Significance
We report an Early Cretaceous bird from 120 My ago that has a completely fused carpometacarpus and pelvis, pushing back the date for these avian traits by over 40 My. We suggest that this taxon grew more rapidly than other basal birds, but the degree of bone fusion is not causally linked with growth pattern in primitive birds. We hypothesize that the surprisingly high degree of bone fusion in basal birds may have been environmentally induced pertinent to flight or alternatively resultant from genetic modifications. Future developmental studies focusing on the development of bone fusion are needed to test these hypotheses and illustrate how the skeletal system of living birds achieves its modern shape.
Keywords: bone fusion, bird, Cretaceous, histology, development
Abstract
Bird skeletons exhibit remarkable modifications that allow for flight. The most distinguishable features are the fusion of the bones in the hand, feet, and pelvis into composite rigid and bony structures. However, the historical origins of these avian bone fusions remain elusive because of the rarity of transitional fossils and developmental studies on modern birds. Here, we describe an Early Cretaceous bird (120 Mya) that has fully fused alular-major metacarpals and pelvis. We discuss the manus and pelvis fusions across Paravian phylogeny and demonstrate that these features evolved independently across nonavian theropods, Enantiornithes, and Ornithuromorpha. The fusions of these bones are rare in known nonavian theropods and Early Cretaceous birds but are well established among Late Cretaceous and modern birds, revealing a complicated evolution pattern unrecognized previously. We posit that the developments of bone fusion were polymorphic close to the origin of birds, resulting in the varying degrees of fusion in Paraves. However, that development polymorphism appears to be fundamentally restricted along the line to modern birds by the Late Cretaceous, where all birds have a completely fused manus and pelvis. Such changes likely correspond to a refinement of flight capability. Alternatively, the degree of bone fusion in this primitive bird may have been related to modifications in genes or developmental paths. Future studies and fossil discoveries are required to clarify these hypotheses and pinpoint the developmental pathways involving the bone fusions in early avian evolution through to their modern pattern.
Enantiornithes, arguably the most diverse clade of Mesozoic birds, have been reported from every continent except Antarctica (1, 2). More than half of the known global diversity of the Enantiornithes were recovered from the Early Cretaceous Jehol Biota, northeastern China (1), with the bird-bearing horizons spanning from 120 to 131 Mya (3, 4). The numerous complete and articulated Jehol enantiornithines, some of which preserve feathers, stomach contents, and even traces of soft tissues (5, 6), have significantly increased our understanding about the early history and biology of this avian group. Here, we describe an enantiornithine specimen (Fig. 1 A and B), referrable to Pterygornis, from this biota. The holotype and previously only known specimen of Pterygornis is incomplete, particularly lacking the cranial and pelvic bones (7), but those are exquisitely preserved in this referred specimen, allowing us to further reconstruct the skeletal morphology for this taxon and determine its phylogenetic position. We performed osteohistological analysis on the referred specimen using a thin section of the long bones and suggests that Pterygornis could reach skeletal maturity in approximately 1 y, a growth strategy that most living birds exhibit (8). In contrast, the growth rate is much slower in most other enantiornithines, and it took them several years to reach adulthood (9). Pterygornis shows fully ankylosed alular-major metacarpals and pelvis, distinguishable from most known Early Cretaceous birds. The developmental mechanism underpinning such rare bone fusion in early birds remains largely unexplored.
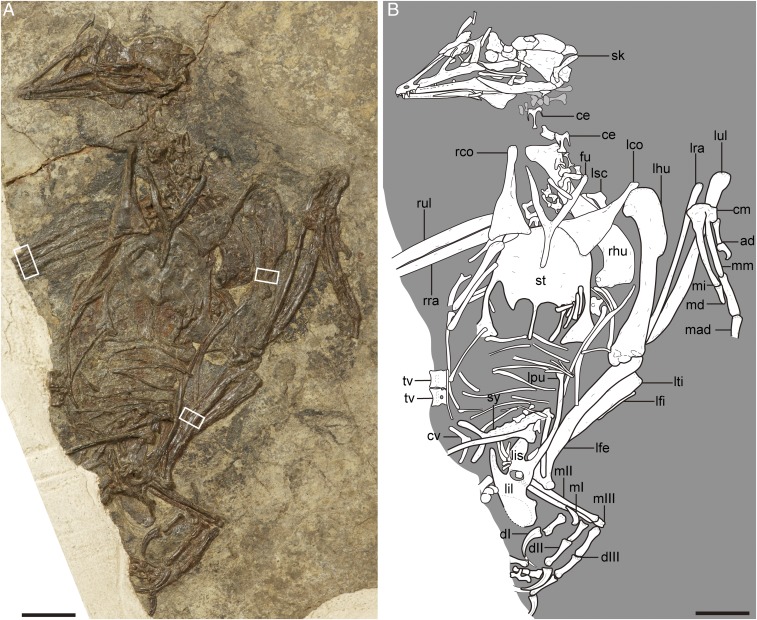
Fig. 1.
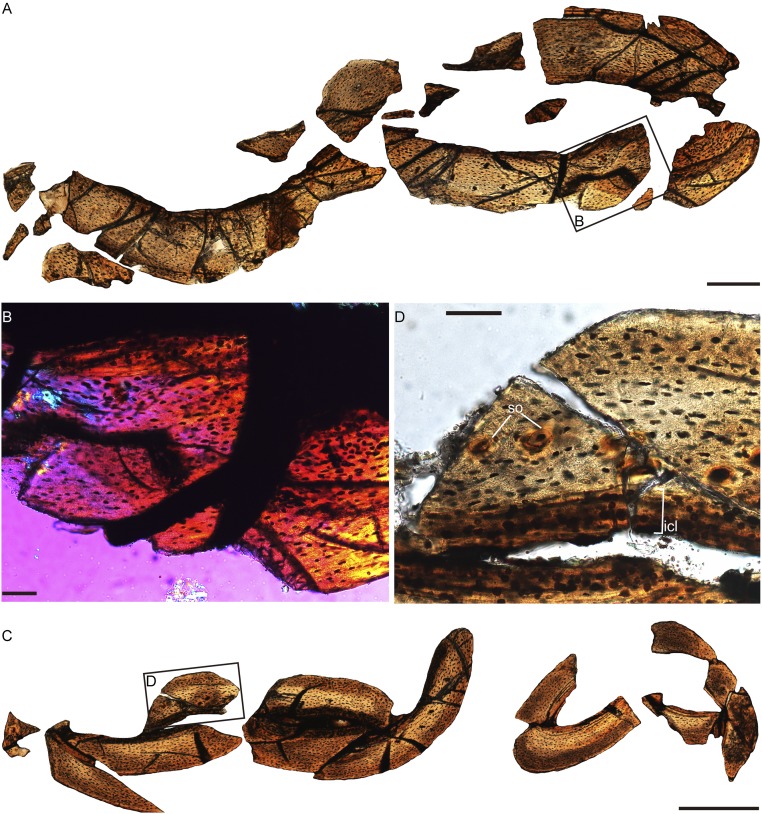
Photograph (A) and interpretative line drawing (B) of the referred specimen of P. dapingfangensis, IVPP V16363. The white boxes in A denote the positions of the bone samples used in the histological analyses. ad, alular digit; ce, cervical vertebra; cm, carpometacarpus; cv, caudal vertebra; dI–III, digit I–III; fu, furcula; lco, left coracoid; lfe, left femur; lfi, left fibula; lhu, left humerus; lil, left ilium; lis, left ischium; lpu, left pubis; lra, left radius; lsc, left scapula; lti, left tibiotarsus; lul, left ulna; mI–III, metatarsal I–III; mad, major digit; md, minor digit; mi, minor metacarpal; mm, major metacarpal; rco, right coracoid; rhu, right humerus; rra, right radius; rul, right ulna; sk, skull; st, sternum; sy, synsacrum; tv, thoracic vertebra. (Scale bar, 10 mm.)
Results
Description and Comparison.
The specimen, Institute of Vertebrate Paleontology and Paleoanthropology (IVPP) V16363 (Table S1), can be referred to as the enantiornithine Pterygornis dapingfangensis in having the following diagnostic characteristics of this taxon: The lateral margin of the coracoid is strongly convex, making the proximal end of the coracoid medially curved; the sternum has an external rostral spine and a pair of craniolateral processes; and the lateral trabeculae of the sternum are robust, bearing large fan-shaped caudal expansions that extend as far caudally as the xiphoid process (7). Descriptions of the skull, forelimb, and the pelvic girdle are presented in the main text, and other body regions are provided in SI Text.
Table S1.
Selected measurements of P. dapingfangensis IVPP V16363
| Element | Length, mm |
| Skull | 32.9 |
| Humerus | 33.5 |
| Ulna | 35.4* |
| Radius | 36.5 |
| Coracoid | 17.5 |
| Carpometacarpus | 14.5 |
| Alular digit-1 | 5.1 |
| Alular digit-2 | 2.5 |
| Major digit-1 | 8.8 |
| Ilium length | 16.9 |
| Pubis length | 21.2 |
| Tibiotarsus | 31.2* |
| Pedal digit I-1, 2 | 5.1, 75 |
| Pedal digit II-1, 2, 3 | 5.0, 6.4, 8.3* |
| Pedal digit III-1, 2, 3, 4 | 7.0, 6.3, 6.3, 4.1* |
Asterisk indicates estimation.
The frontal processes of the premaxillae project caudodorsally, forming a 19° angle with the maxillary processes (Fig. 2 A and B). The most striking feature is that the premaxillary corpus is pierced by a foramen on the cranioventral corner of the external naris (Fig. 2B). The foramen is oval in shape with the long axis craniocaudally oriented, and it is larger than the crown of the premaxillary teeth, precluding it from simply being a nutrient foramen. In addition, the smooth margin of the foramen weakens the possibility of this being a preservational artifact. No comparable structure, to our knowledge, has been reported in stem or crown birds (10, 11), or theropod dinosaurs (12). Therefore, it is considered as an autapomorphy here. The premaxilla has five teeth (Fig. 2B), but most other enantiornithines bear four (4). The jugal process of the maxilla extends caudal to the ventral process of the lacrimal, contributing to the cranioventral corner of the orbital. At least three maxillary teeth are present on each side. The lacrimal is T-shaped, and its ventral process contacts the maxilla medially. The parietals are unfused to each other medially. The otic process of the quadrate is not differentiated into the squamosal and prootic captitula. A mandibular symphysis is absent. The meckelian groove is not completely covered by the splenial. The groove is deep and terminates just short of the rostral end of the dentary. The surangular bears a well-developed retroarticular process.
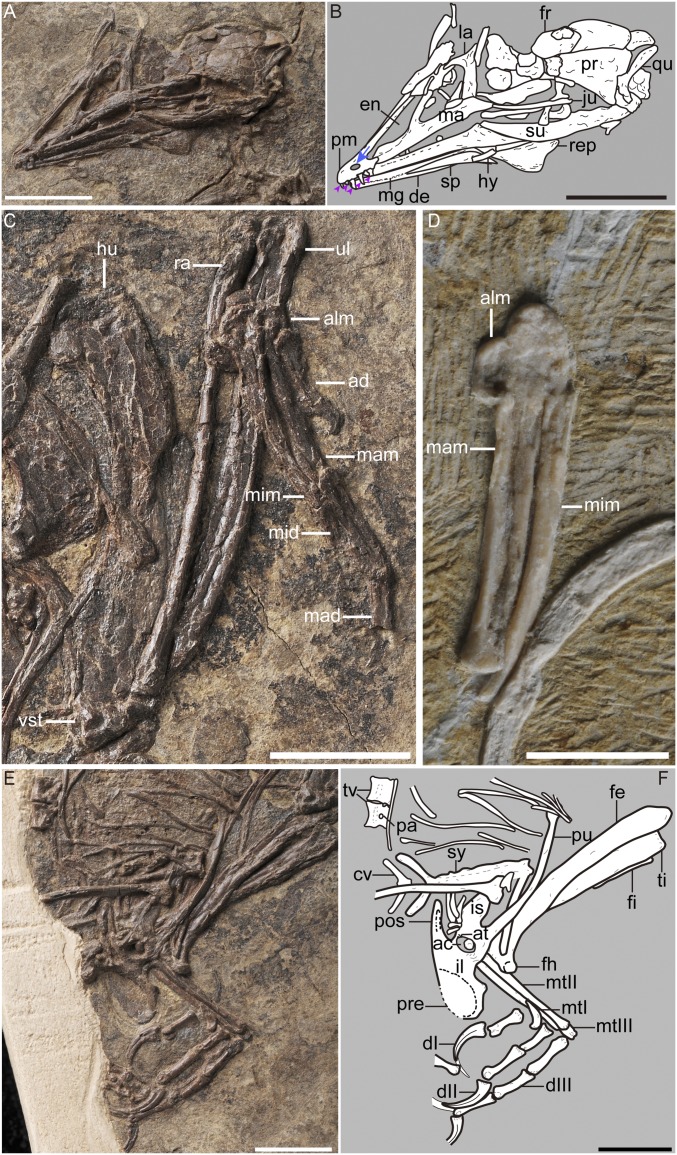
Fig. 2.
Detailed morphologies of P. dapingfangensis showing the fused manus and pelvis. (A and B) Skull in left lateral view of IVPP V16363. (C) Left forelimb of IVPP V16363. (D) Left hand of IVPP V20729 in dorsal view. (E and F) Pelvis and hindlimb of IVPP V16363. ac, acetabulum; ad, alular digit; alm, alular metacarpal; at, antitrochanter; cv, caudal vertebra; dI–III, digit I–III; de, dentary; en, external nasal; fe, femur; fh, femoral head; fi, fibula; fr, frontal; hu, humerus; hy, hyoid; il, ilium; is, ischium; ju, jugal; la, lacrimal; ma, maxilla; mad, major digit; mam, major metacarpal; mg, meckelian groove; mid, minor digit; mim, minor metacarpal; mtI–III, metatarsal I–III; na, nasal; pa, parapophysis; pm, premaxilla; pos, postacetabular wing of ilium; pr, parietal; pre, preacetabular wing of ilium; pu, pubis; qu, quadrate; ra, radius; rep, retroarticular process; sp, splenial; su, surangular; sy, synsacrum; ti, tibiotarsus; tv, thoracic vertebra; ul, ulna; vs.t, ventral supracondylar tubercle. The arrow indicates the presences of a foramen on the premaxilla. The five arrowheads denote the five premaxillary teeth. [Scale bar, 10 mm (A–C, E, and F) and 5 mm (D).]
The bicipital crest of the humerus bears a distinct pit-shaped fossa on its craniodistal surface (Fig. 2C and Fig. S1B). Previous studies hypothesized that the fossa provided the attachment for an unknown muscle (2). Wang et al. (13) further argued that it served as the insertion site of the scapulohumeralis muscle, which originates from the lateral surface of the scapula and is responsible for the retraction of the humerus (14). The dorsal condyle is elliptical and strongly inclined dorsally. The ventral condyle is nearly transversely oriented and projects less cranially than the dorsal condyle. Above the dorsoventral border of the ventral condyle, there is a large process that projects as cranially as the dorsal condyle (Fig. 2D and Fig. S1 A and B). This process is probably homologous to the ventral supracondylar tubercle of modern birds (10). No similar structure is observed in other enantiornithines, basal ornithuromorphs, and more primitive birds (2, 15, 16). In modern birds, the ventral supracondylar tubercle provides the attachment for the ventral collateral ligament of the elbow joint (17). The ulna is bowed proximally and straight distally, and it is more robust than the radius (Fig. 2C). The alular metacarpal is completely fused with the major metacarpal throughout its length (Fig. 2 C and D). In contrast, these two bones are at best only proximally fused in other Early Cretaceous birds (7, 16, 18). As in other enantiornithines, the minor metacarpal is only fused with the major metacarpal proximally, and it extends further distally than the major metacarpal. The alular digit is reduced, terminating proximal to the distal end of the major metacarpal. The minor digit only preserves one phalanx, and the manual phalangeal formula is 2–3–1.
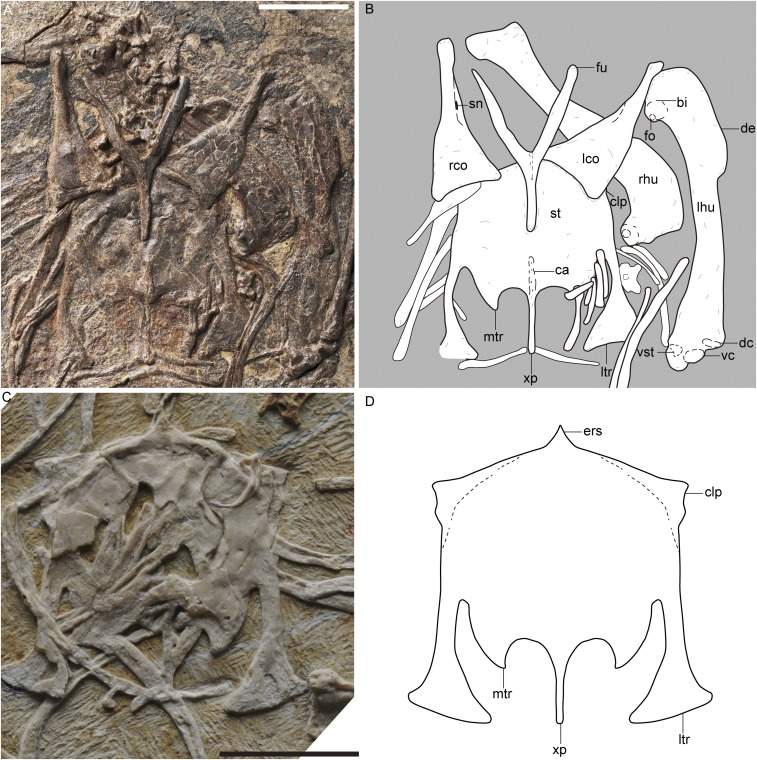
Fig. S1.
Pectoral girdle and sternum of P. dapingfangensis. (A) Photograph and (B) line drawing of IVPP V16363. (C) Holotype, IVPP V20729. (D) Reconstructed sternal morphology. bi, bicipital crest; ca, carina; clp, craniolateral process; dc, dorsal condyle; de, deltopectoral crest; ers, external rostral spine; fo, fossa; fu, furcula; lco, left coracoid; lhu, left humerus; ltr, lateral trabecula; mtr, medial trabecula; rco, right coracoid; rhu, right humerus; sn, supracoracoidal nerve foramen; st, sternum; vc, ventral condyle; vs.t, ventral supracondylar tubercle; xp, xiphoid process. [Scale bar, 10 mm (A and C); line drawings (B and D) are not to scale.]
The pelvic bones, absent in the holotype, are well preserved in IVPP V16363 (Fig. 2 E and F). Surprisingly, the ilium, ischium, and pubis are completely fused with one another, a feature absent in all of the other Early Cretaceous enantiornithines, with the exceptions of Qiliania and Piscivorenantiornis (19), and that absence occurs in many specimens that are widely regarded as fully adult (1, 4, 13). Therefore, the absence of fusion cannot be attributed exclusively to ontogenetic variations given so many examples. An antitrochanter is weakly developed on the caudodorsal corner of the acetabulum. The pubes are caudally directed, forming a 57° angle with the long axis of the ilium that is larger than in some enantiornithines—for example, Qiliania (28°) and Linyiornis (37°). The distal end of the pubis flares into a pubic boot. The lateral surface of the ischium is essentially flat. We performed comprehensive phylogenetic analyses using combined information from both the holotype and IVPP V16363, and Pterygornis is recovered in a derived position within the Enantiornithes (SI Text and Fig. S2).
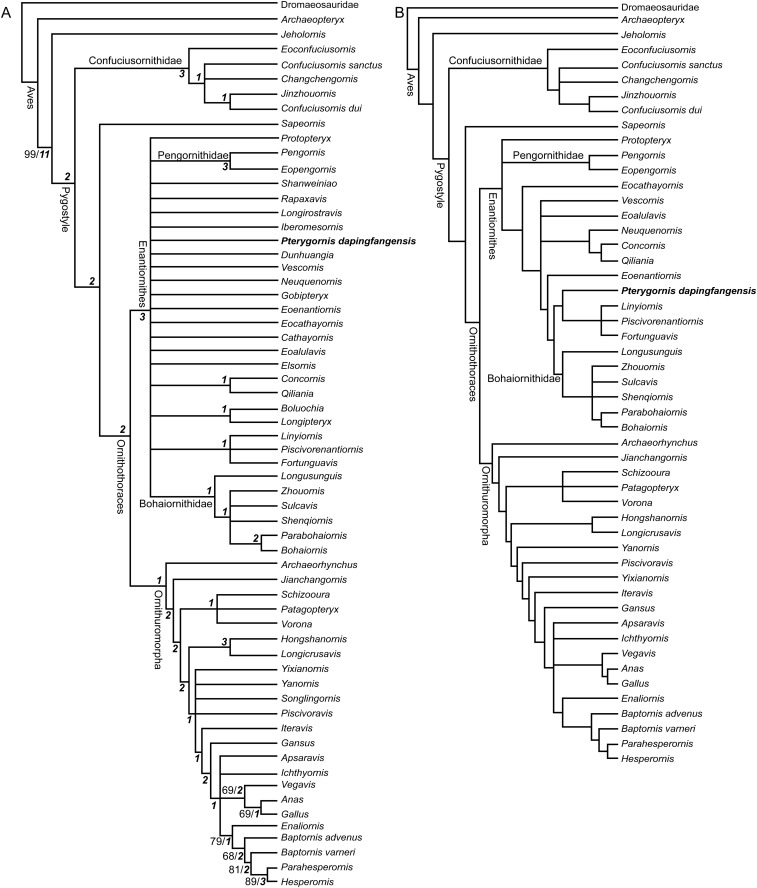
Fig. S2.
Cladogram of Mesozoic birds showing the phylogenetic position of P. dapingfangensis. (A) Strict consensus tree (1,087 steps; Consistency index = 0.338; Retention index = 0.667). (B) Reduced consensus tree when the most unstable taxa are removed. The Bootstrap and Bremer values are indicated near the corresponding nodes in normal and bold italic formats, respectively.
Bone Histological Description.
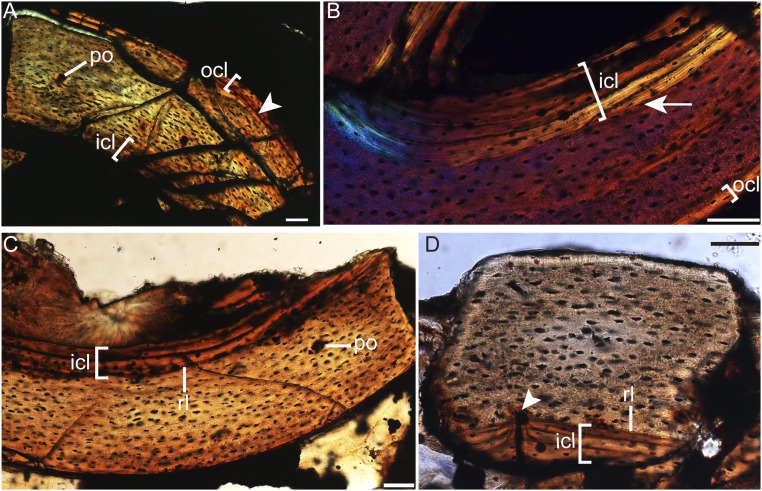
The humeral cross-section is crushed (Figs. S3A and S4 A and B). The cortex comprises parallel-lamellar bone tissue with predominantly longitudinal-oriented canals, although oblique canals are observed. A thin layer of endosteal-derived lamellar bone (the inner circumferential layer, ICL) occurs, riming the medullary cavity (Fig. S3A). The osteocyte lacunae embedded in the bone matrix external to the ICL are flattened and well organized in parallel to the external margin, and their density decreases toward the periosteum (Figs. S3A and S4A). In some areas of the section, the osteocyte lacunae are globular and randomly dispersed (Fig. S4B). Where the periosteal margin of the bone wall is intact, a distinct line of arrested growth (LAG) is present and marks the onset of the deposition of lamellar bone with transversely oriented collagen fibers, designating the presence of an outer circumferential layer (OCL; Fig. S3A). The OCL is avascular and contains flattened osteocyte lacunae. No secondary osteons are visible (SI Text for histological description of the ulna and radius).
Fig. S3.
Osteohistology of P. dapingfangensis (IVPP V16363). (A) Left humerus under normal light. (B) Right radius under linear polarizer with λ-compensator. (C and D) Left femur under normal light. Arrowhead in A indicates the LAG that marks the presences of the OCL. The arrow in B indicates the reversal line that marks the presence of the ICL. The arrowhead in D marks where a secondary osteon interrupts the reversal line. icl, inner circumferential layer; ocl, outer circumferential layer; po, primary osteon; ri, reversal line. (Scale bar, 50 µm.)
Fig. S4.
Forelimb osteohistology of P. dapingfangensis, IVPP V16363. (A) General cross-section of the left humerus under normal light, with detailed features in B under linear polarizer with λ-compensator. (C) General cross-section of the right ulna and radius under normal light, with detailed features in D under normal light. icl, inner circumferential layer; so, secondary osteon. [Scale bars, 400 µm (A and C) and 50 µm (B and D).]
The femoral cortex is stratified into three layers, with a thick middle layer bounded internally by the ICL and externally by the OCL (Fig. S5). The ICL is relatively thick, measuring more than one quarter of the thickness of the preserved bone wall (Figs. S3C and S5). However, this proportion should be treated with caution because the external margin has been worn off to some extent. The ICL is delimited from the external bone tissue by a reversal line as in the radius (Fig. S3D). The middle layer is composed of parallel-lamellar bone tissue with longitudinally and reticular-oriented canals (Fig. S5B), although some areas exhibit fibro-lamellar bone tissue. Secondary osteons are developed. Serendipitously, the sampled cross-section happens to record the phase when a secondary osteon interrupts the reversal line (Fig. S3D). The osteocyte lacunae in the middle layer are flattened and organized parallel to the external margin, and this is most pronounced in areas close to the ICL and OCL. However, in the locally restricted fiber-lamellar bone tissues, the osteocyte lacunae are plump and randomly distributed (Fig. S5B).
Fig. S5.
Femur osteohistology of P. dapingfangensis, IVPP V16363. (A) General cross-section under normal light, with detailed microstructures (B and C) under higher magnifications. icl, inner circumferential layer; ocl, outer circumferential layer; rc, reticular canal. [Scale bars, 200 µm (A) and 50 µm (B and C).]
SI Text
Additional Description of IVPP V16363.
Systematic paleontology.
Aves Linnaeus, 1758; Ornithothoraces Chiappe, 1995; Enantiornithes Walker, 1981; P. dapingfangensis Wang et al. (7).
Holotype.
IVPP V20729, an incomplete and disarticulated specimen.
Referred material.
IVPP V16363, a partially complete and articulated specimen preserved on a single slab (Table S1).
Occurrence and horizon.
IVPP V16363 was unearthed from the Jiufotang Formation near the Lamadong Town, Jianchang City, Liaoning Province.
Emended diagnosis.
A small enantiornithine bird that is distinguishable from other Early Cretaceous enantiornithines in having the following autapomorphies: premaxilla with a foramen; five premaxillary teeth; surangular with cranioventrally sloping rostral end; humerus bearing ventral supracondylar tubercle; alular and major metacarpals fused throughout their length; and ilium, ischium, and pubis fused around the acetabulum. Pterygornis is further diagnosed in having a unique combination of the following features: proximal shaft of coracoid curved medially; sternum with external rostral spine and craniolateral processes; lateral trabeculae of sternum with large fan-shaped caudal expansions that project as far caudally as the xiphoid process; furcula with elongated hypocleidium that accounts for 70% the length of the clavicular ramus; and trochlea of metatarsal II with well-defined ginglymoid articular facet and articular furrow on its planar surface.
Description and comparison.
The cervical vertebrae are elongated, mediolaterally compressed, and their ventral surfaces are keeled (Fig. 1 and Fig. S1A). In none of the visible cervical centra is there any sign of lateral excavation. The thoracic vertebrae bear prominent lateral excavations. The parapophyses, lying dorsal to the lateral excavation, are placed in a midpoint position of the centra (Fig. 2 E and F), a synapomorphy of the Enantiornithes (2). The synsacrum is completely fused (Fig. 2 E and F). The centra of the sacral vertebrae are mediolaterally compressed, making the ventral surface convex without the groove present in Rapaxavis. A single vertebra can be identified caudal to the synsacrum. It is likely the first free caudal vertebra. The transverse processes are nearly twice as long as the mediolateral width of the centrum and strongly project caudolaterally beyond the caudal margin of the centrum.
The furcula is Y shaped with an interclavicular angle of 61° (Fig. S1 A and B). The hypocleidium is notably elongated and accounts for more than 70% of the length of the clavicular ramus. The ratio is greater than other taxa, including Eoenantiornis, Concornis, and Shanweiniao. The lateral margin of the coracoid is strongly convex, rendering the proximal shaft medially curved (Fig. S1 A and B). A procoracoid process is absent. Along the medial margin of the neck runs a longitudinal furrow that houses the supracoracoidal nerve foramen. The strongly vaulted ventral surface evidences the presence of a deep dorsal fossa as in other enantiornithines (13). The sternum bears a pair of craniolateral processes and two pairs of caudal trabeculae (Fig. S1 C and D). The lateral trabeculae are robust, run parallel to the midline of the sternum, and project as far caudally as the xiphoid process. Caudally, the lateral trabeculae carry large fan-shaped expansions. The expansion is so well-developed that its medial projection reaches the level of the caudal end of the medial trabecula. Comparable large caudal expansion is only present in some taxa, including the Bohaiornithidae, Houornis, and Cathayornis. However, the lateral trabeculae end proximal to the xiphoid process in the Bohaiornithidae (11), but in Houornis and Cathayornis it projects farther caudally (4).
The femoral head is situated on a short, robust neck. As in some other Early Cretaceous enantiornithines, a fossa for the femoral origin of ligamentum capitis fermoralis is absent (Fig. 2 E and F). The tibiotarsus lacks a cnemial crest. As in other enantiornithines, the three major metatarsals II–IV are not fused with one another distally. Although the proximal ends are overlain by the pelvis, metatarsals II–IV are most likely fused proximally with reference to the holotype: IVPP V16363 is slightly larger than the holotype, in which metatarsals II–IV are fused proximally. Metatarsal II terminates to the proximal margin of the metatarsal III trochlea. A fossa for metatarsal I is absent in the medioplantar surface of metatarsal II. Metatarsal I is P shaped in medioplantar view. The bone is sharply tapered proximally; distally, the shaft is recurved plantarly and forms a broad articular facet for the hallux. Therefore, a fully reversed hallux is in place. The nonungual phalanges are robust, spool-shaped, and have well-defined articular trochleae with distinct pits for the attachment of the collateral ligament. The phalanx I-1 is stout. The longest nonungual phalanx is phalanx III-1. The pedal claws are strongly recurved and are longer than their preceding phalanges.
Histological Description.
The cortex of the ulna is composed of parallel-lamellar bone tissue (Fig. S4C). The bone is less vascularized than in the humerus, with exclusively longitudinally oriented canals restricted to the internal half of the cortex. A distinct ICL is developed around the medullary cavity and is comprised of endosteal-derived lamellar bone tissue (Fig. S4D). No LAG is visible, and the OCL is very thin. However, both observations may be biased by the erosion of the periosteum, given the scalloped appearance of the external margin of the cortex. Secondary osteons are present (Fig. S4D) but are less common than the primary ones.
The transverse section of the radius is as thick as the preserved bone wall of the ulna (Fig. S4C). The medullary cavity is surrounded by the ICL that accounts for approximately one-third of the diameter of the cortex (Fig. S3B), proportionally thicker than in the humerus and ulna. The ICL consists of circumferential avascular lamellar bone tissue of endosteal origin and contains flattened osteocyte lacunae. The ICL is separated from the primary bone tissue of periosteal origin by a reversal line (Fig. S3B). The cortex external to the ICL comprises parallel-lamellar bone tissue and is poorly vascularized with sparsely distributed longitudinally oriented canals. A thin layer (∼10 µm in thickness) adjacent to the periosteal margin is distinguishable from the internal bone tissue and is interpreted to be the OCL based on the following observations: This layer exhibits lighter color under polarized light (Fig. S3B), indicating that the collagen fibers are better organized, and the osteocyte lacunae are strongly flattened with their long axes parallel to the external margin of the bone, whereas the osteocyte lacunae in the internal bone tissue are more globular.
Phylogenetic Analyses.
A phylogenetic analysis was performed using the revised data matrix in Wang et al. (7). We scored characters for Pterygornis based on the combined information from IVPP V16363 and the holotype. Fifty-three characters can now be coded for this taxon in light of this specimen with respect to the previous study (7). Two recently described enantiornithines Linyiornis and Piscivorenantiornis were included, and their scorings were taken from published literature (5, 13). The revised data matrix consists of 62 taxon and 262 morphological characters, 59 of which are Mesozoic birds (SI Appendix). Phylogenetic analysis was conducted using the TNT software package (48), with following settings: All characters were equally weighted, and 33 of them were treated as ordered; to search for the most parsimonious trees (MPTs), an unconstrained heuristic search with Wager tree as the starting tree was performed, using 1,000 replicates of random stepwise addition (branch swapping: tree-bisection-reconnection, TBR), and 10 trees were held at each step; branches were collapsed to create polytomies if the minimal branch length is zero. Bootstrap and Bremer values were calculated as the support indices. Bootstrap values were obtained by conducting 1,000 replicates using the default settings in TNT. Bremer values were calculated using the “bremer script” included in TNT.
A total of 1,140 MPTs of 1,087 steps were recovered (Consistency index = 0.338; Retention index = 0.667). The strict consensus tree is poorly resolved (Fig. S2A). The Enantiornithes forms a large polytomy with only a few derived clades nested in, including the Bohaiornithidae and the Pengornithidae. Pterygornis is collapsed with other enantiornithines with little resolved interrelationship.
The poorly resolved strict consensus tree largely stems from unstable taxa that show multiple topological positions (49). Consequently, multiple MPTs were recovered that impaired the resolution of the strict consensus. Therefore, we carried out a reduced consensus analysis to retrieve common phylogenetic information from MPTs (49). The reduced consensus study was conducted using the iterative position congruence reduced method (iterative PCR) script included in TNT (48). Eleven taxa were identified as the mots unstable: Elsornis, Cathayornis, Gobipteryx, Iberomesornis, Longirostravis, Rapaxavis, Shanweiniao, Songlingornis, Dunhuangia, Longipteryx, and Boluochia. When these unstable taxa were excluded from the consensus, the reduced consensus tree is nearly completely resolved (Fig. S2B). This topology is essentially comparable with recent analyses in placements of the major clades (11, 19, 21). Within Enantiornithes, Protopteryx and the Pengornithidae emerge in the basalmost positions. Pterygornis is resolved as the outgroup to a triplet comprising Linyiornis, Fortunguavis, and Piscivorenantiornis, which all together form the sister clade to the Bohaiornithidae. Salient synapomorphies that support the Pterygornis + (Linyiornis + Fortunguavis + Piscivorenantiornis) clade include quadrate pneumaticity absent (character 26:0); broad, deep fossa on the dorsal surface of the coracoid (character 91:1); scapular shaft sagittally curved (character 96:2); coracoidal sulci of the sternum widely spaced mediolaterally (character 115:0); proximocranial surface of the humerus without circular fossa (character 124:0); ungual of the major digit larger or subequal to other manual unguals (character 173:0); and tibia and proximal tarsals completely fused (character 209:1).
Ancestral State Reconstructions of the Pelvis and Manual Fusions Across the Paravian Phylogeny.
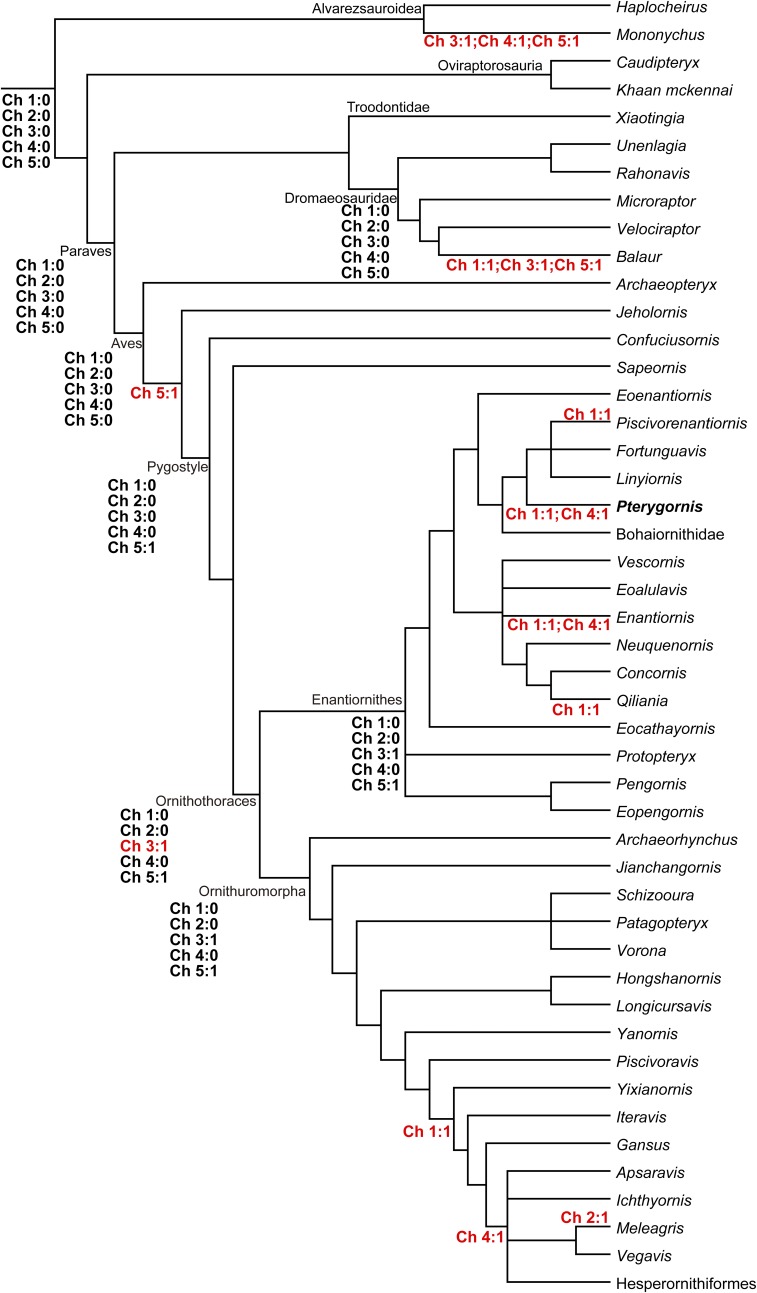
We formulated five binary characters to describe the degrees of pelvis and manus fusions among Mesozoic birds and their close theropod relatives, including Alvarezsauroidea, Oviraptorosauria, Troodontidae, and Dromaeosauridae. A Paraves tree is composited from the reduced consensus of the Mesozoic birds resolved in the present analysis and the nonavian paravian phylogeny in Turner et al. (27) (Fig. 3 and Fig. S2). The Late Cretaceous enantiornithines is represented by Enantiornis, and its systematic position follows previous study (1). A parsimony-based ancestral state reconstruction of these five characters was conducted using the Mesquite software package (47), and the results are given in Fig. 3 and Fig. S6:
Character 1. Ilium, ischium, and pubis fused around the acetabulum in adult: absent (0); present (1).
Character 2. Ilium and ischium fused caudally to enclose the caudal margin of the ilioischiadic foramen in adult: 0 (absent); present (1).
Character 3. Alular and major metacarpals fused proximally in adult: absent (0); present (1).
Character 4. Alular and major metacarpals fused distally in adult: absent (0); present (1).
Character 5. Semilunate carpal fused with proximal end of major metacarpal in adult: absent (0); present (1).
Fig. 3.
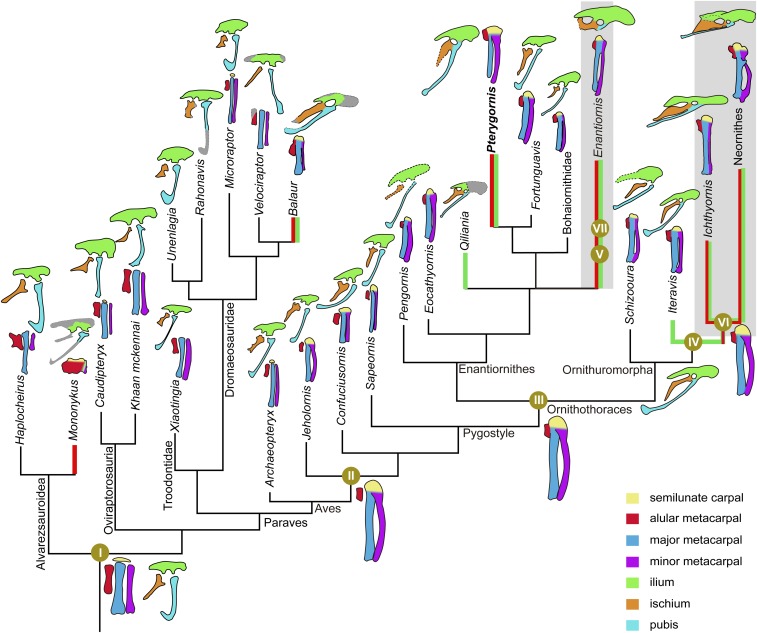
Paravian phylogeny showing the major changes of manus and pelvis fusions. The ancestral conditions of the pelvis and manus fusions of the major nodes were reconstructed using the parsimonious method in Mesquite. Major changes are summarized below: I, the metacarpals and pelvis unfused in adults; II, the semilunate carpal fused with the proximal ends of the major and minor metacarpals, with the alular metacarpals separated; III, the alular metacarpal fused with the major metacarpal proximally but separated distally; IV and V, the ilium, ischium, and pubis fused around the acetabulum; VI and VII, alular and major metacarpals completely fused along their length. The green thick lines denote the taxa with fused pelvis, and the red thick lines indicate taxa with fused alular and major metacarpals. Late Cretaceous birds are denoted in shaded background. The line drawings are not to scale.
Fig. S6.
Ancestral state reconstructions of the manus and pelvis fusions across the paravian phylogeny. Five binary characters (ch 1–5) describing the varying degrees of pelvis and manus fusions (SI Text) were submitted to an ancestral state reconstruction using the parsimonious method in the Mesquites software package (see Materials and Methods). The unequivocally reconstructed states and character changes are denoted near the corresponding nodes and lineages.
Character scorings for selected taxa: Haplocheirus sollers: 0, 0, 0, 0, 0; Mononykus olecranus: 0, 0, 1, 1, 1; Caudipteryx dongi: 0, 0, 0, 0, 0; Khaan mckennai: 0, 0, 0, 0, 0; Xiaotingia zhengi: 0, 0, 0, 0, 0; Unenlagia comahuensis: 0, 0, ?, ?, ?; Rahonavis ostromi: 0, 0, ?, ?, ?; Microraptor zhaoianus: 0, 0, 0, 0, 0; Velociraptor mongoliensis: 0, 0, 0, 0, 0; Balaur bondoc: 1, 0, 1, 0, 1; Archaeopteryx: 0, 0, 0, 0, 0; Jeholornis: 0, 0, 0, 0, 1; Confuciusornis: 0, 0, 0, 0, 1; Sapeornis: 0, 0, 0, 0, 1; Protopteryx: 0, 0, 0, 0, 1; Pengornis: 0, 0, 1, 0, 1; Eopengornis: 0, 0, ?, ?, 1; Eocathayornis: ?, ?, 1, 0, 1; Vescornis: 0, 0, 0, 0, 0; Eoalulavis: ?, ?, ?, ?, ?; Neuquenornis: ?, ?, 1, 1, 1; Concornis: ?, ?, ?, ?, ?; Qiliania: 1, 0, ?, ?, ?; Eoenantiornis: ?, 0, 1, 0, 1; Pterygornis: 1, 0, 1, 1, 1; Linyiornis: 0, 0, ?, ?, 1; Piscivorenantiornis: 1, 0, 1, 0, 1; Fortunguavis: 0, 0, 1, 0, 1; Bohaiornithidae: 0, 0, 1, 0, 1; Enantiornis: 1, 0, 1, 1, 1; Archaeorhynchus: 0, 0, 1, 0, 1; Jianchangornis: 0, ?, 0, 0, 1; Schizooura: 0, 0, 1, 0, 1; Patagopteryx: ?, ?, ?, ?, ?; Vorona: ?, ?, ?, ?, ?; Hongshanornis: ?, ?, 1, ?, 1; Longicrusavis: ?, ?, 1, ?, 1; Yanornis: 1, 0, 1, 0, 1; Piscivoravis: 0, 0, 1, 0, 1; Yixianornis: 1, 0, 1, 0, 1; Iteravis: 1, 0, 1, 0, 1; Gansus: 1, 0, 1, 0, 1; Apsaravis: 1, ?, 1, 1, 1; Ichthyornis: 1, 0, 1, 1, 1; Vegavis: 1, ?, 1, 1, 1; Hesperornis: 1, 0, ?, ?, ?; and Meleagris: 1, 1, 1, 1, 1.
Discussion
Compared with other enantiornithines and basal birds, the most striking feature of Pterygornis is the high degree of skeletal fusion, particularly the manus and pelvis (Fig. 2 C–F). The alular and major metacarpals are fused throughout their length, as in crown birds (Fig. 2 C and D). The alular metacarpal provides the attachment for the M. extensor carpi radialis that originates from the dorsal epicondyle of the humerus (10). The wing can be extended through contraction of that muscle (10), and during that extension, the alular metacarpal undergoes considerable tension. Without fusion to the major metacarpal, the tension on the alular metacarpal can only be transferred to the rest of the manus by means of connective tissue between the bones, which is energetically costly and the alular metacarpal (and manus) could be damaged if the instantaneous force increases beyond a certain point. Instead, by being fused to the carpometacarpus, the alular metacarpal should be stabilized significantly more efficiently in control of the bastard wing, and the tension produced by muscle contraction would be transferred across the whole hand as a unit. The fusion between the alular and major metacarpals are absent in nonornithothoracine birds, including Confuciusornithidae, Sapeornithidae, and Archaeopteryx (refs. 15, 18, and 20 and Fig. 3 and Fig. S6). In more derived Early Cretaceous birds, Enantiornithes and Ornithuromorpha, the alular and the major metacarpals are separated distally, while ankylosed proximally (refs. 1, 11, and 21 and Fig. 3 and Fig. S6). In contrast, these two bones are completely fused to one another in both clades in the Late Cretaceous (2, 22). Fusion of the alular and major metacarpals is rare among nonavian theropods, with some exceptions such as derived alvarezsauroids, the enigmatic Avimimus, and the dromaeosaurid Balaur (Fig. 3). However, the manual morphology of those nonavian theropods differs substantially from the avian hands and did not function in flight. For instance, the unique manus in alvarezsauroids has been regarded as adaptation for digging (23). We performed a parsimony-based ancestral state reconstruction for the alula-major metacarpals and pelvis fusions across the phylogeny of Paraves. The result indicates that the fusion of the alular and major metacarpals evolved convergently in these nonavian dinosaurs, Enantiornithes and Ornithuromorpha (Fig. 3 and Fig. S6 and SI Text).
Before the discovery of IVPP V16363, the three pelvic bones remain unfused in ontogeny in nearly all known Early Cretaceous birds with the exceptions of two enantiornithines, Qiliania and Piscivorenantiornis, and some ornithuromorphs such as Gansus (Fig. 3 and Fig. S6 and SI Text). The pelvic fusion is similar among these taxa, the three pelvic elements ankylosed around the acetabulum. In most modern birds, the ischium and ilium are fused caudally to enclose the caudal margin of the ilioischiadic foramen (10, 24), but this feature is absent in all of the Mesozoic birds. In living birds, the pelvis incorporates individual elements to form an immobile, weight-bearing structure, withstanding strain exerted by hindlimb muscles during locomotion (25). A fused pelvic girdle is occasionally present in some nonparavian theropods, including some ornithomimids, coelophysoids, and ceratosaurs (26). The pelvic girdle is unfused in Oviraptorosauria (27), the immediate outgroup to Paraves. Within Paraves, pelvic fusion is only known in several derived dromaeosaurids such as Balaur and Hesperonychus (27). In contrast, these elements remain separated in basal dromaeosaurids, including Mahakala, Rahonavis, and Unenlagia (27), the known troodontids, and basal Aves (Fig. 3). The ancestor state reconstruction revealed the unfused pelvis as the ancestor condition of Paraves, and the pelvic fusion evolved independently and on multiple occasions in lineages of Dromaeosauridae and Aves (Fig. 3 and Fig. S6).
Embryonic development of living birds has attracted considerable attention recently, and bone ossification sequences for many species are well established (28). Unfortunately, much less attention has been paid to how the compound bones become fused to one another, because much of bone fusion occurs after hatching, and most developmental studies focus only on the embryonic stages. In many living birds, the pelvic fusion completes long before the age of 1 y—for example, around 140 d posthatching in domestic fowls (for carpometacarpus, around 120 d of posthatching; ref. 29). However, osteohistological studies show that the pelvis remains unfused in basal birds that are more than 2 y old—for example, Jeholornis, Confuciusornis, Sapeornis, and Enantiornithes (30–32). A fused pelvis is common among Late Cretaceous Enantiornithes and Ornithuromorpha (22, 33). It appears that the unfused pelvis in adult individuals of Early Cretaceous birds is plesiomorphically inherited from their dinosaurian ancestors and that pelvic fusion evolved convergently in late history of the Enantiornithes and Ornithuromorpha. Early Cretaceous ornithuromorphs generally exhibited a relatively higher degree of bone fusion than contemporaneous enantiornithines (21). For example, the tarsometatarsus is unfused distally in enantiornithines (1, 2) but completely fused in most ornithuromorphs except the most basal ornithuromorph Archaeorhynchus (34). Interestingly, osteohistological studies suggest a similar growth pattern for Archaeorhynchus and enantiornithines that grew much slower than most other ornithuromorphs (34).
Bone microstructure of Pterygornis indicates that its growth rate had slowed considerably before its death, demonstrated by the presence of the ICL, OCL, secondary osteons, and the predominant parallel-lamellar bone tissue (Figs. S3–S5). All of these features generally are accepted to signal the cessation of active growth (9, 35, 36). Enantiornithes typically grew slowly and underwent several instances of growth stoppages before reaching adulthood, a growth pattern reflected in their bone histology where the cortex is mainly formed by parallel-lamellar or lamellar bone tissue containing multiple LAGs in the deep cortex (9, 30). In contrast, Pterygornis only have one LAG, and it is positioned close to the periosteal margin, suggesting that skeletal maturity was reached within 1 y with minimal additional later growth. The growth pattern of Pterygornis is distinct from most other enantiornithines but similar to Cruralispennia and Confuciusornis (31, 37). With all of these observations, one would argue that the manus and pelvis fusions in Pterygornis stem from their rapid growth. This assumption is questionable, because the pelvis is unfused in Confuciusornis, Cruralispennia, and basal ornithuromorphs that grew relatively fast, but a fused pelvis is present in Late Cretaceous enantiornithines that grew much slower than Pterygornis (9). Therefore, we posit that the degree of bone fusion is not closely related with the growth strategy in basal birds.
Although rare, some nonavian coelurosaurian theropods exhibit varying degrees of fusion in the manus and pelvis (27) and probably evolved convergently (earlier in this section). Nonavian dinosaurs had the potential to fuse these bones, but normally they did not. These bones show varying degrees of fusion in Early Cretaceous birds but otherwise are completely fused in adults of Late Cretaceous species. In a Darwinian model of evolution, the origin of a character will not be step-wise but rather be accompanied by varied states resulting from the developmental polymorphism related to that character (38). The varying degrees of fusion (here, in the alular-major metacarpals and pelvis) in theropod dinosaurs and birds indicate that the developmental trajectories in charge of bone fusion are polymorphic. The fact that all birds across clades have a fused hand and pelvis by the Late Cretaceous indicates that such developmental polymorphism was fundamentally “pruned” and that ontogenetic bone fusion became fixed in avian evolution. So what mechanism led to selection in favor of increased fusion among the manus and pelvis to such an extent that it occurs exclusively in later birds and resulted in the loss of other developmental polymorphisms along the line to crown birds, making bone fusion become “locked in” across avian phylogeny?
Biological novelties can stem from the emergence of new genes or regulatory systems, or a combination of both (39, 40). Great progress has been made regarding the phylogeny of crown birds recently from genomic analyses (41), but pinpointing the specific genes–regulatory pathways with corresponding phenotypes is more challenging. The fusion of the manus and pelvis at such an early stage in avian evolution may have been caused by modifications in genes and/or developmental paths. The origin of flight has significantly changed the bauplan of birds (42). Given the potential functional benefits, the complete fusion in the manus and pelvis in some early birds, but only rarely occurring, may reflect the refinement of flight capability, suggesting developmental plasticity (43). This is not unusual, because environmentally (here, terrestrial locomotory changes to flight) induced morphologies are common in animals and some of them actually spur the evolutionary success of certain groups (44, 45). Our study also shows that the manus fusion occurred earlier than the pelvis in avian evolution. We posit that this pattern reflects that the forelimbs underwent greater selection pressure and thus modified more rapidly during the course of flight evolution.
In summation, Pterygornis records the oldest occurrence of a fused carpometacarpus and pelvis in birds, and it is premature to pick which of the abovementioned hypotheses or their combination are most compatible with the evolutionary pattern without additional knowledge about the development of these bones and the molecular–developmental mechanisms of extant birds. Future studies are necessary to test these hypotheses and pinpoint the developmental pathways involving the bone fusion in early avian evolution.
Materials and Methods
Bone Histology Preparation.
The bone cross-sections were prepared following the standard methods (46). Four samples were taken from the positions as close to the middiaphysis as possible from the left humerus and femur, right ulna and radius (Fig. 1A). Samples were embedded in one-component resin (EXAKT Technovit 7200) and hardened in a light polymerization device (EXAKT 520) for 12 h. Histological cross-sections were cut using an accurate circular saw (EXAKT 300CP). Sections were glued to frosted glass slides with adhesive (EXAKT Technovit 7230) and then ground down using the EXAKT 400CS grinding system until the desired optical contrast was obtained. The bone sections were checked by light microscopy under normal and polarized lights (Zeiss AX10). Images were captured using a digital camera (Zeiss AxioCam MRc5).
Phylogenetic Analysis and Ancestral State Reconstruction.
The phylogenetic analysis was performed using the modified dataset of Mesozoic birds in Wang et al. (7). Description and complete results of the phylogenetic analysis are provided in the SI Text and SI Appendix. To trace the evolutionary changes of the manus and pelvis fusion across a broad phylogenetic scale, a paravian tree was constructed (Fig. 3 and Fig. S6). The phylogeny of Mesozoic birds is modified from the reduced strict consensus resolved in this study, and the placements of the major nonavian paravian clades follow Turner et al. (27). We formulated five binary characters to describe the degree of fusion of the pelvis and the alular and major metacarpals in paravians (SI Text). Since the pelvis and manus fusions rarely occur in nonavian paravians and they are likely nonhomologous to these features in Aves, we only selected a few species from among those nonavian paravians and their closest relatives that provide unambiguous information regarding the states of bone fusion in question. The ancestral states of these five characters are reconstructed for the major nodes across the Paravian phylogeny. This analysis was conducted using the parsimonious method in the Mesquite software package (47), and the complete results are given in Fig. 3 and Fig. S6.
Supplementary Material
Acknowledgments
We thank S. Zhang for helping in preparing the bone thin sections; W. Gao for photography; A. O. Vargas, G. Zhang, and C. M. Chung for discussion about the possible genes involved in skeletal fusion in living birds; and T. Stidham for editing and commenting on the manuscript. This study is supported by National Natural Science Foundation of China Grants 41502002 and 41688103.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707237114/-/DCSupplemental.
References
- 1.O’Connor JK. 2009. A systematic review of Enantiornithes (Aves: Ornithothoraces). PhD thesis (University of Southern California, Los Angeles)
- 2.Chiappe LM, Walker CA. Skeletal morphology and systematics of the Cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes) In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. University of California Press; Berkeley, CA: 2002. pp. 240–267. [Google Scholar]
- 3.Zhou Z. The Jehol Biota, an Early Cretaceous terrestrial Lagerstätte: New discoveries and implications. Natl Sci Rev. 2014;1:543–559. [Google Scholar]
- 4.Wang M. 2014. Taxonomical revision, ontogenetic, ecological and phylogenetic analyses of Enantiornithes (Aves: Ornithothoraces) of China. PhD thesis (University of Chinese Academy of Sciences, Beijing)
- 5.Wang M, Zhou Z, Sullivan C. A fish-eating enantiornithine bird from the Early Cretaceous of China provides evidence of modern avian digestive features. Curr Biol. 2016;26:1170–1176. doi: 10.1016/j.cub.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, et al. Preservation of ovarian follicles reveals early evolution of avian reproductive behaviour. Nature. 2013;495:507–511. doi: 10.1038/nature11985. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Hu H, Li Z. A new small enantiornithine bird from the Jehol Biota, with implications for early evolution of avian skull morphology. J Syst Palaeontol. 2015;14:481–497. [Google Scholar]
- 8.Erickson GM, et al. Was dinosaurian physiology inherited by birds? Reconciling slow growth in archaeopteryx. PLoS One. 2009;4:e7390. doi: 10.1371/journal.pone.0007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinsamy A. The Microstructure of Dinosaur Bone: Deciphering Biology with Fine-Scale Techniques. Johns Hopkins Univ Press; Baltimore: 2005. [Google Scholar]
- 10.Baumel JJ, Witmer LM. Osteologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Berge JCV, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. 2nd Ed. Nuttall Ornithological Club; Cambridge, MA: 1993. pp. 45–132. [Google Scholar]
- 11.Wang M, Zhou Z, O’Connor JK, Zelenkov NV. A new diverse enantiornithine family (Bohaiornithidae fam. nov.) from the Lower Cretaceous of China with information from two new species. Vertebr Palasiat. 2014;52:31–76. [Google Scholar]
- 12.Xu X, Wu X. Cranial morphology of Sinornithosaurus millenii Xu et al. 1999 (Dinosauria: Theropoda: Dromaeosauridae) from the Yixian Formation of Liaoning, China. Can J Earth Sci. 2001;38:1739–1752. [Google Scholar]
- 13.Wang Y, et al. A new Jehol enantiornithine bird with three-dimensional preservation and ovarian follicles. J Vertebr Paleontol. 2016;36:e1054496. [Google Scholar]
- 14.Ashley JF. A study of the structure of the humerus in the Corvidae. Condor. 1941;43:184–195. [Google Scholar]
- 15.Zhou Z, Zhang F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can J Earth Sci. 2003;40:731–747. [Google Scholar]
- 16.Zhou S, Zhou Z, O’Connor JK. Anatomy of the basal ornithuromorph bird Archaeorhynchus spathula from the Early Cretaceous of Liaoning, China. J Vertebr Paleontol. 2013;33:141–152. [Google Scholar]
- 17.Baumel J, Raikow R. Arthrologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Berge JCV, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. 2nd Ed. Nuttall Ornithological Club; Cambridge, MA: 1993. pp. 133–187. [Google Scholar]
- 18.Chiappe LM, Ji SA, Ji Q, Norell MA. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull Am Mus Nat Hist. 1999;242:1–89. [Google Scholar]
- 19.Wang M, Zhou Z. A morphological study of the first known piscivorous enantiornithine bird from the Early Cretaceous of China. J Vertebr Paleontol. 2017;37:e1278702. [Google Scholar]
- 20.Campbell KE. The manus of archaeopterygians: Implication for avian ancestry. Oryctos. 2008;7:13–26. [Google Scholar]
- 21.Zhou S, O’Connor JK, Wang M. A new species from an ornithuromorph (Aves: Ornithothoraces) dominated locality of the Jehol Biota. Chin Sci Bull. 2014;59:5366–5378. [Google Scholar]
- 22.Clarke JA, Norell MA. The morphology and phylogenetic position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. Am Mus Novit. 2002;3387:1–46. [Google Scholar]
- 23.Xu X, et al. A monodactyl nonavian dinosaur and the complex evolution of the alvarezsauroid hand. Proc Natl Acad Sci USA. 2011;108:2338–2342. doi: 10.1073/pnas.1011052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker W. On the morphology of birds. Proc R Soc London. 1887;42:52–58. [Google Scholar]
- 25.Hutchinson JR. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes) Zool J Linn Soc. 2001;131:123–168. [Google Scholar]
- 26.Tykoski RS. 2005. Anatomy, ontogeny, and phylogeny of coelophysoid theropods. PhD thesis (University of Texas at Austin, Austin, TX)
- 27.Turner AH, Makovicky PJ, Norell MA. A review of dromaeosaurid systematics and paravian phylogeny. Bull Am Mus Nat Hist. 2012;371:1–206. [Google Scholar]
- 28.Pourlis AF, Antonopoulos J. The ossification of the pelvic girdle and leg skeleton of the quail (Coturnix coturnix japonica) Anat Histol Embryol. 2014;43:294–300. doi: 10.1111/ahe.12076. [DOI] [PubMed] [Google Scholar]
- 29.Hogg DA. Fusions occurring in the postcranial skeleton of the domestic fowl. J Anat. 1982;135:501–512. [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor JK, Wang M, Zheng X, Wang X, Zhou Z. The histology of two female Early Cretaceous birds. Vertebr Palasiat. 2014;52:112–128. [Google Scholar]
- 31.De Ricqlès AJ, Padian K, Horner JR, Lamm ET, Myhrvold N. Osteohistology of Confuciusornis sanctus (Theropoda: Aves) J Vertebr Paleontol. 2003;23:373–386. [Google Scholar]
- 32.Zheng X, et al. On the absence of sternal elements in Anchiornis (Paraves) and Sapeornis (Aves) and the complex early evolution of the avian sternum. Proc Natl Acad Sci USA. 2014;111:13900–13905. doi: 10.1073/pnas.1411070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiappe LM, James JP, Erickson PGP. New enantiornithine bird from the marine Upper Cretaceous of Alabama. J Vertebr Paleontol. 2002;22:170–174. [Google Scholar]
- 34.Wang M, Zhou Z. A new adult specimen of the basalmost ornithuromorph bird Archaeorhynchus spathula (Aves: Ornithuromorpha) and its implications for early avian ontogeny. J Syst Palaeontology. 2016;15:1–18. [Google Scholar]
- 35.Francillon-Vieillot H, et al. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG, editor. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. American Geophysical Union; New York: 1990. pp. 175–234. [Google Scholar]
- 36.Köhler M, Marín-Moratalla N, Jordana X, Aanes R. Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature. 2012;487:358–361. doi: 10.1038/nature11264. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, O’Connor JK, Pan Y, Zhou Z. A bizarre Early Cretaceous enantiornithine bird with unique crural feathers and an ornithuromorph plough-shaped pygostyle. Nat Commun. 2017;8:14141. doi: 10.1038/ncomms14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bever GS, Gauthier JA, Wagner GP. Finding the frame shift: Digit loss, developmental variability, and the origin of the avian hand. Evol Dev. 2011;13:269–279. doi: 10.1111/j.1525-142X.2011.00478.x. [DOI] [PubMed] [Google Scholar]
- 39.Seki R, et al. Functional roles of Aves class-specific cis-regulatory elements on macroevolution of bird-specific features. Nat Commun. 2017;8:14229. doi: 10.1038/ncomms14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhullar BAS, et al. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution. 2015;69:1665–1677. doi: 10.1111/evo.12684. [DOI] [PubMed] [Google Scholar]
- 41.Prum RO, et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 2015;526:569–573. doi: 10.1038/nature15697. [DOI] [PubMed] [Google Scholar]
- 42.Brusatte SL, O’Connor JK, Jarvis ED. The origin and diversification of birds. Curr Biol. 2015;25:R888–R898. doi: 10.1016/j.cub.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Standen EM, Du TY, Larsson HCE. Developmental plasticity and the origin of tetrapods. Nature. 2014;513:54–58. doi: 10.1038/nature13708. [DOI] [PubMed] [Google Scholar]
- 44.Starck JM, Chinsamy A. Bone microstructure and developmental plasticity in birds and other dinosaurs. J Morphol. 2002;254:232–246. doi: 10.1002/jmor.10029. [DOI] [PubMed] [Google Scholar]
- 45.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol. 2007;21:394–407. [Google Scholar]
- 46.Lamm ET. Preparation and sectioning of specimens. In: Padian K, Lamm ET, editors. Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation. University of California Press; Berkeley, CA: 2013. pp. 55–160. [Google Scholar]
- 47.Maddison WP, Maddison DR. 2011 Mesquite: A Modular System for Evolutionary Analysis, Version 3.1. Available at mesquiteproject.org. Accessed July 26, 2017.
- 48.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. [Google Scholar]
- 49.Pol D, Escapa IH. Unstable taxa in cladistic analysis: Identification and the assessment of relevant characters. Cladistics. 2009;25:515–527. doi: 10.1111/j.1096-0031.2009.00258.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.