Significance
The remarkable diversity of trees in tropical forests is thought to be maintained by natural enemies such as fungal pathogens, which must exhibit sufficient host specificity to differentially impact survival of co-occurring host species. Little is known about the specificity of fungi that infect seeds of tropical trees. Here we show that communities of seed-associated fungi are structured more by plant species than by soil type, forest characteristics, or time in soil. These fungi have host-specific impacts on seed viability and germination. In this way, highly diverse communities of soilborne fungi directly impact a critical component of reproduction in tropical trees—seeds—with the potential to contribute to maintaining diversity in some of the richest terrestrial communities on Earth.
Keywords: diversity, pioneer species, Janzen–Connell, soil seed bank, pathogen
Abstract
The Janzen–Connell (JC) hypothesis provides a conceptual framework for explaining the maintenance of tree diversity in tropical forests. Its central tenet—that recruits experience high mortality near conspecifics and at high densities—assumes a degree of host specialization in interactions between plants and natural enemies. Studies confirming JC effects have focused primarily on spatial distributions of seedlings and saplings, leaving major knowledge gaps regarding the fate of seeds in soil and the specificity of the soilborne fungi that are their most important antagonists. Here we use a common garden experiment in a lowland tropical forest in Panama to show that communities of seed-infecting fungi are structured predominantly by plant species, with only minor influences of factors such as local soil type, forest characteristics, or time in soil (1–12 months). Inoculation experiments confirmed that fungi affected seed viability and germination in a host-specific manner and that effects on seed viability preceded seedling emergence. Seeds are critical components of reproduction for tropical trees, and the factors influencing their persistence, survival, and germination shape the populations of seedlings and saplings on which current perspectives regarding forest dynamics are based. Together these findings bring seed dynamics to light in the context of the JC hypothesis, implicating them directly in the processes that have emerged as critical for diversity maintenance in species-rich tropical forests.
Identifying mechanisms that permit species coexistence in diverse biotic communities is a major effort in ecology (1, 2). The Janzen–Connell (JC) hypothesis has long been invoked to explain coexistence of large numbers of plant species in tropical forests (3). This hypothesis posits that natural enemies selectively reduce survival of plants near conspecifics and when local densities of conspecifics are high, facilitating recruitment of heterospecifics and ultimately increasing species richness at the community level (3–12).
A central tenet of the JC framework is that natural enemies exhibit sufficient host specificity to generate negative feedbacks (13, 14). Although herbivores and seed predators can influence plant community composition (15, 16), it is unclear whether they exhibit sufficient specificity in associations or functional interactions to influence relative recruitment in a manner consistent with maintaining diversity (17–23). In contrast, pathogens of seedlings and saplings have been identified as major drivers of mortality in tropical plants, with host-specific impacts that are consistent with JC effects (8, 13, 14, 22, 24, 25). Even polyphagous pathogens can have host-specific effects that, by varying in impact on different species, contribute directly to maintaining diversity (22).
To date, most studies detecting JC effects have focused on spatial factors, either investigating the effects of natural enemies by enumerating seedling or sapling mortality at prescribed densities or distances from conspecific crowns (7, 12) or evaluating survival of seedlings in soils gathered beneath, or at distances from, conspecific and heterospecific adults (8, 9, 14, 22). However, biotic or abiotic modifications to soil under conspecific crowns, such as microbial alteration of soil chemistry, recruitment of mutualists, or mitigation of antagonists via pathogen suppression can alter host susceptibility to infection (26–29). Treatments with fungicides to assess effects of pathogens can remove mutualists, altering the context of plant–microbe interactions (30). Plant susceptibility to infection typically varies over time (29, 31), but the time-course of infection is rarely evaluated, especially for propagules that may persist for long periods of time in soil seed banks (e.g., before periodic openings of canopy gaps, a signal of germination and growth in many forest species; ref. 32). More generally, studies of seedlings or saplings focus on life stages that occur after recruitment, and thus provide limited insight into the fate of one of the most important aspects of reproduction for most tropical trees: the dispersal and survival of seeds (33).
The resulting gaps in our knowledge are profound. Seeds represent a key component of reproduction for most tropical trees and thus are the life phase in which factors relevant to survival experience particularly strong, unfiltered selection (34). Unlike plants after germination, which dynamically adjust resource allocation for defense, seeds defend themselves against antagonists with limited resources that decrease over time (35). For most tropical trees, soilborne fungi are the major cause of seed mortality (36–39). Thus, understanding host affinity and host-specific effects of seed-infecting fungi is critical for understanding the earliest and potentially most powerful stages of species-specific mortality relevant to JC effects.
We established a common garden experiment across multiple soil types and forest ages in a contiguous, mixed lowland tropical forest in Panama (SI Appendix, Fig. S1). We buried seeds of nine tropical pioneer tree species (SI Appendix, Table S1) in mesh bags in five gardens. We evaluated viability and fungal infection of individual seeds retrieved up to 1 y after burial to assess the importance of host species, location, and time-course of infection in shaping communities of fungi that naturally colonize seeds in the soil. We then used inoculation experiments to verify host range and quantify effects of focal fungi on seed germination and survival. We tested the hypotheses that (i) communities of soilborne fungi that infect seeds will be structured predominantly by host plant species, consistent with strong selection on this critical life stage in plant reproduction, and (ii) that such fungi alter germination and seed viability in a species-specific manner.
Results
Detectable fungal infections were five times more common in seeds that were buried in gardens than in fresh seeds (SI Appendix, Table S1; Wilcoxon signed-rank, P = 0.004), confirming that buried seeds were colonized naturally by soilborne fungi. After burial, detectable fungal infections in seeds differed in frequency among plant species and as a function of burial duration, but not as a function of burial location. We detected fungi more frequently in inviable seeds than in viable seeds (SI Appendix, Fig. S2 and Table S2).
Typically, one fungal strain emerged from each infected seed in culture. Overall 1,460 fungal isolates were obtained, including 1,377 from buried seeds (SI Appendix, Table S1). Species accumulation curves show that our sampling adequately represents the richness of these fungal communities (SI Appendix, Figs. S3 and S4). Diversity of fungi associated with buried seeds differed among plant species, but not as a function of burial duration, location, or seed viability [SI Appendix, Fig. S5 and Table S3; generalized linear model (GLM): plant species, P < 0.001; duration, P = 0.115; location, P = 0.600; viability, P = 0.183].
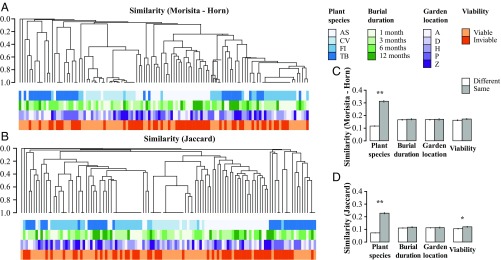
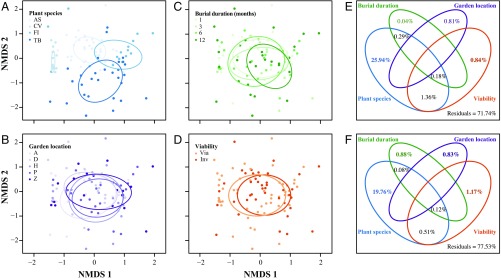
Given variation in the abundance of detectable fungi among plant species, we focused analyses on four plant species that together accounted for 77.8% of sequenced isolates. Complementary analyses consistently revealed the importance of plant species in defining fungal communities, with additional but minor contributions from burial duration, garden location, and seed viability (Figs. 1 and 2 and SI Appendix, Table S4). Pairwise comparisons showed that community similarity was greater among conspecific seeds than heterospecific seeds and between seeds of the same vs. different viability classes (Fig. 1 C and D and SI Appendix, Table S6). When all host species were considered, fungal communities still reflected plant species, location, and viability, but not burial duration (SI Appendix, Fig. S6 C and D and Tables S6 and S7). Plant species explained the majority of the variance in fungal community structure that could be explained in our experiment (Fig. 2 and SI Appendix, Fig. S7 and Table S8).
Fig. 1.
Hierarchical cluster analyses and pairwise similarities of seed-associated fungal communities reveal strong effects of plant species and seed viability on fungal community structure. Dendrograms represent Morisita–Horn (A) and Jaccard (B) similarities among communities of fungi. Tips of dendrograms represent fungal communities defined as the sequenced fungal strains isolated from seeds of a given plant species (AS, Annona spraguei; CV, Cochlospermum vitifolium; FI, Ficus insipida; and TB, Trema micrantha “black”), a specific burial duration (1, 3, 6, or 12 mo), a specific burial location (A, Armour; D, Drayton; H, 25 ha; P, Pearson; and Z, Zetek), and a specific viability class (viable or inviable seeds). Sequence data were obtained from 992 isolates from buried seeds (72%; mean ± SE, 110.2 ± 38.4 isolates per plant species; SI Appendix, Table S5). Together these represent 209 operational taxonomic units (OTU) based on 99% sequence similarity (Fisher’s alpha = 80.8). Overall, 107 OTU were represented by only one isolate (51.2%). Panels on the right summarize pairwise comparisons of fungal community similarity from the cluster diagrams based on abundance (C) and presence–absence data (D). Analysis of the entire dataset (nine plant species) also highlights the strong effect of plant species (SI Appendix, Fig. S6 and Tables S6 and S7). Error bars represent SE. **P < 0.001, *P < 0.05.
Fig. 2.
Nonmetric multidimensional scaling (NMDS) analysis representing similarity among fungal communities (points) isolated from seeds of the four best-sampled tree species. Panels depict the same ordination but different colors represent plant species (A), garden location (B), burial duration (C), and viability (D) (Inv, Inviable; Via, Viable); stress = 0.062; abbreviations match those in Fig. 1. Ellipses represent standard deviation of point scores relative to their centroid. Venn diagrams represent variation in fungal community composition that is explained by each variable and their shared variation, based on abundance (E) and presence–absence data (F). Redundancy analyses show that plant species (F = 14.26, P = 0.001) and viability (F = 2.26, P = 0.029) are significant when fungal abundance is considered, but plant species (F = 10.35, P = 0.001), burial duration (F = 1.42, P = 0.023), garden location (F = 1.30, P = 0.046), and viability (F = 2.63, P = 0.001) are significant when only presence–absence data are considered. In each case, the majority of the variation that can be explained is explained by plant species.
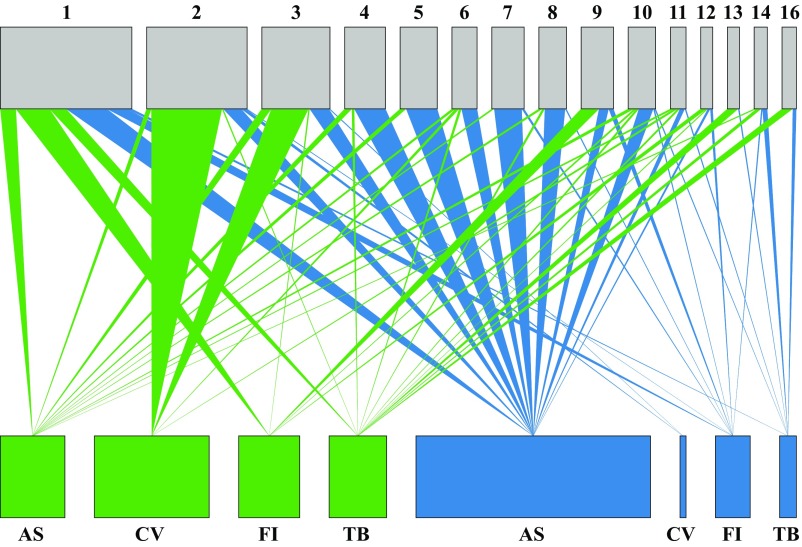
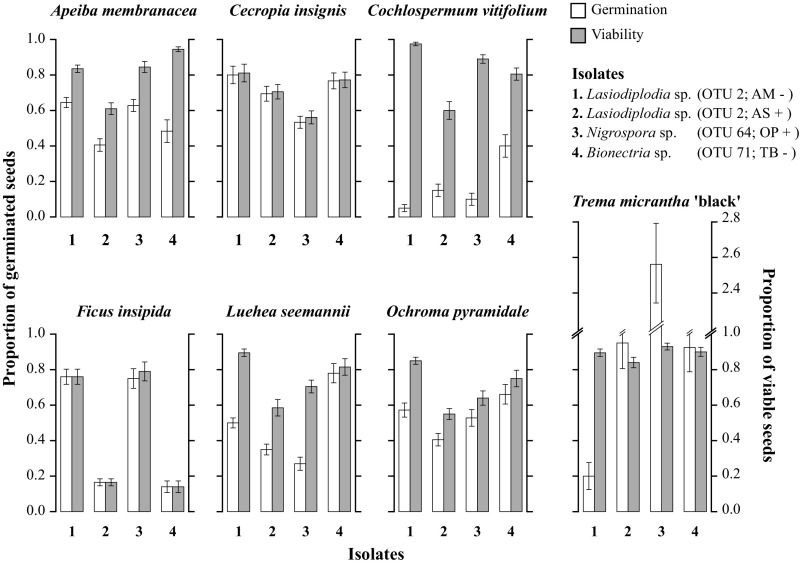
Although communities of seed-associated fungi showed host affinity, these fungi were not strictly host-specific; instead, many fungal strains were polyphagous (Figs. 1 and 3). Polyphagy may provide the context for effective specialization (40), whereby natural enemies associate with multiple host taxa but elicit host-specific effects. Effective specialization was evaluated in two ways. First, we found that in 70% of cases, OTU associated with viable or inviable seeds of one plant species were associated with the opposite viability class in other species (Fig. 3). Second, in vitro experiments verified polyphagy and quantified species-specific effects of representative fungi on seed germination and survival. We successfully inoculated fresh seeds of all tested species, reisolated each fungal strain from inoculated seeds, and detected no fungal infections in controls before the experiment (SI Appendix, Table S9). Fungi differed in their effects on germination and viability within and across plant species (Fig. 4 and SI Appendix, Table S10). Fungi that reduced germination in some plant species had weak negative effects or strong positive effects on others (Fig. 4). Those that had no effect on seed viability in some plant species decreased viability in others (Fig. 4). By decoupling germination and viability measures we confirmed that fungi affected seeds before seedling emergence (Fig. 4).
Fig. 3.
Associations among plant species and seed-associated fungi. Lines depict observed associations between fungal OTU (Top; OTU represented by ≥4 isolates, indicated by OTU number above each gray box) and plant species (Bottom; four best-sampled tree species), with line thickness proportional to the number of sequenced isolates per OTU from each plant species. Plant boxes and lines are colored to indicate viable (green) or inviable (blue) seeds. Box width is proportional to the number of sequences corresponding to each OTU (Top) or the number of sequenced isolates from each plant species (Bottom). OTU: 1, Hypocrea sp.; 2, Lasodiplodia sp.; 3, Lasodiplodia sp. 2; 4, Nectria sp.; 5, Nectria sp. 2; 6, Lasodiplodia sp. 3; 7, Fusarium sp.; 8, Fusarium sp. 2; 9, Hypocrea sp. 2; 10, Hypocrea sp. 3; 11, Nectria sp. 3; 12, Trichoderma sp.; 13, Pseudocercospora sp.; 14, Cylindrocladiella sp.; 16, Cylindrocladiella sp. 2. Plant species: AS, Annona spraguei; CV, Cochlospermum vitifolium; FI, Ficus insipida; and TB, Trema micrantha “black.”
Fig. 4.
Effects of focal fungi on seed germination and viability. Bars represent the proportion of germinated (Left axes) or viable (Right axes) seeds after inoculation with each of four fungal isolates. Viable seeds are defined as the proportion of seeds that were alive (determined via the tetrazolium test, see Methods) but failed to germinate, plus seeds that germinated. Bar heights correspond to values normalized by the maximum number of germinated or viable seeds observed in controls (noninoculated seeds). In some cases, inoculated seeds of Trema micrantha “black” germinated more than control seeds, so proportions exceed 1. Because surface sterilization after inoculation did not influence the germination or viability of seeds (SI Appendix, Table S10), bars include both treatments. Error bars represent SE. Fungal isolates were cultured from buried seeds that were viable (+) or inviable (−) from AM, Apeiba membranacea; AS, Annona spraguei; OP, Ochroma pyramidale; and TB, Trema micrantha “black.” Isolates 1 and 2 represent the same OTU at 99% sequence similarity, but different OTU at 100% sequence similarity (SI Appendix, Table S11).
Discussion
The JC hypothesis provides a powerful framework for explaining the maintenance of tree diversity in tropical forests (3). Seeds are a critical component of reproduction for most tropical trees, such that understanding the scope of JC effects requires an understanding of the fate of seeds in soil. Horizontally transmitted, soilborne fungi are the major agents of mortality for seeds in tropical forests (37, 39), but little is known regarding their host affinity or their host-specific effects.
Through a common garden experiment in a lowland tropical forest we showed that communities of fungi that infect seeds in soil were structured predominantly by plant species, and to a far lesser degree by forest characteristics (together represented by location, which in turn comprised distinctive soil types, forest ages, and canopy species composition) or burial duration (i.e., time in soil, here 1–12 mo). The most common fungi associated with viable or inviable seeds in a given tree species were consistently associated with the opposite viability class in other species. Inoculation experiments revealed host-specific effects of fungi on seed germination and survival and verified that fungi impact seed survival before germination. Thus, this study brings seeds of tropical trees to light in the context of the JC hypothesis, implicating them directly in the processes that are critical for diversity maintenance in species-rich tropical forests.
The central tenet of the JC hypothesis requires host specialization in interactions between plants and natural enemies. Such specialization manifests as plant mortality at high conspecific densities and/or in proximity to conspecific trees (3). Often it is estimated in terms of host range of natural enemies (e.g., ref. 25). However, the capacity to associate with multiple hosts does not translate necessarily to similar effects across those host species (22, 40). Previous studies have failed to find negative effects of conspecific density or proximity in seeds, but most have used seed removal as a proxy of seed mortality (3). Coupling natural infection by fungi with inoculation experiments provides a basis for assessing two major components of ecological specificity in interactions between seeds and fungi in tropical forests: acquisition of particular fungi from soil and host-specific impacts of focal fungi on seed fate.
Fungi that were commonly recruited to seeds were polyphagous, often occurring in seeds of multiple plant species. However, communities as a whole were structured by plant species. Such structure may reflect specific and differential allocation to suites of seed-defensive traits, including chemical (41) and physical defenses (42) relevant to exclusion of pathogens and recruitment of mutualists (34, 37). Importantly, even closely related species in this study harbored distinguishable fungal communities in seeds (e.g., T. micrantha “black” and T. micrantha “brown”; SI Appendix, Table S12). Overall, mean similarities for fungal communities associated with seeds were similar to those observed in culture-based studies of foliar endophytes at the same sites, suggesting that fungal communities undergo host-driven filtering in other plant tissues and at multiple plant life stages (43) (SI Appendix, Table S13). More generally, this study highlights the tractability of using seeds for evaluating the host preference and functional effects of soilborne fungi, setting the stage for future work across diverse plant guilds.
Although we focused on seeds of pioneer trees, which persist in the soil and thus comprise the soil seed bank, the species used in this study belong to six plant families and thus are phylogenetically diverse. Despite differences in primary dispersal and seed dormancy among species (SI Appendix, Table S1), we observed strong host affinity of fungal communities across all taxa regardless of their phylogenetic placement. Given the diversity of hosts evaluated here, we anticipate that similar processes will occur for seeds of shade-tolerant species, particularly those with seed dormancy (44).
The common gardens used in this study were established in areas within a contiguous forest that differ in edaphic characteristics, local canopy composition, and forest ages (SI Appendix, Fig. S1). Even though soil microbial communities can be strongly influenced by edaphic properties (45–48) and may exhibit spatial structure (49, 50), cultivable communities of seed-associated fungi were strikingly consistent across gardens. Importantly, this study was designed to avoid conspecific crowns, thus providing seeds with diverse neighborhoods of co-occurring species. Most seeds of tropical trees are dispersed naturally near maternal crowns (32, 33), such that the host affinity demonstrated here should be even more pronounced when seeds fall near conspecific trees.
Although gardens were established at the same time, seeds of different species were buried at different times of year depending on seed availability. Thus, dates of burial paralleled natural timing of seed production and dispersal. In this context, the effects of burial duration on seed-associated fungal communities were detectable but relatively small. Soil microbial communities can be shaped by environmental factors such as soil water content (51), which change seasonally in this forest (52). More than 50% of OTU were found only once, suggesting variation in time or space among the diverse fungi that share the capacity to infect seeds. However, we found no evidence of an interaction of tree species and burial duration in the diversity of fungi in seeds, implying that the most prevalent seed-associated fungi may remain relatively abundant in these soils over time. Several common genotypes of Fusarium observed here were found among seed-associated fungi at the same site ∼10 y earlier (ref. 38; see also ref. 53). Lasiodiplodia and Trichoderma/Hypocrea also were detected previously as seed-associated fungi at this site (38, 54). Continued prevalence in local soils is consistent with the potential for evolution of specificity in fungal–seed interactions (see ref. 34), even in the context of highly diverse communities. More generally, studies of microbial communities consistently report a high prevalence of rare taxa. We focused on fungi that were sufficiently abundant to be accessible for ecological analyses: by restricting analyses to the most common OTU, we worked with a thoroughly sampled community, focused on genera that are important components of seed-associated fungal communities, and identified strains of interest for inoculation trials.
In most cases considered here, fungi associated with viable or inviable seeds of one species were associated with the opposite viability class in other species. This suggests the potential for strains associated with mortality in some host species to have neutral or positive effects on other host species. Inoculation experiments confirmed that the outcomes of seed–fungal interactions were partner-dependent: fungi differed in their effects on seed germination and survival for individual plant species. Partner-dependent outcomes also are common in interactions between fungi or fungus-like organisms and seedlings of tropical trees (22, 55). Together, such partner-specific interactions are consistent with one parameter of effective specialization (sensu ref. 40), whereby polyphagous natural enemies contribute to processes relevant to plant diversity through host-specific effects relevant to survival. Such interactions might be expected to evolve readily if, as suggested by our experiment, associations between focal species are relatively consistent over ecological time and space.
Overall, the broad framework of effective specialization, central to integrating polyphagous natural enemies into the JC hypothesis, can be applied across multiple life stages and plant strategies to encompass the diverse fungi with which plant propagules and juvenile plants interact. Together these perspectives provide insight into the powerful effects of fungi in shaping forest dynamics in the tropics, thus defining the structure and composition of Earth’s most diverse plant communities.
Methods
We established five common garden plots (each 9 × 15 m) in a lowland tropical forest at Barro Colorado Island, Panama (for soil types and site descriptions, see refs. 56 and 57). Gardens were located under closed-canopy forest in multiple forest and soil types with different slope and aspect, and averaged 800 m apart (SI Appendix, Fig. S1). No adults of the study species occurred within 20 m of the garden edges.
We used seeds of nine species of pioneer trees that remain viable for extended time in the soil seed bank (Annona spraguei, Apeiba membranacea, Cecropia insignis, Cochlospermum vitifolium, Ficus insipida, Luehea seemannii, Ochroma pyramidale, Trema micrantha “black,” and Trema micrantha “brown”; SI Appendix, Table S1). From February 2012 to September 2013, we collected fresh, mature fruits from tree canopies or freshly fallen seeds from at least three trees (i.e., maternal sources) per species at Barro Colorado Nature Monument and Soberanía National Park (∼9.2°N, 79.8°W; 120–160 m above sea level). Seed processing is described in SI Appendix, SI Methods. Conspecific seeds from 3 to 12 maternal sources were pooled, mixed thoroughly, and partitioned into sets of 45 seeds. Each set was mixed with 10 g of sterile forest soil (autoclaved at 121 °C for 2 h) and enclosed in a nylon mesh bag (pore size = 0.2 mm). Each nylon bag was enclosed in aluminum mesh (mesh size = 2 mm) to exclude seed predators. Sixteen bags per plant species were distributed among four subplots per garden, where they were buried 2 cm beneath the soil surface and 40 cm apart. A total of 720 seed bags were retrieved after 1, 3, 6, and 12 mo of burial (i.e., four replicates per species, nine plant species, four burial durations, and five common gardens).
Seeds were removed from bags within 2 h of collection and surface-sterilized as described in ref. 38. Half of each seed was placed on 2% malt extract agar (MEA) in an individual 1.5-mL microcentrifuge tube for fungal isolation (38), with emergent cultures characterized by molecular analysis and vouchered as living strains (SI Appendix, SI Methods). The other half was used for viability assessment via tetrazolium staining, such that infection and viability were scored for each seed (ref. 58 and SI Appendix, SI Methods). We used the same approach to process 200 fresh seeds (i.e., seeds that were not buried) per species. In total, 8,335 seeds were examined, of which 6,535 were retrieved after burial. Representative seed halves that produced no evident fungal growth after >1 y were verified as lacking uncultivable fungi (SI Appendix, SI Methods).
Operational taxonomic units (OTU) were defined by 99% similarity in the Sanger clustering workflow of the Mobyle SNAP Workbench (ref. 59 and snap.hpc.ncsu.edu/cgi-bin/mobyle/portal.py#welcome) based on previously published methods for clustering ITS-LSUrDNA sequence data (60). This OTU definition is consistent with conservative phylogenetic delimitation of seed-associated Ascomycota from Panama (61). Diversity was measured as Fisher’s alpha (FA), which is robust to variation in sample size and is appropriate given the relative abundance of OTU (ref. 62; see also ref. 38).
Communities were defined as sequenced fungal strains obtained from seeds of a given plant species, burial duration, garden, and viability classification, pooling seeds from all four replicates together. Given differences in isolation frequency, we analyzed both the entire dataset (nine plant species) and the four plant species represented by the largest sample of isolates. After singleton OTU were removed, hierarchical cluster analysis was performed for each dataset using the Morisita–Horn dissimilarity index (based on abundance data) and the Jaccard dissimilarity index (presence–absence data). We used a multivariate GLM to test the extent to which each factor (i.e., plant species, burial duration, burial location, or seed viability) was relevant to fungal community structure. This approach addresses limitations of distance-based approaches such as PERMANOVA, which do not account for the mean–variance relationship (63). We used a negative-binomial GLM based on sequence count data and a binomial GLM based on presence–absence data, performed with the R package mvabund (64, 65). These analyses focused only on OTU for which at least nine isolates (full dataset) or four isolates (four-species dataset) were obtained, as rarer OTU could not, by definition, be present in all plant species. Interactions between plant species and fungal OTU were visualized using the R package bipartite (66) and NMDS (three dimensions, Morisita–Horn index), and variation-partitioning analyses were performed using the R package vegan (67). These analyses were complemented by examination of similarity indices (Morisita–Horn and Jaccard), which were calculated using nonsingleton OTU in Estimates v. 9.0 (68), logit-transformed, and compared using linear models.
We determined whether OTU associated with a given viability class in one species were associated with seeds of the opposite viability class in other species in two steps. First, we performed a GLM with quasibinomial errors to determine differences in viability associated with plant species and fungal OTU. We focused on the four best-sampled plant species and the 15 most common OTU. Plant species was treated as a random effect, such that significant effects of OTU corresponded to variation in viability in different species. Second, we evaluated 13 of the 15 most common OTU, which each occurred in ≥2 host species. For each OTU we selected one host species at random and scored the prevalence of isolates from viable vs. inviable seeds. We then evaluated whether the same OTU in the other host species followed the same or opposite pattern (score = 1 or 0, respectively). This yielded 23 scores, of which 69.6% were scored as zero. A one-tailed test was used to compare the mean of all scores against the null (mean = 1). The overall mean (0.30, 95% CI = 0.10–0.51) differed significantly from the null (P < 0.001).
For each OTU we used host range (i.e., the number of plant species from which the OTU was isolated) and potential virulence (i.e., proportion of seeds from which the OTU was isolated that were inviable; SI Appendix, Table S12) to select four fungal strains with contrasting characteristics for inoculation experiments (SI Appendix, SI Methods). Effects on seed germination and viability were tested using a generalized linear mixed-effects model (GLMM) fit by maximum likelihood (Laplace Approximation) with a binomial error family, using the R package lme4 (69). Plant species, fungal isolate, and treatment (surface-sterilized or not) were coded as fixed effects and replicates as random effects. In some cases the number of treated seeds that germinated exceeded the number of control seeds that germinated. Therefore, the response variable was defined as the proportion of germinated seeds normalized by the maximum value of the proportion of seeds that germinated in the controls. Surface sterilization of seeds after inoculation and incubation did not affect germination or viability (SI Appendix, Table S10; χ2 = 0.01, P = 0.938; and χ2 = 0.12, P = 0.732, respectively).
Supplementary Material
Acknowledgments
We thank the Smithsonian Tropical Research Institute for facilities, logistical support, and permission to conduct the project. We especially thank A. Ndobegang, C. Delevich, A. Pérez, and D. Roche for essential assistance in the field and laboratory, and J. Shaffer, I. Quintero, A. Robison, C. Czekala, and K. Arendt for contributing to field- and laboratory work. This research was funded by NSF Grants DEB-1120205 (to J.W.D. and A.S.D.) and DEB-1119758 (to A.E.A.) and by the College of Agriculture and Life Sciences at The University of Arizona (A.E.A.). P.-C.Z. was supported by NSF Grant DEB-1120205 and his work was partially supported by a grant from the Simons Foundation (429440, WTW).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Sequences used in this study are deposited in GenBank under accession numbers KU977534–KU978121 and KY775762–KY776423.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706324114/-/DCSupplemental.
References
- 1.Wright JS. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia. 2002;130:1–14. doi: 10.1007/s004420100809. [DOI] [PubMed] [Google Scholar]
- 2.Bever JD, Mangan SA, Alexander HM. Maintenance of plant species diversity by pathogens. Annu Rev Ecol Evol Syst. 2015;46:305–325. [Google Scholar]
- 3.Comita LS, et al. Testing predictions of the Janzen-Connell hypothesis: A meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J Ecol. 2014;102:845–856. doi: 10.1111/1365-2745.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 5.Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Den Boer PJ, Gradwell GR, editors. Dynamics of Populations. Cent for Agric Publ and Doc; Wageningen, The Netherlands: 1971. pp. 298–312. [Google Scholar]
- 6.Clark DA, Clark DB. Spacing dynamics of a tropical rain forest tree: Evaluation of the Janzen-Connell model. Am Nat. 1984;124:769–788. [Google Scholar]
- 7.Harms KE, Wright SJ, Calderón O, Hernández A, Herre EA. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 2000;404:493–495. doi: 10.1038/35006630. [DOI] [PubMed] [Google Scholar]
- 8.Bell T, Freckleton RP, Lewis OT. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol Lett. 2006;9:569–574. doi: 10.1111/j.1461-0248.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 9.Zheng W, He H, Xiao L, Yu S. Testing pathogen host specificity: A reciprocal field experiment in two types of tropical forest on Hainan, China. J Trop Ecol. 2013;29:541–549. [Google Scholar]
- 10.Liu X, Etienne RS, Liang M, Wang Y, Yu S. Experimental evidence for an intraspecific Janzen-Connell effect mediated by soil biota. Ecology. 2015;96:662–671. doi: 10.1890/14-0014.1. [DOI] [PubMed] [Google Scholar]
- 11.Stump SM, Chesson P. Distance-responsive predation is not necessary for the Janzen-Connell hypothesis. Theor Popul Biol. 2015;106:60–70. doi: 10.1016/j.tpb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Liang M, et al. Adult trees cause density-dependent mortality in conspecific seedlings by regulating the frequency of pathogenic soil fungi. Ecol Lett. 2016;19:1448–1456. doi: 10.1111/ele.12694. [DOI] [PubMed] [Google Scholar]
- 13.Freckleton RP, Lewis OT. Pathogens, density dependence and the coexistence of tropical trees. Proc Biol Sci. 2006;273:2909–2916. doi: 10.1098/rspb.2006.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangan SA, et al. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature. 2010;466:752–755. doi: 10.1038/nature09273. [DOI] [PubMed] [Google Scholar]
- 15.Leigh EG, Jr, Wright SJ, Herre EA, Putz FE. The decline of tree diversity on newly isolated tropical islands: A test of a null hypothesis and some implications. Evol Ecol. 1993;7:76–102. [Google Scholar]
- 16.Terborgh J. Enemies maintain hyperdiverse tropical forests. Am Nat. 2012;179:303–314. doi: 10.1086/664183. [DOI] [PubMed] [Google Scholar]
- 17.Hubbell SP. Seed predation and the coexistence of tree species in tropical forests. Oikos. 1980;35:214–229. [Google Scholar]
- 18.Novotny V, et al. Low host specificity of herbivorous insects in a tropical forest. Nature. 2002;416:841–844. doi: 10.1038/416841a. [DOI] [PubMed] [Google Scholar]
- 19.Paine CET, Beck H. Seed predation by neotropical rain forest mammals increases diversity in seedling recruitment. Ecology. 2007;88:3076–3087. doi: 10.1890/06-1835.1. [DOI] [PubMed] [Google Scholar]
- 20.Theimer TC, Gehring CA, Green PT, Connell JH. Terrestrial vertebrates alter seedling composition and richness but not diversity in an Australian tropical rain forest. Ecology. 2011;92:1637–1647. doi: 10.1890/10-2231.1. [DOI] [PubMed] [Google Scholar]
- 21.Sedio BE, Ostling AM. How specialised must natural enemies be to facilitate coexistence among plants? Ecol Lett. 2013;16:995–1003. doi: 10.1111/ele.12130. [DOI] [PubMed] [Google Scholar]
- 22.Bagchi R, et al. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature. 2014;506:85–88. doi: 10.1038/nature12911. [DOI] [PubMed] [Google Scholar]
- 23.Kurten EL, Carson WP. Do ground-dwelling vertebrates promote diversity in a neotropical forest? Results from a long-term exclosure experiment. Bioscience. 2015;65:862–870. doi: 10.1093/biosci/biv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Augspurger CK, Kelly CK. Pathogen mortality of tropical tree seedlings: Experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia. 1984;61:211–217. doi: 10.1007/BF00396763. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert GS, Webb CO. Phylogenetic signal in plant pathogen-host range. Proc Natl Acad Sci USA. 2007;104:4979–4983. doi: 10.1073/pnas.0607968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bever JD, Platt TG, Morton ER. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol. 2012;66:265–283. doi: 10.1146/annurev-micro-092611-150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becklund KK, Kinkel LL, Powers JS. Landscape-scale variation in pathogen-suppressive bacteria in tropical dry forest soils of Costa Rica. Biotropica. 2014;46:657–666. [Google Scholar]
- 28.Liang M, et al. Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology. 2015;96:562–574. doi: 10.1890/14-0871.1. [DOI] [PubMed] [Google Scholar]
- 29.Record S, Kobe RK, Vriesendorp CF, Finley AO. Seedling survival responses to conspecific density, soil nutrients, and irradiance vary with age in a tropical forest. Ecology. 2016;97:2406–2415. doi: 10.1002/ecy.1458. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Hamel C, Vujanovic V, Gan Y. Nontarget effects of foliar fungicide application on the rhizosphere: Diversity of nifH gene and nodulation in chickpea field. J Appl Microbiol. 2012;112:966–974. doi: 10.1111/j.1365-2672.2012.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: From genes to the field. J Exp Bot. 2012;63:3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 32.Schupp EW, Howe HF, Augspurger CK, Levey DJ. Arrival and survival in tropical treefall gaps. Ecology. 1989;70:562–564. [Google Scholar]
- 33.Nathan R, Muller-Landau HC. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol. 2000;15:278–285. doi: 10.1016/s0169-5347(00)01874-7. [DOI] [PubMed] [Google Scholar]
- 34.Dalling JW, Davis AS, Schutte BJ, Arnold AE. Seed survival in soil: Interacting effects of predation, dormancy and the soil microbial community. J Ecol. 2011;99:89–95. [Google Scholar]
- 35.Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Buylla ER, Martínez-Ramos M. Seed bank versus seed rain in the regeneration of a tropical pioneer tree. Oecologia. 1990;84:314–325. doi: 10.1007/BF00329755. [DOI] [PubMed] [Google Scholar]
- 37.Dalling JW, Swaine MD, Garwood NC. Dispersal patterns and seed bank dynamics of pioneer trees in moist tropical forest. Ecology. 1998;79:564–578. [Google Scholar]
- 38.Gallery RE, Dalling JW, Arnold AE. Diversity, host affinity, and distribution of seed-infecting fungi: A case study with Cecropia. Ecology. 2007;88:582–588. doi: 10.1890/05-1207. [DOI] [PubMed] [Google Scholar]
- 39.Gallery RE, Moore DJP, Dalling JW. Interspecific variation in susceptibility to fungal pathogens in seeds of 10 tree species in the neotropical genus Cecropia. J Ecol. 2010;98:147–155. [Google Scholar]
- 40.Benítez M-S, Hersh MH, Vilgalys R, Clark JS. Pathogen regulation of plant diversity via effective specialization. Trends Ecol Evol. 2013;28:705–711. doi: 10.1016/j.tree.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Gripenberg S, et al. Seed polyphenols in a diverse tropical plant community. J Ecol. June 30, 2017 doi: 10.1111/1365-2745.12814. [DOI] [Google Scholar]
- 42.Fricke EC, Wright SJ. The mechanical defence advantage of small seeds. Ecol Lett. 2016;19:987–991. doi: 10.1111/ele.12637. [DOI] [PubMed] [Google Scholar]
- 43.Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- 44.Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Sci Res. 2004;14:1–16. [Google Scholar]
- 45.Lauber CL, Strickland MS, Bradford MA, Fierer N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem. 2008;40:2407–2415. [Google Scholar]
- 46.Fierer N, et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci USA. 2012;109:21390–21395. doi: 10.1073/pnas.1215210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barberán A, et al. Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol Lett. 2015;18:1397–1405. doi: 10.1111/ele.12536. [DOI] [PubMed] [Google Scholar]
- 48.Prober SM, et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol Lett. 2015;18:85–95. doi: 10.1111/ele.12381. [DOI] [PubMed] [Google Scholar]
- 49.Barberán A, et al. Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol Lett. 2014;17:794–802. doi: 10.1111/ele.12282. [DOI] [PubMed] [Google Scholar]
- 50.Lou Y, et al. An affinity-effect relationship for microbial communities in plant-soil feedback loops. Microb Ecol. 2014;67:866–876. doi: 10.1007/s00248-013-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fierer N, Schimel JP, Holden PA. Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem. 2003;35:167–176. [Google Scholar]
- 52.Goldstein G, et al. Evaluating aspects of water economy and photosynthetic performance with stable isotopes from water and organic matter. In: Mulkey SS, Chazdon RL, Smith AP, editors. Tropical Forest Plant Ecophysiology. Chapman & Hall; New York: 1996. pp. 244–267. [Google Scholar]
- 53.Shaffer JP, et al. Diversity, specificity, and phylogenetic relationships of endohyphal bacteria in fungi that inhabit tropical seeds and leaves. Front Ecol Evol. 2016;4:116. [Google Scholar]
- 54.Kluger CG, et al. Host generalists dominate fungal communities associated with seeds of four neotropical pioneer species. J Trop Ecol. 2008;24:351–354. [Google Scholar]
- 55.Augspurger CK, Wilkinson HT. Host specificity of pathogenic Pythium species: Implications for tree species diversity. Biotropica. 2007;39:702–708. [Google Scholar]
- 56.Leigh EG., Jr . Tropical Forest Ecology: A View from Barro Colorado Island. Oxford Univ Press; New York: 1999. [Google Scholar]
- 57.Baillie I, Elsenbeer H, Barthold F, Grimm R, Stallard R. 2006. A semi-detailed soil survey of Barro Colorado Island (Smithsonian Tropical Research Institute, Panama, Republic of Panama)
- 58.Zalamea P-C, Sarmiento C, Arnold AE, Davis AS, Dalling JW. Do soil microbes and abrasion by soil particles influence persistence and loss of physical dormancy in seeds of tropical pioneers? Front Plant Sci. 2015;5:799. doi: 10.3389/fpls.2014.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monacell JT, Carbone I. Mobyle SNAP Workbench: A web-based analysis portal for population genetics and evolutionary genomics. Bioinformatics. 2014;30:1488–1490. doi: 10.1093/bioinformatics/btu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.U’Ren JM, Lutzoni F, Miadlikowska J, Laetsch AD, Arnold AE. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am J Bot. 2012;99:898–914. doi: 10.3732/ajb.1100459. [DOI] [PubMed] [Google Scholar]
- 61.U’Ren JM, et al. Diversity and evolutionary origins of fungi associated with seeds of a neotropical pioneer tree: A case study for analysing fungal environmental samples. Mycol Res. 2009;113:432–449. doi: 10.1016/j.mycres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Fisher RA, Corbet AS, Williams CB. The relation between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol. 1943;12:42–58. [Google Scholar]
- 63.Warton DI, Wright ST, Wang Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol. 2012;3:89–101. [Google Scholar]
- 64.Wang Y, Naumann U, Wright ST, Warton DI. mvabund- an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol. 2012;3:471–474. [Google Scholar]
- 65.R Core Team 2015. R: A Language and Environment for Statistical Computing (R Found for Statist Comput, Vienna), Version 3.2.3.
- 66.Dormann CF, Fründ J, Blüthgen N, Gruber B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol J. 2009;2:7–24. [Google Scholar]
- 67.Oksanen J, et al. 2016 Vegan: Community Ecology Package, R package Version 2.3-4. Available at https://CRAN.R-project.org/package=vegan. Accessed July 24, 2017.
- 68.Colwell RK. 2013 EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9.1.0. Available at purl.oclc.org/estimates. Accessed July 24, 2017.
- 69.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software. 2015;67:1–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.