Significance
Much recent research into the origins of life focuses on the hypothesis that RNA emerged on early Earth by an abiotic process, and gave Earth its first access to Darwinian evolution. This article provides a key step in this process. Here, we show that the phosphorylated ribonucleoside building blocks for RNA can be made stereoselectively under a prebiotic plausible condition with canonical and noncanonical purines, and with one noncanonical pyrimidine. It also shows that threose nucleoside phosphates can be synthesized in a similar way. This result is significant in terms of numbers of steps, high stereo- and regiochemistry, scope, involvement of minerals, and likelihood of the prebiotic availability of its starting materials.
Keywords: prebiotic synthesis, nucleotide, phosphorylated carbohydrate
Abstract
According to a current “RNA first” model for the origin of life, RNA emerged in some form on early Earth to become the first biopolymer to support Darwinism here. Threose nucleic acid (TNA) and other polyelectrolytes are also considered as the possible first Darwinian biopolymer(s). This model is being developed by research pursuing a “Discontinuous Synthesis Model” (DSM) for the formation of RNA and/or TNA from precursor molecules that might have been available on early Earth from prebiotic reactions, with the goal of making the model less discontinuous. In general, this is done by examining the reactivity of isolated products from proposed steps that generate those products, with increasing complexity of the reaction mixtures in the proposed mineralogical environments. Here, we report that adenine, diaminopurine, and hypoxanthine nucleoside phosphates and a noncanonical pyrimidine nucleoside (zebularine) phosphate can be formed from the direct coupling reaction of cyclic carbohydrate phosphates with the free nucleobases. The reaction is stereoselective, giving only the β-anomer of the nucleotides within detectable limits. For purines, the coupling is also regioselective, giving the N-9 nucleotide for adenine as a major product. In the DSM, phosphorylated carbohydrates are presumed to have been available via reactions explored previously [Krishnamurthy R, Guntha S, Eschenmoser A (2000) Angew Chem Int Ed 39:2281–2285], while nucleobases are presumed to have been available from hydrogen cyanide and other nitrogenous species formed in Earth’s primitive atmosphere.
Life on Earth is thought to have begun with the emergence of an informational molecule that could be replicated, with errors, where those errors are themselves replicable. These are believed to be necessary and perhaps sufficient features to support Darwinian evolution, which in turn is believed to be the only mechanism by which organic matter can spontaneously self-assemble to give properties that we value in life (1, 2). Based on an analysis of its role in modern biology and its presumed increased role in ancient biology, RNA is one of the most prominently sought first informational molecules, although threose nucleic acid (TNA) (3) and peptide nucleic acid (4) have both been proposed as alternative candidates. However, considering their watery environment, the first genetic polymers likely had repeating charges in their backbones (5). TNA and RNA both have these, and are polyelectrolytes.
Therefore, most current efforts in prebiotic chemistry have concentrated on seeking pathways to make RNA (or TNA) from materials that were formed without life on early Earth (6). Nucleosides are subunits of these biopolymers, and multiple prebiotic routes to these have been proposed.
For example, the direct condensation of ribose itself with purine nucleobases (adenine and hypoxanthine) is known to provide the corresponding ribonucleosides (7). However, such procedures, as previously reported, suffer from low yields for β-furanonucleosides and the formation of mixtures as a result of multiple nucleophilic centers on the heterocycle, multiple ring sizes (furanose and pyranose) of the ribose, and reactions that lack stereo- and/or regioselectivity.
Accordingly, a second approach considers condensation of fragments of the nucleobase and/or the carbohydrate to form a composite, with the nucleoside “finished” after the glycosyl bond is formed. For example, Carell and coworkers (8) reported the reaction of ribose (and ribose-borate) with formylated aminopyrimidine nucleobase fragments that, after coupling, further react to produce purine nucleosides. This reaction is regioselective with respect to the nucleobase, giving N-9 purine nucleosides in high yield. With respect to the carbohydrate, both furanoses and pyranoses were formed.
Pyrimidine nucleosides have always been more difficult to obtain under prebiotic conditions. However, Sanchez and Orgel (9) reported many years ago the formation of cytidine derivative by the reaction of a ribose derivative with cyanamide to give an aminooxazoline; the synthesis of the heterocycle was finished by reaction with cyanoacetylene and phosphate-assisted ring opening to make cytidine nucleotide (10). More recently, Powner et al. (11) produced pyrimidine nucleoside phosphates by a process where both the carbohydrate and the nucleobase were introduced as fragments, and finished after the two precursor fragments were coupled.
We return here to a search for pathways to nucleos(t)ides that used completed heterocycles as starting materials (Fig. 1). This would avoid the requirement for prebiotic availability of the reactive cyanamide and cyanoacetylene. Nucleobases as metastable units may have been prebiotically available by either polymerization of hydrogen cyanide (HCN) (12, 13) or by thermal condensation of formamide in the presence of borate minerals (14).
Fig. 1.
Prebiotic pathway to ribo- and threofuranosyl nucleotides. The reaction of phosphorylated ribose (5) and threose (7) with nucleobases yielded ribo- and threofuranosyl nucleoside 2′-phosphates. Phosphorylated carbohydrates 5 and 7 can be available from ribose 4 and threose (6) and amidotriphosphate (3) in prebiotic plausible conditions.
We were further inspired by work of Krishnamurthy et al. (15) that used an amidotriphosphate (16) derived from cyclic trimetaphosphate (17) as a prebiotic reagent to add phosphate to prebiotic carbohydrates to give cyclic sugar phosphates. Previous work on the “Discontinuous Synthesis Model” (DSM) (18) for the prebiotic formation of RNA suggested that such carbohydrates might have been present prebiotically through the borate-moderated condensation of glycolaldehyde, glyceraldehyde (19, 20). Threose might have been present, perhaps even in enantiomerically enriched form, by the peptide-catalyzed condensation of glycolaldehyde alone (21, 22). Glycolaldehyde, in turn, may have been prebiotically available by electrical discharge of humid CO2 atmospheres (23), or by the reaction of formaldehyde with HCN by photoreduction (24). It is also found around solar-type young stars (25).
Results
As the first step, we tested the reaction of ribose-1,2-cyclic phosphate (5), a product reported as a possible prebiotic intermediate by Krishnamurthy et al. (15), with adenine (8). This reaction, done by mixing the components and heating at 85 °C, gave adenosine-2′-phosphate (11) in 15% yield as a major product (Fig. 2). The identity of 11 was confirmed by the comparison with authentic commercial adenosine-2′-phosphate, which showed identical behavior in HPLC mobility. Further proof of the structure was obtained using NMR spectroscopy with isolated material (SI Appendix, sections 3.13–3.15).
Fig. 2.
Synthesis of purine and pyrimidine ribonucleoside-2′-phosphates by the reaction of ribose-1,2-cyclic phosphate (5) and nucleobases in the presence of metal ions (calcium or magnesium). The reaction was analyzed by reversed-phase HPLC. (A) HPLC of the reaction of 5 and adenine (8). (B) HPLC of adenosine 2′-monophosphate. (C) HPLC of coinjection of A and B. (D) Extracted UV spectrum of the peak at 12.2 min of B. For the detailed characterization of the reaction of 5 with 8, 9, 10, 11, see SI Appendix.
However, entirely dispositive with respect to the particular regioisomer formed with respect to the heterocycle was UV spectroscopy, as the N-9 purine nucleoside (adenosine) shows UV absorption maximum at 256 nm at pH 3.3 and 259 nm at pH 7. This is different from a nucleoside analog connected through exocyclic NH2 of adenine, which has UV absorption maxima at 264 nm at pH 3.3 and 267 nm at pH 7 (SI Appendix, section 2.10). UV absorption spectroscopy (Fig. 2) clearly establishes this product as the N-9 purine nucleoside. The synthesis conditions also yielded two other purine nucleoside phosphates, hypoxanthine phosphate (9) and 2,6-diaminopurine phosphates (10) (SI Appendix, sections 2.2 and 2.3).
Interestingly, whereas the reaction between ribose-1,2-cyclic phosphate (5) gave purine nucleotides fairly well, it did not provide the analogous pyrimidine nucleotides with the standard pyrimidine bases, uracil or cytosine. However, the reaction did work quite well with pyrimidin-2-one (14) as a nucleobase. Although pyrimidin-2-one nucleoside is not standard in modern RNA, it may have existed in the first RNA-like polymers, to be subsequently replaced by uracil or cytosine (26).
We then asked about the position of the phosphate groups, which ends up this process at the 2′- position. This is not the side of linkage in standard RNA, where the phosphate is presented at either the 3′- or the 5′-position. However, we found that ribonucleoside-2′-phosphates can be converted to the corresponding 5′-phosphates by incubation in the presence of borate, which preferentially coordinates free 2′- and 3′-hydroxyl groups. Accordingly, heating adenosine-2′-phosphate with urea, borate, and phosphate provided adenosine-5′-phosphate and adenosine (Fig. 3A). The resulting adenosine-5′-phosphate would then feed into the next step of the DSM to be used, once activated, for oligomeric RNA synthesis (27).
Fig. 3.
Reversed-phase HPLC profile of the incubation of adenosine-2′-phosphate (11). (A) Incubation of adenosine-2′-phosphate in the presence of urea, borate, and sodium phosphate. (B) Incubation of adenosine-2′-phosphate in the presence of urea, sodium phosphate, and no borate.
Further experiments showed that borate was important to this process. Without borate, incubation of adenosine-2′-phosphate with urea resulted in the cleavage of 2′-phosphate group to give free phosphate, adenosine, and the nucleoside, as well as a complex mixture of phosphorylation products that included monophosphates (2′-, 3′-, 5′-, and 2′,3′-cyclic, etc.) and diphosphate (Fig. 3B). This suggests that borate plays an important role not to waste prebiotic material by controlling the reactivity of hydroxyl groups by coordinating to cis-diol of ribonucleotide and forces phosphorylation on the 5′-OH (28).
TNA is an alternative potential prebiotic informational polymer that might have appeared on Earth before the RNA world (3). Accordingly, we examined the direct condensation of threose and adenine under the same dry conditions. The reaction provided two products having together as much as a 70% yield (SI Appendix, section 2.9). Those products showed the same mass spectra as synthetic threofuranosyl adenine (40 in SI Appendix). However, their HPLC mobilities did not match those of 40. This suggested that those products have nucleosidic bonds on exocyclic NH2 group of adenine (SI Appendix, section 1.9). This suggestion was confirmed by UV spectroscopy.
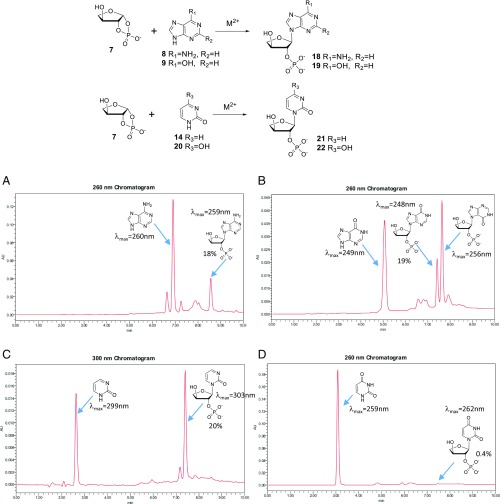
However, reaction of threose-1,2-cyclic phosphate (7) and purine and noncanonical pyrimidine nucleobases gave threose nucleoside-2′-phosphates (Fig. 4 and SI Appendix, section 5.3). These structures were proven by comparison with authentic synthetic compounds (SI Appendix, sections 1 and 2). Here, the glycosidic bond is formed to a ring nitrogen, not to an exocyclic amino group, as judged by UV spectroscopy.
Fig. 4.
Synthesis of purine and pyrimidine threofuranosyl nucleoside 2′-phosphates by the reaction of threose 1,2-cyclic phosphate (7) and nucleobases in the presence of metal ions (calcium or magnesium). The reaction was analyzed by reversed-phase HPLC. (A) HPLC of the reaction of 7 and adenine (8). (B) HPLC of the reaction of 7 and hypoxanthine (9). (C) HPLC of the reaction of 7 and pyrimidin-2-one (14). (D) HPLC of the reaction of 7 and uracil (20).
Threose-1,2-cyclic phosphate proved to be under these conditions a slightly more receptive carbohydrate than ribose. Thus, whereas ribose-1,2-cyclic phosphate (5) does not react with uracil (20) to any detectable extent, threose-1,2-cyclic phosphate (7) reacted with uracil (20) to produce the corresponding nucleotide in 0.4% yield as its 2′-phosphorylated derivative. Here, of course, the phosphate is at a site involved in internucleotides in TNA. Thus, TNA remains an interesting alternative to RNA, if only as the sequential process that eventually generates RNA on a prebiotic Earth (29).
Discussion
These results show that the reaction of ribose-1,2-cyclic phosphate (5) and threose-1,2-cyclic phosphate (7) with a range of purine nucleobases gives ribonucleosides or threonucleosides first as their 2′-phosphates. The condensation reaction is stereoselective, giving β-nucleotides only, and regioselective; N-9 nucleotides predominate in the case of adenine, and 2,6-diaminopurine and N-3 nucleotides are major products for 2-pyrimidinone. Thus, they suggest that the direct coupling of preformed carbohydrates and preformed heterocycles need not be considered a prebiotic dead-end.
The stereoselectivity of the reaction can be rationalized by the cyclic structure of the cyclic carbohydrate phosphates 5 and 7. Since these reactions are not catalyzed by any enzyme-like macromolecules, the reactive intermediate is not thought to be involvement of an oxocarbenium ion, but rather to proceed by concerted mechanism (30). Under this rationalization, reaction from the α-face is blocked by the cyclic phosphate, which activates the 1-position of the carbohydrates. This reactivity and stereoselectivity resemble the modern organic synthetic method that uses Vorbrüggen reaction conditions, which also has a cyclic intermediate as a reactive species (31).
The coupling reaction proceeds under dry state at elevated temperature (≥70 °C). Further, the reaction requires divalent metal ions, magnesium or calcium. In some cases, the presence of ammonium formate increases the reaction yield (Methods and SI Appendix, section 5).
Finally, although borate need not be present for the coupling reaction, it does control subsequent reactivity of the 2′-phosphorylated nucleoside derivatives. In the presence of borate, these 2′-phosphorylated nucleoside derivatives undergo rearrangement in urea in the presence of inorganic phosphate to give the 5′-phosphorylated nucleoside derivatives. Absent borate, complex mixtures are seen. Borate is also useful in making the precursor carbohydrates without substantial decomposition.
Whether the coupling of carbohydrate-1,2-cyclic phosphate and nucleobases is a feasible prebiotic reaction pathway to nucleoside phosphates therefore rests on the availability of various precursor components. These include cyclic trimetaphosphate, ammonia, and ribose (or threose), which generate the cyclic phosphate precursors. These in turn rely on the availability of glyceraldehyde and glycolaldehyde, or glycolaldehyde with formaldehyde in the presence of borate. For the nucleobases, this presumes a hydrogen cyanide-type reaction to generate them, either directly or indirectly by way of formamide.
Methods
Reaction of Ribose-1,2-Cyclic Phosphate (5) and Nucleobases.
Ribose-1,2-cyclic phosphate (5) was prepared following the published method (15, 32). The reaction of 5 and nucleobases was conducted in an Eppendorf tube containing nucleobase [adenine (8), hypoxanthine (9), 2,6-diaminopurine (10), or 2-hydroxypyrimidine hydrochloride (14), each 5 μL of 3.75 mM], ribose 1,2-cyclic phosphate (5) (5 μL of 15 mM), either MgCl2 (5 μL of 3.75 mM) or CaCl2 (5 μL of 3.75 mM), with/without ammonium formate (5 μL of 3.75 mM). The tube was placed in an oven at 85 °C for 18 h with the lid open. It was resuspended in water (0.3 mL) and analyzed by reversed-phase HPLC with 20 μL of injection. The yield (based on the amount of starting nucleobase) of the reactions is summarized in SI Appendix, section 5.1.
Preparative Synthesis of Adenosine-2′-Phosphate (11).
An aqueous mixture containing adenine (7.5 mM, 0.8 mL), ribose-1,2-cyclic phosphate (15 mM, 0.8 mL), and CaCl2 (150 mM, 0.16 mL) was dried and heated at 85 °C for 18 h. It was dissolved in water (6 mL) and purified on ion-exchange prep HPLC to give white solid after lyophilization (∼0.3 mg, 12% based on adenine). Preparative HPLC purification was done using an ion-exchange column (22 mm i.d., 250 mm length, 5 µm; DNAPac PA-100; Thermo Fisher Scientific) on a Waters Delta 600 module. The column was eluted with a gradient of (A) water and (B) 1 M ammonium bicarbonate. The elution program created a linear gradient started from 100% A to 70% A at 15 min with flow rate of 10 mL/min. Peak detection was conducted using the 260-nm absorbance.
HPLC Analysis of the Reaction Products of Ribose-1,2-Cyclic Phosphate (5) and Nucleobases.
HPLC analysis was done with a C-18 reversed-phase narrow-bore column (3 mm i.d., 150 mm length, 5 μm; SunFire; Waters) on a Waters 2695 separation module equipped with 996 photodiode array detector. The column was eluted with a gradient of (A) aqueous 20 mM KH2PO4 with 5 mM tetrabutylammonium bromide (pH 3.3, adjusted by phosphoric acid) and (B) 100% acetonitrile. The elution program created a linear gradient that started from 99% A to 2.5 min, 97% at 5.5 min, 89.5% at 17.5 min, and 65.0% at 23.5 min with total flow rate of 0.8 mL/min. Peak detection and integration were conducted with the signal at 260 nm for adenine, hypoxanthine, 2,6-diaminopurine and 300 nm for pyrimidin-2-one. Full UV spectra (230 ∼ 400 nm) were also obtained.
The yield of the coupling reaction was determined by the peak integration of the products compared with the integration of nucleosides having the same nucleobases (SI Appendix, section 5.2).
Reaction of Threose-1,2-Cyclic Phosphate (7) and Nucleobases.
Threose-1,2-cyclic phosphate (7) was prepared following the published method (15). The reaction was conducted in an Eppendorf tube containing nucleobase [adenine (8), hypoxanthine (9), 2-hydroxypyrimidine hydrochloride (14), each 5 μL of 3.75 mM], threose-1,2-cyclic phosphate (7) (5 μL of 15 mM), either MgCl2 (5 μL of 3.75 mM) or CaCl2 (5 μL of 3.75 mM) with/without ammonium formate (5 μL of 3.75 mM). The tube was placed in an oven at 70 °C for 18 h with the lid open. It was resuspended in water (0.3 mL) and analyzed by reversed-phase HPLC with 20 μL of injection. The yield of the reactions is summarized in SI Appendix, section 5.3.
Reaction of Threose-1,2-Cyclic Phosphate (7) and Uracil.
The reaction was conducted in an Eppendorf tube containing uracil (5 μL of 3.75 mM), threose-1,2-cyclic phosphate (7) (5 μL of 15 mM), MgCl2 (5 μL of 3.75 mM), and sodium hydroxide (5 μL of 100 mM). The tube was placed in an oven at 70 °C for 18 h with the lid open. It was resuspended in 1 M TEAA buffer (0.3 mL) and analyzed by reversed-phase HPLC with 20 μL of injection.
HPLC Analysis of the Reaction Products of Threose-1,2-Cyclic Phosphate (7) and Nucleobases.
HPLC analysis was done with a C-18 reversed-phase narrow-bore column (3 mm i.d., 150 mm length, 5 μm; SunFire; Waters) on a Waters 2695 separation module equipped with 996 photodiode array detector. The column was eluted with a gradient of (A) aqueous 25 mM triethylammonium acetate and (B) 100% acetonitrile. The elution program created a linear gradient started from 100% (by volume) A to 85% A at 10 min with flow rate of 0.5 mL/min. Peak detection and integration were conducted with the signal at 260 nm for adenine, hypoxanthine, uracil and 300 nm for 2-hydroxypyrimidine. Full UV spectra (230 ∼ 400 nm) were also obtained.
The yield of the coupling reaction was determined by the peak integration of the products compared with the integration of nucleosides having the same nucleobases (SI Appendix, section 5.4).
Supplementary Material
Acknowledgments
We thank Professor Andrew Ellington and one unnamed referee for calling our attention to specific literature. This publication was made possible through the support of John Templeton Foundation Grant 54466. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710778114/-/DCSupplemental.
References
- 1.Woese C. The Genetic Code. Harper & Row; New York: 1967. pp. 179–195. [Google Scholar]
- 2.Crick FHC. The origin of the genetic code. J Mol Biol. 1968;38:367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- 3.Schöning K, et al. Chemical etiology of nucleic acid structure: The alpha-threofuranosyl-(3′–>2′) oligonucleotide system. Science. 2000;290:1347–1351. doi: 10.1126/science.290.5495.1347. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 5.Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- 6.Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol. 2004;39:99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 7.Fuller WD, Sanchez RA, Orgel LE. Studies in prebiotic synthesis: VII. Solid-state synthesis of purine nucleosides. J Mol Evol. 1972;1:249–257. doi: 10.1007/BF01660244. [DOI] [PubMed] [Google Scholar]
- 8.Becker S, et al. A high-yielding, strictly regioselective prebiotic purine nucleoside formation pathway. Science. 2016;352:833–836. doi: 10.1126/science.aad2808. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez RA, Orgel LE. Studies in prebiotic synthesis. V. Synthesis and photoanomerization of pyrimidine nucleosides. J Mol Biol. 1970;47:531–543. doi: 10.1016/0022-2836(70)90320-7. [DOI] [PubMed] [Google Scholar]
- 10.Tapiero CM, Nagyvary J. Prebiotic formation of cytidine nucleotides. Nature. 1971;231:42–43. doi: 10.1038/231042a0. [DOI] [PubMed] [Google Scholar]
- 11.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 12.Oro J. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature. 1961;191:1193–1194. doi: 10.1038/1911193a0. [DOI] [PubMed] [Google Scholar]
- 13.Orgel LE. Prebiotic adenine revisited: Eutectics and photochemistry. Orig Life Evol Biosph. 2004;34:361–369. doi: 10.1023/b:orig.0000029882.52156.c2. [DOI] [PubMed] [Google Scholar]
- 14.Saladino R, Barontini M, Cossetti C, Di Mauro E, Crestini C. The effects of borate minerals on the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. Orig Life Evol Biosph. 2011;41:317–330. doi: 10.1007/s11084-011-9236-3. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy R, Guntha S, Eschenmoser A. Regioselective α-phosphorylation of aldose in aqueous solution. Angew Chem Int Ed. 2000;39:2281–2285. [PubMed] [Google Scholar]
- 16.Feldmann W, Thilo E. Zur chemie der kondensierten phosphate und arsenate. XXXVIII. Amidotriphosphat. Z Anorg Allg Chem. 1964;328:113–126. [Google Scholar]
- 17.Pasek MA, Kee TP, Bryant DE, Pavlov AA, Lunine JI. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew Chem Int Ed Engl. 2008;47:7918–7920. doi: 10.1002/anie.200802145. [DOI] [PubMed] [Google Scholar]
- 18.Benner SA, Kim HJ, Carrigan MA. Asphalt, water, and the prebiotic synthesis of ribose, ribonucleosides, and RNA. Acc Chem Res. 2012;45:2025–2034. doi: 10.1021/ar200332w. [DOI] [PubMed] [Google Scholar]
- 19.Ricardo A, Carrigan MA, Olcott AN, Benner SA. Borate minerals stabilize ribose. Science. 2004;303:196. doi: 10.1126/science.1092464. [DOI] [PubMed] [Google Scholar]
- 20.Neveu M, Kim HJ, Benner SA. The “strong” RNA world hypothesis: Fifty years old. Astrobiology. 2013;13:391–403. doi: 10.1089/ast.2012.0868. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, et al. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J Am Chem Soc. 2011;133:9457–9468. doi: 10.1021/ja201769f. [DOI] [PubMed] [Google Scholar]
- 22.Weber AL, Pizzarello S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc Natl Acad Sci USA. 2006;103:12713–12717. doi: 10.1073/pnas.0602320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löb W. Über das verhalten des formamids unter der wirkung der stillen entladung ein beitrag zur frage der stickstoff-assimilation. Ber Dtsch Chem Ges. 1913;46:684–697. [Google Scholar]
- 24.Ritson DJ, Sutherland JD. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew Chem Int Ed Engl. 2013;52:5845–5847. doi: 10.1002/anie.201300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jørgensen JK, et al. Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA. ApJL. 2012;757:L4. [Google Scholar]
- 26.Bean HD, et al. Formation of a β-pyrimidine nucleoside by a free pyrimidine base and ribose in a plausible prebiotic reaction. J Am Chem Soc. 2007;129:9556–9557. doi: 10.1021/ja072781a. [DOI] [PubMed] [Google Scholar]
- 27.Walton T, Szostak JW. A highly reactive imidazolium-bridged dinucleotide intermediate in nonenzymatic RNA primer extension. J Am Chem Soc. 2016;138:11996–12002. doi: 10.1021/jacs.6b07977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, et al. Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: Borate as a multifaceted problem solver in prebiotic chemistry. Angew Chem Int Ed Engl. 2016;55:15816–15820. doi: 10.1002/anie.201608001. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Zhang S, Chaput JC. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat Chem. 2012;4:183–187. doi: 10.1038/nchem.1241. [DOI] [PubMed] [Google Scholar]
- 30.Unrau PJ, Bartel DP. An oxocarbenium-ion intermediate of a ribozyme reaction indicated by kinetic isotope effects. Proc Natl Acad Sci USA. 2003;100:15393–15397. doi: 10.1073/pnas.2433147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vorbrüggen H, Ruh-Pohlenz C. Synthesis of nucleosides. Org React. 2000;55:1. [Google Scholar]
- 32.Fathi R, Jordan F. α-D-Ribofuranosyl 1,2-cyclic monophosphate. Isolation, NMR spectroscopic properties, and rates and mechanism of acid and alkaline hydrolysis. J Org Chem. 1986;51:4143–4146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.