Significance
Failure to maintain mtDNA integrity can lead to a wide variety of neuromuscular disorders. Despite its central role in the development of these disorders, many mechanistic details of mtDNA maintenance are still unclear. In the present work, we have studied the role of PrimPol, an unusual primase-polymerase, in mammalian mtDNA maintenance. We report here that PrimPol is specifically required for replication reinitiation after DNA damage. PrimPol synthesizes DNA primers on an ssDNA template, which can be elongated by the mitochondrial replicative polymerase γ, a solution to reprime replication beyond DNA lesions and to facilitate lagging-strand replication. Our findings show that PrimPol has biological relevance for mtDNA maintenance.
Keywords: DNA repair, fork rescue, mtDNA damage, mtDNA replication
Abstract
Eukaryotic PrimPol is a recently discovered DNA-dependent DNA primase and translesion synthesis DNA polymerase found in the nucleus and mitochondria. Although PrimPol has been shown to be required for repriming of stalled replication forks in the nucleus, its role in mitochondria has remained unresolved. Here we demonstrate in vivo and in vitro that PrimPol can reinitiate stalled mtDNA replication and can prime mtDNA replication from nonconventional origins. Our results not only help in the understanding of how mitochondria cope with replicative stress but can also explain some controversial features of the lagging-strand replication.
PrimPol is an unusual mammalian primase-polymerase belonging to the archaeo-eukaryotic primase superfamily of primases (1, 2). The superfamily includes all known replicative primases in Archaea and Eukaryotes and is evolutionarily unrelated to the bacterial topoisomerase-primases (TOPRIMs) (3, 4). Similarly to the related archaeal PriS/L replicative primases (5, 6), PrimPol has a clear preference for dNTPs over NTPs, allowing it to synthesize DNA primers and function as a DNA-dependent DNA polymerase (2). It has been suggested that the priming, as well as primer extension activities, are required for DNA damage tolerance, such as translesion synthesis (TLS) across lesions such as 8-oxo-7-hydrodeoxyguanosine (2, 7), abasic sites, and UV lesions (8–10). Concordantly, PrimPol-KO cells are viable (1, 2, 9), but have an increased sensitivity to DNA-damaging agents such as UV and hydroxyurea (11). In addition, PrimPol contributes to the repriming of replication forks that are arrested at G-quadruplex structures in the template (12).
Like many DNA repair proteins (13), PrimPol is known to be localized in the nucleus and mitochondria (2), suggesting that it may play similar roles in the maintenance of mtDNA as it does with nuclear DNA. Although PrimPol has been proposed to be involved in multipriming events on mtDNA (2), no specific role for PrimPol in mtDNA maintenance has been experimentally demonstrated to our knowledge. In contrast to the nucleus, mitochondria are thought to have a limited set of DNA repair pathways; for example, they are unable to repair cyclobutane pyrimidine dimers caused by UV damage (14). Repair of DNA lesions represents only a subset of genome maintenance mechanisms, and the most dangerous types of DNA damage can result from complications during DNA replication. This seems to also be the case in mitochondria, as replication fork stalling has been implicated as the main cause of pathological mtDNA rearrangements (15, 16). mtDNA replication can stall as a result of mutations in TWNK helicase and the catalytic subunit of DNA polymerase γ (Pol γA) (16–18), chain-terminating nucleoside analogs such as 2′-3′-dideoxycytidine (ddC) (19), and DNA template damage (20). Unlike some catalytic mutations or ddC interference, oxidative or UV damage-induced stalling does not result in mtDNA copy number depletion, indicating that mitochondria have effective mechanisms to cope with such damage (21).
To our knowledge, nothing is known about the fate of stalled replication forks in mitochondria, and evidence suggests that the outcomes might be different in different tissues (15). Mitotic cells, like those used in tissue culture, mainly employ a highly strand-asymmetric replication mechanism, whereby the lagging-strand DNA is synthesized with a considerable delay (22). This replication mechanism results in typical patterns on 2D agarose gels used in DNA replications studies (23, 24). Although there is still debate about the details, the two proposed models for strand-asymmetric replication mechanism are very alike with the exception of the displaced strand being coated with preformed RNA (23) or the mitochondrial single-strand binding protein mtSSB (25). As there is evidence for (24) and against (26) RNA covering the displaced mtDNA strand in vivo, we use “strand-asymmetric mechanism” as a general term without differentiating between the two models. The main origin of leading-strand replication in the strand-asymmetric mechanism is the origin of heavy-strand replication (OH) in the noncoding region (NCR) of mtDNA (27). Replication from OH is assumedly primed by mitochondrial RNA polymerase (MTRPOL) transcribing from one of the two light-strand promoters (28). A major origin for the lagging-strand synthesis is at the origin of light-strand replication (OL), two thirds of the genome downstream of OH, and is also initiated by MTRPOL (16, 29), although there is evidence for other light-strand origins (25).
In the present work, we examine the role of PrimPol in the restart of stalled mtDNA replication forks and its significance for mtDNA maintenance after damage. We find that PrimPol is not only responsible for the replication reinitiation downstream of DNA lesions, but that it is also involved in the completion of partially ss mtDNA molecules. We conclude that, even though PrimPol is not essential for mtDNA maintenance, it provides an adaptational mechanism against genotoxic stress in mitochondria and might also enable origin-independent initiation of lagging-strand synthesis.
Results
PrimPol Is Required for Repriming of Stalled mtDNA Replication.
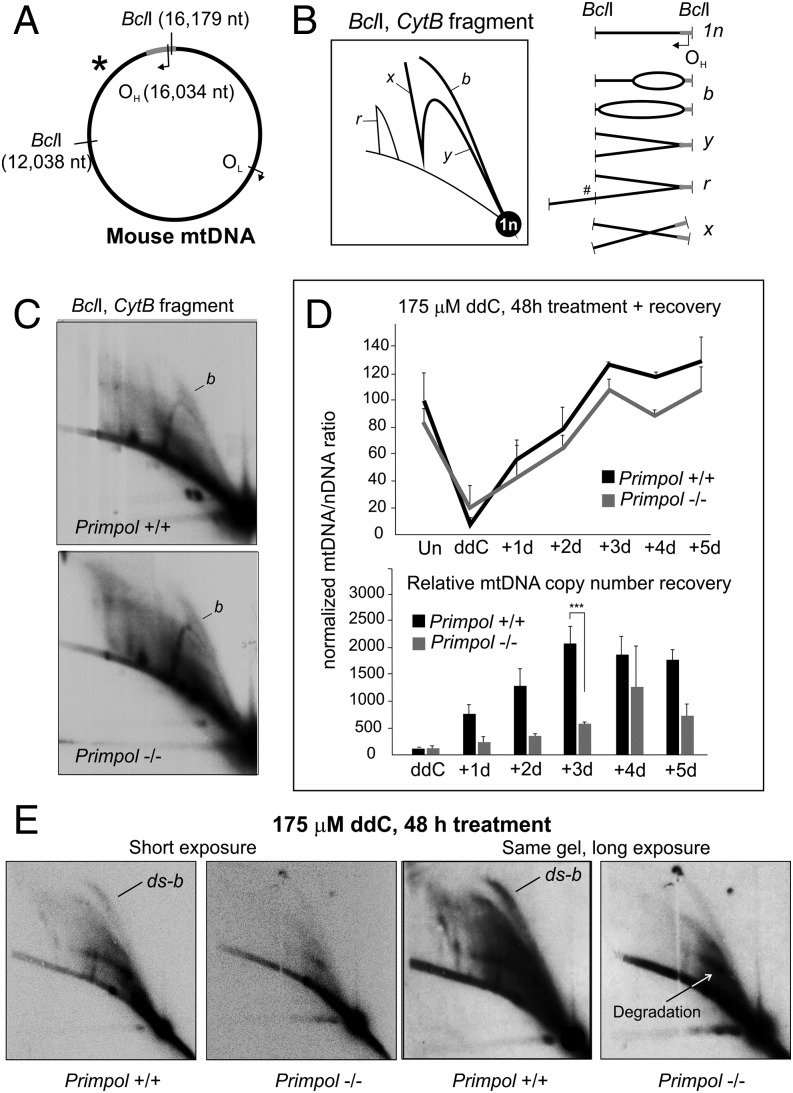
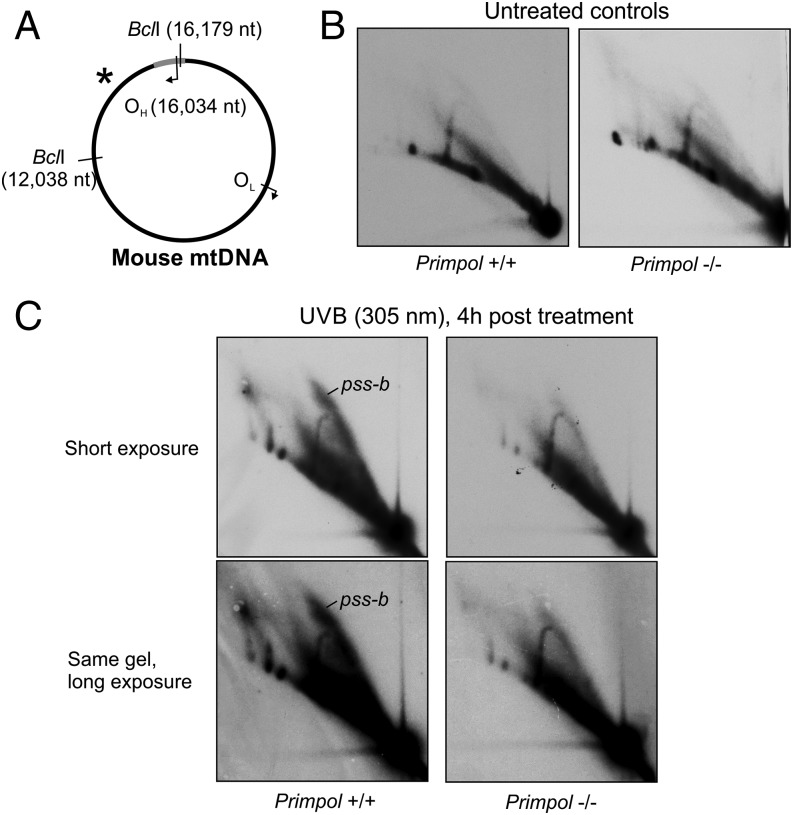
Chain-terminating nucleoside analogs (CTNAs) represent a very specific type of problem for DNA replication that can be mainly resolved via repriming and not, for example, by a TLS-type of activity. As PrimPol was recently shown to be important in maintaining nuclear DNA replication in the presence of CTNAs (11), we wanted to investigate whether this is also the case for mtDNA replication. In fact, Pol γ is the only mammalian replicative polymerase capable of incorporating ddC during DNA synthesis (30), resulting in specific blockage of mtDNA replication that can be detected by using 2D agarose gel electrophoresis (AGE; Fig. 1 and Fig. S1; further details are provided in SI Results and Discussion). Although Primpol-KO MEFs do not show any obvious replication phenotype under normal conditions (Fig. 1C), a 48-h treatment with 175 μM ddC results in complete replication stalling and depletion of mtDNA copy number (Fig. 1D and Fig. S2), revealing major differences in the replication responses between the WT and KO cells (Fig. 1E). WT MEFs showed a strong increase in replication bubbles, indicating increased replication initiation within the OH-containing region of mtDNA (explained in Fig. 1B and Fig. S1). In contrast, Primpol−/− cells seem to lose their replication intermediates upon ddC treatment (Fig. 1E), demonstrating that the compensatory increase in replication initiation is fully dependent on the presence of PrimPol. As replication cannot be completed in either cell type as a result of the recurrent ddC incorporation, the WT MEFs show a similar depletion of mtDNA copy number under ddC as Primpol−/− (Fig. 1D). However, when ddC is removed from the growth medium, mtDNA copy number recovery is significantly delayed in Primpol−/− cells compared with WT MEFs (Fig. 1D). No difference in mtDNA integrity was observed between the two cell lines (Fig. S2C).
Fig. 1.
PrimPol is required for replication restart in mitochondria. (A) A schematic illustration of mouse mtDNA showing the canonical replication origins (OH, OL) and BclI restriction sites adjacent to the probed fragment, marked with an asterisk. (B) An interpretation of 2D-AGE patterns from BclI-digested mouse mtDNA with an illustration of the corresponding replication intermediates. Linear fragments corresponding to the restriction fragment size (1n) will constitute the majority of the molecules. Replication that has initiated within the fragment forms replication bubbles, migrating as a bubble arc (marked as “b”), whereas replication that has exited the fragment (or initiated elsewhere) will form the Y-arc (“y”). The bubble arcs can be partially single-stranded or fully double-stranded (SI Results and Discussion). Replication intermediates resistant against restriction digestion (#) migrate in high molecular weight arcs (“r”) and recombination intermediates on the x-arc (“x”). (C) Two-dimensional AGE of BclI-digested mtDNA from WT (Primpol+/+) and Primpol-KO (Primpol−/−) MEFs, probed for the OH-containing fragment as illustrated in A. No differences in the replication intermediates can be observed. Signal intensities were quantified with a PhosphorImager, and autoradiographic films shown here represent comparable exposures. (D) ddC treatment causes a dramatic depletion in the mtDNA copy number in both cell types, with Primpol−/− cells still showing delayed recovery at 72 h after the removal of the drug [n = 9; P < 0.01, one-way ANOVA with post hoc Tukey honest significant difference (HSD) test; error bars represent SD]. (Lower) Quantification of the copy number recovery, standardized against mtDNA copy number, after 48 h ddC treatment to demonstrate the differences in recovery kinetics. Note that mtDNA depletion in Primpol−/− cells is not as strong, and that mtDNA steady-state levels are lower than in WT. (E) A 48-h treatment of cells with 175 μM ddC causes an accumulation of replication intermediates in WT cells, but a notable decrease in all replicative forms in Primpol−/− MEFs. Note especially the strong increase in dsDNA replication bubbles (ds-b) in the WT MEFs after ddC treatment and the degradation of stalled intermediates. Longer exposure of the same gel is given as a comparison.
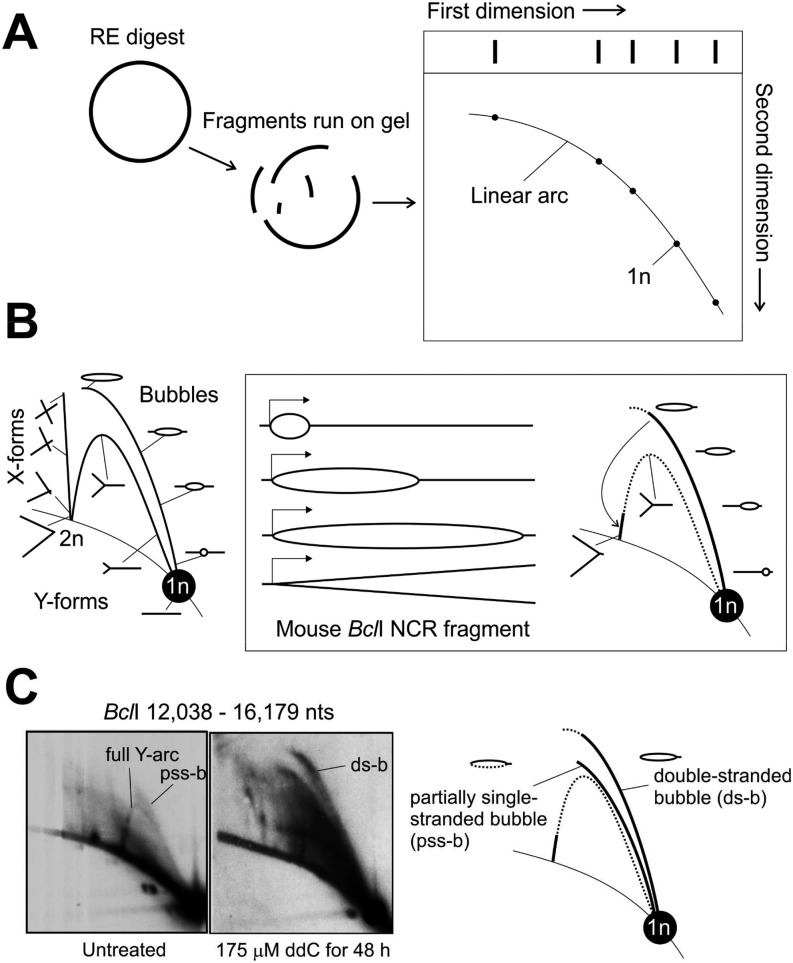
Fig. S1.
A brief introduction to 2D-AGE methodology and interpretation. (A) Isolated mtDNA is digested with a restriction enzyme, separated in first dimension by size and in second dimension by size and shape. A majority of the restriction fragments will be linear molecules, traveling on the so-called linear arc. (B) After Southern blotting, the fragment of interest (e.g., origin containing NCR fragment) is detected by using a radiolabeled probe, exposing a pattern of linear and nonlinear DNA species associated with the fragment of interest. Restriction fragment sized linears (1n) will give the most intense signal and replication, and recombination intermediates will light up relative to their abundance in sample. As the first dimension has separated the molecules by size and second by their shape (i.e., complexity), replication forks arising from replication that have traveled through the fragment will give rise to the characteristic Y-arc, whose peak represents molecules with equally long arms that return back to the linear arc at 2n position when the molecule is almost fully replicated. Similarly, recombination junctions containing two intertwined restriction fragments will align on the X-arc starting from the 2n position. In a simplified scenario for the mouse BclI NCR fragment, replication initiating at OH, close to the end of the fragment, will traverse as a replication bubble through the molecule, generating almost fully replicated Y-forms only when replication exits the fragment. (C) Interestingly, under normal conditions replication bubbles are as abundant as Y-forms in NCR containing fragments (SI Results and Discussion). Furthermore, the observed replication bubbles are typically flat and truncated, consistent with partially single-stranded replication intermediates, which are also highly sensitive to sample preparation procedures (24, 27). Under replication stress, erect, full-length bubble arcs—representing double-stranded replication intermediates—become the dominant forms.
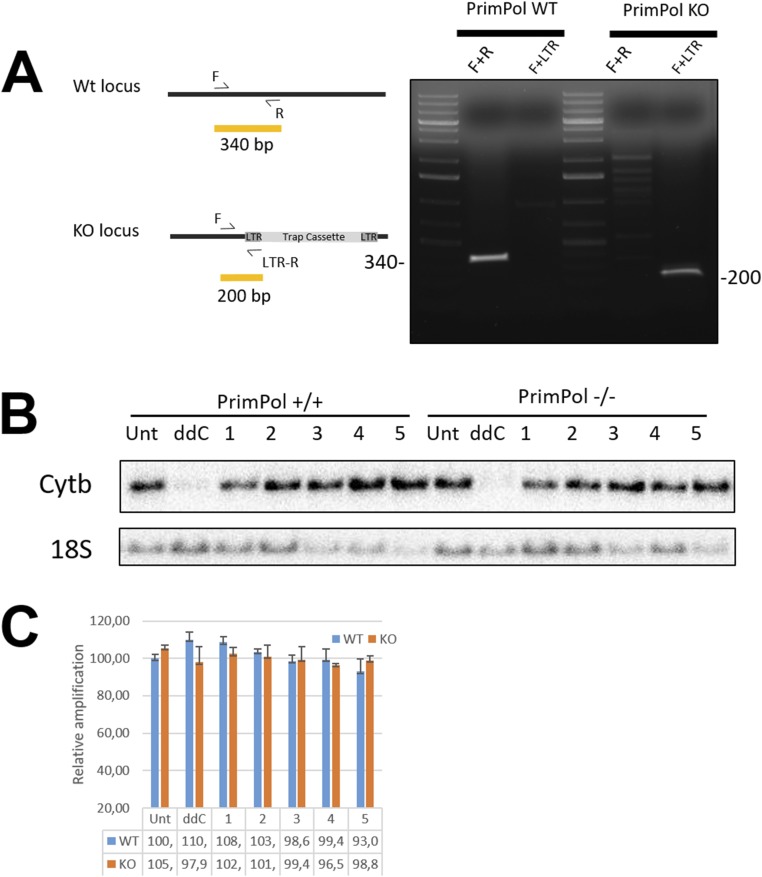
Fig. S2.
mtDNA copy number recovery is impaired in Primpol-KO cells. Characterization of the cell lines used in this study. (A) Verification of the Primpol KO in MEF used in the study (2). (B) Representative Southern blot showing mtDNA (Cytb) depletion and recovery after ddC treatment. Numbers indicate days after removal of the drug. Nuclear DNA marker 18S is used as the loading control. (C) qPCR performance during 48 h of ddC treatment and recovery. mtDNA was quantified by using long- and short-amplicon qPCR, and the long-amplicon result was normalized against the short and compared with the values obtained from untreated samples. Damage to the template DNA should hinder the performance of long-amplicon PCR.
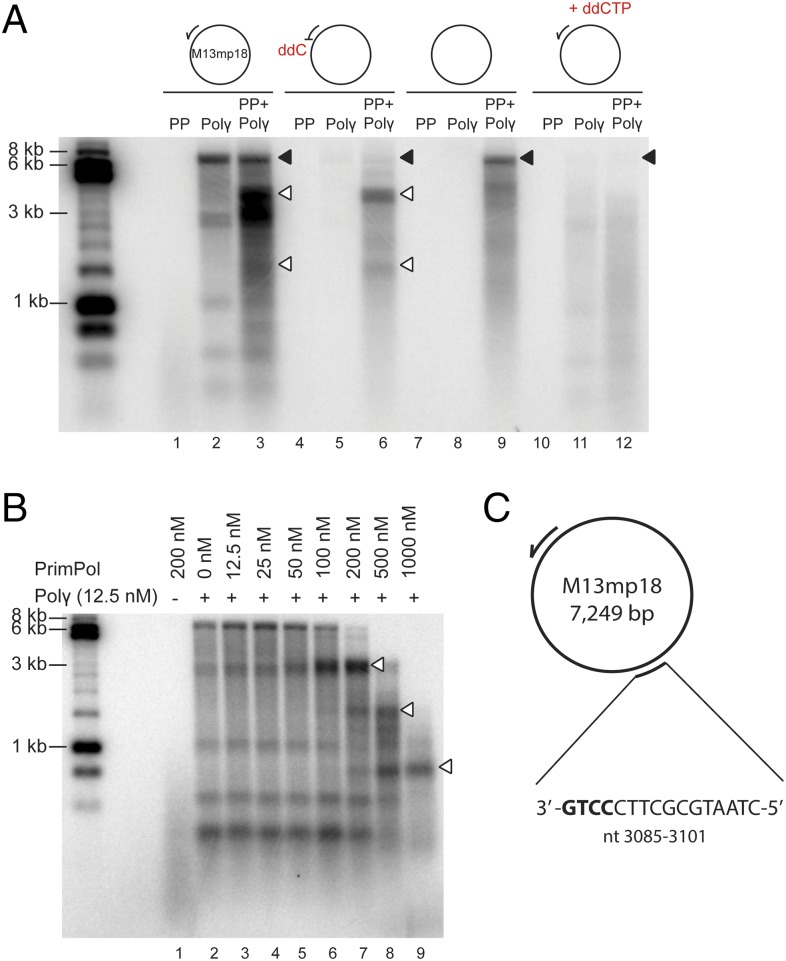
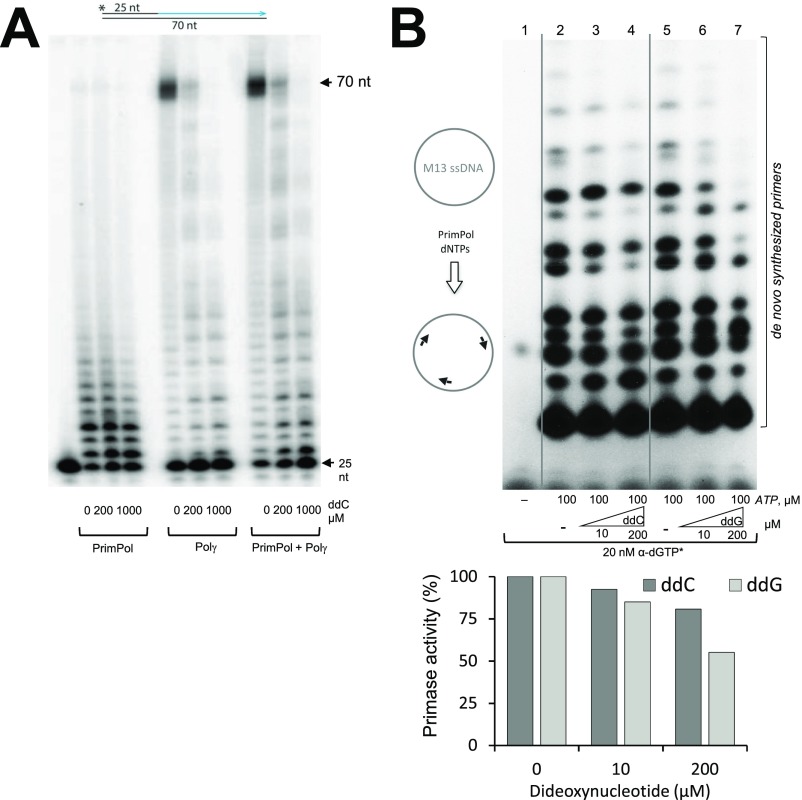
To confirm that PrimPol can reinitiate replication when strand elongation is blocked at the mtDNA replication fork, we simulated the situation in vitro by using M13mp18 ssDNA template, purified recombinant Pol γAB2 (for simplicity, hereafter referred to as Pol γ), and PrimPol. When provided with a normal oligonucleotide primer, Pol γ was able to efficiently synthesize [α-32P]dGTP-labeled full-length (7.3-kb) product from M13mp18 template (Fig. 2A, lane 2, black arrowhead). The shorter DNA bands observed in this reaction are the result of Pol γ pausing in front of secondary DNA structures, which form on this ssDNA template in the absence of single-strand binding proteins. The addition of PrimPol to the reaction resulted in additional DNA products (Fig. 2A, lane 3, white arrowheads), which differ from the ones caused by Pol γ pausing (Fig. S3). PrimPol alone cannot generate these species because PrimPol is unable to synthesize long DNA fragments as a result of its low processivity (Fig. 2A, lane 1). In agreement with the published data on inefficient removal of chain terminators by Pol γ proofreading activity (30), Pol γ was unable to elongate a primer with a 3′ ddC-monophosphate (ddCMP) (Fig. 2A, lane 5). The observed faint full-length DNA product is likely to result from ddCMP-oligonucleotide impurity. When PrimPol is added to the reaction, it will provide new primers for Pol γ and enables DNA synthesis, with the majority of products arising from specific priming events on the M13mp18 template (Fig. 2A, lane 6, white arrowheads). It should be noted that Pol γ is unable to initiate DNA synthesis in the absence of a primer (Fig. 2A, lane 8), but the addition of PrimPol to the reaction enables the synthesis of full-length DNA products (Fig. 2A, lane 9), further corroborating that PrimPol can provide DNA primers for Pol γ (2).
Fig. 2.
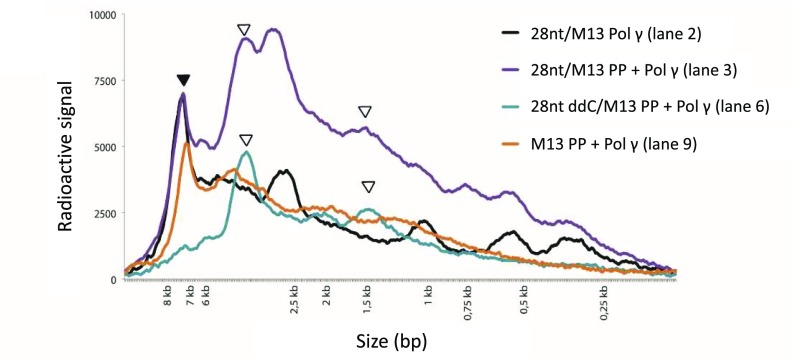
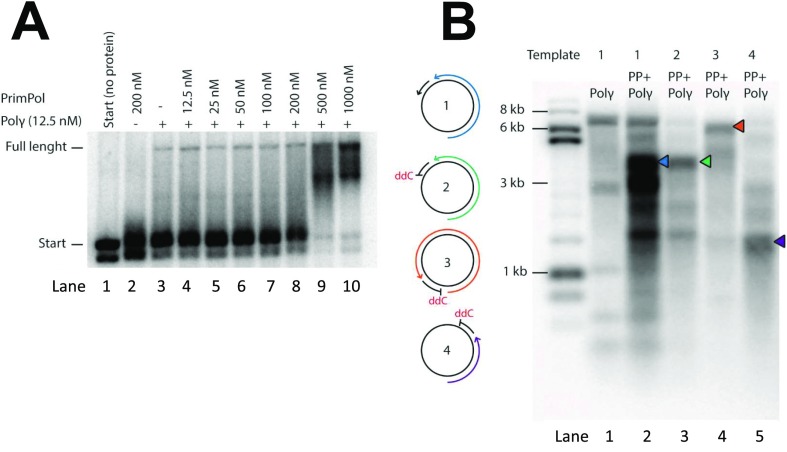
PrimPol can prime Pol γ-dependent DNA synthesis. (A) In vitro DNA synthesis incorporating 32P-radiolabeled dGTP on M13ssDNA. PrimPol alone is unable to synthesize long DNA products in the absence of Mn2+, even when provided with a synthetic annealed DNA primer (lane 1). In contrast, Pol γ (lanes 2 and 3) can effectively utilize the annealed primer and synthesize a full-length product (black arrow). The addition of PrimPol results in additional DNA products (white arrows), assumedly originating from sequence-specific priming downstream of the provided primer. As expected, Pol γ is unable to elongate an annealed primer containing a 3′ ddCMP (lane 5), but can synthesize long DNA products on this template in the presence of PrimPol (lane 6). Similarly, PrimPol can prime DNA synthesis in the absence of additional synthetic annealed primers (lane 9). The addition of ddCTP inhibits Pol γ DNA synthesis, which can partially be rescued by PrimPol (lanes 11 and 12). (B) Pol γ extension of 5′ radiolabeled primer in the presence of increasing PrimPol concentrations visualized on a denaturing alkaline gel. Whereas PrimPol can synthesize only products shorter than 500 nt (lane 1), Pol γ can extend the annealed primer to give rise to full-length DNA products (lane 2). Shorter DNA products are also visible as a result of Pol γ stalling at assumed secondary DNA structures. Pol γ-dependent primer extension is unaffected at low PrimPol concentrations (12.5–50 nM; lanes 3–5). At 100–200 nM PrimPol, the synthesis of the 7.3-kb full-length DNA product is reduced, but an abundant DNA product migrating at ∼3.7 kb (lanes 6 and 7) is generated. At high PrimPol concentrations (500–1,000 nM), synthesis from the annealed primer decreases and new stalling sites appear (lanes 8 and 9, white arrows). (C) Schematic view of the preferred PrimPol priming site on M13ssDNA, positioned relative to the 28-nt primer used in A. The preferred PrimPol priming site (3′-GTCC-5′; nucleotides 3,086–3,089) was mapped by using an adapted 5′-RACE method.
Fig. S3.
Distribution plot from the replication reaction shown in Fig. 2A. Radioactive signal from lanes 2 (black), 3 (purple), 6 (blue), and 9 (orange) in Fig. 2A are plotted on y-axis against size marker on x-axis. Full-length 7.3-kb products are marked with a black arrow, and abundant PrimPol-specific peaks are marked with white arrows (3.7 kb and 1.5 kb).
During in vivo experiments, addition of ddC to the cell growth media results, after several phosphorylation steps, in a mixed deoxycytide/di-deoxycytidine triphosphate (dCTP/ddCTP) cellular pool. To recapitulate the situation in vitro, we next performed replication reactions with a mixed population of free ddCTP and dCTP. Under these conditions, DNA synthesis by Pol γ is weak as a result of the frequent termination of polymerization when ddCTP is incorporated (Fig. 2A, lane 11). The addition of PrimPol leads to substantially more DNA synthesis in the presence of ddCTP (Fig. 2A, lane 12). Unlike Pol γ, PrimPol’s polymerase activity is not significantly inhibited by ddCTP (Fig. S4A). More importantly, primer synthesis by PrimPol is only slightly affected by the presence of ddNTPs (Fig. S4B). Such a strong discrimination in favor of a 3′-OH group in the incoming nucleotides guarantees that the primers made by PrimPol do not abort and can be elongated by the Pol γ replicase. The ability of PrimPol to assist Pol γ by reinitiation of efficient DNA polymerization is hampered only when relatively high concentrations of ddCTP are used (Fig. S4B). In summary, Pol γ cannot continue DNA synthesis after ddC insertion and requires the priming activity of PrimPol for replication restart (Fig. 2A). As long as ddCTP is present, recurrent replication stalling will occur despite the presence of PrimPol, resulting in accumulation of partially replicated molecules in vitro and depletion of mtDNA copy number in vivo (Fig. 1 D and E).
Fig. S4.
Effect of ddNTPs on PrimPol synthetic activities. (A) PrimPol is less prone to incorporate ddC during DNA primer extension than Pol γ. Primer elongation by PrimPol and Pol γ on a 70-nt template in the presence of increasing amounts of ddCTP (0, 200, and 1,000 μM). Note that, in our in vitro conditions (without Mn2+; SI Materials and Methods), PrimPol can synthesize only short DNA products. The full-length DNA product (70 nt) and input primer (25 nt) are indicated. (B) Synthesis of DNA primers by PrimPol is weakly affected by ddNTPs. We explored the effect of ddNTPs on the DNA primase activity of PrimPol, a unique feature that allows the synthesis of a DNA primer to rescue a stalled replication fork, analyzing the generation of multiple DNA primers on a large ssDNA template as M13ssDNA. To maximize the sensitivity of the analysis, we used Mn2+ activating ions and provided only two purine nucleotides, in agreement with the preference of PrimPol to form dimers (2). rATP (100 µM) was provided at a high concentration to boost occupancy at the 5′ site (having no sugar discrimination), whereas [α-32P]-labeled dGTP was provided at low concentration, and it could be used by PrimPol as a valid nucleotide for first and second positions of the dimer. As shown in the autoradiograph, the collection of labeled primers formed span from 2 to approximately 20 nt (lanes 1 and 5). As a negative control, when adding ddCTP (a noncomplementary nucleotide for these assay conditions) at 10 or 200 µM, the pattern of primer synthesis was very slightly affected (lanes 3 and 4). Moreover, when ddGTP was provided (that could potentially compete with the [α-32P]-labeled dGTP), the pattern and efficiency of primer synthesis was only moderately affected when the ddGTP/dGTP concentration ratio was 20,000 fold (lane 7).
Sequence-Specific Priming by PrimPol.
The concentration of PrimPol used (200 nM) equals approximately 40 monomers of the enzyme per M13mp18 template with a potential priming site every 180 nt. As noted earlier, instead of observing a range of elongation products, only a major DNA species was observed, indicating a preferred priming site on the offered template. This priming site became dominant over a 32P-labeled synthetic primer at PrimPol concentrations greater than 50 nM, whereas, at higher concentrations (500–1,000 nM), more and smaller replication products were obtained (Fig. 2B, lanes 8 and 9). However, this does not mean that excess of PrimPol is inhibiting DNA synthesis in these reactions. When the same DNA products are separated over a neutral Tris-borate-EDTA (TBE) gel, instead of a denaturing gel, it is possible to visualize all DNA products, regardless of gaps between labeled primer and PrimPol-primed DNA synthesis, demonstrating that high PrimPol levels actually support more DNA synthesis (Fig. S5A). The observed main priming site on M13mp18 was highly specific, as the size of the main PrimPol-primed product was dependent on the position of the ddCMP-oligonucleotide primer (Fig. S5B). To map this site, we used a modified method for 5′-RACE and discovered that DNA priming started opposite the 3′-GTCC-5′ sequence (Fig. 2C), indicating that PrimPol has an identical sequence preference as HSV1 primase (31) and similar to known prokaryotic primases (32).
Fig. S5.
Priming specificity of PrimPol. (A) Extension of radiolabeled primer annealed to M13mp18 by Pol γ in the presence of increasing PrimPol concentrations. DNA products from the primer extension reactions of Fig. 2B were also run on a nondenaturing (i.e., TBE) gel to visualize the total amount of DNA synthesis. Higher molecular weight products, and thus more DNA synthesis, can be seen when adding PrimPol at high concentrations (500–1,000 nM, lanes 9 and 10), compared with no or low concentrations of PrimPol (lanes 3–8). (B) Schematic view of the four different templates used in this experiment (Left). Template 1 contains a regular primer annealed to M13mp18. Pol γ together with PrimPol can extend the primer to full-length product of ∼7 kb (lanes 1 and 2; Fig. 2A, lanes 2 and 3). In lane 2, an additional abundant PrimPol-specific band can also be seen at ∼3.5 kb (blue arrow). This abundant DNA product is still visible when a ddC primer was used instead of a regular primer (template no. 2, lane 3, green arrow; Fig. 2A, lane 6). Reactions using ddC primers that anneal to different regions of M13mp18 (template 3) showed an altered size of this PrimPol-abundant DNA product to ∼6.5 kb (template 3, lane 4, orange arrow) and 1.5 kb (template 4, lane 5, purple arrow). The lengths of these DNA products (lanes 3–5) correspond to the distance between the major PrimPol priming site (Fig. 2C) and the 5′ end of the annealed ddC primer. DNA replication products were visualized on a denaturing alkaline gel.
PrimPol Is Required for UV-Induced mtDNA Replication Initiation.
Because UV damage induces similar accumulation of replication intermediates as ddC treatment (21), we sought to identify whether this is also a consequence of PrimPol-dependent replication reinitiation. Whereas UV exposure caused a rapid increase in replication bubbles in normal MEFs, this reaction was completely absent in Primpol−/− cells (Fig. 3C). Our interpretation is that UV exposure induces a PrimPol-dependent increase of replication initiation in OH-containing fragments and must be regulated by some damage-response pathway. In contrast to the ddC treatment, in which replication intermediates were lost (Fig. 1E), UV exposure had no effect on the replication intermediates in Primpol−/− cells (Fig. 3C) or mtDNA copy number (Fig. S6).
Fig. 3.
PrimPol is responsible for increased mtDNA replication initiation after UV exposure. (A) Schematic illustration of mouse mtDNA as in Fig. 1. (B) Two-dimensional AGE of BclI-digested mtDNA from untreated Primpol WT (+/+) and Primpol-KO (−/−) MEFs. Comparative exposures of the gels are shown. (C) After 4 h recovery from a 30-s exposure to 305 nm UVB, the control WT cells show a significant increase in partially single-stranded replication bubbles (pps-b; SI Results and Discussion), whereas Primpol-KO MEFs are unaffected. Longer exposure (Bottom) was given to illustrate the qualitative and quantitative differences in the replicative forms.
Fig. S6.
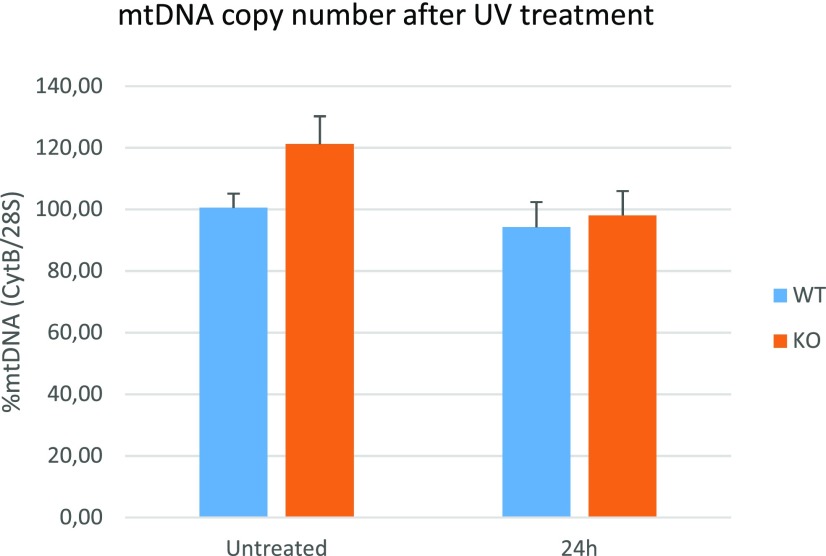
mtDNA copy number is not affected by UV treatment. No differences were observed in the mtDNA copy number 24 h after a single 30-s dose of 30 J/m2 UVB (n = 3; P value not significant by one-way ANOVA with post hoc Tukey HSD test; error bars represent SD).
PrimPol Overexpression Increases Lagging-Strand Initiation During mtDNA Replication.
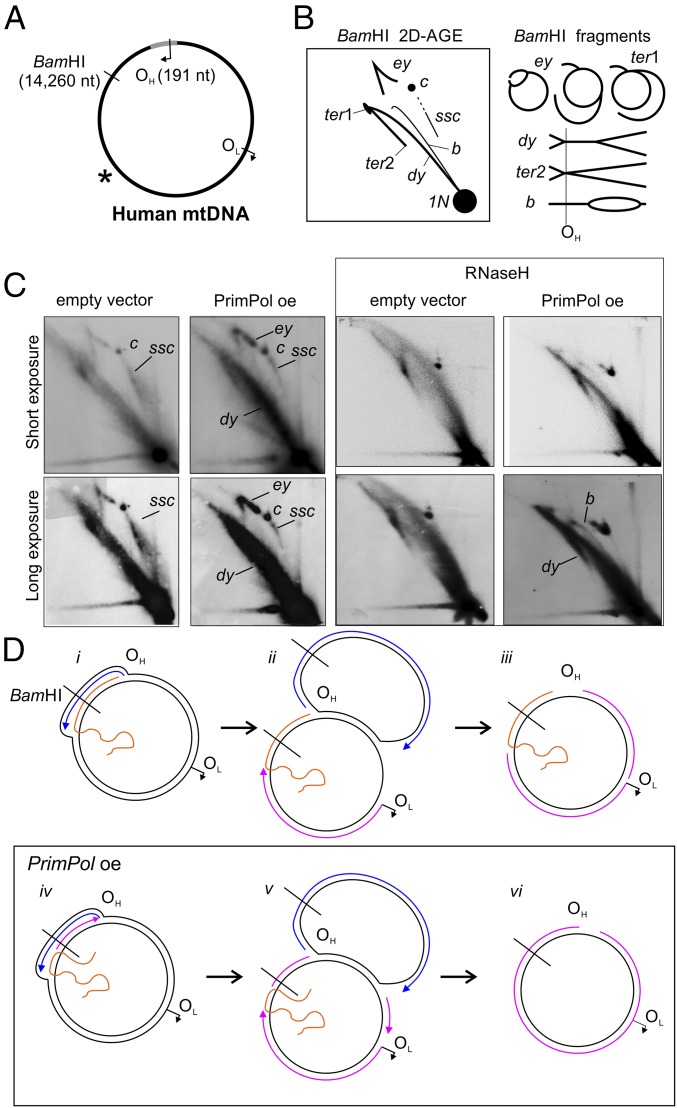
As PrimPol is able to synthesize primers on ssDNA, which Pol γ can elongate (Fig. 2A, lane 9), we decided to next analyze whether PrimPol expression alone could modify ss mtDNA replication intermediates in vivo. We found this plausible, as PrimPol interacts with TWNK and Pol γ in mitochondria (Fig. S7). Furthermore, TWNK is also reported as a PrimPol (as CCDC111) partner in the BioPlex database (33). To get a view of the whole mitochondrial genome, long-range 2D-AGE analysis of full-length mtDNA, cut once downstream or upstream of OH, was performed (Fig. 4 and Fig. S8). Although PrimPol overexpression notably decreased the levels of the partly single-stranded circles (“ssc” in Fig. 4), it also generally increased all replicative molecules and, more specifically, dsDNA intermediates, such as the double-y forms (“dy” in Fig. 4). These changes in replication intermediates are dependent on mitochondrially localized PrimPol and, more specifically, on its primase activity, as demonstrated by a primase-deficient variant of PrimPol (Fig. S8). Taken together, PrimPol overexpression increases ds mtDNA forms at the expense of partially single-stranded forms (Fig. S9C), demonstrating that PrimPol can also prime DNA synthesis on a single-stranded template in vivo.
Fig. S7.
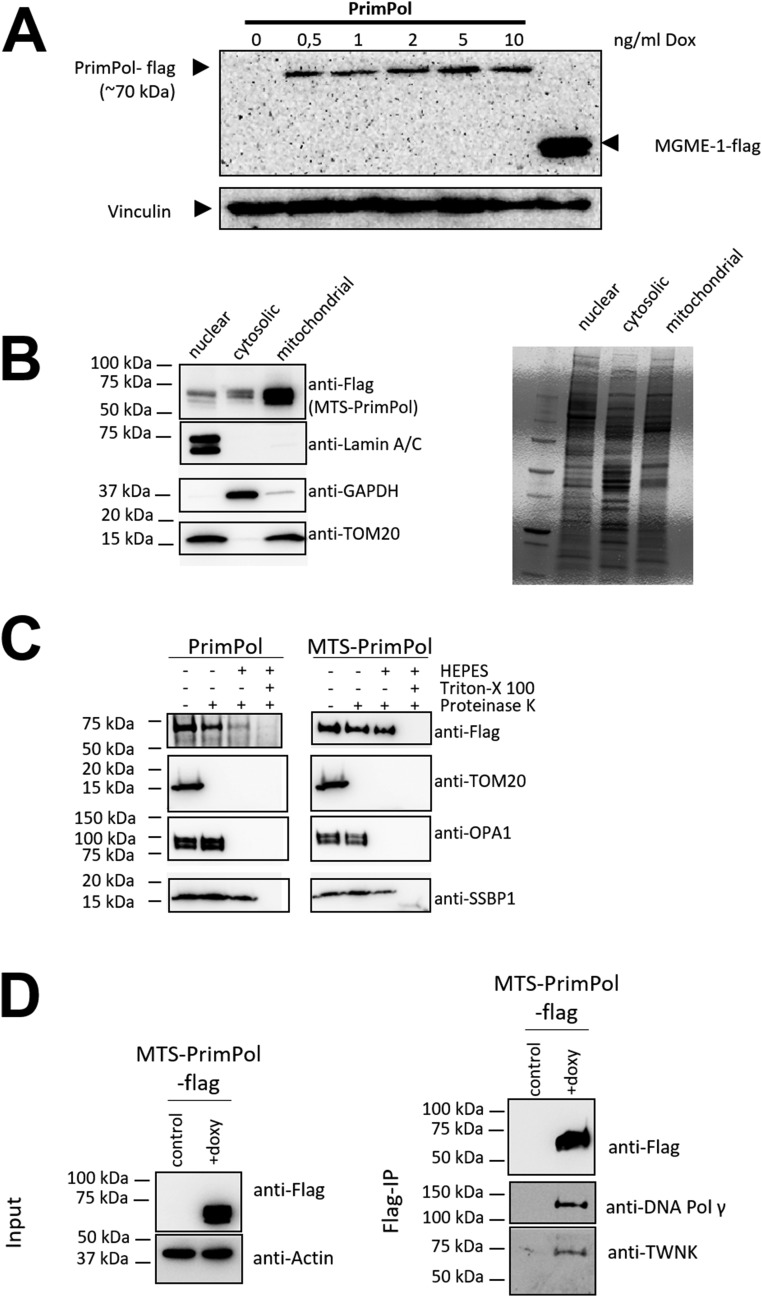
Mitochondrial localization of PrimPol and MTS-PrimPol. (A) Confirmation of Dox-dependent PrimPol-flag expression in Flp-InTM T-RExTM 293 cells. The cells were treated for 48 h with the indicated concentration of Dox in the growth medium. Recombinant PrimPol was detected from the total cell protein preparations with an anti-flag antibody, with MGME1-flag used as a positive control and vinculin as a loading control. (B) Mitochondrially targeted PrimPol is highly enriched in the mitochondrial fraction from induced MTS-PrimPol Flp-InTM T-RExTM 293 cells. (C) Mitochondrial fractions from PrimPol WT and MTS-PrimPol–overexpressing cells. MTS-PrimPol is exclusively mitochondrially localized as demonstrated by Proteinase K treatment of intact mitochondria, capable of digesting outer membrane proteins such as TOM20. When detergent (Triton X-100) is added, Proteinase K will also digest the mitochondrial proteins, including single-strand binding protein SSBP1. Note that the TFAM-targeting peptide is cleaved upon mitochondrial import and therefore does not influence the protein size. (D) Immunoprecipitation of mitochondrial-targeted PrimPol pulls down central mitochondrial replisome components, Pol γ and TWNK helicase. TWNK was also found to be a PrimPol interaction partner in the BioPlex study (33). Noninduced cells were used as the control input.
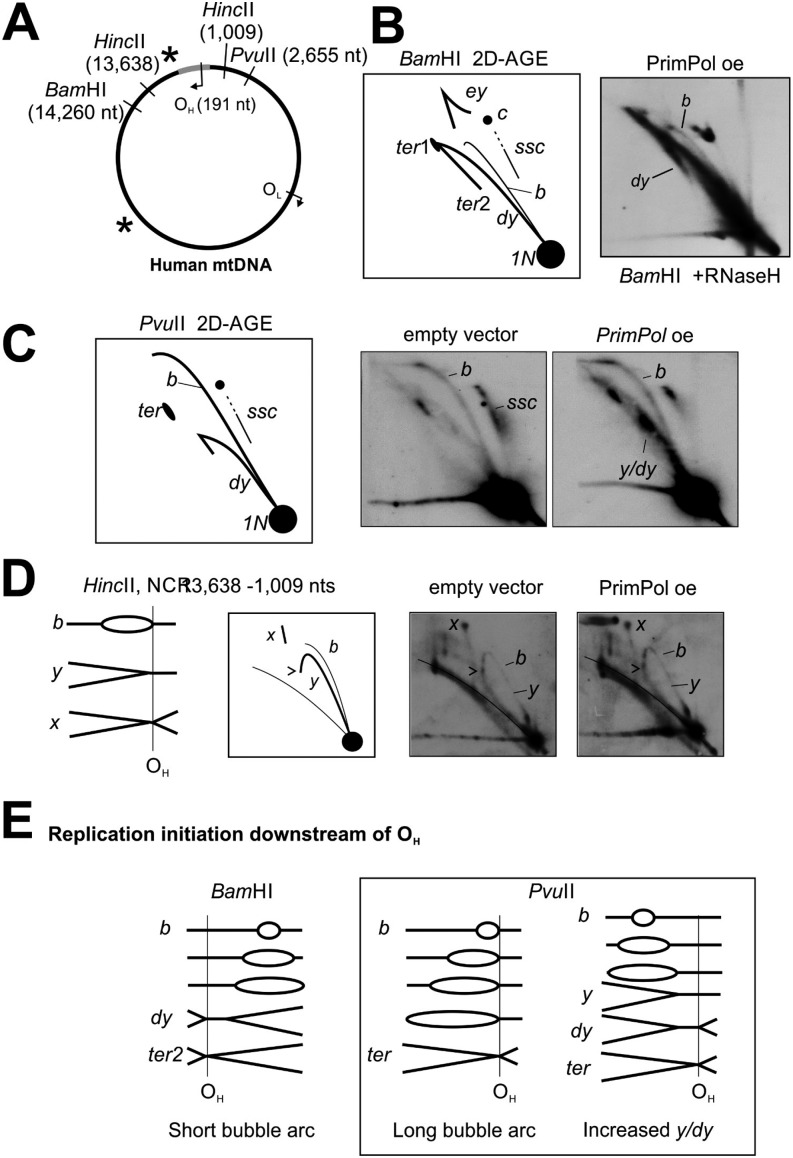
Fig. 4.
PrimPol overexpression in human T-REx 293 cells induces specific changes in the replication intermediates, corresponding to increased lagging-strand priming. (A) Schematic illustration of human mtDNA showing the canonical replication origins, BamHI (downstream of OH) restriction site, and probe location (asterisk). (B) Illustration of BamHI-restriction fragments resulting from replicating mtDNA. BamHI cuts directly downstream of OH, resulting in molecules migrating on the so-called eyebrow arc (ey), which represent replication intermediates that are cut only on the leading strand as a result of RNA coverage or single-strandedness of the lagging strand. After the leading-strand replication is completed, the displaced strand template will remain uncut until the lagging strand is entirely replicated (ter1). Fully dsDNA intermediates are cut on both strands and result in y forms (y) and double-y forms (dy), which convert to termination intermediates (ter2) after the replication is completed. Note that the distances where the replication bubble is converted to y and then double-y forms are fairly short, resulting in poor separation of the latter. Partially single-stranded circles (ssc) streak southeast from the uncut double-stranded circles (c, arrows). (C) A 48-h overexpression of PrimPol (5 ng/mL Dox) depletes the partially single-stranded circles (ssc) while increasing the dsDNA intermediates (dy, y). Host cell line with an integrated empty expression vector represents the control. (D) Under normal conditions (i–iii), the majority of mtDNA replication intermediates are partially ssDNA as a result of only a few active lagging-strand origins, such as OL. When PrimPol is overexpressed (iv–vi), lagging-strand synthesis is initiated at multiple loci, resulting in more dsDNA intermediates that can be cut by the restriction enzyme (e.g., BamHI). Note that, despite the general increase in dsDNA, some restriction sites remain uncut.
Fig. S8.
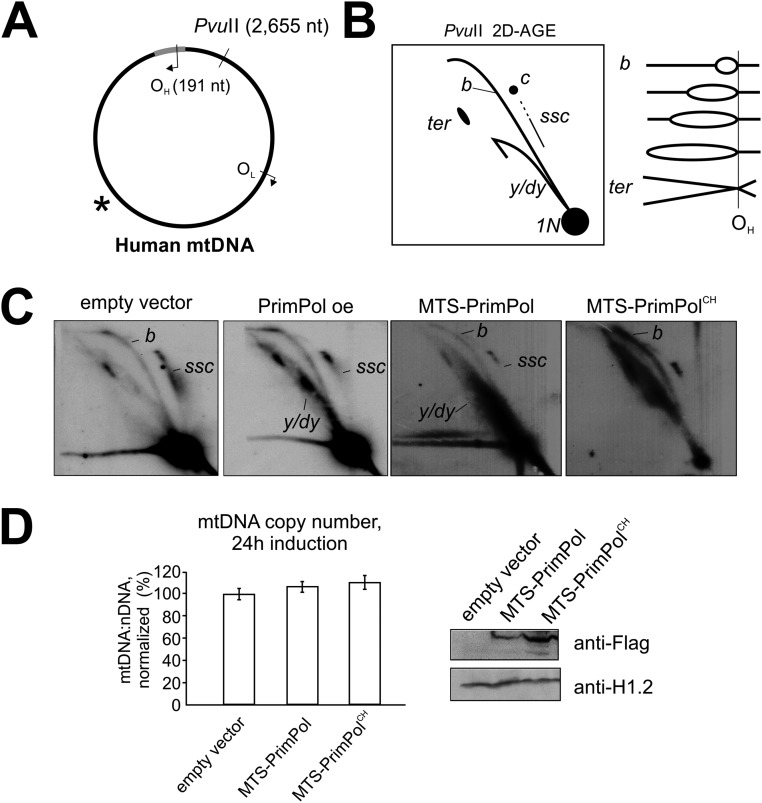
PrimPol overexpression phenotype is dependent on primase activity. (A) Location of PvuII restriction site and ND2 probe (asterisk) on mtDNA. (B) Interpretation of PvuII 2D-AGE. Because the PvuII site is located upstream of OH, a majority of replication intermediates will migrate on the bubble arc (marked as “b”), which convert to an x-like arc when replication is almost completed (ter). The y or double-y forms (y/dy) will be present only when replication is initiating further downstream of OH. Further details are provided in Fig. S9. Uncut circular molecules will migrate as an elongated spot (marked as “c”), trailed by partially single-stranded circular molecules (ssc). (C) Overexpression of mitochondrially targeted recombinant MTS-PrimPol induces similar, but more extreme, mtDNA replication phenotype as the WT PrimPol, including the accumulation of Y-forms (y/dy), while reducing partially single stranded circles (ssc). Interestingly, expression of the primase-dead MTS-PrimPolCH increases all types of replication intermediates without much changing their relative proportions. (D) MTS-PrimPol and MTS-PrimPolCH maintain normal mtDNA copy number 24 h after the induction of the transgene expression, indicating that the accumulation of replication intermediates in these cells does not result from replication stalling.
Fig. S9.
PrimPol-overexpression alterations in mtDNA replication intermediates. (A) Restriction sites and probe locations (asterisk) on mtDNA; only the relevant HincII sites are shown. (B and C) Representative BamHI and PvuII panels with interpretation, as in Fig. 4 and Fig. S8. Note the bubble arc in RNaseH-treated BamHI digest of PrimPol-overexpressor mtDNA. (D) Because of mtDNA replication terminating around OH, y-forms (marked as “y”) in HincII NCR fragment do not reach the linear arc (arrow). Truncated x-forms (“x”) in this fragment represent replication termini generated by meeting replication forks. The termination is unaltered in PrimPol-overexpressing cells. (E) Replication bubbles in BamHI-digested human mtDNA samples can be generated only by replication initiation downstream of OH, with the same being true for dY-forms in PvuII-digested samples. These forms are likely to arise from bidirectional replication, as OH is also maintained as the main replication terminus in PrimPol-overexpressing cells (D).
SI Results and Discussion
Interpretation 2D-AGE Patterns and mtDNA Replication.
Two-dimensional Brewer–Fangman agarose electrophoresis separates nucleic acids according to their size and shape (46). The first dimension separates DNA molecules primarily by size and the second dimension by size and shape. Two-dimensional AGE results are typically presented in orientation whereby size increases from right to left and complexity from bottom to top (Fig. S1). For standard 2D-AGE, a DNA sample is digested with a restriction enzyme of choice, although uncut molecules can also be separated to study DNA topology (40, 47). After Southern blotting, the membrane is probed against the fragment of interest. A majority of the molecules will be one restriction fragment size linear molecules (1n), and replication as well as recombination intermediates will form typical arc-like patterns (Fig. S1B). Curiously, OH containing mtDNA fragments always yield Y-arcs, representing replication forks with gradually increasing length of arms (Fig. 1 and Fig. S1C). Unidirectional replication originating from OH should produce only a bubble arc, which converts abruptly to a truncated Y-arc (Fig. S1B). The full Y intermediates have been interpreted to arise from bidirectional replication, which has initiated downstream of OH and terminates in the noncoding region of mtDNA (27, 41). Aside from the 2D-AGE data and a single transmission EM observation (39), no other evidence exists on replication initiation outside of OH. In contrast, unidirectional and highly strand-asymmetric replication originating from OH is supported by numerous in vivo (16, 24, 25, 27) and in vitro (28, 29, 48) studies. Convincing support for single leading-strand origin comes also from in organello studies that demonstrated that only the bubble structures in OH-containing fragments incorporate radiolabeled nucleotides (23). At present, the exact identity of the Y-forms seen in these fragments is unknown. In yeast, Y-forms might represent strand-invasion structures (49), but their existence in animal mitochondria is highly controversial (22, 39).
Relationship of PrimPol and mtDNA Replication Priming.
Assignment of OH as the leading strand origin is based on mapping the free 5′-ends in the mitochondrial noncoding region (27, 50). Interestingly, the leading-strand primers synthesized by POLRMT in vitro terminate specifically at CSBII (28) approximately 200 bp upstream of OH. Even if termination at CSBI is taken into account (51), the proposed primer ends fall 100 bp short from the observed 5′-ends of DNA (27, 50, 52). In fact, it has been speculated that efficient primer-removing activity in mitochondria could also degrade the nascent DNA molecules to a limited extent (51), but this is somewhat an unsatisfactory explanation. Unfortunately, PrimPol cannot fix this gap in understanding the leading-strand priming. Although PrimPol is required for increased priming from the noncoding region under ddC-induced replication stress (Fig. 1E), it is expendable for mtDNA maintenance under normal conditions (2) and is unlikely to play a major role in mtDNA leading-strand replication initiation. Under PrimPol overexpression, specific classes of mtDNA replication intermediates arise, which could correspond to increased priming of the lagging-strand replication, such as the nearly complete Y/dY-arc seen in PvuII 2D-AGE (Fig. 4 and Fig. S9) and the appearance of a partial bubble arc in BamHI 2D-AGE (Fig. S9). Although it is an interesting observation, at present we cannot say whether this represents a biologically significant mechanism or an artifact caused by excess PrimPol priming any accessible ssDNA during mtDNA replication.
To confirm that the mtDNA replication phenotype observed in PrimPol-overexpressing cells was specific for PrimPol activity in mitochondria and not caused by, for example, arbitrary secondary effects of possible changes in nuclear DNA, we created a mitochondrially targeted PrimPol (MTS-PrimPol) construct. In addition, an MTS-PrimPol variant with double point mutations in two conserved Zn finger residues (MTS-PrimPolCH) was generated to test whether the primase activity was required for the replication phenotype. The CH mutants are similar to Zn finger deletion variants and are primase-dead but polymerase-competent, enabling the separation of the two functions (9). Although the transgenic expression of WT MTS-PrimPol resulted in the similar (albeit slightly stronger) phenotype as regular PrimPol overexpression, MTS-PrimPolCH increased the levels of all replication intermediates (Fig. S8C). Although similar to the hallmarks of replication stalling, no decrease in mtDNA copy number was observed (Fig. S8D). As it is unlikely that a repair polymerase could replace replicative polymerase at the replication fork (53), a likely explanation is that primase-deficient PrimPol binds mtDNA and interferes with replication, slowing down its progress but not enough to cause copy number depletion. Similar accumulation of mtDNA replication intermediates, without an effect on copy number, is seen also after UV (Fig. 3 and Fig. S6) or oxidative damage (21). As Primpol-KO cells do not show signs of mtDNA replication defect, we regard this as an artificial situation caused by expression of the mutant protein.
ddC and Mitochondrial Gap Repair.
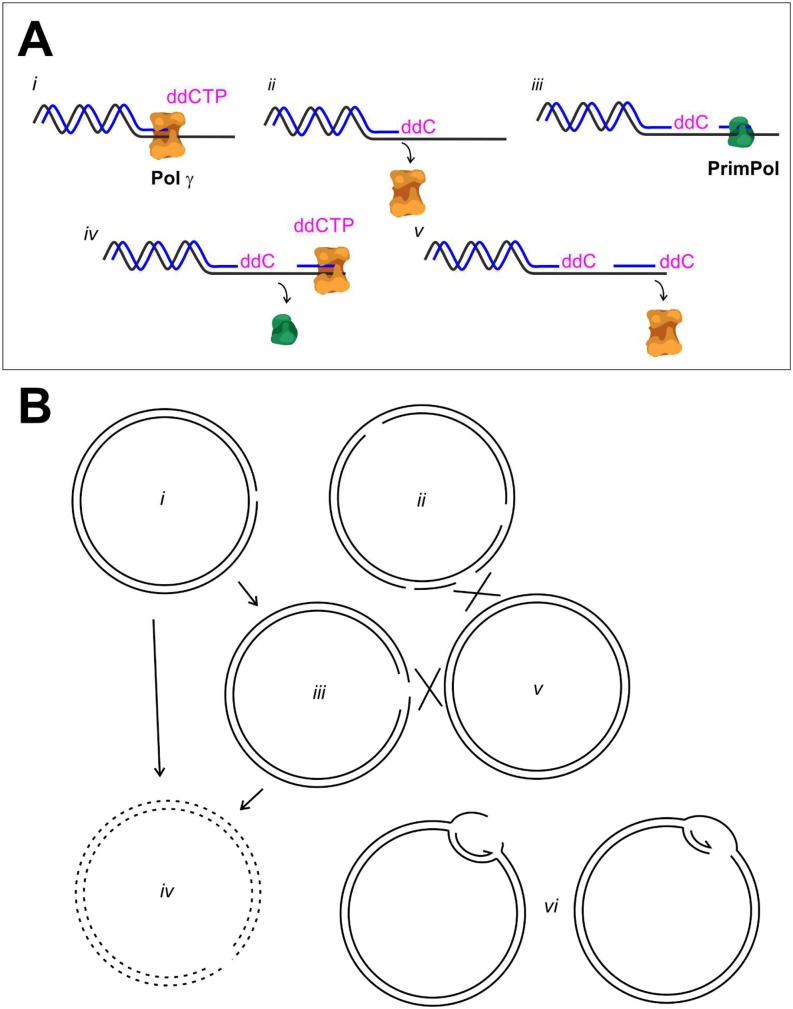
At the mitochondrial replication fork, insertion of a chain-terminating nucleotide is highly unlikely to happen under normal physiological conditions. Even if replication is rescued by repriming, the initial problem will persist as a result of Pol γ’s inherent insufficiency to remove such nucleotides (30). Replication restart caused by ddC insertion or more natural DNA lesions on the template molecule will result in daughter-strand gap lesions that need to be repaired. In prokaryotes (54) and eukaryotic nucleus (53), the main mechanism for gap repair is homology-mediated repair by recombination. The other option would be to increase the turnover of gapped molecules by targeting them for destruction, possibly facilitated by their tendency to form double-strand breaks. In fact, increased turnover of mtDNA has been suggested to contribute to clonal expansion of deletions in patients treated with chain-terminating nucleotide analogs used in antiretroviral therapy (55). However, replication restart by PrimPol clearly enhances the mtDNA copy number recovery after ddC treatment (Fig. S2C). If the gapped molecules were to be turned over, any fork rescue would be futile, as the replicated copies would be lost similarly as in aborted replication (Fig. S10). As recombinational repair of mtDNA remains a controversial topic, we can only speculate about the gap repair mechanisms in mitochondria at present.
Fig. S10.
Repriming and gap repair would be essential for mtDNA copy number recovery after ddC treatment. (A) Pol γ stalls after it has inserted ddC (i), leaving behind a chain-terminated daughter strand (ii). PrimPol can restart replication downstream of the ddC insertion (iii and iv), leaving behind a single-stranded gap. High levels of ddC will cause recurrent stalling of Pol γ (v) and result in newly replicated molecules with multiple gaps. (B) As the exonuclease activity of Pol γ cannot remove ddC (30), replication restart will result in mtDNA molecules with one (i) or multiple single-strand gaps (ii), which are sensitive to further nicking, resulting in double-strand breaks (iii). The gapped or broken molecules can be turned over (iv) or repaired by homology-dependent repair mechanisms (v). As gaps can exist on either strand, further replication rounds will result in double-strand breaks (vi). Without functional gap repair, mtDNA molecules resulting from rescued replication are not viable, and no differences in copy number recovery would be observed between Primpol-KO and WT cells.
Discussion
Almost all recently discovered mtDNA maintenance proteins are shared with the nucleus (13), making it difficult to dissect their specific importance for the mitochondrial compartment. Furthermore, nuclear DNA damage activates a number of signaling pathways that block cell proliferation or target the cell for apoptosis, making any simultaneous damage in the mitochondrial compartment trivial. The same also applies for the role of PrimPol. Although not essential for life (9), PrimPol is beneficial for nuclear genome maintenance, as its loss influences the mitotic checkpoint responses after damage (34). However, mitochondria also require efficient DNA repair and damage-tolerance mechanisms for long-term survival. Mitochondria have formidable intrinsic sources of DNA damage, most notably reactive oxygen species originating from the electron transport system (35), capable of causing oxidative damage that can block mtDNA replication (20, 21). mtDNA replication can also stall as a result of impaired replisome proteins (17, 18) or sequence-specific replication pause sites (36). Unless the replication is reinitiated, stalling can lead to replication fork collapse and double-strand breaks, resulting in the formation of pathological deletions (16). Our experiments provide direct evidence that PrimPol is required for replication reinitiation in mitochondria, identifying it as a central player in mtDNA replication fork rescue.
PrimPol Is Required for Increased Replication Initiation After DNA Damage.
The increase in replication intermediates, especially when accompanied by a decrease in mtDNA copy number, has been treated as a hallmark of replication stalling in a number of studies that used 2D-AGE (17, 18, 37). However, if the stalled replication forks are actively processed or turned over, an increase in replication intermediates can be obtained only through a concomitant increase in replication initiation. In the case of ddC, the accumulation of replication intermediates is caused by recurrent initiation and stalling events (Figs. 1 and 2). In the absence of PrimPol, no reinitiation occurs, resulting in the loss of replication intermediates (Fig. 1C), further demonstrating that replication reinitiation is required for replication maintenance in the presence of ddCTP. As shown here, the primase and polymerase activity of PrimPol are not significantly affected by the presence of ddNTPs, precluding the formation of abortive primers that could compromise fork restart and progression. Despite PrimPol’s ability to reprime replication after a CTNA, mtDNA will eventually be lost because of the repeated incorporation of ddCTP by Pol γ (Fig. S10).
Similar to the situation of treatment with ddC, PrimPol is responsible for the increased replication initiation after UV exposure (Fig. 3), although the damage seems to be tolerated in cells lacking PrimPol. It is likely that PrimPol is only one of several players involved in mtDNA damage response and its activities. Despite PrimPol contributing to the defense against a range of genotoxic insults, it is not essential for cell survival after acute damage. This is apparent also from the fact that PrimPol can facilitate recovery after UV damage to nuclear DNA, but does not influence cell survival (34). As stalled replication forks pose a great risk for genome integrity as a result of their tendency to collapse and form double-strand breaks with potentially catastrophic consequences, cells have evolved a number of partially redundant mechanisms to avoid such damage (38).
PrimPol Primes mtDNA Synthesis Independent of the Replication Origin.
Fully dsDNA replication intermediates exist in mitochondria (39, 40) and have been suggested to originate from bidirectional replication that is initiated downstream of OH (41) (SI Results and Discussion). Under normal conditions, dsDNA replication represents the minority of replicative molecules in mitotic cells, and it remains unsettled to which extent these intermediates represent an independent replication and not just more frequent lagging-strand initiation during strand-asymmetric replication. Interestingly, overexpression of PrimPol results in the generation of fully dsDNA replication intermediates (Fig. 4). As PrimPol can provide primers for Pol γ on single-stranded substrate (Fig. 2A, lanes 7–9), it is also likely to prime the lagging strand during asymmetric replication. Increased lagging-strand priming would be the easiest explanation for the reduction of partially single-stranded circles (“ssc” in Fig. 4C) as well as the increase in fully dsDNA replication intermediates in PrimPol-overexpressing cells (e.g., “dy” in Fig. 4 and Fig. S9).
Although replication stalling is acknowledged as an important culprit behind pathological mtDNA deletions (15, 16), almost nothing is known about the fate of stalled replication intermediates, such as their subsequent processing, and which enzymatic players are involved. Our finding that PrimPol is required for the reinitiation of replication in mitochondria will hopefully pave the way for further understanding of mtDNA replication mechanisms and how the mitochondrial genome is protected against various intrinsic and extrinsic stressors.
Materials and Methods
Immortalization of MEFs and Generation of Flp-In T-REx 293 PrimPol Cell Line.
WT Primpol (+/+) and Primpol-KO (−/−) cells (Fig. S1A) were generated from primary MEFs (2) that were immortalized by transfection with an SV40T antigen expression vector using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s recommendation. After transfection, the cells were grown to confluence and passaged onto 10-cm tissue culture dishes, followed by another five 1/10 passages (i.e., a 1/100,000-fold splitting of the original cells), which exposes the cells to a strong negative selection against nontransformed cells. Cells that continue growing after 6–10 passages are considered immortalized. Additionally, an inducible cell line expressing WT PrimPol was established by using Flp-In T-REx 293 cells. The cloning of PrimPol cDNA with a C-terminal flag-tag into the pcDNA5FRTO and the generation of the cell line was performed essentially as in the work of Wanrooij et al. (18). In this system, the transgene is expressed upon addition of doxycycline (Dox) to the growth medium. Dox 5–10 ng/mL was determined to give a stable long-term expression of the transgene (Fig. S7). At these concentrations, Dox is nontoxic for mitochondrial functions (42).
Cell Culture, ddC, and UV Light Treatment.
Flp-In T-REx 293 and MEF cells were cultured in DMEM containing 4.5 g/L glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 μg/mL uridine, and 10% FBS at 37 °C in a humidified atmosphere with 8.5% CO2 atmosphere. No antibiotic agents were added to the growth medium. After the indicated times, cells were pelleted and DNA samples were extracted. To induce mtDNA replication stalling, cells were treated for 48 h with 175 μM of ddC (Sigma-Aldrich) (37). As previously described for UV damage (21), cells were exposed on the tissue culture dish with DMEM to a single 30-s dose of 30 J/m2 UVB using a Benchtop 2UV Transilluminator 302 nm instrument (UVP). UV light doses were controlled by using a handheld UV radiometer (UM-25; Minolta).
mtDNA Copy Number Analysis.
Total cellular DNA was isolated by using proteinase K and SDS lysis followed by phenol:chloroform extraction and ethanol precipitation (40). mtDNA levels were analyzed by separating 2 μg HindIII-digested total DNA on a 0.4% agarose gel in 1× TBE 1.2 V/cm for 16 h at room temperature. Southern blotting and DNA hybridization were carried out as previously described (40) by using a Cytb (mouse; nucleotides 14,783–15,333) probe for mtDNA, and an 18S rDNA probe (nucleotides 24–772; National Center for Biotechnology Information accession no. M10098) as loading control. Radioactive signal was captured on Kodak storage phosphor screen SO230, detected by using a Molecular Imager FX (BioRad), and quantified by using the associated QuantityOne software.
mtDNA Isolation and Analysis of mtDNA Replication Intermediates.
mtDNA was isolated by using 1 h 20 µg/mL cytochalasin (Sigma-Aldrich) treatment for MEF cells or 30 min for T-Rex 293 cells before cell breakage, followed by differential centrifugation and sucrose gradient purification (43). The 2D-AGE analysis was performed essentially as in the work of Pohjoismäki et al. (37). Further details are provided in SI Materials and Methods.
In Vitro Replication Assays.
M13mp18 ssDNA (M13ssDNA) was used as a template to assay replication by PrimPol and Pol γ in the presence of dideoxynucleotides. Pol γA, Pol γB (forming the holoenzyme Pol γAB2), and PrimPol were expressed and purified as described previously (20, 44). Standard reaction mixtures contained 10 mM Bis-Tris propane, 10 mM MgCl2, 1 mM DDT, 200 μM dNTPs, 1 mM ATP, [α-32P]dGTP as radioactive tracer, and the indicated amount of ddCTP. When indicated, 12.5 nM Pol γA, 18.75 nM Pol γB (as a dimer), and 200 nM PrimPol was added. The reaction was performed by using 5 nM of singly- or nonprimed M13ssDNA (the 28-mer primer is complementary to nucleotides 6,218–6,245). Reactions were incubated for 60 min at 37 °C, stopped with 0.5% SDS/25 mM EDTA, purified with G-25 columns (GE Healthcare), and loaded on a 1% alkaline (30 mM NaOH, 1 mM EDTA) agarose gel. The gel was run at 20 V for 16 h (4 °C), dried, exposed to a storage phosphor screen, and scanned with a Typhoon 9400 device (Amersham Biosciences).
Mapping of PrimPol Priming Site.
Mapping of PrimPol priming site was performed by an adapted protocol for 5′-RACE (45). Further details are provided in SI Materials and Methods.
SI Materials and Methods
MEF Genotyping.
PCR-based genotyping of the Primpol KO was carried out as described previously (9) by using the following primers:
Primpol forward, 5′-cctacatctgcaagaagacttagc-3′; Primpol reverse, 5′-acactgggtccctttacagatgg-3′acac LTR reverse, 5′-ataaaccctcttgcagttgcatc-3′
In Vitro DNA Primer Extension Assay.
DNA polymerization was carried out with a 25-nt 5′ 32P-radiolabeled primer annealed to a 70-mer linear template. Reactions were performed in a buffer containing 10 mM Bis-Tris Propane HCl (pH 7.0), 10 mM MgCl2, and 1 mM DTT. Radiolabeled DNA substrate (5 nM) and 200 µM dNTPs were added to the reaction mixture (10 μL total volume). Reactions were performed at 37 °C and started by addition of 200 nM PrimPol and/or 12.5 nM Pol γA and 18.75 nM Pol γB (as a dimer) as indicated. Where indicated, 0, 200 μM, or 1,000 μM of ddCTP was added to the reaction mix. Reactions were stopped by addition of 1.1 μL of termination mixture (5% SDS, 250 mM EDTA) and analyzed on a 10% polyacrylamide gel containing 8 M urea.
In Vitro Primase Assay.
M13ssDNA was used as template to assay primase activity of PrimPol in the presence of dideoxynucleotides. Human WT PrimPol was overexpressed and purified as previously described (2). Reaction mixtures (20 µL) contained 50 mM Tris⋅HCl, pH 7.5, 25 mM NaCl, 1 mM MnCl2, 1 mM DTT, 2.5% glycerol, 0.1 mg/mL BSA, 20 nM [α-32P]dGTP (3,000 Ci/mmol), 100 μM ATP, and the indicated amounts of ddCTP or ddGTP, in the presence of 5 nM M13ssDNA and 400 nM PrimPol. After 60 min at 30 °C, reactions were stopped by addition of formamide loading buffer [25 mM EDTA, 95% (vol/vol) formamide, and 0.3% (wt/vol) xylene cyanol], and loaded on a 8-M urea-containing 20% polyacrylamide sequencing gels. After electrophoresis, de novo synthesized oligonucleotides (primers) were detected by autoradiography.
In Vitro Replication Assays.
M13ssDNA was used as template to assay replication by PrimPol and Pol γ in the presence of dideoxynucleotides. Pol γA, Pol γB (forming the holoenzyme Pol γAB2), and PrimPol were expressed and purified as described previously (13, 44). Standard reaction mixtures contained 10 mM Bis-Tris propane, 10 mM MgCl2, 1 mM DDT, 200 μM dNTPs, 1 mM ATP, [α-32P]dGTP as radioactive tracer, and the indicated amount of ddCTP. When indicated, 12.5 nM Pol γA, 18.75 nM Pol γB (as a dimer), and 200 nM PrimPol was added. The reaction was performed by using 5 nM of single- or nonprimed ss M13mp18 DNA. The primers are complementary to nucleotides 6,218–6,245 (#1), 3,837–3,864 (#2), or 1,470–1,496 (#3). Reactions were incubated for 60 min at 37 °C, stopped with 0.5% SDS and 25 mM EDTA, purified with G-25 columns (GE Healthcare), and loaded on a 1% alkaline (30 mM NaOH, 1 mM EDTA) agarose gel. The gel was run at 20 V for 16 h (4 °C), dried, exposed to a storage phosphor screen, and scanned with the Typhoon 9400 device (Amersham Biosciences).
Quantitative Real-Time PCR.
To assess whether the copy number recovery in Primpol-KO and WT cells was influenced by mtDNA integrity, long- and short -amplicon quantitative PCR (qPCR) was performed essentially as in the work of Furda et al. (56). Total DNA was isolated as described in Materials and Methods, but the mtDNA digestion step was skipped. A total of 100 ng of the total DNA samples were measured in quadruplicate. The short PCR thermoprofile is as follows.
A single 3-min cycle at 95 °C was followed by 40 cycles of a two-step amplification: 95 °C for 10 s and 60 °C for 20 s. The long PCR thermoprofile is as follows. A single 5-min cycle at 94 °C was followed by 40 amplification cycles: desaturating at 94 °C for 30 s, annealing at 64 °C for 30 s, and elongation at 70 °C for 7 min. The signal from the FAM mtDNA probe and HEX nDNA probe were detected with the AriaMx Real-Time system (Agilent Technologies), and the data were analyzed with Agilent AriaMx 1.0 software. The quantification value (Cq) was used in the analysis. Short and long mitochondrial Cq values were standardized with the nuclear Cq value to correct the amount of sample in each well. The short-amplicon qPCR was used for regular mtDNA copy number determination where stated.
Primers were as follows:
NDUFV1 nuclear gene [UniProtor, the UniProt Knowledgebase (UniProtKB), accession no. U3BG69]: forward, 5′ ATC CAG GAT CCC ACA GAG CT; reverse, 5′ CCT TTC CAG CAG ATG TGG GT; and probe, 5′ VIC- GAG CCT TAG GGA AGA GGC AG –MGB.
6.5-kb mitochondrial fragment: 1978 forward, 5′-TGC CTG CCC AGT GAC TAA AG; 8496 reverse, 5′-GGT AGC TGT TGG TGG GCT AA; probe 2017 reverse, 5′ Fam-TGA CCG TGC AAA GGT AGC AT-BHQ1; 108-bp mitochondria fragment: 2086 reverse, 5′-GAC CCT CGT TTA GCC GTT CA; 1978 forward, 5′-TGC CTG CCC AGT GAC TAA AG; and probe 2017 reverse, 5′ Fam-TGA CCG TGC AAA GGT AGC AT-BHQ1.
Pol γ Processivity in the Presence of PrimPol.
A 28-mer primer (nucleotides 6,218–6,245) was 5′-labeled with [γ-32P]ATP using T4 polynucleotide kinase (Thermo Scientific) and annealed to M13ssDNA. A total of 2.5 nM of the template was used in the standard reaction mixture described here earlier. When indicated, 12.5 nM Pol γA and 18.75 nM Pol γB was added together with an increasing concentration of PrimPol (0 nM, 12.5 nM, 25 nM, 50 nM, 100 nM, 200 nM, 500 nM, 1,000 nM). Reactions were incubated for 90 min at 37 °C, stopped with 0.5% SDS and 25 mM EDTA, and loaded on a 1% denaturing alkaline (30 mM NaOH, 1 mM EDTA) agarose gel (Fig. 2B) or a 0.8% 1× TBE agarose gel (Fig. S5A). The gel was run at 20 V for 17 h (4 °C), dried, exposed to a storage phosphor screen, and scanned with the Typhoon 9400 device (Amersham Biosciences).
SDS/PAGE and Western Blotting.
Total protein lysate for SDS/PAGE was extracted from cell pellets by using Totex extraction buffer (20 mM Hepes, pH 7.9, 400 mM NaCl, 20% glycerol, 1% Nonidet P-40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 10 mM β-glycerophosphate, 10 mM NaF, 5 mM DTT, and protease inhibitors) and quantified by using Bradford assay. A total of 40 µg samples of total proteins were separated by SDS/PAGE by using a 8% Laemmli gel and blotted onto nitrocellulose membrane in Towbin buffer. The membranes were blocked in 5% nonfat milk in Tris-buffered saline Tween 20 (TBS-T) for 1 h, incubated with primary antibody overnight at 4 °C, and then incubated for 1 h with the peroxidase-coupled secondary antibody. Chemiluminescent detection and quantification was performed by using a BioSpectrum 810 imaging system detection instrument (UVP). Primary antibodies used were rabbit anti-flag (no. ABIN871107; Antibodies Online), 1:5,000; mouse anti-vinculin (no. V9264; Sigma), 1:10,000; rabbit anti-PrimPol (2), 1:5,000; mouse anti-HSP60 (no. ABIN361784; Antibodies Online), 1:20,000; rabbit anti-H1.2 (no. ab181973; Abcam), 1:5,000; and goat anti-Lamin A/C (no. sc-6215; Santa Cruz), 1:500. Secondary antibodies used were Novex goat anti-rabbit IgG HRP (no. A16104; Life Technologies), goat anti-mouse IgG HRP (no. ABIN101744; Antibodies Online), and bovine anti-goat IgG HRP (no. sc-2352; Santa Cruz).
Mitochondrial Targeted PrimPol Constructs.
The different variants of PrimPol were cloned with a C-terminal flag-tag into the pcDNA5FRTO vector as done previously. For MTS-PrimPol, the mitochondrial targeting sequence of human TFAM (aa 1–50) was inserted at the N terminus of PrimPol-flag using the Acc65I and BamHI restriction sites. Next, the MTS-hPrimPol-Flag insert was digested with AflII (BsptI) and ApaI and ligated into the pcDNA5-FRT-TO plasmid. A primase-dead variant of MTS-PrimPol was made by using site-directed mutagenesis to generate a double point mutation (C419G/H426Y) in the zinc finger motif (9). The generation of the cell lines was performed essentially as in a previous work (18). In this system, the transgene is expressed upon addition of Dox to the growth medium. The generated recombinant proteins were determined to be exclusively mitochondrially targeted (Fig. S7C) and to give a stable, long-term expression with 5–10 ng/mL of Dox.
The following oligos were used for mutagenesis:
CH-sense (C419G/H426Y), 5′GATATTTGTAAATATCGGTGGGGTGAAAACATTGGAAGAGCCTATAAGAGTAATAATATAATG-3′AAd CH-antisense, 5′CATTATATTATTACTCTTAATAGCTCTTCCAATGTTTTCACCCCACCGATATTTACAAATATC-3′
Two-Dimensional AGE and Southern Blotting.
A total of 5 μg of mitochondrial nucleic acid was digested according to the manufacturer’s recommendation with BclI or HincII (both from Thermo Scientific) and run on a 0.4% agarose gel in 1× TBE until the fragments of interest had migrated 10 cm into the gel. The gel slab was rotated 90°, and a 0.95% agarose gel was cast around it. The second dimension was run until the fragment of interest had migrated ∼10 cm. For long-range 2D AGEs, 5 μg of mitochondrial nucleic acids was digested with FastDigest BamHI or PvuII (Thermo Scientific), which cut mtDNA only once, and run on a 0.28% agarose gel in 1× TBE until the linearized mtDNA fragment had migrated 10 cm into the gel. The gel slab was rotated 90°, and a 0.58% agarose gel was cast around it. The second dimension was run at 2.6 V/cm until the fragment of interest had migrated ∼10 cm from the top. Southern blotting was performed by using the standard procedure, and the nucleic acid hybridization was performed by using a PCR probe spanning nucleotides 14,783–15,333 (Cytb) of the mouse mtDNA and 4,470–5,511 (ND2) or 35–611 (NCR) of the human mtDNA. Probes were labeled by using a Rediprime II random prime labeling kit (GE Healthcare) and [α-32P]dCTP (3,000 Ci/mmol; PerkinElmer). The autoradiographs were exposed on a Kodak MS film (Sigma-Aldrich) or were captured on a Kodak storage phosphor screen SO230 and detected by using Molecular Imager FX (BioRad).
Mapping of PrimPol Priming Site.
Mapping of PrimPol priming site was performed by an adapted protocol for 5′-RACE (45) using the following primers:
P1 CATTGTCGGCGCAACTATCG
P2 GTGGAATGCTACAGGCGTTG
P2 TGGCGGTACTAAACCTCCTG
PT CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGCTTTTTTTTTTTTTTTTTTTTVN
PO CCAGTGAGCAGAGTGACG
PI GAGGACTCGAGCTCAAGC
PS AAACGCGCTACAGTCTGACG
PrimPol-dependent Pol γ products were obtained by using the standard reaction on nonprimed M13ssDNA (as described earlier) and purified by using a spin column-based DNA purification system. A complementary strand was then synthesized by using a template-specific primer P1; a 5-min denaturation at 95 °C, followed by 30 s annealing at 53 °C and one-step extension at 72 °C for 30 min with Taq polymerase (Promega GoTaq G2 Hot Start Polymerase) were performed by using the manufacturer’s recommended reaction mixture composition. The reaction products were spin-column purified, and a poly(A)-tail was appended by a 15-min incubation with terminal transferase (NEB) in the recommended buffer. After deactivation of terminal transferase, the products were diluted fourfold with dH2O. The first round of PCR amplification was performed by using 1 µL of diluted template, primers P2, PT, and PO, and 30 cycles of amplification. The obtained products were run on 1% agarose gel and spin-column purified. A total of 1 µL of 100-fold diluted products were used as a template for the second round of PCR amplification (30 cycles). The reactions were run according to the manufacturer’s instructions by using primers P3 and PI. Both PCRs were performed with Promega GoTaq G2 Hot Start Polymerase. The obtained products were run on a 1% agarose gel, spin-column purified, and sent for sequencing by using primer PS.
Mitochondrial Fractionation and PrimPol Coimmunoprecipitation.
A 10-cm dish of T-REx 293 MTS-PrimPol cells was harvested 26 h after induction with 100 ng/mL Dox (Sigma-Aldrich). After washing the cell pellet with ice-cold PBS solution and centrifugation (500 × g, 3 min), the cell pellet was resuspended in 1 mL of ice-cold 0.1× homogenization buffer (HG buffer; 10× HG buffer, 400 mM Tris⋅HCl, pH 7.8, 250 mM NaCl, and 50 mM MgCl2) containing 1× HALT protease inhibitor mixture (Thermo Scientific) and incubated on ice for 10 min. Cells were disrupted with 60–80 strokes in a glass-glass homogenizer. The cell disruption was monitored with Trypan Blue staining under a light microscope, and 125 µL of 10× HG buffer was added to stop the hypotonic lysis. Unbroken cells and nuclei were removed by two centrifugation steps at 1,000 × g for 3 min at 4 °C. The nuclear pellet of the first centrifugation step was incubated in 995 µL of 1× HG buffer plus 5 µL of 10% Nonidet P-40 for 10 min on ice to lyse unbroken cells and centrifuged (1,200 × g, 3 min, 4 °C). Mitochondria in the postnuclear supernatant were pelleted at 17,000 × g for 10 min at 4 °C, washed with 1× HG buffer, and centrifuged again to obtained a crude mitochondrial fraction. The postmitochondrial supernatant was kept as cytosolic fraction. The nuclear and crude mitochondrial fractions were lysed in RIPA buffer [50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1% (vol/vol) Nonidet P-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, and 2 mM EDTA] containing 1× HALT protease inhibitor mixture. From each fraction, equal amounts of proteins were analyzed by SDS/PAGE and Western blotting by using the following primary antibodies: anti-Flag M2 (1:1,000; Sigma), anti-Pol γ (1:1,000; Abcam), anti-OPA1 (1:1,000; no. 612606; BD Biosciences), anti-GAPDH (1:2,500; no. 2118; Cell Signaling), anti-histone H2B (1:1,000; no. 12364; Cell Signaling), anti-lamin A/C (1:1,000; no. 4777; Cell Signaling), anti-Tom20 (1:1,000; no. sc-11415; Santa Cruz), and anti-SSBP1 (1:1,000; no. 12212–1-AP; Proteintech).
Mitochondria for Proteinase K accessibility assays were isolated according to the procedure described by Frezza et al. (57), and the subsequent protocol for the Proteinase K accessibility assay was adapted from Dimmer et al. (58) In brief, cells from a 15-cm dish were harvested 24 h after induction. Cells were washed with ice-cold PBS solution and centrifuged (500 × g, 3 min). The cell pellet was resuspended in 2 mL of ice-cold IBc buffer (10 mM Tris⋅Mops, pH 7.4, 1 mM EGTA⋅Tris, pH 7.4, 0.2 M sucrose, pH adjusted to 7.4) with 1× HALT protease inhibitor mixture (Thermo Scientific), and cells were disrupted in a glass homogenizer with a Teflon pestle. The cell disruption was monitored with Trypan Blue. After centrifugation at 600 × g for 10 min at 4 °C, the pellet was subjected to a second round of homogenization. The supernatants of both rounds of homogenization were then centrifuged at 7,000 × g for 10 min at 4 °C to obtain crude mitochondria. Mitochondria were washed with IBc buffer, centrifuged again (7,000 × g, 10 min, 4 °C), and finally resuspended in 200 µL of IBc buffer before the mitochondrial concentration was determined with the Pierce BCA Protein Assay Kit. Subsequently, 50 µg of mitochondria was incubated with 100 µg/mL Proteinase K (Thermo Scientific) by resuspending the mitochondrial pellet in a reaction volume of 100 µL of IBc buffer (protease-treated mitochondria), 2 mM Hepes, pH 7.4 (protease-treated mitoplasts), or 2 mM Hepes, pH 7.4, with a final concentration of 0.1% (vol/vol) Triton X-100 (protease- and detergent-treated mitoplasts). After addition of Proteinase K, samples were incubated for 30 min on ice, and the reaction was inactivated by adding 0.2 µL of 1 M PMSF. Samples were precipitated with 15% trichloroacetic acid, washed with ice-cold acetone, and resuspended in 50 µL of 4× Laemmli sample buffer containing 4% (vol/vol) β-mercaptoethanol following an analysis of 10 µL of each sample by SDS/PAGE and Western blotting.
For the coimmunoprecipitation, Flp-In T-REx 293 HEK MTS-PrimPol cells were harvested 24 h after induction with 10 ng/mL Dox (no. D9891; Sigma-Aldrich). As a control, uninduced cells were carried along. Cells were solubilized in Huttlin lysis buffer [50 mM Tris⋅HCl, pH 7.5, 300 mM NaCl, 0.5% (vol/vol) Nonidet P-40] (33) containing 1× HALT protease inhibitor mixture (no. 78430; Thermo Scientific). After incubating 30 min on ice, cell lysates were cleared by centrifugation (17,000 × g, 10 min, 4 °C) and the protein concentration was quantified by using the Pierce BCA Protein Assay Kit (no. 23225; Thermo Scientific). As input control, 25 µg total cell lysate was collected, while an equal amount of 1 mg of total cell lysate was incubated with 50 µL of anti-FLAG M2 magnetic beads (50% slurry; no. M8823; Sigma) and incubated while rotating for 4–5.5 h at 4 °C. After washing three times with 500 µL Huttlin lysis buffer (33), the immunoprecipitation was eluted with 50 µL of 4× Laemmli sample buffer (no. 1610747; Bio-Rad) containing 4% (vol/vol) β-mercaptoethanol (10 min, 95 °C). Eluates and input samples were subsequently analyzed by SDS/PAGE and immunoblotting using the following primary and HRP-conjugated secondary antibodies: anti–β-actin (1:5,000; no. A5441; Sigma), anti-Flag M2 (1:1,000; no. F1804; Sigma), anti-DNA Pol γ (1:1,000; no. ab128899; Abcam), anti-Twinkle (1:1,000; no. ab187517; Abcam), anti-rabbit IgG HRP (1:5,000; no. 31460; Invitrogen), anti-mouse IgG HRP (1:5,000; no. 31430; Invitrogen), mouse TrueBlot anti-mouse Ig HRP (1:1,000; no. 18–8817-33; Rockland), and rabbit TrueBlot anti-rabbit IgG HRP (1:1,000; no. 18–8816-33; Rockland). For detection, the SuperSignal West Pico Chemiluminescent Substrate (no. 34080; Thermo Scientific) or SuperSignal West Femto Maximum Sensitivity Substrate (no. 34096; Thermo Scientific) was used and detected with the ChemiDoc Touch Imaging System (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Sandra Chocrón and Maria Martínez-Jiménez for valuable PrimPol-related discussions and work and Mr. Craig Michell (University of Eastern Finland) for language editing. This work was supported by the Jane & Aatos Erkko (JAE) Foundation (R.T.-M. and J.L.O.P.), the Finnish Academy (S.G.), the Wallenberg Foundation (S.W., J.M.E.F., and A.P.), Olle Engkvist Byggmästare Foundation (G.S.), Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (G.C.), and Spanish Ministry of Economy and Competitiveness Grant BFU2015-65880-P (to L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705367114/-/DCSupplemental.
References
- 1.Wan L, et al. hPrimpol1/CCDC111 is a human DNA primase-polymerase required for the maintenance of genome integrity. EMBO Rep. 2013;14:1104–1112. doi: 10.1038/embor.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Gómez S, et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol Cell. 2013;52:541–553. doi: 10.1016/j.molcel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilliam TA, Keen BA, Brissett NC, Doherty AJ. Primase-polymerases are a functionally diverse superfamily of replication and repair enzymes. Nucleic Acids Res. 2015;43:6651–6664. doi: 10.1093/nar/gkv625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer LM, Koonin EV, Leipe DD, Aravind L. Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: Structural insights and new members. Nucleic Acids Res. 2005;33:3875–3896. doi: 10.1093/nar/gki702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocquier AA, et al. Archaeal primase: Bridging the gap between RNA and DNA polymerases. Curr Biol. 2001;11:452–456. doi: 10.1016/s0960-9822(01)00119-1. [DOI] [PubMed] [Google Scholar]
- 6.Matsui E, et al. Distinct domain functions regulating de novo DNA synthesis of thermostable DNA primase from hyperthermophile Pyrococcus horikoshii. Biochemistry. 2003;42:14968–14976. doi: 10.1021/bi035556o. [DOI] [PubMed] [Google Scholar]
- 7.Zafar MK, Ketkar A, Lodeiro MF, Cameron CE, Eoff RL. Kinetic analysis of human PrimPol DNA polymerase activity reveals a generally error-prone enzyme capable of accurately bypassing 7,8-dihydro-8-oxo-2′-deoxyguanosine. Biochemistry. 2014;53:6584–6594. doi: 10.1021/bi501024u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi J, et al. PrimPol bypasses UV photoproducts during eukaryotic chromosomal DNA replication. Mol Cell. 2013;52:566–573. doi: 10.1016/j.molcel.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourón S, et al. Repriming of DNA synthesis at stalled replication forks by human PrimPol. Nat Struct Mol Biol. 2013;20:1383–1389. doi: 10.1038/nsmb.2719. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Jiménez MI, et al. Alternative solutions and new scenarios for translesion DNA synthesis by human PrimPol. DNA Repair (Amst) 2015;29:127–138. doi: 10.1016/j.dnarep.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K, et al. Repriming by PrimPol is critical for DNA replication restart downstream of lesions and chain-terminating nucleosides. Cell Cycle. 2016;15:1997–2008. doi: 10.1080/15384101.2016.1191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavone D, et al. PrimPol is required for replicative tolerance of G quadruplexes in vertebrate cells. Mol Cell. 2016;61:161–169. doi: 10.1016/j.molcel.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazak L, Reyes A, Holt IJ. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 14.Clayton DA, Doda JN, Friedberg EC. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci USA. 1974;71:2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan KJ, et al. What causes mitochondrial DNA deletions in human cells? Nat Genet. 2008;40:275–279. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- 16.Phillips AF, et al. Single-molecule analysis of mtDNA replication uncovers the basis of the common deletion. MolCell. 2017;65:527–538.e526. doi: 10.1016/j.molcel.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Goffart S, et al. Twinkle mutations associated with autosomal dominant progressive external ophthalmoplegia lead to impaired helicase function and in vivo mtDNA replication stalling. Hum Mol Genet. 2009;18:328–340. doi: 10.1093/hmg/ddn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanrooij S, Goffart S, Pohjoismäki JL, Yasukawa T, Spelbrink JN. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007;35:3238–3251. doi: 10.1093/nar/gkm215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JL, Brown CE, Matthews-Davis N, Reardon JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob Agents Chemother. 1994;38:2743–2749. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stojkovič G, et al. Oxidative DNA damage stalls the human mitochondrial replisome. Sci Rep. 2016;6:28942. doi: 10.1038/srep28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torregrosa-Muñumer R, Goffart S, Haikonen JA, Pohjoismäki JL. Low doses of ultraviolet radiation and oxidative damage induce dramatic accumulation of mitochondrial DNA replication intermediates, fork regression, and replication initiation shift. Mol Biol Cell. 2015;26:4197–4208. doi: 10.1091/mbc.E15-06-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pohjoismäki JL, Goffart S. Of circles, forks and humanity: Topological organisation and replication of mammalian mitochondrial DNA. BioEssays. 2011;33:290–299. doi: 10.1002/bies.201000137. [DOI] [PubMed] [Google Scholar]
- 23.Reyes A, et al. Mitochondrial DNA replication proceeds via a ‘bootlace’ mechanism involving the incorporation of processed transcripts. Nucleic Acids Res. 2013;41:5837–5850. doi: 10.1093/nar/gkt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohjoismäki JL, et al. Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA hybrid. J Mol Biol. 2010;397:1144–1155. doi: 10.1016/j.jmb.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown TA, Cecconi C, Tkachuk AN, Bustamante C, Clayton DA. Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Genes Dev. 2005;19:2466–2476. doi: 10.1101/gad.1352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miralles Fusté J, et al. In vivo occupancy of mitochondrial single-stranded DNA binding protein supports the strand displacement mode of DNA replication. PLoS Genet. 2014;10:e1004832. doi: 10.1371/journal.pgen.1004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasukawa T, et al. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006;25:5358–5371. doi: 10.1038/sj.emboj.7601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham XH, et al. Conserved sequence box II directs transcription termination and primer formation in mitochondria. J Biol Chem. 2006;281:24647–24652. doi: 10.1074/jbc.M602429200. [DOI] [PubMed] [Google Scholar]
- 29.Fusté JM, et al. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol Cell. 2010;37:67–78. doi: 10.1016/j.molcel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Lim SE, Copeland WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem. 2001;276:23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- 31.Cavanaugh NA, Kuchta RD. Initiation of new DNA strands by the herpes simplex virus-1 primase-helicase complex and either herpes DNA polymerase or human DNA polymerase alpha. J Biol Chem. 2009;284:1523–1532. doi: 10.1074/jbc.M805476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frick DN, Richardson CC. DNA primases. Annu Rev Biochem. 2001;70:39–80. doi: 10.1146/annurev.biochem.70.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Huttlin EL, et al. The BioPlex network: A systematic exploration of the human interactome. Cell. 2015;162:425–440. doi: 10.1016/j.cell.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey LJ, Bianchi J, Hégarat N, Hochegger H, Doherty AJ. PrimPol-deficient cells exhibit a pronounced G2 checkpoint response following UV damage. Cell Cycle. 2016;15:908–918. doi: 10.1080/15384101.2015.1128597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohjoismäki JL, Goffart S. The role of mitochondria in cardiac development and protection. Free Radic Biol Med. 2017;106:345–354. doi: 10.1016/j.freeradbiomed.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Hyvärinen AK, et al. The mitochondrial transcription termination factor mTERF modulates replication pausing in human mitochondrial DNA. Nucleic Acids Res. 2007;35:6458–6474. doi: 10.1093/nar/gkm676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohjoismäki JL, et al. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006;34:5815–5828. doi: 10.1093/nar/gkl703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander JL, Orr-Weaver TL. Replication fork instability and the consequences of fork collisions from rereplication. Genes Dev. 2016;30:2241–2252. doi: 10.1101/gad.288142.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohjoismäki JL, Goffart S, Spelbrink JN. Replication stalling by catalytically impaired Twinkle induces mitochondrial DNA rearrangements in cultured cells. Mitochondrion. 2011;11:630–634. doi: 10.1016/j.mito.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Pohjoismäki JL, et al. Human heart mitochondrial DNA is organized in complex catenated networks containing abundant four-way junctions and replication forks. J Biol Chem. 2009;284:21446–21457. doi: 10.1074/jbc.M109.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowmaker M, et al. Mammalian mitochondrial DNA replicates bidirectionally from an initiation zone. J Biol Chem. 2003;278:50961–50969. doi: 10.1074/jbc.M308028200. [DOI] [PubMed] [Google Scholar]
- 42.Goffart S, Spelbrink H. Inducible expression in human cells, purification, and in vitro assays for the mitochondrial DNA helicase Twinkle. Methods Mol Biol. 2009;554:103–119. doi: 10.1007/978-1-59745-521-3_7. [DOI] [PubMed] [Google Scholar]
- 43.Yang MY, et al. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell. 2002;111:495–505. doi: 10.1016/s0092-8674(02)01075-9. [DOI] [PubMed] [Google Scholar]
- 44.Atanassova N, et al. Sequence-specific stalling of DNA polymerase γ and the effects of mutations causing progressive ophthalmoplegia. Hum Mol Genet. 2011;20:1212–1223. doi: 10.1093/hmg/ddq565. [DOI] [PubMed] [Google Scholar]
- 45.Scotto-Lavino E, Du G, Frohman MA. 5′ end cDNA amplification using classic RACE. Nat Protoc. 2006;1:2555–2562. doi: 10.1038/nprot.2006.480. [DOI] [PubMed] [Google Scholar]
- 46.Brewer BJ, Fangman WL. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987;51:463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- 47.Kolesar JE, Wang CY, Taguchi YV, Chou SH, Kaufman BA. Two-dimensional intact mitochondrial DNA agarose electrophoresis reveals the structural complexity of the mammalian mitochondrial genome. Nucleic Acids Res. 2013;41:e58. doi: 10.1093/nar/gks1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23:2423–2429. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerhold JM, Aun A, Sedman T, Jõers P, Sedman J. Strand invasion structures in the inverted repeat of Candida albicans mitochondrial DNA reveal a role for homologous recombination in replication. Mol Cell. 2010;39:851–861. doi: 10.1016/j.molcel.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Crews S, Ojala D, Posakony J, Nishiguchi J, Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979;277:192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- 51.Xu B, Clayton DA. RNA-DNA hybrid formation at the human mitochondrial heavy-strand origin ceases at replication start sites: An implication for RNA-DNA hybrids serving as primers. EMBO J. 1996;15:3135–3143. [PMC free article] [PubMed] [Google Scholar]
- 52.Fish J, Raule N, Attardi G. Discovery of a major D-loop replication origin reveals two modes of human mtDNA synthesis. Science. 2004;306:2098–2101. doi: 10.1126/science.1102077. [DOI] [PubMed] [Google Scholar]
- 53.Livneh Z, et al. High-resolution genomic assays provide insight into the division of labor between TLS and HDR in mammalian replication of damaged DNA. DNA Repair (Amst) 2016;44:59–67. doi: 10.1016/j.dnarep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Pagès V. Single-strand gap repair involves both RecF and RecBCD pathways. Curr Genet. 2016;62:519–521. doi: 10.1007/s00294-016-0575-5. [DOI] [PubMed] [Google Scholar]
- 55.Payne BAI, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet. 2011;43:806–810. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furda AM, Bess AS, Meyer JN, Van Houten B. Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods Mol Biol. 2012;920:111–132. doi: 10.1007/978-1-61779-998-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frezza C, Cipolat S, Scorrano L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 58.Dimmer KS, et al. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17:201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]