plastochromanol-8: fifty years of research. Phytochemistry 108: 9–16 [DOI] [PubMed] [Google Scholar]
- Kruk J, Szymańska R, Nowicka B, Dłużewska J (2016) Function of isoprenoid quinones and chromanols during oxidative stress in plants. New Biotechnol 33: 636–643 [DOI] [PubMed] [Google Scholar]
- Ksas B, Becuwe N, Chevalier A, Havaux M (2015) Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci Rep 5: 10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler DC, Kling DS, Reed DJ (1986) Antioxidant protection of phospholipid bilayers by alpha-tocopherol: control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J Biol Chem 261: 12114–12119 [PubMed] [Google Scholar]
- Luis JC, Johnson CB (2005) Seasonal variations of rosmarinic and carnosic acids in rosemary extracts: analysis of their in vitro antiradical activity. Span J Agric Res 3: 106–112 [Google Scholar]
- Martini D, Iacazio G, Ferrand D, Buono G, Triantaphylidès C (1994) Optimization of large scale preparation of 13-(S)-hydroperoxy-9Z,11E-octadecadienoic acid using soybean lipoxygenase: application to the chemoenzymatic synthesis of (+)-coriolic acid. Biocatalysis 11: 47–63 [Google Scholar]
- Masuda T, Kirikihira T, Takeda Y (2005) Recovery of antioxidant activity from carnosol quinone: antioxidants obtained from a water-promoted conversion of carnosol quinone. J Agric Food Chem 53: 6831–6834 [DOI] [PubMed] [Google Scholar]
- Masuda T, Kirikihira T, Takeda Y, Yonemori S (2004) Thermal recovery of antioxidant activity from carnosol quinone, the main antioxidation product of carnosol. J Sci Food Agric 84: 1421–1427 [Google Scholar]
- Montillet JL, Cacas JL, Garnier L, Montané MH, Douki T, Bessoule JJ, Polkowska-Kowalczyk L, Maciejewska U, Agnel JP, Vial A, et al. (2004) The upstream oxylipin profile of Arabidopsis thaliana: a tool to scan for oxidative stresses. Plant J 40: 439–451 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2001) Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol 125: 1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2003) Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol 131: 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Schwarz K, Alegre L (1999) Enhanced formation of alpha-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol 121: 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani N, Inatani R (1984) Two antioxidative diterpenes from rosemary (Rosmarinus officinalis L.) and a revised structure for rosmanol. Agric Biol Chem 48: 2081–2085 [Google Scholar]
- Naveena BM, Vaithiyanathan S, Muthukumar M, Sen AR, Kumar YP, Kiran M, Shaju VA, Chandran KR (2013) Relationship between the solubility, dosage and antioxidant capacity of carnosic acid in raw and cooked ground buffalo meat patties and chicken patties. Meat Sci 95: 195–202 [DOI] [PubMed] [Google Scholar]
- Pearson DA, Frankel EN, Aeschbach R, German JB (1997) Inhibition of endothelial cell-mediated oxidation of low-density lipoprotein by rosemary and plant phenolics. J Agric Food Chem 45: 578–582 [Google Scholar]
- Pérez-Fons L, Aranda FJ, Guillén J, Villalaín J, Micol V (2006) Rosemary (Rosmarinus officinalis) diterpenes affect lipid polymorphism and fluidity in phospholipid membranes. Arch Biochem Biophys 453: 224–236 [DOI] [PubMed] [Google Scholar]
- Pérez-Fons L, Garzón MT, Micol V (2010) Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J Agric Food Chem 58: 161–171 [DOI] [PubMed] [Google Scholar]
- Romo Vaquero M, Garcia Villalba R, Larrosa M, Yáñez-Gascón MJ, Fromentin E, Flanagan J, Roller M, Tomás-Barberán FA, Espín JC, García-Conesa MT (2013) Bioavailability of the major bioactive diterpenoids in a rosemary extract: metabolic profile in the intestine, liver, plasma, and brain of Zucker rats. Mol Nutr Food Res 57: 1834–1846 [DOI] [PubMed] [Google Scholar]
- Schmid-Siegert E, Stepushenko O, Glauser G, Farmer EE (2016) Membranes as structural antioxidants: recycling of malondialdehyde to its source in oxidation-sensitive chloroplast fatty acids. J Biol Chem 291: 13005–13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KB, Heber U (1984) Thylakoid membrane stability in drought-tolerant and drought-sensitive plants. Planta 161: 37–45 [DOI] [PubMed] [Google Scholar]
- Song Y, Yan H, Chen J, Wang Y, Jiang Y, Tu P (2014) Characterization of in vitro and in vivo metabolites of carnosic acid, a natural antioxidant, by high performance liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal 89: 183–196 [DOI] [PubMed] [Google Scholar]
- Szarka A, Tomasskovics B, Bánhegyi G (2012) The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci 13: 4458–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavadyan L, Khachoyan A, Martoyan G, Kamal-Eldin A (2007) Numerical revelation of the kinetic significance of individual steps in the reaction mechanism of methyl linoleate peroxidation inhibited by alpha-tocopherol. Chem Phys Lipids 147: 30–45 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellwood CR, Cole RA (2004) Relevance of carnosic acid concentrations to the selection of rosemary, Rosmarinus officinalis (L.), accessions for optimization of antioxidant yield. J Agric Food Chem 52: 6101–6107 [DOI] [PubMed] [Google Scholar]
- Wijeratne SSK, Cuppett SL (2006) Lipid hydroperoxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem 54: 4476–4481 [DOI] [PubMed] [Google Scholar]
- Wijeratne SSK, Cuppett SL (2007) Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J Agric Food Chem 55: 1193–1199 [DOI] [PubMed] [Google Scholar]
- Zeng HH, Tu PF, Zhou K, Wang H, Wang BH, Lu JF (2001) Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol Sin 22: 1094–1098 [PubMed] [Google Scholar]
- Zhang Y, Smuts JP, Dodbiba E, Rangarajan R, Lang JC, Armstrong DW (2012) Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J Agric Food Chem 60: 9305–9314 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang L, Zu Y, Chen X, Wang F, Liu F (2010) Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem 118: 652–662 [DOI] [PubMed] [Google Scholar]