Significance
The host interferon system is a potent antiviral system, responsible for protecting vertebrate organisms against virus infection. In this paper we demonstrate that the N terminus of the vaccinia virus innate immune evasion protein, E3, is necessary to inhibit the action of interferon. Interferon-treated cells infected with virus encoding an E3 deleted of the N-terminal domain die rapidly. This death is dependent on the host RIPK3 protein, which is a mediator of programmed necrotic death, and on DAI, which is a sensor of virus infection. Since both E3 and DAI contain Z-DNA binding domains, we hypothesize that the cellular DAI virus sensor and the viral antiinterferon E3 protein compete for binding to a vaccinia virus-induced Z-nucleic acid, pathogen-associated molecular pattern.
Keywords: necroptosis, vaccinia, RIPK3, type 1 interferon, Z-DNA binding domain
Abstract
Vaccinia virus (VACV) encodes an innate immune evasion protein, E3, which contains an N-terminal Z-nucleic acid binding (Zα) domain that is critical for pathogenicity in mice. Here we demonstrate that the N terminus of E3 is necessary to inhibit an IFN-primed virus-induced necroptosis. VACV deleted of the Zα domain of E3 (VACV-E3LΔ83N) induced rapid RIPK3-dependent cell death in IFN-treated L929 cells. Cell death was inhibited by the RIPK3 inhibitor, GSK872, and infection with this mutant virus led to phosphorylation and aggregation of MLKL, the executioner of necroptosis. In 293T cells, induction of necroptosis depended on expression of RIPK3 as well as the host-encoded Zα domain-containing DNA sensor, DAI. VACV-E3LΔ83N is attenuated in vivo, and pathogenicity was restored in either RIPK3- or DAI-deficient mice. These data demonstrate that the N terminus of the VACV E3 protein prevents DAI-mediated induction of necroptosis.
Vaccinia virus (VACV) is a double-stranded DNA virus that replicates in the cytoplasm of the host cell. Of the 200 genes encoded by VACV, one-third are dedicated to host immune evasion. One of the prime VACV immune evasion genes is E3L. The E3L-encoded protein has two conserved domains that are essential for pathogenesis, presumably by preventing the activation of the type I IFN pathway (1, 2). E3 contains a C-terminal double-stranded RNA (dsRNA)-binding domain that functions by sequestering dsRNA to avoid activation of IFN-inducible, dsRNA-dependent antiviral enzymes such as PKR (2–5). The N terminus of E3 has a Z-form nucleic acid binding (Zα) domain whose function is not well understood. While the N terminus is required for pathogenesis in mice, it is dispensable for replication and IFN resistance (IFNR) in most cell culture settings. Among N-terminal mutants of VACV E3, pathogenesis correlates with binding to Z-DNA in vitro. Virulence of N-terminal mutants was restored in IFN receptor 1-deficient mice (Ifnar−/−), indicating that the N terminus is necessary to inhibit type I IFN action in vivo (6). The N terminus is also necessary for PKR-dependent IFNR in 129 mouse embryonic fibroblasts (MEFs). However, virulence of a VACV N-terminal deletion mutant was not restored in PKR-deficient mice, indicating a potential distinct requirement for this domain to block another consequence of IFN signaling in vivo (6).
Necroptosis plays a significant role in limiting the pathogenesis of wild-type (WT) VACV in mouse models. A previous study demonstrated that WTVACV-infected cells become sensitized to TNF-driven necroptosis (7). Cells infected with WTVACV, and then treated with TNF, undergo necroptosis at late times postinfection, suggesting that unlike herpesviruses, VACV does not encode a protein that can directly inhibit TNF-mediated necroptosis (7). Additionally, mice deficient in RIPK3 are more susceptible to VACV and succumb to lethal infections faster than do WT mice.
Necroptosis is an inflammatory form of programmed cell death that is an alternative host defense pathway initiated during the course of some viral infections (8–10). Activation of necroptosis leads to a signal cascade that is dependent on the receptor interacting protein kinase 3 (RIPK3) and the downstream pseudokinase, mixed lineage kinase-like protein (MLKL) (11). This signaling pathway leads to a breakdown in membrane integrity in a caspase-independent manner (12, 13). Necroptosis induced through the utilization of classical death receptors (DRs) results in the RIPK homotypic interaction motif (RHIM)-dependent RIPK1–RIPK3 complex, called a necrosome (7, 14–16). Both RIPK1 and RIPK3 are protein kinases, and the kinase activity of these proteins is required in the classic TNF-driven death where RIPK3 activation is dependent on RIPK1 (15, 17). RIPK3 not only plays a central role in transducing signals that drive this DR-induced process, but is also involved in signals for necroptosis initiation through nonclassical pathways such as pattern recognition receptor (PRR) activation, independent of RIPK1 (18). RIPK1-independent activation of RIPK3 has been demonstrated by other adaptor proteins, including DNA-induced activator of IFN (DAI, also known as DLM and ZBP1) (16) and TIR-domain-containing adaptor-inducing IFN β (TRIF) (18). Exposure of RIPK3 to a RHIM adaptor (RIPK1, TRIF, or DAI) induces a conformational change that activates both autophosphorylation kinase activity and transphosphorylation of MLKL, the downstream executor of necroptosis (11, 19, 20).
Necroptosis is a potentially critical host defense mechanism to limit virulence by inducing death of host cells. Some viruses have adapted to regulate necroptosis, allowing the infectious cycle to continue as demonstrated in murine cytomegalovirus (MCMV) (16, 21). Normally, necroptosis is initiated through RHIM domain interactions between RIPK3 and an adaptor protein. MCMV interferes with necroptosis signaling by utilizing the M45 protein, which acts as a viral RHIM signaling inhibitor, as a viral inhibitor of RIPK activation (vIRA). The RHIM domain of vIRA binds to and sequesters RIPK3 (18, 22–25). Similar to MCMV, both herpes simplex virus (HSV)-1 and HSV-2 have the ability to regulate necroptosis by encoding homologs to M45 that also function as vIRAs in human cells (26). Studies with HSV-1 also revealed the indication that necroptosis may present a barrier to cross-species infection (27, 28).
In this study, we show that the N-terminal Zα domain of the E3 protein is necessary for conferring resistance to IFN in murine L929 cells, preventing the rapid induction of RIPK3-dependent necroptosis. Necroptosis was independent of RIPK1 kinase activity and dependent on the host DNA sensor, DAI/DLM/ZBP1, which like RIPK1, functions to activate RIPK3-dependent necroptosis. Attenuation of VACV-E3LΔ83N was reversed in both RIPK3−/− mice and ZBP1−/− mice, suggesting that DAI-dependent necroptosis plays a role in limiting the virulence of VACV-E3LΔ83N. Notably, VACV likely does not encode a direct necroptosis inhibitor, but the VACV E3 protein likely represents a mechanism to inhibit sensing of virus infection by DAI. Since the N terminus of both E3 and DAI encode Z-NA binding domains, E3 protein may function as a competitor of Z-form nucleic acid sensing or signaling.
Results
The N Terminus Is Required for Type I IFN Resistance in L929 Cells.
The VACV E3 protein plays an essential role in counteracting the host innate immune system. While the C-terminal dsRNA binding domain has been extensively characterized, the role of the N-terminal Z-NA BD in innate immune evasion has been difficult to characterize, due to the lack of a cell culture system where the phenotype of N-terminal E3 mutants in mice can be reproduced. Virulence of VACV in mice is dependent on the presence of a full-length E3 protein. A mutant virus encoding an N-terminal Z-NA BD truncation (VACV-E3LΔ83N) is highly attenuated in WT mice (1, 4, 6) but not in Ifnar−/− mice, implicating the N terminus in subverting type I IFN signaling (6). While characterizing VACV mutants in several mouse cell lines, we identified L929 cells as having a phenotype consistent with the IFN-sensitive (IFNS) phenotype seen in vivo.
L929 cells were pretreated with increasing doses of mouse IFN, then infected with equivalent plaque forming units (pfu) of WTVACV or VACV-E3LΔ83N. As shown in Fig. 1A, plaque formation by WTVACV was IFNR, while plaque formation of VACV-E3LΔ83N was IFNS. This is consistent with rescue of VACV-E3LΔ83N pathogenesis in Ifnar−/− mice.
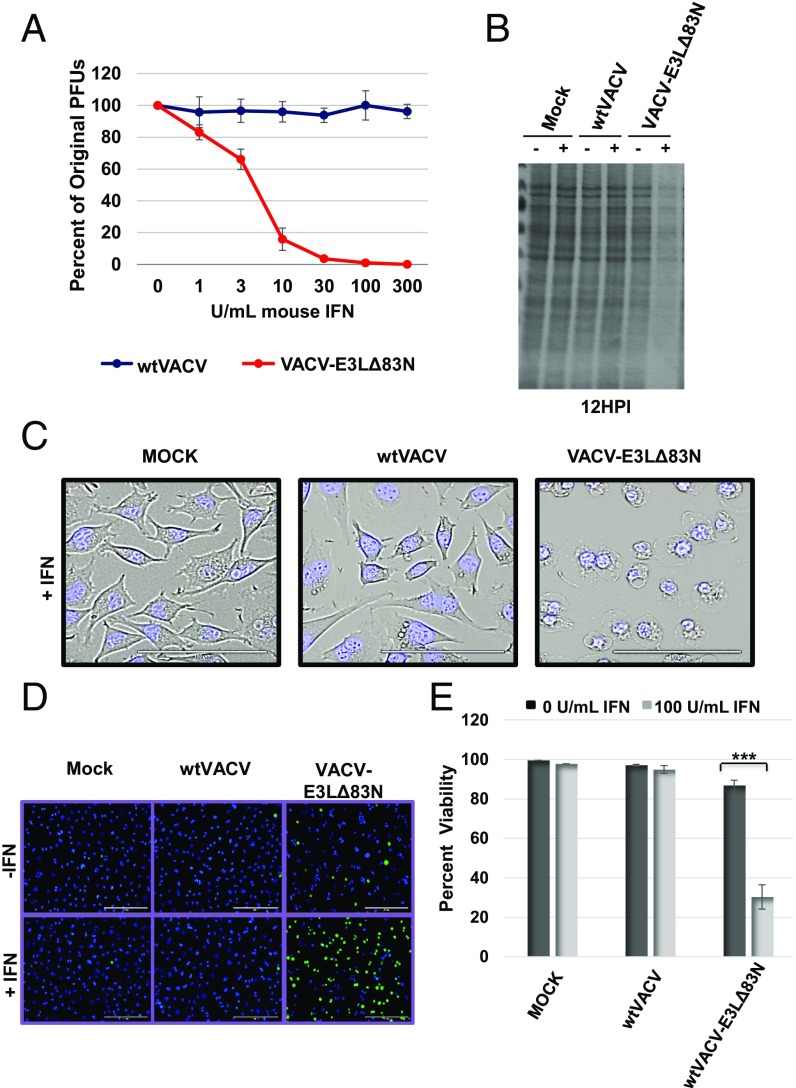
Fig. 1.
E3 N-terminal truncations result in IFN sensitivity and rapid cell death in L929 cells. (A) Plaque reduction assay was performed by pretreating a monolayer of L929 cells with increasing concentrations of mouse IFN for 18 h and infecting with 100 pfu of the indicated viruses. (B) Total protein present in cell lysates was evaluated with Coomassie stain. Lysates were harvested at 12 HPI from L929 cells pretreated with IFN for 18 h and subsequently infected at an MOI of 5. (C) Transmitted live imaging at 6 HPI of L929 cells pretreated with 100 units/mL of mouse IFN and then infected with an MOI of 5. (D and E) IFNS N-terminal mutants undergo a rapid loss of membrane integrity and cellular death. Cell viability was determined using SYTOX exclusion by taking the average percentage of viable cells in 10 fields. Loss of membrane integrity and cell death is indicated by the uptake of both dyes. ***P < 0.001.
We began characterizing IFN sensitivity of VACV-E3LΔ83N in L929 cells by performing a [35S]-methionine labeling experiment to determine if viral protein translation was altered in IFN-treated cells. Viral protein synthesis appeared reduced in IFN-treated, VACV-E3LΔ83N–infected cells (Fig. S1). However, visualization of the Coomassie blue-stained gel revealed a strong reduction in total protein on the gel compared with controls (Fig. 1B and Fig. S1), suggesting that protein was lost from the mutant virus-infected cells. This pattern suggested that VACV-E3LΔ83N virus-infected cells, but not WTVACV-infected cells, might leak their contents, leading to a reduced recovery of proteins from VACV-E3LΔ83N–infected cells.
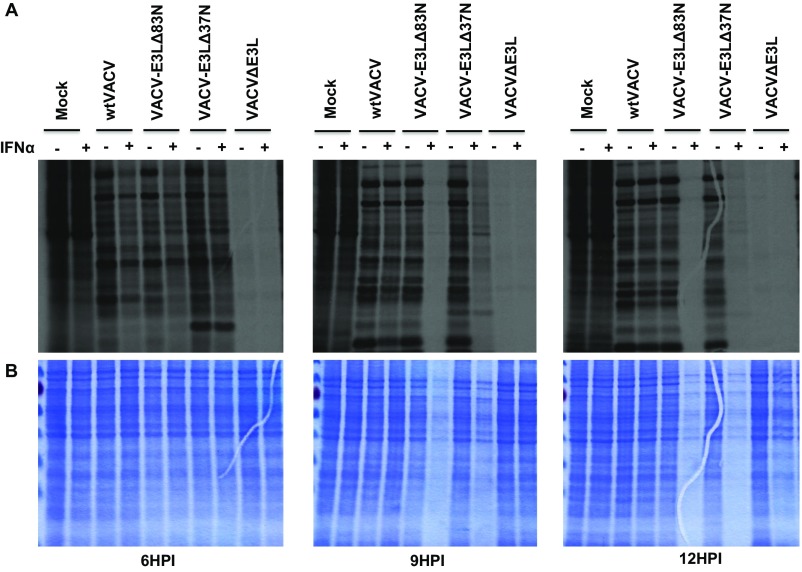
Fig. S1.
(A) Autoradiograph of newly synthesized proteins. L929 cells were mock or pretreated with IFN for 18 h and then infected with the indicated virus at an MOI of 5. At 5, 8, and 11 HPI, the cells were starved of methionine and pulsed with [35S] methionine-containing media to label newly synthesized protein at 5.5, 8.5, and 11.5 HPI. Cell lysates were harvested at 6, 9, and 12 HPI and separated by SDS gel electrophoresis. (B) Total protein present in cell lysates was evaluated with Coomassie stain. Cells were infected and harvested as described above. Samples pretreated with IFN and infected with VACV-E3LΔ83N demonstrated a significant global reduction in protein.
Type I IFN Treatment Leads to an Alteration in Morphology in VACV-E3LΔ83N–Infected Cells.
To assess morphological changes in mutant virus-infected cells, live imaging was conducted. L929 cells treated with mouse IFN and then infected with VACV-E3LΔ83N showed distinct morphological changes not seen in cells infected with WTVACV (Fig. 1C and Movies S1 and S2). Starting at ∼4 h postinfection (HPI), VACV-E3LΔ83N–infected cells underwent progressive cytoplasmic enlargement and plasma membrane disruption, patterns that were not observed in cells infected with WTVACV, irrespective of IFN treatment (Fig. 1C and Movies S1 and S2). Such a pattern of cellular swelling and membrane disruption suggests that a rapid death occurs in cells infected with VACV-E3LΔ83N, where leakage may underlie the global loss of protein recovery seen in Fig. 1B and Fig. S1.
IFN Sensitivity Results in a Rapid Death Characterized by Membrane Permeability.
To establish that leakage was occurring in VACV-E3LΔ83N–infected cells, we evaluated cellular permeability using a membrane-impermeable nuclear stain. This assay revealed that L929 cells pretreated with IFN and infected with VACV-E3LΔ83N became permeable, while the uninfected control cells or cells infected with WTVACV did not (Fig. 1 D and E). The induction of death was dependent on both the cells being primed through IFN treatment and being infected with VACV-E3LΔ83N. Combined with the live imaging results, this pattern suggests that mutant virus in IFN-primed cells is triggering a programmed cell death pathway.
IFN-Primed Death Is Dependent on RIPK3 and Is Caspase Independent.
Programmed cell death pathways can be broadly separated into those that are caspase dependent (apoptosis and pyroptosis) and those that are caspase independent (necroptosis, or programmed necrosis). To determine whether VACV-E3LΔ83N–induced death was caspase dependent, we employed a pan-caspase inhibitor, Z-VAD-FMK (zVAD). zVAD globally inhibits all known caspases (21). However, zVAD failed to reverse IFN sensitivity of VACV-E3LΔ83N (Fig. 2A). Similarly, zVAD treatment failed to reverse the inability to recover proteins from infected cells (Fig. 2B) and did not reverse IFN-primed cell death in VACV-E3LΔ83N–infected L929 cells (Fig. 2 C and D). This suggests that the cell death induced by VACV-E3LΔ83N in IFN-treated L929 cells was independent of caspases and therefore was neither apoptosis nor pyroptosis.
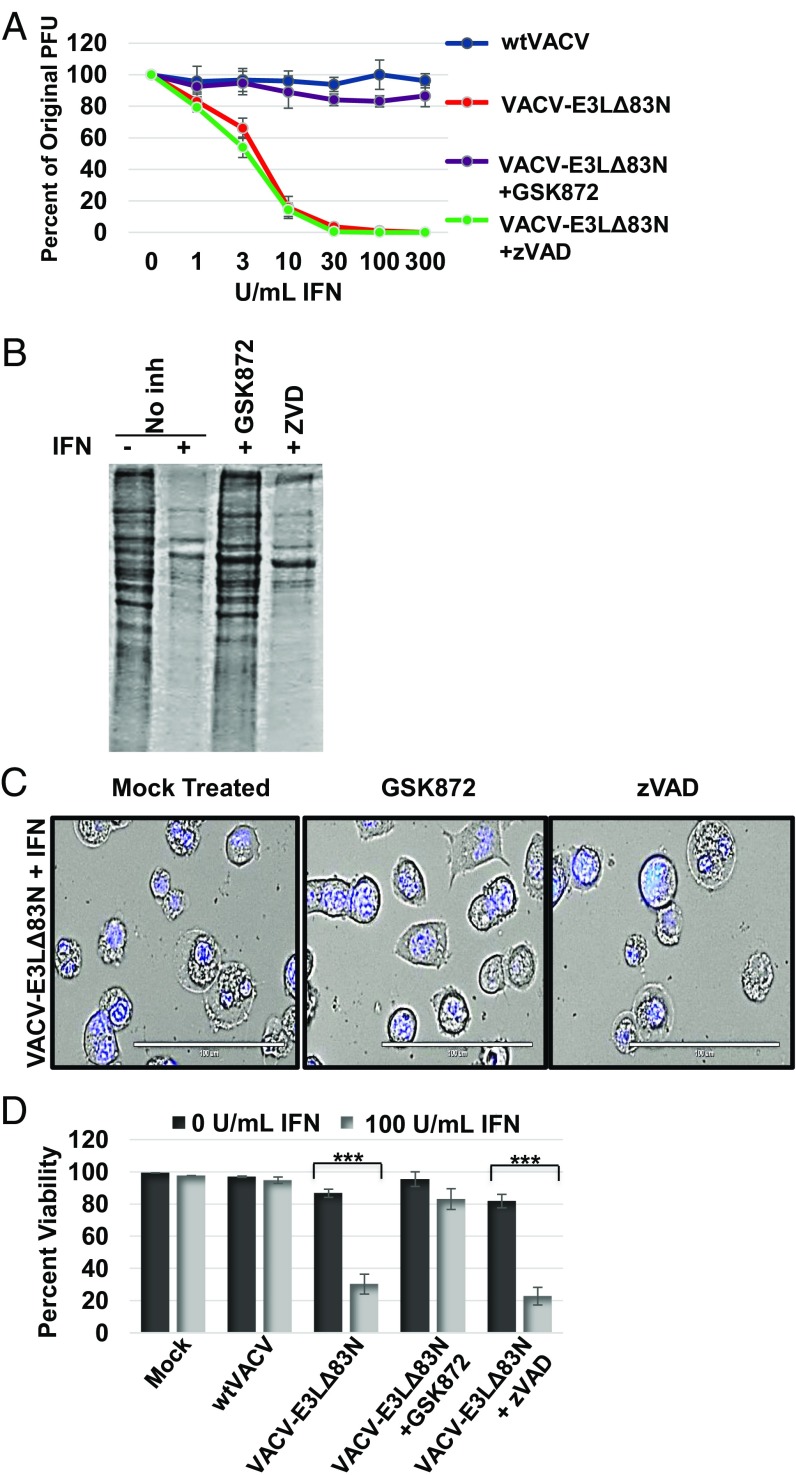
Fig. 2.
IFN-primed cell death is reduced by a RIPK3 kinase inhibitor. (A) Plaque reduction assay was performed by pretreating L929 cells with increasing doses of mouse IFN for 18 h and then treating with a RIPK3 inhibitor (GSK872) at 3 μM, or a pan-caspase inhibitor (zVAD) at 50 μM, or mock treatment (DMSO) for 1 h before infection. Cells were infected with equivalent pfu of WTVACV or VACV-E3LΔ83N. Once plaques formed, the percent of pfu reduction compared with mock IFN treated was calculated. (B) Total protein present in cell lysates was evaluated with Coomassie stain. Lysates were harvested at 12 HPI from L929 cells pretreated with IFN for 18 h and subsequently infected at an MOI of 5 in the presence of GSK872, zVAD, or mock treatment. (C) Transmitted live imaging at 6 HPI of L929 cells pretreated with 100 units/mL of mouse IFN for 18 h and for 1 h with either mock, zVAD, or GSK872 and then infected with VACV-E3LΔ83N at an MOI of 5. (D) Cell viability was determined using SYTOX exclusion by taking the average percentage of viable cells in 10 fields at 5 HPI in the presence of GSK872 or zVAD or mock treated. ***P < 0.001.
Necroptosis occurs independently of caspase activity and depends on the protein kinase, RIPK3. Thus, we asked if a RIPK3-specific inhibitor, GSK872, could reverse the cell death induced in IFN-treated VACV-E3LΔ83N–infected L929 cells. Treatment with GSK872 inhibited E3LΔ83N-induced cell death in IFN-treated cells (Fig. 2 C and D), reversed the failure to recover proteins from infected cells (Fig. 2B), and restored the IFNR of plaque formation (Fig. 2A).
VACV-E3LΔ83N Infection in IFN-Treated L929 Cells Results in MLKL Activation.
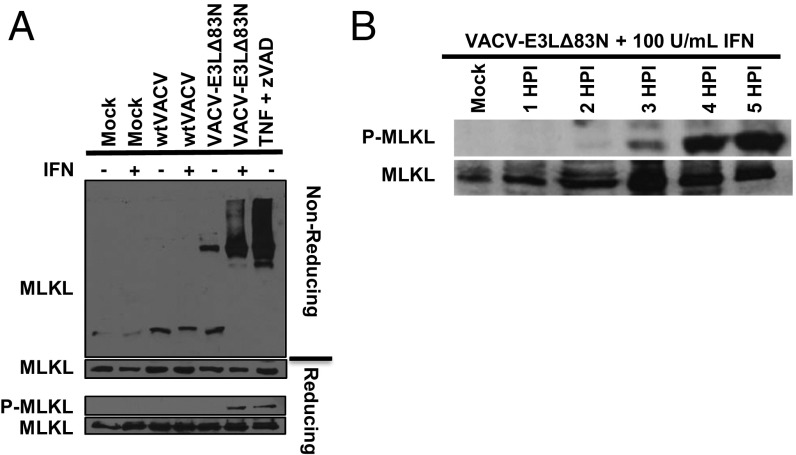
MLKL phosphorylation and aggregation have been shown to be the downstream consequences of RIPK3 activation (10, 11). To confirm that the cell death in IFN-treated, VACV-E3LΔ83N–infected cells was from programmed necrosis, MLKL activation was examined in mock-, WTVACV-, or VACV-E3LΔ83N–infected L929 cells, and in TNF/zVAD-treated cells. Western blots were performed to assay for the presence and phosphorylation of MLKL (Fig. 3). Phosphorylation of MLKL was specific to IFN-treated, VACV-E3LΔ83N–infected cells and cells treated with the positive control, TNF+zVAD (Fig. 3A). Phosphorylation of MLKL was detectable by 3 HPI (Fig. 3B). In nonreducing conditions, MLKL also migrated as an aggregate in extracts from IFN-treated VACV-E3LΔ83N–infected cells (Fig. 3A), consistent with MLKL activation. These results indicate that necroptosis, involving the aggregation and phosphorylation of MLKL, was occurring in IFN-treated VACV-E3LΔ83N–infected L929 cells. We have seen similar death and MLKL phosphorylation in human HT29 cells infected with VACV-E3LΔ83N (Fig. S2).
Fig. 3.
VACV-E3LΔ83N infection leads to MLKL activation. (A) Aggregation and phosphorylation of MLKL evaluated by Western blotting. Aggregation was detected by SDS/PAGE under nonreducing conditions. (B) Western blot detection of MLKL phosphorylation. P-MLKL was detected as early as 3 HPI.
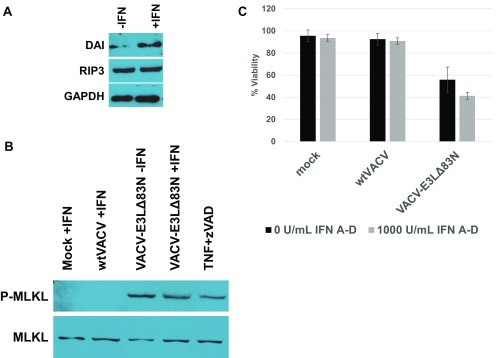
Fig. S2.
The human cell line, HT29, undergoes DAI-dependent necroptosis in response to infection with VACV-E3LΔ83N. (A) Untreated or IFN-treated cells were analyzed by Western blot for expression of DAI, RIP3, and GAPDH. (B) Cells were prepared as described above (or treated with human TNF-α and zVAD as in Fig. S2) but were harvested as lysates for Western blot analysis at 18 HPI. (C) Cells were mock or pretreated with 1,000 units/mL of recombinant human IFN A-D for 18 h and then infected with WTVACV or VACV-E3LΔ83N at an MOI of 5. Cells were overlaid with medium containing SYTOX and Hoechst dye and imaged at 8 HPI. Cells were counted for dye inclusion as described in the text.
RIPK3 Activity Is Required for IFN-Sensitive Plaque Reduction but Is Independent of RIPK1.
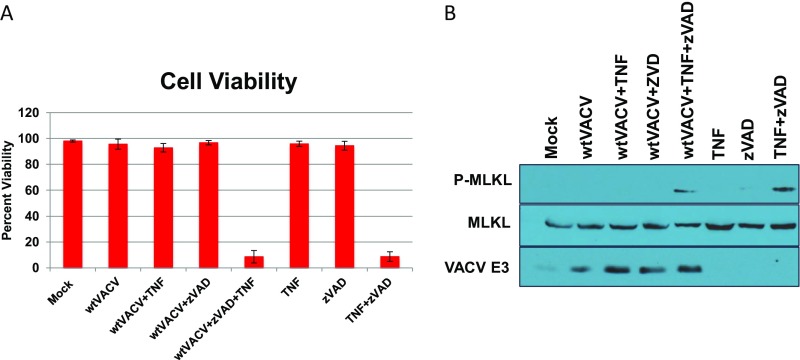
To investigate the adaptor that leads to RIPK3 activation in IFN-treated, VACV-E3LΔ83N–infected cells, we evaluated the requirements for RIPK1 and RIPK3 kinase activities using specific inhibitors. The RIPK1 kinase inhibitor, GSK963, was unable to rescue plaquing efficiency (Fig. 4A), cell viability (Fig. 4B), or MLKL activation (Fig. 4C) in L929 cells pretreated with IFN and infected with VACV-E3LΔ83N, whereas, treatment with the RIPK3 inhibitor, GSK872, restored IFNR in all assays. These results suggest that the necroptotic cell death induced by VACV-E3LΔ83N in IFN-treated L929 cells was RIPK1 independent. WTVACV-infected cells were in fact susceptible to death induced by TNF+zVAD treatment, which is RIPK1 dependent, at early times postinfection (Fig. S3), consistent with previously published results (7, 13). Thus, VACV-E3LΔ83N–induced death occurs independently of either RIPK1 or TNF in contrast to the necroptosis seen previously with WTVACV (7).
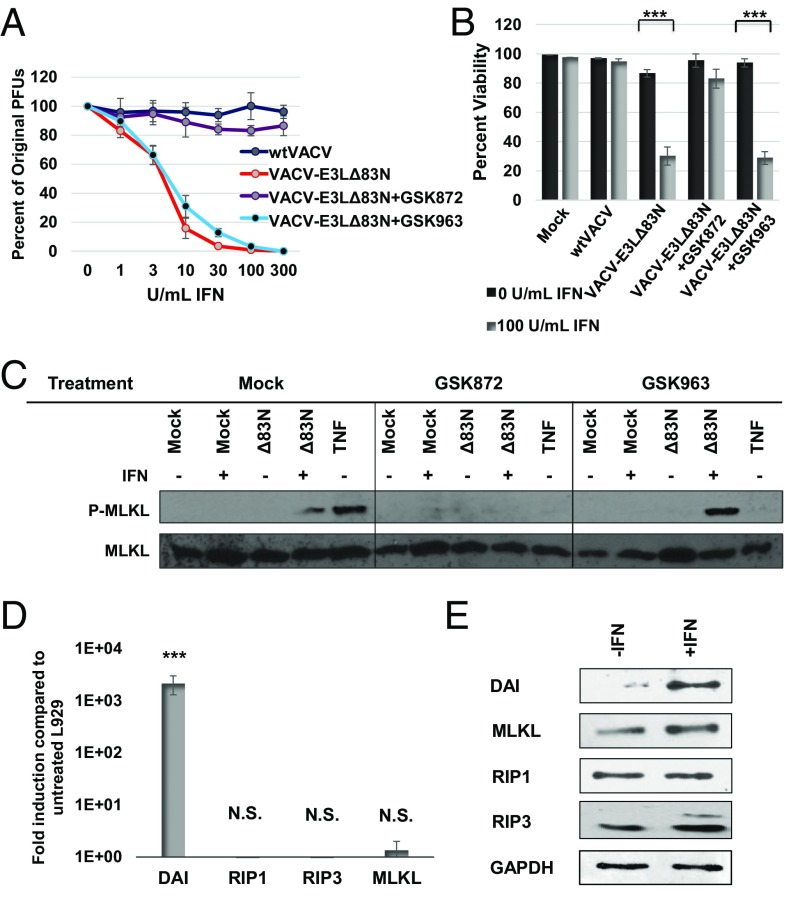
Fig. 4.
IFN-primed necroptosis is independent of RIPK1. (A) Plaque reduction assay was performed by pretreating L929 cells with increasing doses of mouse IFN for 18 h and then treating with a RIPK3 inhibitor (GSK872) at 3 μM, or a RIPK1 inhibitor (GSK963) at 3 μM, or mock treatment (DMSO) for 1 h before infection. Cells were infected with equivalent pfu of WTVACV or VACV-E3LΔ83N. (B) L929 cell viability was determined using SYTOX exclusion by taking the average percentage of viable cells in 10 fields at 5 HPI in the presence of GSK872, GSK963, or mock treatment. (C) L929 cells were pretreated with IFN for 18 h and either infected with VACV-E3LΔ83N or treated with TNF and zVAD in the presence of GSK872, GSK963, or mock treatment, and evaluated for P-MLKL by Western blot. (D) qPCR evaluation of necroptosis-associated gene induction in L929 cells following 18-h treatment of 100 units/mL of IFN. (E) Western blot of protein expression level of necroptosis-associated proteins following 18-h treatment of 100 units/mL of IFN. ***P < 0.001. NS, no significance (>0.05).
Fig. S3.
VACV is not a direct inhibitor of necroptosis. (A) L929 cells were infected with WTVACV at an MOI of 5. At 5 HPI, cells were treated with 50 µM zVAD and subsequently treated with 20 µg/mL of mouse TNF-α at 6 HPI. Cell viability was measured by Sytox exclusion at 11 HPI by taking the average percent of viable cells in a total of 10 fields, imaged at 20×. (B) Cells were infected and treated as described above, but were prepared as lysates for Western blot analysis at 11 HPI.
DAI Is Up-Regulated in L929 Cells Following IFN Treatment.
As the IFN-dependent cell death induced by VACV-E3LΔ83N is RIPK1 independent, we sought to identify the adaptor protein being utilized in its place. Several other RHIM-containing proteins have been shown to interact with RIPK3 to induce necroptosis. DAI was a potential candidate because it contains a Zα domain homologous to the one absent in VACV-E3LΔ83N. Induction of necroptosis in L929 cells by VACV-E3LΔ83N requires the treatment of IFN. Given that DAI is one of the most highly induced proteins following IFN treatment (29), we evaluated this transcript as well as transcripts for other important proteins in the necroptosis signaling pathway. No significant changes were seen in transcript levels of RIPK1, RIPK3, or MLKL following IFN treatment, but a 1,000-fold induction of DAI transcripts was observed (Fig. 4D). When RIPK1, RIPK3, MLKL, and DAI levels were directly evaluated by Western blotting, an increase only in DAI levels was observed in IFN-treated L929 cells (Fig. 4E).
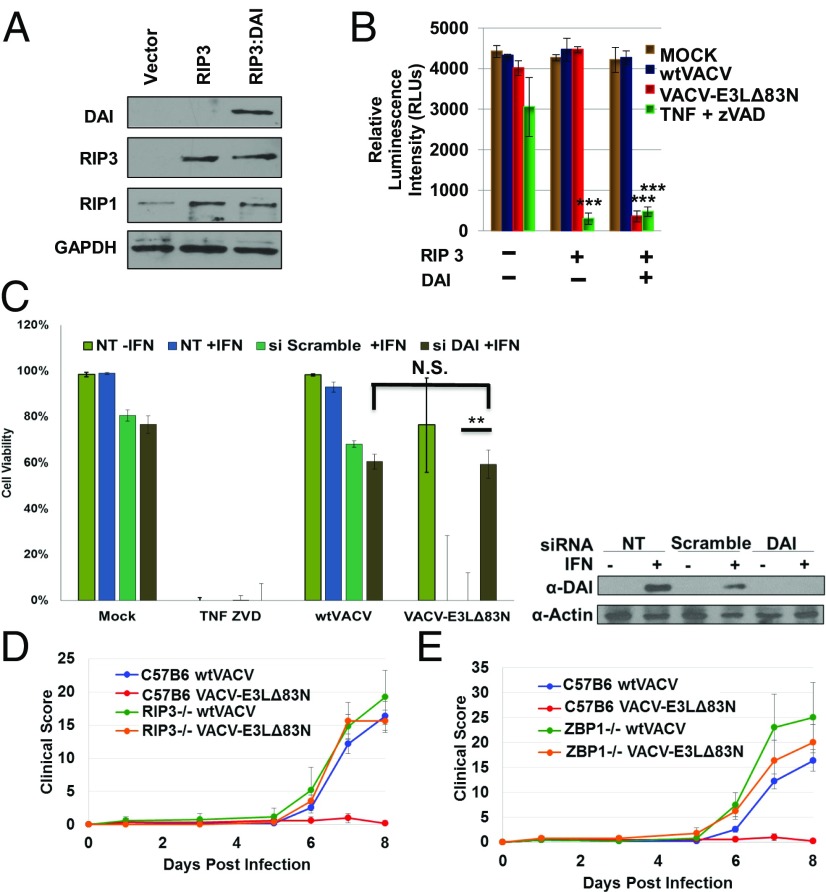
DAI Contributes to Induction of Necroptosis by VACV-E3LΔ83N.
Since DAI was up-regulated by IFN treatment of L929 cells, we sought to determine if DAI expression was sufficient to induce necroptosis in cells infected with VACV-E3LΔ83N. Utilizing a plasmid expression system, we ectopically expressed human RIPK3 alone or in combination with human DAI in HEK293T cells, which do not express DAI or RIPK3 (Fig. 5A). Following transfections, cells were either treated with TNF+zVAD or infected with WTVACV or VACV-E3LΔ83N (Fig. 5B). Expression of RIPK3 was sufficient to sensitize the HEK293T cells to necroptosis following treatment with TNF+zVAD. This is consistent with the endogenous expression of RIPK1 in 293T cells (Fig. 5A). Expression of RIPK3 alone was not sufficient to sensitize the cells to VACV-E3LΔ83N–induced necroptosis; the expression of both RIPK3 and DAI was required (Fig. 5B). WTVACV failed to induce death in any condition tested. We have also evaluated the effects of knockdown of DAI expression in L929 cells, where VACV-E3LΔ83N induces necroptotic cell death (Fig. 3). Transfection of L929 cells with siRNA to DAI led to reduced induction of DAI after IFN treatment (Fig. 5C). Knockdown of DAI restored cell viability in IFN-treated, VACV-E3LΔ83N–infected cells to the levels of viability seen in IFN-treated cells that were transfected with either siRNA to DAI or a scrambled siRNA, and subsequently infected with WTVACV (Fig. 5C). Thus, DAI expression was required for VACV-E3LΔ83N–induced cell death, and DAI is likely the adaptor protein for RIPK3 in necroptosis induced by VACV-E3LΔ83N.
Fig. 5.
Both RIPK3 and DAI are required for VACV-induced necroptosis. (A) 293T cells express RIPK3 and DAI only after transfection. (B) Viability of human 293T cells under indicated conditions was measured by Cell Titer-Glo assay. (C) L929 cells were untransfected (NT) plus or minus IFN, or transfected with either scrambled siRNA plus treated with IFN, or transfected with siRNA targeted to knockdown DAI expression, plus treated with IFN; cells were then either mock infected, treated with TNF-zVAD, infected with WTVACV, or infected with VACV-E3LΔ83N. (Right) Western blot of the cell lysates, showing the presence or absence of DAI. (D) Eight- to 10-wk-old RIPK3−/− or WT C57BL/6 mice were inoculated by intranasal route with 106 pfu of the indicated viruses (five mice per group). (E) Eight- to 10-wk-old ZBP1(DAI)−/− or WT C57BL/6 mice were infected by intranasal route with 106 pfu of the indicated viruses (five mice per group). **P < 0.01. N.S., no significance (>0.05).
Deficiency of RIPK3 or ZBP1 Rescues VACV-E3LΔ83N Virulence in Mice.
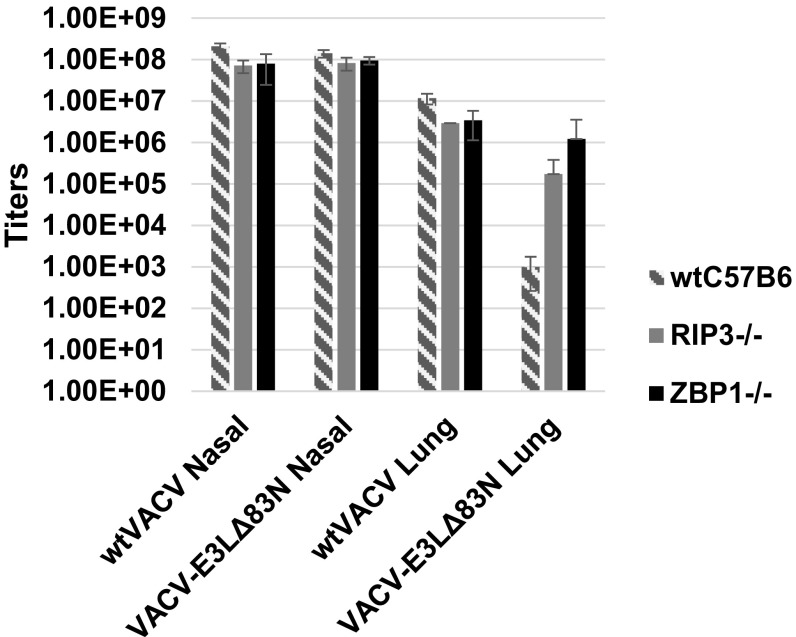
Given the importance of mouse studies that have defined the N terminus in subverting type I IFN signaling and virulence (6), we sought to pursue in vivo studies in WT C57BL/6, RIPK3−/−, and ZBP1(DAI)−/− mice. Mice were infected intranasally with 106 pfu of either WTVACV or VACV-E3LΔ83N [in the mouse-adapted, neurovirulent Western Reserve (WR) strain and monitored for clinical symptoms]. WTVACV infections resulted in significant pathology in WT, RIPK3−/−, and ZBP1−/− mice. As previously described, at this dose the VACV-E3LΔ83N mutant was apathogenic in WT C57BL/6 mice (6). Pathogenesis of this mutant was restored in RIPK3−/− (Fig. 5D) and ZBP1−/− (Fig. 5E) mice to levels comparable to that of WTVACV. We have also isolated virus from tissues of infected animals (Fig. S4). WTVACV and VACV-E3LΔ83N replicated to equivalent levels in the nose, while lower levels of VACV-E3LΔ83N were detected in the lungs of infected WTC57Bl6 mice. VACV-E3LΔ83N replication was increased in the lungs of RIP3−/− or ZBP1−/− mice, consistent with the increase in pathogenesis seen in VACV-E3LΔ83N–infected knockout mice, although the increased titers were not statistically significant because few transgenic animals were available (Fig. S4). These results together indicate that the N terminus of E3 is important to block RIPK3-dependent host pathways that can limit the virulence of VACV.
Fig. S4.
Indicated tissues were harvested from the infected animals (five mice per group) and virus extracted from the tissue was titered on BSC-40 cells.
Discussion
The VACV E3 protein has long been established as an essential innate immune evasion protein that confers resistance to IFN (5, 6). Although the consequences of E3 C-terminal dsRNA sequestration have been clear for decades (2–4), the dramatic IFN sensitivity of VACV E3 N-terminal deletion mutants has remained unresolved. Previous work suggested that the N terminus of E3 was essential for IFNR by preventing the phosphorylation of PKR in MEF129 cells. However, the highly attenuated phenotype of VACV-E3LΔ83N was not reversed in PKR-deficient mice, implicating an alternative mechanism for the IFNS in vivo phenotype of the mutant virus (6).
In this study, we resolve the role of the E3 N terminus, providing evidence that this domain competes with DAI to prevent DAI-dependent activation of RIPK3 and consequent necroptosis. Infection of L929 cells with VACV-E3LΔ83N led to a rapid IFN-dependent cell death characterized by cellular swelling and loss of plasma membrane integrity. This cell death was caspase independent but dependent on the kinase activity of RIPK3. Infection of IFN-treated cells with VACV-E3LΔ83N led to MLKL phosphorylation and aggregation, indicative of necroptotic cell death (20). VACV could not inhibit TNF-induced cell death, which is RIPK1 dependent (7, 30), suggesting an alternative activator for RIPK3. Since expression of DAI/DLM/ZBP1 is highly inducible in L929 cells by IFN treatment, and since DAI/DLM/ZBP1 contains a Zα domain that can substitute for the Zα domain of the E3 protein in supporting pathogenesis in mice (31), we asked if cell death was dependent on DAI/DLM/ZBP1 expression. Knockdown of DAI in L929 cells restored viability in IFN-treated, VACV-E3LΔ83N–infected cells. HEK293T cells do not express RIPK3 or DAI and are resistant to virus- or TNF-induced necroptosis. Ectopic expression of RIPK3 restored TNF-induced cell death in HEK293T cells, but expression of both RIPK3 and DAI was needed to restore death in VACV-E3LΔ83N–infected cells.
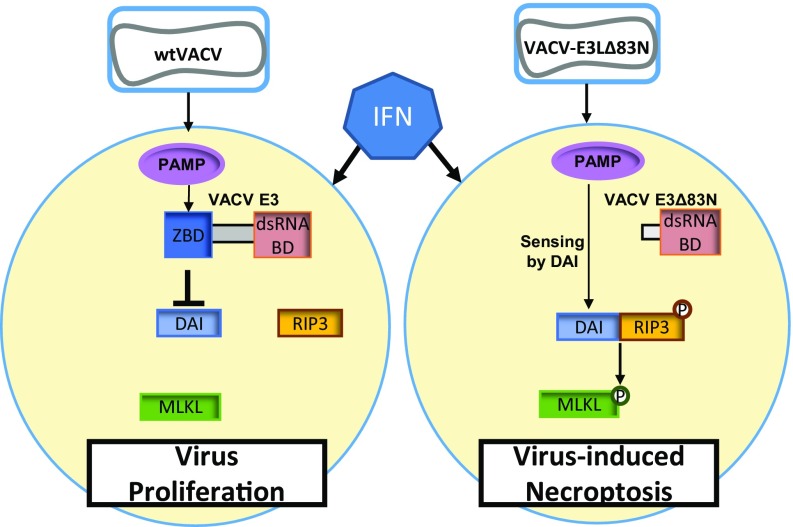
E3 and DAI are members of the same Zα binding domain-containing family of proteins. Despite the E3 and DAI Zα domains sharing less than 20% sequence homology, the DAI domain functionally replaced the E3 domain (31–33). This suggests that both DAI and the N terminus of E3 can bind to the same nucleic acid molecules in a VACV-infected cell. We therefore propose a model (Fig. 6) in which IFN priming of cells enhances the expression of DAI, which acts as a sensor of a VACV-induced Z-form nucleic acid pathogen-associated molecular pattern (PAMP). During WTVACV infection, the Zα domain of E3 is presumed to bind to this putative PAMP and mask it, preventing sensing by DAI. In VACV-E3LΔ83N–infected cells, the absence of the N-terminal Zα domain of E3 allows the homologous Zα domain of DAI to sense the VACV-induced PAMP and act as a RIPK3 adaptor protein to initiate necroptosis.
Fig. 6.
Model for mechanism of VACV inhibition of necroptosis.
Virus-induced necroptosis has been shown to be an important innate defense mechanism against viral infections with three distinct viruses: poxviruses, herpesviruses, and influenza A virus (IAV) (8–10). WTVACV has previously been shown to sensitize cells to TNF-driven necroptosis at late times postinfection, suggesting that VACV does not produce any proteins that can inhibit TNF- and RIPK1-dependent necroptosis (7, 13). Thus, it appears that VACV can only inhibit DAI-mediated necroptosis but not RIPK1-mediated necroptosis. These earlier studies also reported that pathogenesis in mice infected with WTVACV was exacerbated in RIPK3−/− mice. We did not detect an increase in pathogenesis in RIPK3−/− mice infected with WTVACV. The difference in results between previous studies and the current study may be due to the different modes of infection, IP in the previous work, vs. IN in the work described in this manuscript.
The herpesviruses have also been shown to encode an inhibitor of necroptosis (26, 34, 35). MCMV is the most well characterized of the herpesviruses that can inhibit induction of necroptosis as an innate immune defense mechanism (35). The MCMV M45 protein contains a RHIM domain that can interact with the RHIM domain of RIPK3 and inhibit TNF-, TRIF- and DAI-induced necroptosis (16, 18, 21). In the absence of M45, MCMV-infected cells become susceptible to DAI-mediated necroptosis (16, 23). Unlike RHIM-dependent viral inhibition of necroptosis in the herpesvirus system, E3 inhibition is likely a different mechanism of inhibition, as E3 does not contain an identifiable RHIM domain (21).
Two groups have recently reported that IAV infection leads to DAI-mediated cell death (8, 29). IAV infection can induce RIPK3-dependent necroptosis or apoptosis, depending on the nature of proteins recruited to the complex, or it can induce pyroptosis. Unlike the poxviruses and herpesviruses, IAV does not appear to encode a necroptosis inhibitor. The consequence of DAI-mediated cell death in IAV pathogenesis is unclear, in that differing results were seen in IAV-infected ZBP1−/− mice (8, 29).
The nature of the molecules sensed by DAI in infected cells remains unclear. DAI was initially identified as a DNA sensor, and as a Zα domain-containing protein. It is capable of binding Z-form nucleic acid in vitro. DAI has been reported to sense either IAV RNA or IAV proteins in IAV-infected cells (8, 29). The nature of the molecules being sensed during herpesvirus and VACV infection are likewise unclear, although the fact that the DAI Zα domain can functionally replace the Zα domain of E3 suggests that these proteins are competing for binding to a VACV-induced Z-form nucleic acid. Further characterization of this system may lead to the identification of physiologically relevant Z-form nucleic acid in the cell.
Materials and Methods
Cells and Viruses.
L929 cells and HEK293T cells were obtained from ATCC. The WR strain of WTVACV and mutant virus deleted of the N-terminal 83 amino acids of E3 (VACV-E3LΔ83N) were described previously (4).
IFN Resistance Evaluation by Plaque Reduction.
L929 cell monolayers were treated with 0–300 units/mL of mouse IFN-α and then infected with either WTVACV or VACV-E3LΔ83N virus. Plaque formation was detected by staining with crystal violet (SI Materials and Methods for more details).
L929 Viability Assay.
Viability was assessed with a SYTOX nuclear stain exclusion assay or by a CellTiter-Glo luminescent cell viability assay (Promega) (SI Materials and Methods for more details).
Inhibitors and Treatment of Cells.
All inhibitor treatments were applied for 1 h preinfections. GSK872 and GSK963 were kindly provided by GlaxoSmithKline (SI Materials and Methods for more details).
RT-PCR Methods.
RNA was isolated from mock- or IFN-treated cells, reverse transcribed into cDNA, and amplified. cDNA was detected on a CFX Connect Real-Time PCR detection system (SI Materials and Methods for more details).
Expression of DAI and RIP3 in HEK293T.
The human RIP3 and DAI proteins were expressed from plasmid expression systems (pLV-EF1a-cDNA-IRES-Puro) in HEK293T cells. At 48 h posttransfection, if indicated, the cells were treated as described and infected at a multiplicity of infection (MOI) of 5 with the indicated virus.
siRNA Transfections.
L929 cells were transfected with either ON-TARGET plus Nontargeting Pool or SMARTpool: ON-TARGET plus Zbp1 siRNA (SI Materials and Methods for more details). The experiments were performed 18 h later as described.
Intranasal Infections of Mice.
Anesthesia and infections were as previously described (6). Mice were infected with either WTVACV or VACV-E3LΔ83N virus intranasally (IN). C57BL/6 mice were from The Jackson Laboratory, Ripk3−/− mice were from Genentech (36), and Zbp1−/− mice (37) were from Shizuo Akira, Osaka University, Osaka, Japan. All procedures were approved by the Emory University Institutional Animal Care and Use Committee (SI Materials and Methods for more details).
Western Immunoblot Analysis.
L929 cells were pretreated with mouse IFN-α and infected with viruses at an MOI of 5. Lysates were harvested as previously described (16). For samples used for MLKL aggregation, cells were prepared as described above but lysed in 100 μL of 1× SDS without 2-mercaptoethanol. Proteins were separated by SDS/PAGE (SI Materials and Methods for more details).
Total Protein Staining.
L929 cell monolayers were infected with WR strains of WTVACV or E3LΔ83N at an MOI of 5. Lysates were prepared and separated on denaturing gels and visualized following SDS/PAGE (SI Materials and Methods for more details).
Live Imaging of L929-Infected Cells.
L929 cells were pretreated with 100 units/mL of mouse IFN-α for 18 h before infection. A live nuclear stain was applied 15 min before infection. Cells were infected with either WTVACV or VACV-E3LΔ83N as described above. For images used to evaluate alterations in morphology, infected cells were incubated for an additional 5 h and imaged with an EVOS FL auto imaging system. For time-lapse imaging, cells were overlaid with minimum essential medium (MEM) containing 1 µM SYTOX Green nucleic acid stain. Images were taken every 2 min for 5 h (SI Materials and Methods for more details).
Statistics.
Statistical analysis was done by using a two-tailed, unpaired t test. ***P < 0.001, **P < 0.01, *P < 0.05. No significance (N.S.) was used to represent P > 0.05. Error bars represent SE.
SI Materials and Methods
Cells.
HT29 cells from ATCC were maintained according to ATCC recommendation in McCoy’s 5a Medium Modified, catalog no. 30–2007, with 10% heat-inactivated FBS.
IFN Resistance Evaluation by Plaque Reduction.
L929 cell monolayers were treated with 0–300 units/mL of mouse IFN-α (Calbiochem) and then infected with 1 × 102 pfu of WR strain of either WTVACV or VACV-E3LΔ83N virus. Plaque formation was detected by staining with crystal violet at 3–4 d postinfection.
siRNA Transfections.
L929 cells were transfected using DharmaFECT 4 (T-2004-01) in serum-free MEM and either ON-TARGET plus Nontargeting Pool (d-001810-10) or SMARTpool: ON-TARGET plus Zbp1 siRNA (l-048021-00-0005). Cells were plated at 80% confluency in 12-well dishes. siRNA (5 µL of 5 µM/well) and DharmaFECT Reagent 4 (2 µL/well) were diluted in 200 µL of serum-free MEM. After 20 min, the media were added to the aspirated L929 cells and they were rocked. One hour later, 800 µL of MEM with 5% FBS and either 100 or 0 units of IFN was added. The experiments were performed 18 h later as described.
Intranasal Infections of Mice.
Anesthesia and infections were carried out as previously described (1). Anesthetized mice were infected with 106 pfu of WR strain of either WTVACV or VACV-E3LΔ83N virus intranasally (five animals per group). Mice were monitored daily for clinical signs. C57BL/6 mice were from The Jackson Laboratory, Ripk3−/− mice (Ripk3tm1Vmd) were from Genentech (2), and Zbp1−/− mice (Zbp1tm1Aki) (3) were from Shizuo Akira, Osaka University. Mice were maintained by the Emory University Division of Animal Resources, and all procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Titers from Animal Tissues.
At 8 d postinfection, mice were killed and tissues were harvested [lung and sinus (nasal turbinates)] and immediately frozen in liquid nitrogen. Tissues were homogenized with electric tissue homogenizer in a 10% tissue suspension using MEM containing 5% FBS and antibiotic-antimycotic (Gibco). Nasal tissue was additionally ground in liquid nitrogen with mortar and pestle prior to the electric homogenization; all electric homogenizing was followed by three freeze/thaw cycles, and tissues were then titered on BSC40 cells.
Live Imaging of L929 Infected Cells.
L929 cells were seeded in 12-well CytoOne tissue culture-treated plates (USA Scientific) and pretreated with 100 units/mL of mouse IFN-α (Calbiochem) for 18 h before infection. A live nuclear stain was applied using Hoechst and incubated 15 min before infection. Cells were infected with an MOI of 5 with either WTVACV or VACV-E3LΔ83N as described above. For images used to evaluate alterations in morphology, infected cells were incubated for an additional 5 h and imaged with an EVOS FL auto imaging system (Invitrogen). For time-lapse imaging, cells were overlaid with MEM media containing 1 µM SYTOX Green nucleic acid stain (Thermo Fisher Scientific). Cells were incubated at 37 °C with 5% CO2-supplemented air on an EVOS FL auto imaging system (Invitrogen) with onstage incubator system and an image was taken every 2 min for 5 h.
L929 Viability Assay.
Viability was assessed with a SYTOX nuclear stain exclusion assay or by a CellTiter-Glo luminescent cell viability assay (Promega). For SYTOX nuclear stain, Hoechst 33342 {2′-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5′-bi-1H-benzimidazole} and SYTOX were applied for 15 min before infection and throughout the time following the infection incubation. Cells were imaged as described above. CellTiter-Glo luminescent cell viability assay was performed at 12 h postinfection (HPI) according to manufacturer’s recommendations.
HT29 Viability Assay.
Cells were plated to maintain a confluency below 50% at time of infection. Cells were mock or pretreated with 1,000 units/mL of recombinant human IFN A-D for 18 h. Cells were then infected at an MOI of 5 with rocking for an hour. Cells were overlaid with medium containing SYTOX and Hoechst dye and imaged at 8 HPI. Cells were counted for dye inclusion as described in the main text.
Inhibitors and Treatment of Cells.
All inhibitor treatments were applied for 1 h before infections and throughout the time following the infection incubation. Caspase involvement was evaluated by treating cells with the pan-caspase inhibitor zVAD-FMK (zVAD) (ApexBio) at 50 µM. RIP proteins were evaluated with the use of GSK inhibitors at 3 µM. N-(6-(isopropylsulfonyl)quinolin-4-yl)benzo[d]thiazol-5-amine (GSK872; GlaxoSmithKline) was used to inhibit RIP3 activity and 2,2-dimethyl-1-(5(S)-phenyl-4,5-dihydro-pyrazol-1-yl)-propan-1-one (GSK963; GlaxoSmithKline) was used to determine the requirement of RIP1 activity. Cells used as a positive control for necroptosis were treated with mouse TNF-α (Sigma) at 20 ng/mL following a 1-h pretreatment with 100 µM zVAD.
RT-PCR Methods.
RNA was isolated from mock- or IFN-treated cells at 18 h posttreatment using Qiagen’s Rneasy Mini Kit (74104). RNA was then reverse transcribed into cDNA using PrimeScript RT reagent kit (RR037A, Takara). Samples were amplified using PrimeTime Gene Expression Master Mix (1055772, IDT) on a CFX Connect Real-Time PCR detection system (1855201, BioRad). Primer sets were purchased from IDT. Fold induction of genes was determined using the ΔΔCq method with GAPDH as the control gene and untreated cells as mock.
Protein Extraction and Western Immunoblot Analysis.
L929 cells were pretreated with 100 units/mL of mouse IFN-α (Calbiochem) for 18 h before infection, and were infected with viruses at an MOI of 5. Lysates were harvested at 4 HPI as previously described (4). For samples used for MLKL aggregation, cells were scraped and pelleted as described above but lysed in 100 μL of 1× SDS without 2-mercaptoethanol. Rabbit polyclonal antibodies were used to detect P-MLKL (Abcam) and total MLKL (Abcam) and mouse monoclonal antibodies for GAPDH (Santa Cruz). Secondary antibodies were goat anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz) or anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz). Immunoreactive bands were visualized by chemiluminescence with SuperSignal West Dura Duration substrate (Pierce Biotechnology). Primary antibodies to detect DAI and RIP3 were from Abcam, with the exception of Fig. 5C, in which the anti-DAI primary was Zippy-1 Adipogen.
Total Protein Staining.
Fifty percent confluent L929 cell monolayers in 60-mm dishes were infected with WR strains of WTVACV or E3LΔ83N at an MOI of 5. Cells were washed twice with PBS then pelleted and lysed in 100 μL of 1× SDS with 1× Halt Protease and Phosphotase Inhibitor Mixture (Pierce Thermo Scientific). Protein lysates were isolated utilizing QIAshredder columns (Qiagen) according to manufacturer’s recommendations. The samples were boiled and separated on denaturing 10% SDS/PAGE gels. The gels were stained for total protein levels with Coomassie staining solution (0.1% Coomassie Brilliant Blue R-250, 40% methanol, and 10% glacial acetic acid) for 30 min. The gels were then destained with destaining solution (40% methanol and 10% glacial acetic acid) three times, each for 20 min, and incubated overnight in 10% glacial acetic acid to remove any residual stain.
Protein Synthesis Shut-Off and Protein Loss Assay.
Fifty percent confluent L929 cell monolayers in 60-mm dishes were infected with WR strains of WTVACV or VACVΔE3L or E3L N-terminal mutants (VACV-E3LΔ83N or VACV-E3LΔ37N) at an MOI of 5. At 30 min before indicated time of radiolabeling, cells were washed twice with PBS to remove all media containing methionine and were subsequently overlaid with methionine-free DMEM (CellGro) for 30 min at 37 °C. Following starvation of methionine, the cells were incubated for 30 min at 37 °C with labeling media containing 50 μCi [35S]-methionine/mL (800 Ci/mmol; Perkin-Elmer) to label newly synthesized proteins. Cells were washed, pelleted, and lysed as described above for total protein staining followed by SDS/PAGE. Protein visualization was done either as described above (staining) or in the case of radiolabeling, proteins were visualized by autoradiography with a 24-h film exposure at −20 °C.
Supplementary Material
Acknowledgments
We thank Nobuko Fukushima and Hamed Alattar for their technical assistance in performing assays. This manuscript is dedicated to the memory of Alex Rich and Julius Youngner. NIH Grants supported this work (R01 AI095394 to B.L.J. and R01 AI118853 to E.S.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700999114/-/DCSupplemental.
References
- 1.Brandt T, et al. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology. 2005;333:263–270. doi: 10.1016/j.virol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Chang HW, Jacobs BL. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993;194:537–547. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- 3.Chang HW, Uribe LH, Jacobs BL. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J Virol. 1995;69:6605–6608. doi: 10.1128/jvi.69.10.6605-6608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt TA, Jacobs BL. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001;75:850–856. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie E, et al. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J Virol. 1995;69:499–505. doi: 10.1128/jvi.69.1.499-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White SD, Jacobs BL. The amino terminus of the vaccinia virus E3 protein is necessary to inhibit the interferon response. J Virol. 2012;86:5895–5904. doi: 10.1128/JVI.06889-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogusa S, et al. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton JW, Chan FK. Staying alive: Cell death in antiviral immunity. Mol Cell. 2014;54:273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Curr Opin Virol. 2013;3:296–306. doi: 10.1016/j.coviro.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: Novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 16.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XN, et al. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser WJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebsamen M, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omoto S, et al. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem. 2015;290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuriakose T, et al. ZBP1/DAI is a innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1:aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Kim YG, et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci USA. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YG, Lowenhaupt K, Oh DB, Kim KK, Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci USA. 2004;101:1514–1518. doi: 10.1073/pnas.0308260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahmann JD, et al. The solution structure of the N-terminal domain of E3L shows a tyrosine conformation that may explain its reduced affinity to Z-DNA in vitro. Proc Natl Acad Sci USA. 2004;101:2712–2717. doi: 10.1073/pnas.0308612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Kaiser WJ, Mocarski ES. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol (Berl) 2015;204:439–448. doi: 10.1007/s00430-015-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocarski ES, Guo H, Kaiser WJ. Necroptosis: The Trojan horse in cell autonomous antiviral host defense. Virology. 2015;479-480:160–166. doi: 10.1016/j.virol.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.