Significance
We show that the timing of alternating-current stimulation can couple or decouple low-frequency brain rhythms between segregated frontal cortical areas in a highly selective fashion without changing other neural frequencies, synchronization across the opposite cerebral hemisphere, or local neural activity. The up- and down-regulation of interareal neural coupling caused bidirectional changes in adaptive control and learning measured behaviorally. It was even possible to induce behavioral deficits and then immediately rescue this behavior in the same individuals in a matter of minutes. The findings suggest it is possible to intervene in the neural integration between frontal cortical structures that govern complex human behavior. The development of drug-free interventions for addressing disorders of excessive and deficient cortical connectivity is implicated.
Keywords: high-definition transcranial alternating current stimulation, adaptive control, phase synchronization, medial frontal cortex, lateral prefrontal cortex
Abstract
Rescuing executive functions in people with neurological and neuropsychiatric disorders has been a major goal of psychology and neuroscience for decades. Innovative computer-training regimes for executive functions have made tremendous inroads, yet the positive effects of training have not always translated into improved cognitive functioning and often take many days to emerge. In the present study, we asked whether it was possible to immediately change components of executive function by directly manipulating neural activity using a stimulation technology called high-definition transcranial alternating current stimulation (HD-tACS). Twenty minutes of inphase stimulation over medial frontal cortex (MFC) and right lateral prefrontal cortex (lPFC) synchronized theta (∼6 Hz) rhythms between these regions in a frequency and spatially specific manner and rapidly improved adaptive behavior with effects lasting longer than 40 min. In contrast, antiphase stimulation in the same individuals desynchronized MFC-lPFC theta phase coupling and impaired adaptive behavior. Surprisingly, the exogenously driven impairments in performance could be instantly rescued by reversing the phase angle of alternating current. The results suggest executive functions can be rapidly up- or down-regulated by modulating theta phase coupling of distant frontal cortical areas and can contribute to the development of tools for potentially normalizing executive dysfunction in patient populations.
Adaptive control refers to the dynamic processing that coordinates goal pursuit, allowing us to adjust our actions to changing situations and improve performance after events such as negative feedback from the environment (1, 2). Impaired adaptive control is observed in many neurological and neuropsychiatric disorders such as schizophrenia, autism, Alzheimer’s disease, attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, Parkinson’s disease, and epilepsy (3–9). Thus, it is not surprising that efforts to improve adaptive control have long characterized the fields of psychology and neuroscience.
Computerized training has proven to be effective in the domains of language, motor function, and vision (10, 11). However, in other cognitive domains such as attention, working memory, and adaptive control, the effects of computer training interventions have been contradictory and less clear (12–14). One significant drawback to cognitive training is the duration of task practice required, often on the order of days and weeks, before desired results are achieved. Here, we asked whether it was possible to use brain stimulation technology to induce immediate and lasting neuroplastic changes in the functional connectivity hypothesized to underlie the adaptive control of behavior and learning in humans.
We targeted the phase coupling or synchronization of electroencephalographic (EEG) rhythms in the theta frequency band (∼6 Hz) between medial frontal cortex (MFC) and lateral prefrontal cortex (lPFC) because correlative studies have suggested that functional cortical circuits for adaptive control may emerge by theta phase coupling between these regions, specifically following control-related prompts such as motor errors, conflict, or negative performance feedback (15, 16). We sought to isolate and alter the spectral and spatial properties of connectivity, using nine-channel high-definition transcranial alternating current stimulation (or HD-tACS), which promises an unprecedented degree of anatomical precision and the capability to conduct multifocal modifications of neural activity in a frequency-specific and bidirectional manner. The stimulation protocols delivered 6-Hz HD-tACS simultaneously to MFC and lPFC with a relative 0° (inphase) or 180° (antiphase) phase difference between targeted areas (Fig. 1A). We predicted that the different stimulation protocols (i.e., inphase/antiphase) might bias error feedback-related network synchronization in opposite directions, and thus facilitate or impede the neural integration of MFC and lPFC. Second, if MFC-lPFC theta coupling elicited by error feedback represents a causal mechanism underlying flexible behavior, then a bidirectional manipulation of this connectivity should cause bidirectional changes (i.e., improvements and impairments) in performance related to adaptive control and learning.
Fig. 1.
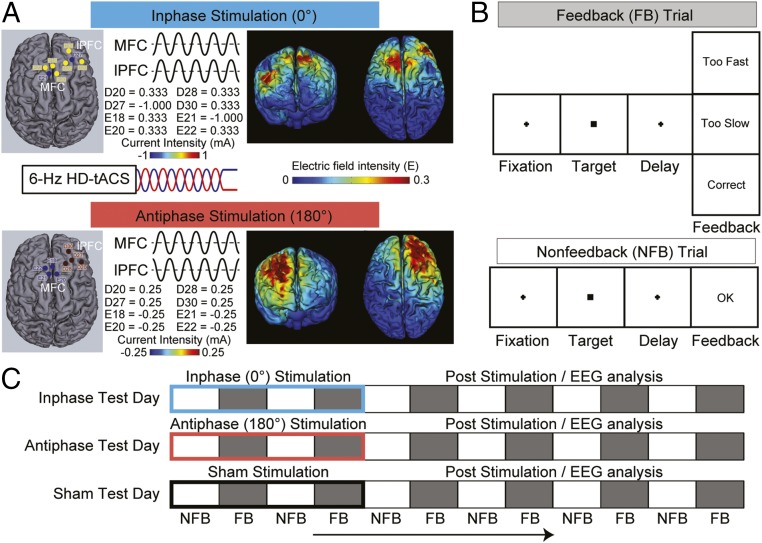
Stimulation and task procedures of Experiment 1. (A) The right-lateralized eight-channel 6-Hz inphase (Top) and antiphase (Bottom) HD-tACS protocols and current-flow models are shown on 3D reconstructions of the cortical surface. The location and current intensity value of each stimulating electrode are shown. Target regions were the medial frontal cortex (MFC) and right lateral prefrontal cortex (lPFC). Each stimulation site used four electrodes in a center-surround, source-sink pattern to achieve focality. (B) The sequence of events on feedback and nonfeedback trials in the time-estimation task. (C) Schematic illustration of the experimental design. Each subject underwent three separate test days (inphase, antiphase, and sham). On each day, EEG was recorded for 60 min while subjects performed the time-estimation task. The first 20 min consisted of inphase (blue), antiphase (red), or sham (black) HD-tACS, depending on the test day, and was followed by 40 min in which no stimulation was applied. The task alternated between blocks of feedback (FB, gray) and nonfeedback (NFB, white) trials. Critically, EEG data were analyzed only after the HD-tACS during the poststimulation period to avoid stimulation-related artifacts.
Results
In Experiment 1, each subject participated in three different stimulation conditions (i.e., 6-Hz inphase, 6-Hz antiphase, and sham) on different days with stimulation order counterbalanced across subjects. Inphase/antiphase stimulation allowed us the unique opportunity to gain bidirectional control over the nature of the theta interaction between MFC and lPFC. We targeted right lPFC in Experiment 1. During and after the delivery of HD-tACS, on each day, subjects performed a modified version of a classic time-estimation task (17) while we recorded their EEG brain rhythms (Fig. 1B). Since the original discovery of human feedback-related electrophysiological activity, this task has been rigorously used to study feedback-guided learning, and thus is a model task for examining interareal neural communication during adaptive behavior and learning.
In the task, participants were instructed to respond when they had estimated a time-lapse of 1.7 s. Feedback at the end of the trial indicated whether the estimation was “too fast,” “too slow,” or “correct.” A response ±200 ms around target time was considered correct. Six blocks of 80 trials each with valid feedback were interleaved with six blocks of 20 trials each without valid feedback (Fig. 1C, see SI Appendix, SI Materials and Methods for details). This manipulation allowed us to examine adaptive behavior and learning using performance measures in nonfeedback blocks that would reflect the maintenance of the internal representation of the time interval learned during preceding feedback trials, as no external feedback was available to guide later adjustment. We used established measures of learning (i.e., error magnitude and response variability) (18) and adaptive control (i.e., adjustment efficiency) (19) to evaluate the effects of stimulation on behavior.
Experiment 1.
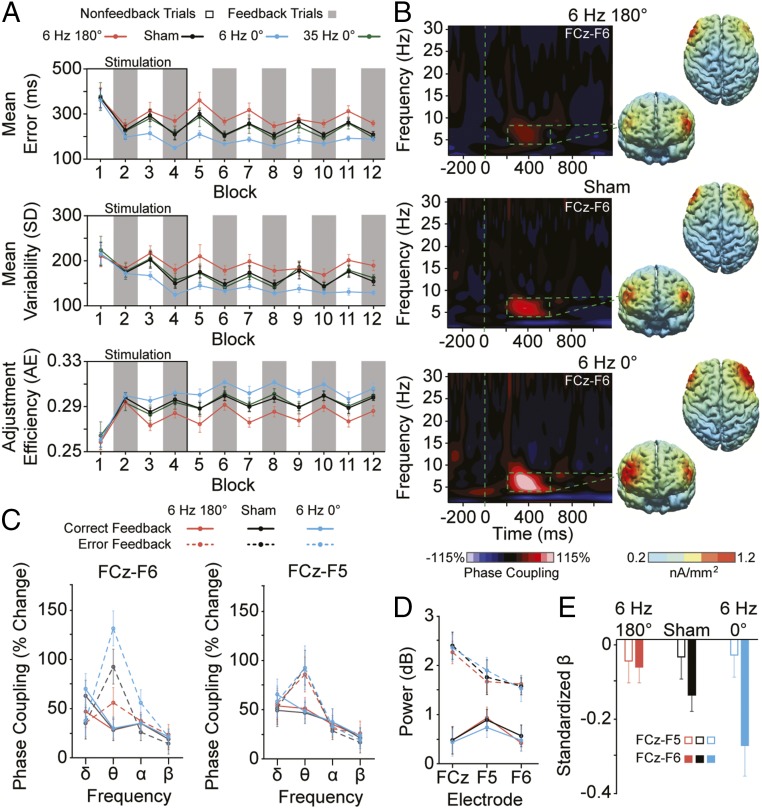
Shifting the phase of the alternating current applied simultaneously to MFC and right lPFC, switched the direction of the causal effects on learning and adaptive behavior. As shown in Fig. 2A, antiphase stimulation increased error magnitude [F(1, 29) = 6.833; P = 0.014] and response variability [F(1, 29) = 4.871; P = 0.035] and decreased adjustment efficiency [F(1, 29) = 13.149; P < 0.01] relative to sham. By stimulating subjects during their performance of the task, we could observe the emergence of the behavioral deficits that occurred roughly after the third initial block (i.e., ∼18 min) and outlasted the 40-min poststimulation period. The consistency in behavioral deficits over time prevented subjects from learning from feedback in the antiphase condition [feedback × time interactions, Fs(5, 145) < 2.039; Ps > 0.142], unlike during the sham baseline, when the same subjects showed evidence of learning and adaptive efficiency [feedback × time interactions, Fs(5, 145) > 2.716; Ps < 0.042].
Fig. 2.
Experiment 1 results. (A) Performance measures of absolute error magnitude (Top), response variability (Middle), and adjustment efficiency (Bottom) across blocks of feedback (gray) and nonfeedback (white) trials during and after antiphase (6 Hz 180°, red), sham (black), and inphase (6 Hz 0°, blue) stimulation within the same subjects. The solid green lines show data from a subset of subjects who participated in the follow-up behavioral condition in which 35-Hz inphase stimulation was administered. (B) Time-frequency representations of phase coupling between electrodes overlaying medial frontal cortex (MFC) (i.e., the frontocentral midline, FCz) and right lateral prefrontal cortex (lPFC) (i.e., F6) from error-minus-correct feedback trials recorded after antiphase (180°), sham, or inphase (0°) stimulation. Cortical source reconstruction of FCz-seeded theta (4–8 Hz) interelectrode phase coupling at peak values between 200 and 600 ms after error relative to correct feedback shown across antiphase, sham, and inphase conditions. (C) FCz-F6 (Left) and FCz-F5 (Right) phase coupling from 200 to 600 ms after correct (solid line) and error (dashed line) feedback shown across delta (1–3 Hz), theta (4–8 Hz), alpha (9–12 Hz), and beta (13–30 Hz) frequency bands, and antiphase (red), sham (black), and inphase (blue) stimulation conditions. (D) Local theta (4–8 Hz) total power, 200–600 ms after correct (solid) or error (dashed) feedback shown across stimulation conditions and electrodes of interest. (E) Aggregated individualized β-weights from bivariate regressions between error feedback-locked peak theta phase coupling and the degree of error magnitude on the trial immediately after error feedback shown across antiphase (red), sham (black), and inphase (blue) conditions. The analytic window was 4–8 Hz and 200–600 ms postfeedback. Solid bars show phase coupling measured between FCz-F6, and outlined bars show coupling from FCz-F5. Phase coupling was calculated as the percentage change from baseline (i.e., −200–0 ms before feedback onset). Error bars in A, C–E show ±1 SEM.
In contrast, inphase stimulation caused the opposite pattern of results: decreasing error magnitude [F(1, 29) = 61.058; P < 0.01] and response variability [F(1, 29) = 51.746; P < 0.01], and increasing adjustment efficiency [F(1, 29) = 43.625; P < 0.01], relative to sham. These behavioral benefits began roughly after the second initial block (i.e., ∼10 min) and continued through the full recording session. The learning improvements after inphase stimulation were so dramatic that subjects appeared to immediately reach peak levels of performance, no longer requiring explicit feedback to perform the task at high proficiency (Fig. 2A, blue lines, blocks 5–12). This observation was supported by stimulation × feedback interactions for error magnitude [F(1, 29) = 4.527; P = 0.042] and response variability [F(1, 29) = 5.368; P = 0.028] during the poststimulation period. To rule out artifacts related to the 6-Hz stimulation protocol, we administered the same inphase HD-tACS protocol except using 35-Hz alternating current. We observed no significant effects of the 35-Hz stimulation on behavior [Fs(1, 20) < 1.117; Ps > 0.303], suggesting a degree of frequency specificity in the frontal mechanism augmented by the 6-Hz stimulation driving changes in adaptive behavior (see Fig. 1C and SI Appendix, SI Results for details). Thus, by reversing the relative phase difference of the alternating current applied over medial and right lateral frontal cortices, we could rapidly and bidirectionally control components of adaptive efficiency and learning.
To test the hypothesis that the behavioral impairments/improvements caused by 6-Hz stimulation were a result of phase desynchronization/synchronization of theta rhythms between MFC and right lPFC, we examined feedback-related interareal phase coupling on valid feedback trials immediately after the 20-min stimulation period (i.e., blocks 5–12). Critically, we focused only on data collected after the stimulation period (i.e., after blocks 1–4) to avoid any confounding effects of stimulation-induced artifacts (20). We calculated interelectrode phase coupling, a measure of the consistency of phase angles between two regions averaged over trials (unweighted by magnitude information) (6, 21), focusing on electrodes overlaying the MFC (i.e., FCz) and right lPFC (i.e., F6). The surface Laplacian was used to improve spatial precision and filter out distant effects resulting from volume conduction (see SI Appendix, SI Materials and Methods for methodological details and additional analyses controlling for volume conduction) (6, 22).
We found evidence to support the hypothesis that the precise timing of MFC-right-lPFC theta phase coupling governs the flexible control of behavior. The bidirectional behavioral effects of stimulation closely mirrored the bidirectional changes in theta phase synchronization (Fig. 2 B and C). Relative to sham, 6-Hz antiphase stimulation reduced theta phase coupling over MFC and right lPFC on error versus correct feedback trials [F(1, 29) = 7.597; P = 0.01]. In contrast, inphase stimulation in the same subjects more tightly aligned the phases of the theta rhythms between these regions [F(1, 29) = 25.446; P < 0.01]. The phase-dependent stimulation effects on theta coupling showed a high degree of frequency and hemispheric (or spatial) specificity. First, no between-stimulation condition differences in phase coupling on error minus correct feedback trials were observed at other frequency bands over the right [FCz-F6, Fs(2, 58) < 2.197; Ps > 0.133] or left [FCz-F5, Fs(2, 58) < 0.630; Ps > 0.476; Fig. 2C] hemisphere. Second, theta phase coupling between electrodes overlaying MFC (FCz) and left lPFC (F5) was not modulated by stimulation [F(2, 58) = 0.666; P = 0.508; Fig. 2C]. Moreover, stimulation effects in connectivity were not accompanied by changes in local (i.e., intraelectrode intertrial) signal power in the theta band over regions of interest: MFC [FCz, F(2, 58) = 0.100; P = 0.864], right lPFC [F6, F(2, 58) = 0.409; P = 0.657], left lPFC [F5, F(2, 58) = 0.666; P = 0.508] (Fig. 2D). In sum, antiphase stimulation applied concurrently to medial and right lateral frontal cortices appeared to disrupt theta connectivity in a selective fashion, causing behavioral impairments in adaptive efficiency and learning. However, by reversing the phase angle of alternating current, we could completely flip the results, causing a preferential boost to right-lateralized theta connectivity and improvements in measures of adaptive behavior (see SI Appendix, SI Discussion for details).
The results in Fig. 2 A–D support the view that theta phase coupling mediates an MFC–right–lPFC interaction critical to components of adaptive control and learning. However, to provide a more rigorous quantification of the long-range theta dynamics underlying adaptive behavior, we performed single-trial regression analyses. As illustrated in Fig. 2E, at baseline, one-sample t tests of the individual standardized β-weights revealed that peak theta phase coupling between MFC (i.e., FCz) and right lPFC (i.e., F6) after error feedback predicted greater posterror accuracy (i.e., smaller error magnitude on the trial after error feedback; t29 = 3.277; P < 0.01). The effect was right lateralized. Theta phase coupling across the left hemisphere (i.e., from FCz to F5) did not predict single-trial fluctuations in posterror accuracy (t29 = 0.782; P = 0.441). Critically, antiphase/inphase stimulation weakened/strengthened, respectively, the single-trial relationships between right-lateralized theta phase coupling and posterror accuracy, while having no effect on connectivity–behavior relationships involving the left hemisphere [stimulation × hemisphere interaction, F(2, 58) = 6.953; P < 0.01]. Parsing this interaction revealed that after antiphase stimulation, β-weights related to FCz-F6 connectivity–behavior correlations were no longer significant (t29 = 1.259; P = 0.218) and significantly reduced relative to sham (t29 = 2.203; P = 0.036), whereas after inphase stimulation, β-weights were highly significant (t29 = 6.237; P < 0.01) and enhanced relative to sham (t29 = 2.824; P < 0.01). Thus, by applying multifocal alternating current to MFC and right lPFC, it appeared we could causally manipulate the connectivity underlying aspects of adaptive behavioral control in a bidirectional and selective manner at the single-trial level.
Experiment 2.
The results from Experiment 1 offer a striking demonstration for how high-resolution neuromodulation can be used to isolate and augment right-lateralized MFC-lPFC theta connectivity, with bidirectional effects on adaptive behavior based on the phase angle of the alternating current used. Next, we asked whether the same was true for left-hemisphere theta connectivity. Based on the lateralized nature of the single-trial brain–behavior correlations observed at baseline (Fig. 2E), and previous reports of right-lateralized feedback-related theta rhythms during reward processing, feedback learning, and action adjustments (23–26), we hypothesized that MFC–right–lPFC connectivity would be preferentially used in the causal implementation of flexible learning behavior, whereas MFC–left–lPFC connectivity would not directly influence cognitive performance. Of note, little is known about the nature of the theta dynamics that link MFC to different hemispheres of the lPFC and whether these different spatial streams of theta connectivity across the cerebral hemispheres serve different functions necessary for adaptive behavior and learning. In brief, we found that we could synchronize or desynchronize the theta rhythms over MFC and left lPFC according to the timing of the alternating current applied over these regions, paralleling the electrophysiological results from Experiment 1, using right-lateralized stimulation. However, surprisingly, this causal manipulation had no effect on behavior (see SI Appendix, SI Results and Fig. S1 for details). The findings across Experiments 1 and 2 suggest a functional asymmetry in frontal cortex, whereby left-lateralized theta connectivity, although potentially involved in the communication of the adjusted action plan, does not appear to function like right-lateralized connectivity in bringing about the actual implementation of the flexible behavior. These results are consistent with neuroimaging studies suggesting a dominant role for right lPFC in cognitive action control (27, 28), but future work is needed to clarify the functional relevance of left-lateralized theta connectivity, which may be revealed using tasks that elicit greater left-hemisphere synchronization, such as those involving positive affect or linguistic processing (see SI Appendix, SI Discussion for additional information).
Experiment 3.
In Experiment 3, we sought a more rigorous demonstration of causal control over adaptive human behavior with greater potential real-world and clinical applicability. We asked whether it was possible to pit the opposing causal effects of the stimulation protocols we developed in Experiment 1 against each other. Specifically, could we use antiphase HD-tACS to induce performance deficits, and then immediately rescue behavior using inphase stimulation? Various forms of frontal cortical dysconnectivity and maladaptive control are consistently observed in neurological and neuropsychiatric disorders (9, 29, 30), and thus efforts to build novel drug-free tools for restoring neurocognitive function and behavior have important implications for human health and disease.
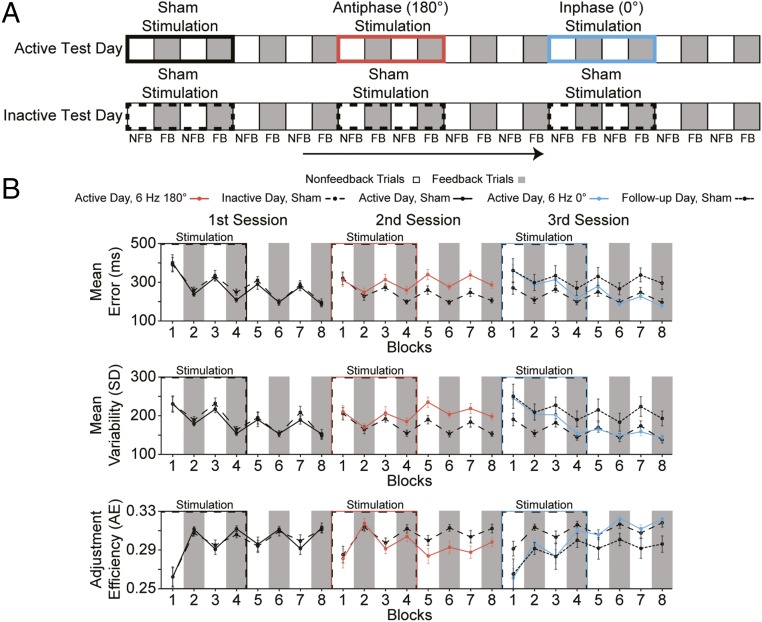
In Experiment 3, all subjects underwent two different test days, each with three consecutive stimulation sessions administered while subjects performed the time-estimation task (Fig. 3A). On one test day, subjects received a sequence of sham, antiphase, and inphase stimulation, whereas on another day, the same subjects received three consecutive sham stimulation sessions. The order of active and sham test days were counterbalanced across subjects (see SI Appendix, SI Materials and Methods for details).
Fig. 3.
Experiment 3 design and results. (A) Schematic illustration of the experimental design. Each participant underwent an active test day consisting of three consecutive stimulation sessions (i.e., sham, antiphase, inphase) and an inactive test day consisting of three consecutive sham stimulation sessions. On each day, behavior was recorded for ∼120 min while subjects performed the time-estimation task in which blocks of feedback (FB, gray) and nonfeedback (NFB, white) trials were interleaved. (B) Performance measures of absolute error magnitude (Top), response variability (Middle), and adjustment efficiency (Bottom) across blocks of feedback (gray) and nonfeedback (white) trials are shown during and after the sham (solid black), antiphase (6 Hz 180°, red), and inphase (6 Hz 0°, blue) stimulation sessions on the active test day, and the three sham (dashed black) stimulation sessions on the inactive test day within the same subjects. The dotted black lines in the third session show data from the final sham session of the follow-up test in which a subset of subjects from Experiment 3 received the sequence of sham, antiphase, and sham stimulation to determine whether the deleterious effects of antiphase stimulation would continue over a more protracted time course or naturally improve and return to baseline levels without the assistance of inphase stimulation. Error bars show ±1 SEM.
The results from Experiment 3 showed that we could not only replicate the behavioral results from Experiment 1, using antiphase stimulation, but that the inphase protocol could correct all antiphase induced behavioral deficits and instantly return subjects’ performance to baseline levels in less than 20 min. Consistent with Experiment 1 (Fig. 2A), antiphase stimulation increased error magnitude [F(1, 29) = 19.150; P < 0.01] and response variability [F(1, 29) = 21.540; P < 0.01] and decreased adjustment efficiency [F(1, 29) = 14.621; P < 0.01] compared with the chronologically equivalent session on the sham test day (Fig. 3B). Surprisingly, after three blocks (i.e., ∼18 min) of inphase stimulation, all performance deficits were effectively normalized, such that measures of error magnitude [F(1, 29) = 0.332; P = 0.569], response variability [F(1, 29) = 0.181; P = 0.673], and adjustment efficiency [F(1, 29) = 0.730; P = 0.400] recorded from blocks 4–8 were statistically indistinguishable from the chronologically equivalent sham session. Experiment 3 demonstrated the plasticity of a phase-sensitive mechanism in frontal cortex and how neuromodulation technology could exploit this mechanism’s plasticity to disrupt and then rapidly repair adaptive control and learning in the same individuals. The results suggest that functionally relevant adult brain plasticity can be augmented on the timescale of minutes with an immediate effect on adaptive behavior.
To test whether the behavioral effects of Experiment 3 could be a result of fluctuations in baseline behavior across days, creating a potential confound in the results related to the electrical brain stimulation, we performed test–retest reliability analyses by comparing data during the first sham sessions across days. For this analysis, we compared error magnitude, response variability, and adjustment efficiency data collected during the first session of each test day in which sham stimulation was administered (Fig. 3B, first session, blocks 1–8, solid vs. dashed black lines). We found evidence of successful learning exhibited by significant feedback × time interactions [Fs(3, 87) > 5.734; Ps < 0.01]. However, critically, no main effects of test day [Fs(1, 29) < 0.027; Ps > 0.872] or interactions involving test day [Fs(3, 87) < 0.585; Ps > 0.596] were observed. In addition, individual behavioral values in these sham conditions across test days were significantly correlated (Spearman’s rhos > 0.621; Ps < 0.01). The results indicate that the primary performance metrics on this classic time estimation task were rather stable over a period of approximately 1 wk (average time between testing, 8.3 ± 2.5 d) and further strengthen confidence in the validity of the stimulation-induced behavioral results obtained across Experiments 1–3.
In Experiment 3, we found that inphase stimulation could recover adaptive behavior that was artificially impaired, using antiphase stimulation. We based this interpretation on the observation that subjects’ measures of adaptive control and learning improved after inphase stimulation, eventually returning to baseline sham levels of performance. However, it is also possible that inphase stimulation had no effect and the behavioral measures simply improved on their own as a function of time and further practice on the task, as the deleterious effects of antiphase stimulation gradually wore off. To test this alternative hypothesis, we invited back all 30 participants from Experiment 3 to participate in an additional test day in which we applied the sequence of sham, antiphase, and sham stimulation. On the basis of data from the 19 subjects who returned, we found that the impairments induced by antiphase stimulation were not naturally resolved with time and further practice, but continued throughout the full sham session after antiphase stimulation (Fig. 3B, third session, solid black lines). This was demonstrated by significant effects of stimulation, increasing error magnitude [F(1, 18) = 14.217; P < 0.01] and response variability [F(1, 18) = 20.719; P < 0.01], and decreasing adjustment efficiency [F(1, 18) = 13.642; P < 0.01] in the sham session after an antiphase session (Fig. 3B, third session, solid black lines), relative to the sham session after a sham session (Fig. 3B, third session, dashed black lines). These results are consistent with the enduring impairments we observed in Experiment 1 after antiphase stimulation (Fig. 2A) and support the interpretation that by reversing the relative phase difference of HD-tACS applied over MFC and right lPFC, we could effectively rescue impaired adaptive control in healthy individuals.
Discussion
Here, we provide evidence for a causal relation between interareal theta phase synchronization in frontal cortex and multiple components of adaptive human behavior. The results support the idea that the precise timing of rhythmic population activity spatially distributed in frontal cortex conveys information to direct behavior (31–35). Given previous work showing that phase synchronization can change spike time-dependent plasticity (35–37), together with our findings showing stimulation effects on neural activity and behavior can outlast a 20-min period of electrical stimulation (Fig. 2A), it is reasonable to suppose that the externally modulated interareal coupling changed behavior by causing neuroplastic modifications in functional connectivity. That is, the results suggest the striking conclusion that we may be able to noninvasively intervene in the temporal coupling of distant rhythmic activity in the human brain to optimize (or impede) the postsynaptic effect of spikes from one area on the other, improving (or impairing) the cross-area communication necessary for cognitive action control and learning (see SI Appendix, SI Discussion for additional theorizing). Moreover, these neuroplastic alterations in functional connectivity were induced with a 0° phase, suggesting that inducing synchronization does not require a meticulous accounting of the communication delay between regions such as MFC and lPFC to effectively modify behavior and learning. This finding conforms to empirical and modeling work showing that despite long axonal conduction delays between distant brain areas, theta phase synchronizations at 0° phase lag can occur between these regions and underlie meaningful functions of cognition and action (35, 38, 39). It is also possible that a third subcortical or posterior region with a nonzero time lag interacted with these two frontal areas to drive changes in goal-directed behavior.
Finally, the results showing that patterns of synchronization in frontal cortex can be exogenously isolated and enhanced are potentially relevant to the understanding and treatment of brain disorders associated with cortical hypoconnectivity, such as Alzheimer’s disease, autism, and schizophrenia (8, 9), whereas the results showing that synchronization can be effectively reduced might be useful in addressing the hyperconnectivity impairments observed in disorders such as epilepsy and Parkinson’s disease (9). Future work is needed in basic and clinical science to determine the true applicability of the HD-tACS protocols as potential therapeutic tools. This includes more fully investigating the effects of stimulation on other components of executive function, tracking the full time course of the neural and behavioral gains and losses associated with each protocol, and determining whether the effects can be prolonged and made more potent by modifying stimulation parameters, such as stimulation duration and intensity, repeated stimulation sessions, and the pairing of stimulation with cognitive training. The potential seems high for capitalizing on the experience-dependent plasticity underlying training- and stimulation-induced cognitive enhancement and for maximizing intervention strategies to rescue cognitive functions in patient populations.
Materials and Methods
Materials and methods used in this study are discussed in SI Appendix, SI Materials and Methods. Briefly, all participants gave written informed consent approved by the Boston University Institutional Review Board and were paid. All experiments were within-subjects, sham-controlled, and double blind, in which subjects received 20 min of HD-tACS while performing a time estimation task (Fig. 1B). Subjects’ EEG was continuously recorded (2,048-Hz sampling rate, 0.05–200-Hz bandpass filter) while they performed this task. The electrophysiological and behavioral data were analyzed offline. Debriefing questions confirmed that subjects were blind to the nature of the stimulation conditions.
Supplementary Material
Acknowledgments
I thank John Nguyen for help with data collection and the editor and reviewers for their valuable comments and thoughtful suggestions. This work was supported by National Institutes of Health Grant R01 MH 114877.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710257114/-/DCSupplemental.
References
- 1.Bellman R, Kalaba R. A mathematical theory of adaptive control processes. Proc Natl Acad Sci USA. 1959;45:1288–1290. doi: 10.1073/pnas.45.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 3.Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal systems behavior scale in schizophrenia: Relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald KD, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 5.van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Res. 2007;151:211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart RMG, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci USA. 2015;112:9448–9453. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhart RMG, Zhu J, Park S, Woodman GF. Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction-error signaling. J Neurosci. 2015;35:12232–12240. doi: 10.1523/JNEUROSCI.1717-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Kurland J, Baldwin K, Tauer C. Treatment-induced neuroplasticity following intensive naming therapy in a case of chronic wernicke’s aphasia. Aphasiology. 2010;24:737–751. [Google Scholar]
- 11.Thrane G, Friborg O, Anke A, Indredavik B. A meta-analysis of constraint-induced movement therapy after stroke. J Rehabil Med. 2014;46:833–842. doi: 10.2340/16501977-1859. [DOI] [PubMed] [Google Scholar]
- 12.Anguera JA, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen AM, et al. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett A, et al. The effect of an online cognitive training package in healthy older adults: An online randomized controlled trial. J Am Med Dir Assoc. 2015;16:990–997. doi: 10.1016/j.jamda.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MX, Wilmes K, Vijver Iv. Cortical electrophysiological network dynamics of feedback learning. Trends Cogn Sci. 2011;15:558–566. doi: 10.1016/j.tics.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 18.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 19.Luft CDB, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. J Neurosci. 2013;33:2029–2038. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noury N, Hipp JF, Siegel M. Physiological processes non-linearly affect electrophysiological recordings during transcranial electric stimulation. Neuroimage. 2016;140:99–109. doi: 10.1016/j.neuroimage.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Reinhart RMG, Woodman GF. Oscillatory coupling reveals the dynamic reorganization of large-scale neural networks as cognitive demands change. J Cogn Neurosci. 2014;26:175–188. doi: 10.1162/jocn_a_00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal oscillatory dynamics predict feedback learning and action adjustment. J Cogn Neurosci. 2011;23:4106–4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- 24.Marco-Pallares J, et al. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie GJ, Tata MS. Right frontal cortex generates reward-related theta-band oscillatory activity. Neuroimage. 2009;48:415–422. doi: 10.1016/j.neuroimage.2009.06.076. [DOI] [PubMed] [Google Scholar]
- 27.Shallice T, Gazzaniga MS. The Cognitive Neuroscience. MIT Press; Cambridge, MA: 2004. The fractionation of supervisory control; pp. 943–956. [Google Scholar]
- 28.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 29.Dalley JW, Robbins TW. Fractionating impulsivity: Neuropsychiatric implications. Nat Rev Neurosci. 2017;18:158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- 30.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 31.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 32.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 33.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 34.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 36.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: A network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- 39.Vicente R, Gollo LL, Mirasso CR, Fischer I, Pipa G. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc Natl Acad Sci USA. 2008;105:17157–17162. doi: 10.1073/pnas.0809353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.