Significance
Retinal vGluT3 amacrine cells (GACs) release glutamate and glycine to enhance and suppress contrast/motion sensitivity, respectively. GACs' relatively compact size and diffuse ramification pattern suggest global dendritic processing of visual signals across ON and OFF sublayers of the inner plexiform layer. Surprisingly, we find that signal spread in GAC dendrites is limited in both vertical and lateral dimensions. Vertical restriction preserves the fundamental ON-OFF segregation scheme, while lateral restriction enables high-resolution visual processing within a sublayer. Both types of local processing are achieved mainly by synaptic inhibition, though facilitated by intrinsic electrotonic decay. The ability to encode visual signals locally in dendritic segments allows GACs to function in a multiplexed circuit, where the outputs are fine-tuned for diverse, circuit-specific targets.
Keywords: vGluT3 amacrine cell, retinal processing, synaptic integration
Abstract
A basic scheme of neuronal organization in the mammalian retina is the segregation of ON and OFF pathways in the inner plexiform layer (IPL), where glutamate is released from ON and OFF bipolar cell terminals in separate inner (ON) and outer (OFF) sublayers in response to light intensity increments and decrements, respectively. However, recent studies have found that vGluT3-expressing glutamatergic amacrine cells (GACs) generate ON-OFF somatic responses and release glutamate onto both ON and OFF ganglion cell types, raising the possibility of crossover excitation in violation of the canonical ON-OFF segregation scheme. To test this possibility, we recorded light-evoked Ca2+ responses from dendrites of individual GACs infected with GCaMP6s in mouse. Under two-photon imaging, a single GAC generated rectified local dendritic responses, showing ON-dominant responses in ON sublayers and OFF-dominant responses in OFF sublayers. This unexpected ON-OFF segregation within a small-field amacrine cell arose from local synaptic processing, mediated predominantly by synaptic inhibition. Multiple forms of synaptic inhibition compartmentalized the GAC dendritic tree and endowed all dendritic varicosities with a small-center, strong-surround receptive field, which varied in receptive field size and degree of ON-OFF asymmetry with IPL depth. The results reveal a form of short-range dendritic autonomy that enables a small-field, dual-transmitter amacrine cell to process diverse dendritic functions in a stratification level- and postsynaptic target-specific manner, while preserving the fundamental ON-OFF segregation scheme for parallel visual processing and high spatial resolution for small object motion and uniformity detection.
In the mammalian retina, ON and OFF bipolar cells release glutamate from their axon terminals in the inner half and the outer half of the inner plexiform layer (IPL), respectively (1–3), except for the rare cases of ectopic, en passant glutamate release from the axonal shaft (4, 5). This anatomically segregated processing of ON and OFF light-evoked excitatory signals serves as a fundamental building block for subsequent image processing in the visual system (6, 7). However, recent studies have found that a small-field, diffusely ramifying amacrine cell type containing the vesicular glutamate transporter 3 (vGluT3) (8–13) releases glutamate (14–16) in addition to a conventional amacrine cell transmitter, glycine (16, 17). This glutamatergic amacrine cell (GAC) is believed to release glutamate from its neurites in both ON and OFF IPL sublayers, because both ON direction-selective and OFF alpha ganglion cells receive glutamatergic input from it (14, 16). Interestingly, patch-clamp recordings from the GAC soma show depolarization to both the onset and the offset of center light stimulation (14, 18, 19). If GACs are electrotonically compact, as is generally believed for small-field amacrine cells (20), a parsimonious prediction would be that GACs release glutamate in both ON and OFF IPL sublayers in response to both center light ON and OFF (14). An ON release of glutamate in the OFF layer or vice versa (“cross-layer” glutamate release) would introduce serious signal contamination between ON and OFF excitatory channels in the IPL and challenge the basic organizational scheme of ON-OFF parallel processing. This paradox motivated us to investigate the basic dendritic physiology of GACs.
In this study, we used two-photon Ca2+ imaging from dendrites of individual GACs infected with the genetically encoded Ca2+ indicator GCaMP6s (21) to address three questions. First, does glutamate release from GACs violate the ON-OFF segregation scheme in the IPL? Second, can a small-field amacrine cell like GAC support short-range, isolated dendritic processing? Third, if GACs can support local processing, what are the underlying mechanisms and functional consequences? Preliminary results of this study have been reported previously in abstract form (22).
Results
Asymmetric ON-OFF Ca2+ Responses from Local Dendritic Regions of GACs.
To understand how various parts of a GAC process visual signals, we sparsely infected GACs with GCaMP6s in vGluT3-cre-tdTomato mice (Fig. 1A, Fig. S1, and Movie S1). Individual GACs expressing both tdTomato and GCaMP6s in the INL were imaged in the whole-mount retina under a two-photon microscope. These cells ramified mainly between 20% and 60% of the IPL depth, with a minor portion of dendrites found outside of this main band, and showed typically one to two relatively thick primary dendrites that descended from the soma and branched off both radially and laterally into secondary and higher-order dendritic segments, as reported previously (8, 9, 13, 14, 18, 19). The dendritic field was irregularly shaped, with an equivalent field diameter (defined as the diameter of a circle that has the same area as the dendritic field) of 87 ± 13 µm and 91 ± 8 µm (n = 5 cells; P = 0.36) in the ON and the OFF sublamina, respectively (Fig. S1). The entire dendritic tree, except for initial sections of the primary dendrites, was decorated with numerous varicosities, which were often separated by thin dendritic segments (Movie S1).
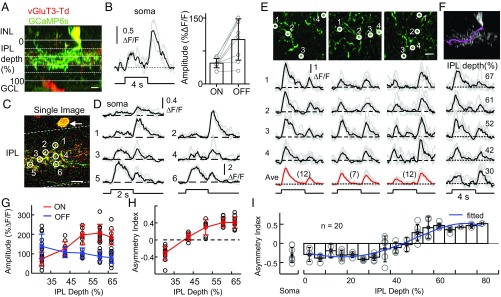
Fig. 1.
Layer-dependent asymmetric ON-OFF Ca2+ responses from single GACs. (A) Cross-sectional reconstruction of a GAC infected with GCaMP6s (green) in a vGluT3-Cre/tdTomato (red) mouse retina. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer. (Scale bar: 10 μm.) (B, Left) An example of somatic Ca2+ response to a center spot flash (100 μm diameter); black, averaged response; gray, individual trails. (B, Right) Mean peak ON and OFF somatic response amplitudes (n = 8 cells). (C) Image of a GCaMP6-expressing GAC in a titled retinal area, showing the soma (arrow) and dendrites at different IPL depths on a single two-photon focal plane. Dashed lines indicate the IPL boundaries. (Scale bar: 10 μm.) (D) Ca2+ responses from the cell in C, showing IPL depth-dependent asymmetric ON-OFF responses from the soma and six varicosities selected from different IPL depths. (E, Top) Two-photon images of the cell in A in flat-mount, showing (from left to right) dendrites at IPL depths of 61%, 42%, and 30%. (E, Middle) Ca2+ responses from four randomly selected varicosities (black, averaged responses; gray, individual trials). (E, Bottom) Responses from all analyzed varicosities (number indicated in parentheses) at each of the three IPL depths. Red, averaged responses, gray, individual trials. (F, Top) Reconstruction of a continuous GAC dendrite coursing through different IPL depths (pink). (F, Bottom) Responses from varicosities at different IPL depths of the reconstructed dendrite in the top panel, showing changes in ON-OFF asymmetry along the dendrite. (G) Peak ON-OFF response amplitudes of varicosities located at different IPL depths of the cell shown in A. Black circles, individual varicosities; color circles and error bars, mean and SD, respectively. (H) Asymmetry index of varicosities at different IPL depths for the cell in A. (I) Asymmetry index of dendritic varicosities as a function of IPL depth (n = 20 cells) and of soma (n = 8 cells). Circles, individual cells; bars and error bars, mean and SD, respectively; blue line, polynomial fit.
Fig. S1.
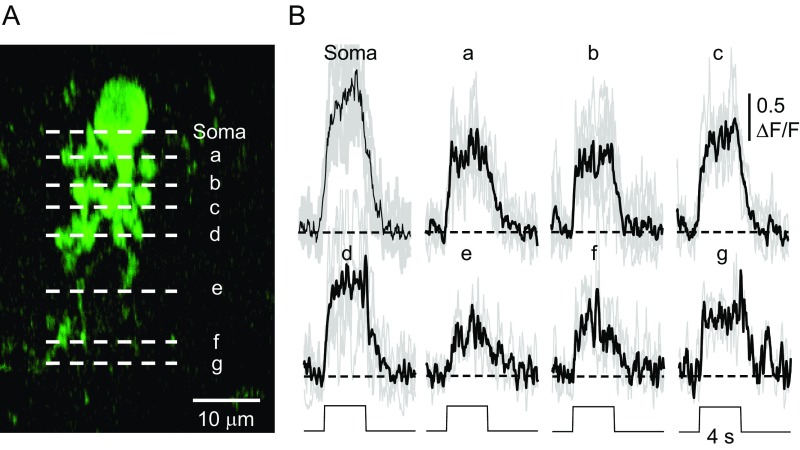
Measurement of GAC dendritic field size in the ON and OFF IPL sublayers. (A) Cross-sectional reconstruction of a vGluT3 amacrine cell (soma, white arrow) infected with GCaMP6s (green) in a vGluT3-tdTomato mouse retina (red). The white line in the middle of the IPL indicates 45% of IPL depth, the border between ON and OFF sublayers. (B) Dendritic morphology in ON (Left) and OFF (Right) sublayers shown in collapsed two-photon z-stack images taken from the ON (45–100% IPL) and OFF (0–45% IPL) layers, respectively. The polygons indicate areas covered by the dendrites, which are used to estimate the equivalent dendritic field diameter in ON and OFF sublayers.
When stimulated with a center light spot (100 µm diameter), a GAC generated somatic ON-OFF Ca2+ responses, showing a smaller ON component and a larger OFF component (Fig. 1B), consistent with previously reported patch-clamp results (14, 18, 19). However, when the retina was tilted at an angle with respect to the horizontal plane so that the soma and dendritic segments from different IPL depths could be imaged simultaneously on a single two-photon focal plane, different regions of the GAC dendritic tree exhibited markedly different responses to the same center spot illumination, showing OFF-dominant responses in the outer IPL, ON-OFF responses in the mid IPL, and ON-dominant responses in the inner IPL (Fig. 1 C and D). Sequential, layer-by-layer imaging of GAC dendrites throughout the IPL depths in horizontally placed flat-mount retinas confirmed the sublayer-specific ON-OFF asymmetry in GAC responses to center light stimulation (Fig. 1E). Notably, the differences in response polarity and asymmetry were found not only between dendritic sectors belonging to different primary or secondary dendrites, but also between sectors belonging to different IPL depths of a same continuous dendrite (Fig. 1F; n = 11 continuous dendrites chosen at random from five cells). As the dendritic ramification depth increased in the IPL, the ON response amplitude increased, whereas the OFF response amplitude decreased (Fig. 1G). The ON-OFF response asymmetry index (AI; defined as [RON − ROFF]/[RON + ROFF], where RON and ROFF are ON and OFF response amplitude, respectively) changed monotonically from approximately −0.4 at 30% IPL depth to approximately +0.4 at 70% IPL depth (Fig. 1H). A plot of AI (averaged from 20 GACs) as a function of ramification depth revealed a reversal of asymmetry (AI = 0) at ∼45% IPL depth, corresponding to the demarcation between ON and OFF BC axon terminals (Fig. 1H). The finding of a sublayer-specific ON-OFF response asymmetry contradicts the previous assumption that narrow-field amacrine cells like GACs have a consistent light response polarity throughout their dendritic trees, as in the case of AII amacrine cells (23, 24) (Fig. S2).
Fig. S2.
Light-evoked Ca2+ responses in an AII amacrine cell. (A) Cross-sectional reconstruction of an AII amacrine cell infected with GCaMP6s. Dotted lines indicate dendritic layers from which Ca2+ responses were examined. (B) Ca2+ responses from regions marked in A to a center spot (100 μm diameter), showing a uniform response polarity from all levels of dendritic stratification. Similar results were obtained in three cells.
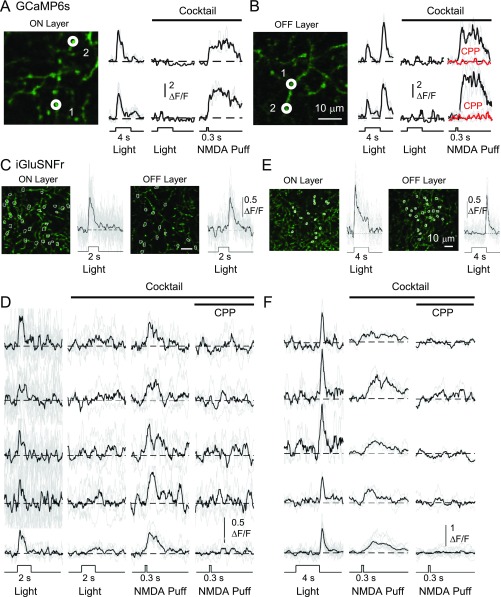
Consistent with the asymmetric Ca2+ responses in ON and OFF sublayers, two-photon imaging from GAC dendrites that were selectively infected with the glutamate sensor, iGluSnFR (25), showed no detectable glutamate release from ON dendrites at the offset of center light stimulation, or from OFF dendrites at the onset of center light stimulation, even though glutamate release was detected from these dendrites in response to exogenous application of NMDA (n = 8; Fig. S3).
Fig. S3.
Glutamate imaging from iGluSnFR-expressing GAC dendrites. (A and B) Two-photon imaging of Ca2+ signals from ON (A) and OFF (B) varicosities of a GCaMP6s-expressing GAC, showing Ca2+ responses to NMDA puffs (2 mM, 300 ms, through a 2–5 MΩ pipette at 15 psi) in the presence of an antagonist mixture, containing 20 µM L-AP4, 10 µM ACET, 50 µM CNQX, 17 µM SR, 1 µM strychnine, and 25 µM 18-βGA. The NMDA-evoked responses had similar amplitudes as those responses to a light spot (100 µm diameter) recorded in the control solution. CPP (20 µM) completely blocked the NMDA-evoked responses (B). Note that the GAC response to the NMDA puff is presumably due to direct activation of NMDA receptors on the GAC, because the mixture was present to block indirect effects of NMDA on the GAC, and because bipolar cells do not express NMDA receptor. (C) Two-photon imaging of glutamate signals at dendritic varicosities of a GAC infected with iGluSnFR in a vGluT3-cre mouse, showing ON-only (Left) and OFF-only (Right) glutamate responses to light stimulation (100 µm diameter center spot) in the ON (Left) and OFF (Right) sublayers, respectively. (D) NMDA puff in the presence of the mixture evoked CPP-sensitive glutamate release from four representative varicosities in C (Left), which showed an ON, but no detectable OFF, glutamate response to light stimulation in the control solution. (E and F) Similar iGluSnFr imaging results as in C and D, except from four representative varicosities in the OFF layer of a different GAC (F). These OFF varicosities exhibited an OFF, but no detectable ON, glutamate response to light stimulation, even though they could be evoked to release glutamate by the NMDA puff. In D and F, the top four rows of traces are individual responses from the representative varicosities, and the bottom row shows averaged responses from the four varicosities. Note that only a fraction (∼25%) of the varicosities in the ON and OFF layers showed clearly detectable glutamate releases in response to NMDA puffs. In A, B, D, and F, black represents averaged responses from three to four trials, and gray represents individual trials. In C and E, black represents averaged responses from selected varicosities, and gray represents responses from individual varicosities.
Layer-Specific Receptive-Field Properties of GAC Dendrites.
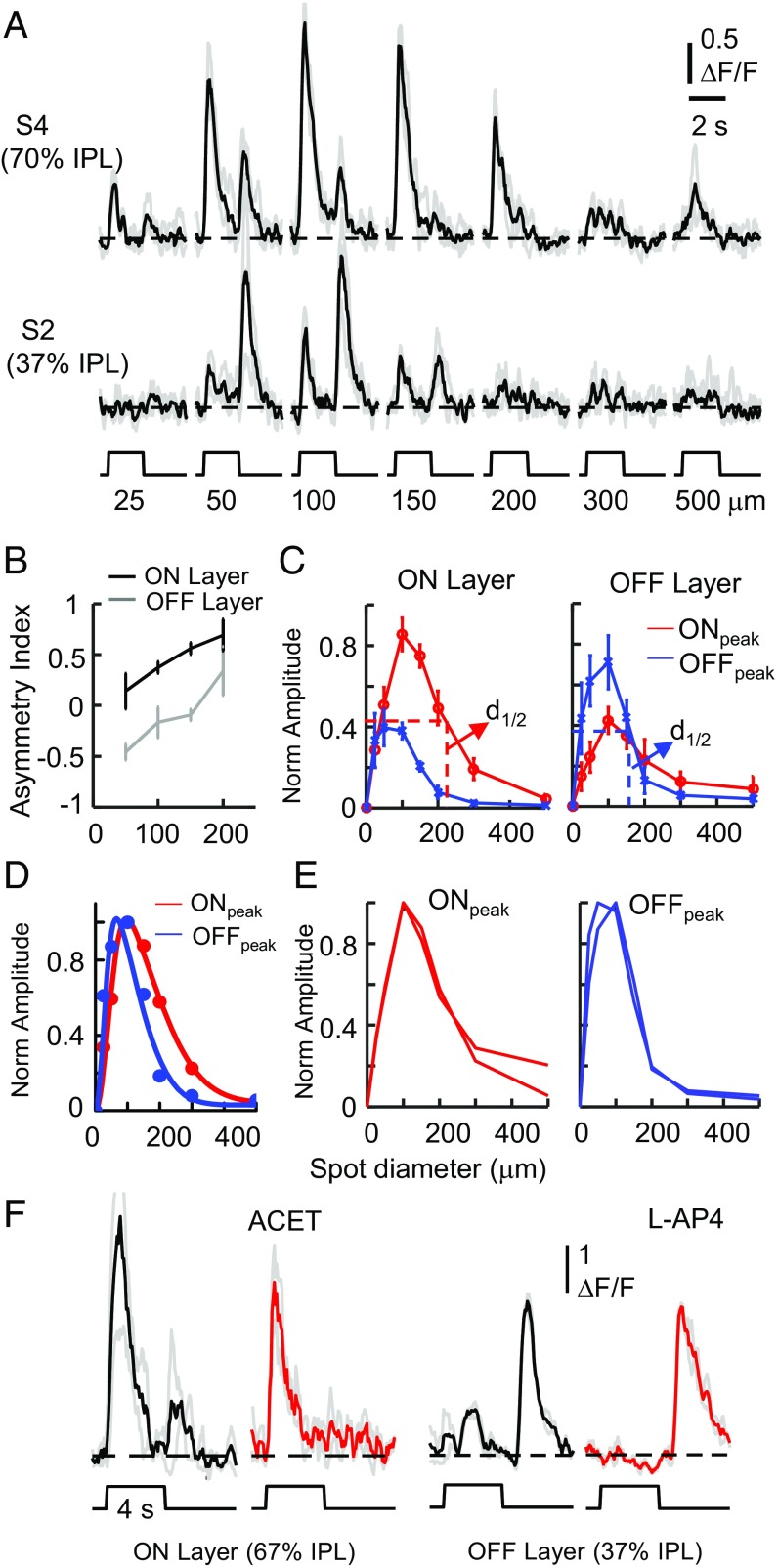
A layer-by-layer analysis of dendritic responses of GACs to light spots of various diameters (25–500 µm) revealed some distinct receptive field properties of local dendrites. First, all dendritic varicosities in both ON and OFF sublayers had a strong inhibitory surround, such that spots larger than 200 µm in diameter nearly completely suppressed the responses (Fig. 2A), indicating that the release of glutamate and glycine is activated by local, but not global, stimulation at each release site. Second, as the spot diameter increased, the degree of asymmetry (i.e., absolute value of AI) increased for responses in the ON sublayer, but decreased for responses in the OFF sublayer (Fig. 2 A and B). Third, although the dendritic field size was similar in the ON and OFF sublayers (Fig. S1), the spatial profile of the response to spot stimulation (averaged from varicosities within a ∼50 × 50 μm2 area) showed a larger receptive field size in the ON sublayer than in the OFF sublayer, with a spot diameter at half-maximum response amplitude (d1/2) of 241 ± 36 μm for the ON response and 151 ± 30 μm for the OFF response (n = 7 cells; P < 0.0001; Fig. 2 C and D). A difference-of-Gaussian fit of the averaged receptive field profile yielded an excitatory receptive field center with a diameter of ∼160 µm for ON responses and ∼105 µm for OFF responses, and an inhibitory receptive field surround with a diameter of ∼619 µm for ON responses and ∼442 µm for OFF responses (Materials and Methods and Fig. 2D). Thus, differences in presynaptic and postsynaptic inhibition from the surround contributed to the difference in ON and OFF receptive field size, which may in turn explain the increase (or decrease) in the absolute value of the asymmetric index in the ON (or OFF) sublayer with increasing light spot size (Fig. 2B). Fourth, while the ON and OFF receptive field center size was different, the spatial profile of the ON response was similar in the ON and OFF sublayers, as was that of the OFF response (Fig. 2E), indicating that the small ON response in the OFF dendrites of a GAC originated from the ON dendrites, and vice versa. Indeed, L-AP4, which blocks ON bipolar cell input to the ON sublayer, completely blocked the ON responses from the OFF dendrites of GACs (n = 4 cells), whereas ACET, which blocks OFF bipolar input to the OFF sublayer, blocked the OFF response of ON GAC dendrites nearly completely (n = 4 cells; Fig. 2F), confirming that the ON response originated in the ON sublayer and decayed significantly as it spread to the OFF sublayer, and vice versa for the OFF response.
Fig. 2.
Receptive field properties of GAC dendrites. (A) Averaged Ca2+ responses from varicosities in a ∼50 × 50 μm2 area at ON (S4) and OFF (S2) sublayers to light spots of increasing diameters. (B) Asymmetry index of ON (black) and OFF dendrites (gray) as a function of spot size. (C) Spatial profile of averaged ON (red) and OFF (blue) peak response amplitudes from ON sublayer (Left, normalized to the maximum ON peak response amplitude) and OFF sublayer (Right, normalized to maximum OFF peak response amplitude). n = 7 cells. Error bars represent SEM. (D) Spatial profile of normalized average ON response from the ON sublayer (red) and OFF response from the OFF sublayer (blue), fitted by a difference-of-Gaussian model (Materials and Methods). (E) Overlay of normalized ON response profiles from ON and OFF sublayers (Left), and normalized OFF response profiles from ON and OFF sublayers (Right). (F) Blockade of OFF response in ON sublayers by ACET (10 μM) and ON response in OFF sublayers by l-AP4 (20 μM). For statistical analysis, data from ON and OFF sublayers are pooled from all IPL depths in the ON and OFF sublayers, respectively.
Synaptic Contributions to the ON-OFF Response Asymmetry of GAC Dendrites.
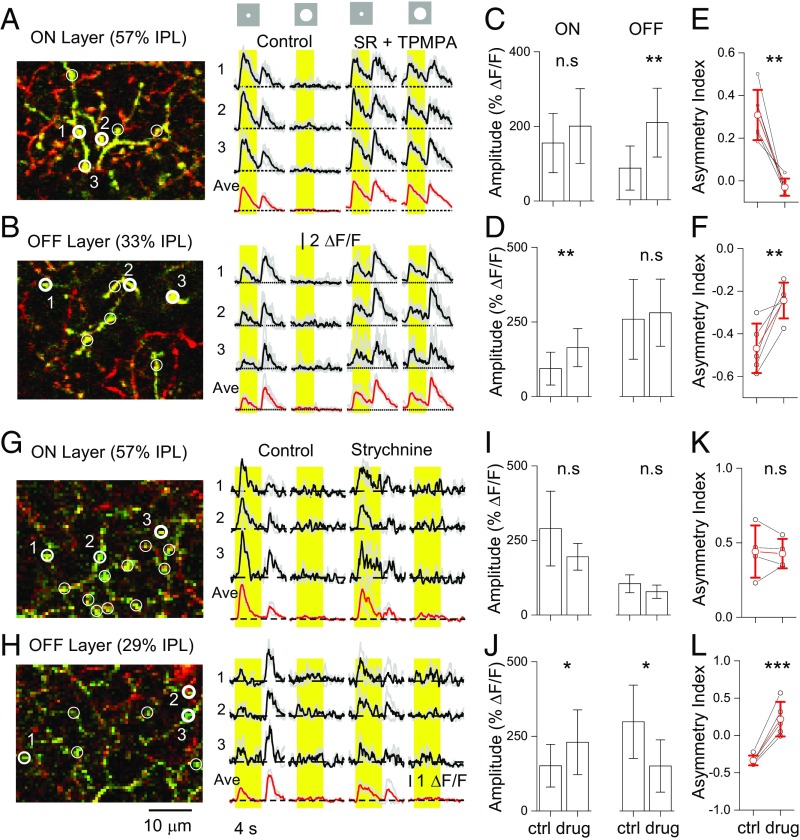
To determine whether the sublayer-specific ON-OFF response asymmetry is shaped predominantly by local synaptic inhibition or by intrinsic electrotonic isolation of the dendrites, we examined the effects of inhibitory synaptic receptor antagonists on the responses of GAC dendrites to center spot (100 μm diameter) and large-field (500 μm diameter) illumination. Blocking GABAA and GABAC receptors with SR-95531 (SR; 17 μM) and TPMPA (50 μM) brought out strong responses to large-field illumination in both ON and OFF sublayers, eliminating the characteristically strong surround inhibition of the cell (Fig. 3 A and B). In the ON sublayer, SR + TPMPA significantly increased the amplitude of the OFF response to the center spot (ΔF/F increase from 85 ± 59% to 207 ± 92%; P = 0.002; n = 5 cells), but had a much smaller effect on the ON response amplitude (ΔF/F increase from 155% ± 79% to 201 ± 100%; P = 0.06; n = 5 cells) (Fig. 3 A and C), resulting in nearly complete elimination of ON-OFF response asymmetry (AI decrease from 0.31 ± 0.12 to −0.03 ± 0.04; P = 0.003; n = 5 cells) (Fig. 3E). In the OFF sublayer, SR + TPMPA increased the ON response amplitude to the center spot (ΔF/F increase from 94 ± 55% to 164 ± 64%; P = 0.005; n = 5 cells), but did not significantly affect the OFF responses (ΔF/F increase from 259 ± 134% to 281 ± 112%; P = 0.42; n = 5 cells) (Fig. 3 B and D), leading to a significant reduction in the degree of response asymmetry (decrease in absolute value of AI from 0.47 ± 0.12 to 0.24 ± 0.08; P = 0.01; n = 5 cells) (Fig. 3F). Thus, GABAergic inhibition played a critical role in shaping the ON-OFF asymmetric responses in GAC dendrites. GABAergic inhibition was also responsible for the strong surround inhibition of both ON and OFF dendrites of GACs.
Fig. 3.
Role of GABAergic and glycinergic inhibition in asymmetric ON-OFF responses of GAC dendrites. (A and B) Responses from the ON (A) and OFF (B) sublayers of a GAC to center (100 μm diameter) and large-field (500 μm diameter) light stimulation in control and in the presence of SR (17 µM) and TPMPA (50 µM). (C and D) Effects of SR + TPMPA on the peak response amplitude in the ON (C) and OFF (D) sublayers. (E and F) Effects of SR + TPMPA on AI in the ON (E) and the OFF (F) sublayers. (G and H) Responses from the ON (G) and OFF (H) sublayers of a GAC to center (100 μm diameter) and large-field (500 μm diameter) light stimulation in control and in the presence of strychnine (1 µM). (I and J) Effects of strychnine on the peak response amplitude in the ON (I) and OFF (J) sublayers. (K and L) Effects of strychnine on the AI in the ON (K) and OFF (L) sublayers. Black traces, responses from three numbered varicosities; red traces, average responses from all circled varicosities as shown on the left. Error bars represent SD. n.s., not significant (P > 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. For statistical analysis, data from ON and OFF sublayers are pooled from all IPL depths in the ON and OFF sublayers, respectively.
In contrast, blocking glycine receptors with strychnine (1 μM) did not bring out a response to the large-field light stimulation from either ON or OFF dendrites (Fig. 3 G and H), suggesting that the strong surround inhibition came predominantly from GABAergic amacrine cells (Fig. 3 A and B). For the center light stimulation, in the ON sublayer, strychnine did not have a consistent, statistically significant effect on the ON response amplitude (ΔF/F: 290 ± 125% to 195 ± 45%; P = 0.16; n = 4 cells) and reduced the OFF response amplitude only slightly (ΔF/F: 104 ± 30% to 78 ± 22%; P = 0.08; n = 4 cells), resulting in no statistically significant change in response asymmetry (AI: 0.44 ± 0.18 to 0.43 ± 0.1; P = 0.79; n = 4 cells) (Fig. 3 G, I, and K), although strychnine seemed to slow the decay of ON responses. However, in the OFF sublayer, strychnine significantly enhanced the amplitude of the ON response to a center spot (ΔF/F: 152 ± 72% to 230 ± 109%; P = 0.02; n = 5 cells), suggesting a strong glycinergic ON-to-OFF crossover inhibition (Fig. 3 H and J). Curiously, strychnine also reduced the amplitude of the OFF response to the center spot (ΔF/F: 299 ± 123% to 121 ± 88%; P = 0.02; n = 5 cells) (Fig. 3 H and J), resulting in reversal of the polarity of asymmetry (Fig. 3L). Suppression of OFF excitatory activity by strychnine has been previously reported for retinal ganglion cells and attributed to a possible enhancement (disinhibition) of GABAergic feedback inhibition of OFF bipolar cells and/or an interference with crossover inhibition of bipolar, amacrine, and ganglion cells (26). These two possibilities might be further tested by sequentially applying SR/TPMPA and strychnine; however, in the presence of all these drugs, GACs generated so much spontaneous baseline activity so as to make light responses no longer distinguishable, precluding such experiments.
Taken together, the foregoing results suggest that the asymmetry in the ON-OFF response of GAC dendrites to center light stimulation was largely a result of synaptic inhibition mediated by a combination of glycinergic and GABAergic interactions. The suppression of signal spread from the ON to OFF dendrites was mediated by both glycinergic and GABAergic inhibition, whereas the suppression of signal spread from the OFF to ON dendrites seemed dominated by GABAergic interactions. In addition, GABAergic, but not glycinergic, interactions played a dominant role in shaping the strong surround inhibition of GAC dendrites in all sublayers (Discussion).
Localized Ca2+ Responses in GAC Dendritic Branches.
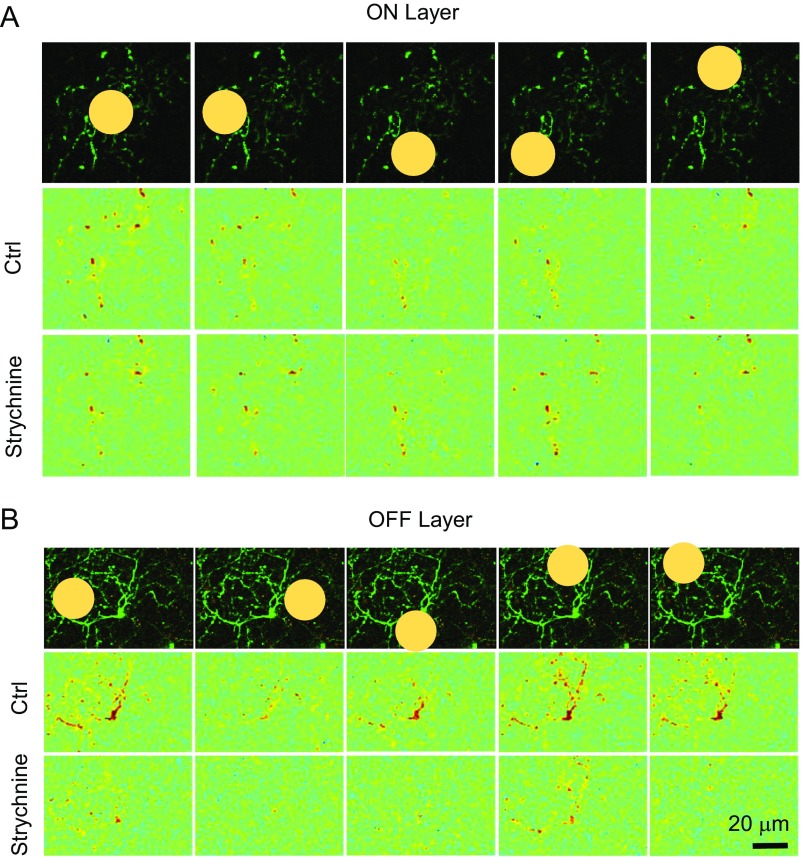
To investigate whether dendritic signal processing is also isolated laterally within each IPL sublayer, we used a small light spot (25 μm diameter) to stimulate local dendritic regions and varicosities within the dendritic field of a GAC. Unlike the 100-μm-diameter center spot, which activated nearly all varicosities in each sublayer of GAC dendrites, a small light spot was effective in activating varicosities only in a local region within the vicinity of the stimulus (Fig. 4A). Varicosities located at a distance from the stimulus usually gave no clear responses or much smaller responses, indicating that focal excitation was processed locally, with limited lateral spread. Blocking GABAergic inhibition with SR (17 μM) and TPMPA (50 μM) expanded the lateral spread of the response to the small spot stimulation in both ON (n = 2 cells) and OFF (n = 3 cells) sublayers (Fig. 4B and Fig. S4). However, strychnine (1 µM) did not have a clear effect on the lateral spread of local responses to small spot stimulation in either ON (n = 3 cells) or OFF sublayer (n = 4 cells), although it significantly enhanced local ON responses in the OFF sublayer (Fig. 4C and Fig. S5), in agreement with the results shown in Fig. 3 H and J. Thus, GABAergic, but not glycinergic, inhibition seemed to play a dominant role in restricting the lateral spread of excitation in GAC dendrites. Notably, even in the presence of SR + TPMPA, there was still a considerable reduction in response amplitude from varicosities distant from the site of local stimulation (Fig. 4B), suggesting a possible role of intrinsic electrotonic decay along GAC dendrites as well.
Fig. 4.
Local processing of Ca2+ signal within GAC dendrites. (A) Responses of individual varicosities to a center light spot (Left; shaded yellow disk, 100 μm diameter) and a small spot (Right; small shaded disk, 25 μm diameter) delivered to four different locations within the dendritic field. Note that the small light spot activated only varicosities near the vicinity of the stimulus, suggesting limited lateral communication among varicosities, and a spatial resolution finer than the dendritic field size. (B), SR (17 µM) + TPMPA (50 µM) expanded the lateral spread of the response to small spot illumination within GAC dendrites in both ON and OFF sublayers. (C) Strychnine (1 µM) had no clear effect on the lateral spread of Ca2+ responses to focal spots within the GAC dendritic field in either ON or OFF sublayers.
Fig. S4.
Effects of GABA receptor antagonists on the spatial extent of dendritic responses to small light spots. Focal light spots evoked localized Ca2+ responses around the vicinity of the stimulus in both ON (A, Middle) and OFF (B, Middle) sublayers. Application of SR (17 μM) and TPMPA (50 μM) expanded the lateral spread of the Ca2+ signal in both ON (A, Bottom) and OFF (B, Bottom) sublayers. (Top) Spot diameter: 25 μm in A, 40 μm in B.
Fig. S5.
Glycine receptor antagonist does not enhance the spatial extent of dendritic responses to small light spots. Focal light spots evoked localized Ca2+ responses around the vicinity of the stimulus in both ON (A, Middle) and OFF (B, Middle) sublayers. Strychnine (1 μM) did not enhance the lateral spread of Ca2+ signal in either ON (A, Bottom) or OFF (B, Bottom) sublayers. Spot size, 25 μm diameter.
Discussion
Asymmetric ON-OFF Ca2+ Responses and Dual Transmitter Releases from Local Dendrites of GACs.
Our results demonstrate that GAC dendrites process visual signals locally and generate ON-OFF asymmetric responses in a stratification depth-specific manner. Because GACs release both glutamate and glycine in a Ca2+-dependent manner (14, 16), and because vesicular release probability is a supralinear function of Ca2+ concentration (proportional to the third–fourth power of Ca2+ concentration; ref. 27), the asymmetric ON-OFF Ca2+ signals seen at the varicosities can be expected to lead to more asymmetric ON vs. OFF release probabilities. A rectified release of glutamate from GAC dendrites will effectively avoid crossover excitation, thereby preserving the ON-OFF segregation scheme in the IPL, as supported by the iGluSnFR imaging results (Fig. S3). Similarly, the release of glycine from GAC dendrites is also expected to be segregated between the ON and OFF sublayers, although the degree of rectification may differ between glutamate and glycine releases, depending on the nature of the interactions between local Ca2+ dynamics and the release machinery at glutamate and glycine output synapses. Thus, unlike the “prototype” of diffuse, small-field amacrine cells (e.g., the AII amacrine), GACs do not seem to play a major role in glycinergic crossover inhibition, and instead may be involved primarily in local glycinergic inhibition within individual IPL sublayers. Nonetheless, because the precise Ca2+ dynamics and the exact Ca2+ thresholds for glutamate and glycine releases at the synapses remain unclear, the observation of ON-OFF asymmetric Ca2+ responses at GAC varicosities does not exclude the possibility of incomplete ON-OFF segregation of glutamate and/or glycine release at some synapses under certain stimulation conditions. Moreover, our results also suggest that those varicosities exhibiting symmetric ON-OFF Ca2+ responses in the middle of the IPL (Fig. 1 C and E) may have ON-OFF glutamate and/or glycine release, perhaps onto target cells that show ON-OFF light responses, such as uniformity detectors (16, 17).
Synaptic Mechanisms Underlying ON-OFF Asymmetric Responses from Local GAC Dendrites.
In contrast to wide-field nonspiking amacrine cells, such as the starburst, which is known to generate local direction-selective response at its distal dendrites (28–31), narrow-field, diffusely stratified amacrine cells are generally believed to be electrotonically compact and to have a uniform response polarity within the cell. Thus, it is surprising to see segregated ON-OFF responses from a GAC dendrite spanning different IPL sublayers, which can be separated by a dendritic distance as short as ∼10 µm. Compartmentalized computation within such a short dendritic distance is not likely attributable to pure intrinsic electrotonic isolation. We found that this short-range, intersublayer segregation of dendritic signals was achieved primarily by synaptic inhibition, most likely involving glycinergic/GABAergic ON-to-OFF and GABAergic OFF-to-ON crossover inhibition, and/or local GABAergic and glycinergic inhibitions that suppress the spread of excitation along local dendritic segments (Fig. 3). Other forms of dendritic inhibition, including tonic GABAergic baseline inhibition, also may contribute to the suppression of dendritic signal spread between ON and OFF sublayers by, for example, shunting dendritic segments, while allowing local Ca2+ entry through NMDA receptors (Fig. S3).
Receptive Field Structure and Functions of GAC Dendrites.
We found that all varicosities in the GAC dendritic tree had a small-center, strong GABAergic-surround receptive field. However, the receptive field size of local dendritic varicosities was larger in the ON layer than in the OFF layer, while the size of the dendritic field was non-statistically significantly different between the ON and OFF layers (Fig. S1), suggesting layer-specific differences in dendritic computation. The ON-OFF asymmetry index also varied with dendritic ramification depth and had a differing dependency on spot size in the ON sublayers and OFF sublayers. In addition to the segregation of dendritic signals between different stratification depths, our results show that signals from varicosities located at the same stratification depth were isolated laterally as well. Individual dendritic varicosities responded differently to a small stimulus spot (25 µm diameter) appearing at different locations within the dendritic field, suggesting that each varicosity had a spatial resolution finer than the dendritic field size. The lateral isolation of signals was dependent largely on GABAergic, but not glycinergic, inhibition and to a lesser degree on electrotonic isolation as well, although the precise underlying mechanism for this remains unclear. Thus, GAC dendrites support local computation in both vertical (radial) and lateral dimensions.
Taken together, our results reveal a previously undescribed form of short-range dendritic autonomy within the relatively small dendritic tree of GAC that enables this diffusely stratified cell to process visual signals locally at different stratification levels where different parts of the dendritic tree interact with different target neurons in a stratification-specific manner. This form of local dendritic processing, together with the dual transmitter system, allows a single GAC to play diverse functional roles in multiple synaptic circuits, while maintaining two binding features of the cell: ON-OFF segregation and a small-center, strong-surround receptive field. The former feature preserves the basic ON-OFF parallel processing scheme in the visual system, and the latter ensures the high spatial resolution and spatial contrast sensitivity essential not only for local/differential object motion detection, but also for uniformity detection and other forms of visual computation in the IPL.
Materials and Methods
All animal procedures were performed in accordance with National Institutes of Health guidelines. We used either vGluT3-Cre mice (19) or vGluT3-tdTomato mice generated by crossbreeding vGluT3-Cre mice with tdTomato mice, strain B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (The Jackson Laboratory). Mouse eyes were injected intravitreally with the virus construct (AAV1 Syn Flex GCaMP6s, WPRE SV40; Penn Vector Core). Single GCaMP6s-expressing GACs were imaged under a two-photon microscope. The light stimulus had an intensity of 3.4 × 10−13 W/µm2 over a uniform background light of 3.4 × 10−14 W/µm2 at photoreceptors. The materials and methods used in this study are described in detail in SI Materials and Methods.
SI Materials and Methods
Virus Injection and Whole-Mount Retina Preparation.
Mice aged 3–6 wk were used for intravitreal injection of the virus construct GCaMP6s (AAV1 Syn Flex GCaMP6s, WPRE SV40) and iGluSnFr (AAV1 hsyn Flex iGluSnFr, WPRE SV40; University of Pennsylvania Vector Core) after being anesthetized with ketamine and xylazine. A small incision was made in the sclera with a 30-gauge needle, and the virus construct (∼1 μL) was then injected into the vitreous near the dorsal retina through the incision via a sharp glass pipette back connected to a hand-held Hamilton syringe. Imaging experiments were performed at 5–6 wk after injection of the construct.
To prepare whole-mount retinas, mice of either sex were dark-adapted for 1–3 h and then killed by an injection of Euthasol (>2 mL/kg, i.p.) (32). The eyes were quickly enucleated and hemisected. The retinas were isolated from the retinal pigment epithelium under a dissection microscope in artificial cerebrospinal fluid (ACSF; composition, 120 mM NaCl, 3.1 mM KCl, 1.1 mM CaCl2, 1.2 mM MgSO4, 0.5 mM KH2PO4, 0.5 mM l-glutamine, 26 mM NaHCO3, 0.1 mM ascorbic acid, 0.1 mM Na-pyruvate, and 20 mM d-glucose) equilibrated with 95% O2-5% CO2 at room temperature (20–25 °C). A piece of retina was placed in a recording chamber, with the ganglion cell side facing up, and held down at the chamber bottom with a platinum tissue holder. The recording chamber was placed on the stage of a two-photon microscope and continuously perfused with ACSF at ∼32 °C at a rate of ∼4 mL/min. The entire tissue preparation process was completed under dim red light illumination.
Two-Photon Imaging and Visual Stimulation.
We used a two-photon microscope system (Ultima; Prairie Technologies) equipped with a Ti:Sapphire pulsed laser (MaiTai), configured on an Olympus upright microscope (BX51WI) with a 60×, 1.0 NA objective lens (LUMPlanFL/IR). Dendrites of single GCaMP6s-expressing GACs were imaged under the control of Prairie View software (Prairie Technologies) at a resolution of 256 × 256 pixels at a rate of 10–30 frames/s, with the scanning laser tuned to a 920-nm wavelength (laser dwell time, 2 µs/pixel). GCaMP6s signals were collected by one photomultiplier tube (green channel) after being bandpass filtered at 520 ± 18 nm (FF01-520/35–25; Semrock), while the timing of the visual stimulus was recorded simultaneously by another photomultiplier tube (red channel) bandpass filtered at 607 ± 23 nm (HQ607/45; Chroma).
Visual stimuli were generated on a miniature transmissive TFT LCD (model XGAP01; CRL OPTO; 36.9 mm × 27.6 mm dimension, 1,024 × 768 pixels, contrast ratio >1:100), using Vision Works software (Vision Research Graphics). The LCD image was transilluminated by collimated light from a halogen lamp, bandpass filtered at 577 ± 10 nm (FF01-577/20; Semrock), and projected to the photoreceptor outer segment layer of the retina through the microscope condenser lens. The maximum intensity of the LCD image at the retina was 3.4 × 10−13 W/µm2 (measured with a radiometer; model S470; UDT Instruments). The light stimulus, usually consisting of a stationary light spot of variable diameters (25–1,000 µm), was presented on the retina at an intensity of 3.4 × 10−13 W/µm2 over a uniform background light of 3.4 × 10−14 W/µm2. The constant background light helped the retina adapt to the scanning IR laser light used for two-photon imaging.
Image Processing and Data Analysis.
Following the recording of Ca2+ responses from a GAC, a stack of Z-series images was collected from the same area with higher laser intensity at a z-step size of 0.5–1 µm and a resolution of 512 × 512 pixels. The 3D reconstruction and tracing of the dendrites were done in Fiji with the plug-in Simple Neurite Tracer (https://fiji.sc/). Regions of interest (ROIs) were drawn manually over varicosities and local dendritic segments. Ca2+ signals from each ROI were extracted as ΔF/F after application of a moving-average filter to the raw data. Fluorescence signals from ROIs were analyzed with a custom-written GUI (https://github.com/chenmg04/brightnessOverTime) in MATLAB (MathWorks). To fit the spatial profile of the responses to spots of different diameters (Fig. 3D), a difference-of-Gaussians model (33, 34) was used according to the equation: R = kc (1 − exp(−d2/2sc2)) − ks(1 − exp(−d2/2ss2)), where kc and ks are the amplitudes and sc and ss are the SDs for the center and surround receptive field profiles, respectively, and d is the diameter of the light spot. The diameters of the center and surround receptive fields are defined as 4sc and 4ss, respectively, which cover 95% of the areas under the Gaussian distributions. Statistical significance was determined at the level of P = 0.05 by the paired-sample t test.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants EY026065 (to Z.J.Z.) and EY17353 (to Z.J.Z.), an unrestricted grant from Research to Prevent Blindness to Yale Eye Center, and the Marvin L. Sears Professorship at Yale University (to Z.J.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11268.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711622114/-/DCSupplemental.
References
- 1.Famiglietti EV, Jr, Kolb H. Structural basis for ON- and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- 2.Famiglietti EV, Jr, Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198:1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- 3.Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- 4.Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: Bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: Elementary building blocks of vision. Nat Rev Neurosci. 2014;15:507–519. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- 7.Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson J, et al. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477:386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haverkamp S, Wässle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468:251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- 10.Fremeau RT, Jr, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong J, et al. Distribution of vesicular glutamate transporters in rat and human retina. Brain Res. 2006;1082:73–85. doi: 10.1016/j.brainres.2006.01.111. [DOI] [PubMed] [Google Scholar]
- 12.Stella SL, Jr, Li S, Sabatini A, Vila A, Brecha NC. Comparison of the ontogeny of the vesicular glutamate transporter 3 (VGLUT3) with VGLUT1 and VGLUT2 in the rat retina. Brain Res. 2008;1215:20–29. doi: 10.1016/j.brainres.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshak DW, et al. Synaptic connections of amacrine cells containing vesicular glutamate transporter 3 in baboon retinas. Vis Neurosci. 2015;32:E006. doi: 10.1017/S0952523815000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, et al. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron. 2014;84:708–715. doi: 10.1016/j.neuron.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnaswamy A, Yamagata M, Duan X, Hong YK, Sanes JR. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature. 2015;524:466–470. doi: 10.1038/nature14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Zhang Y, Chen M, Zhou ZJ. Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron. 2016;90:27–34. doi: 10.1016/j.neuron.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tien NW, Kim T, Kerschensteiner D. Target-specific glycinergic transmission from VGluT3-expressing amacrine cells shapes suppressive contrast responses in the retina. Cell Rep. 2016;15:1369–1375. doi: 10.1016/j.celrep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T, Soto F, Kerschensteiner D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. Elife. 2015;4:e08025. doi: 10.7554/eLife.08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimes WN, Seal RP, Oesch N, Edwards RH, Diamond JS. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis Neurosci. 2011;28:381–392. doi: 10.1017/S0952523811000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masland RH. The tasks of amacrine cells. Vis Neurosci. 2012;29:3–9. doi: 10.1017/s0952523811000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Chen L, Zhou Z. Synaptic properties of vGluT3 amacrine cells in the mouse retina. Invest Ophthalmol Vis Sci. 2015;56:4377. [Google Scholar]
- 23.Borghuis BG, et al. Imaging light responses of targeted neuron populations in the rodent retina. J Neurosci. 2011;31:2855–2867. doi: 10.1523/JNEUROSCI.6064-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balakrishnan V, Puthussery T, Kim MH, Taylor WR, von Gersdorff H. Synaptic vesicle exocytosis at the dendritic lobules of an inhibitory interneuron in the mammalian retina. Neuron. 2015;87:563–575. doi: 10.1016/j.neuron.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marvin JS, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werblin FS. Six different roles for crossover inhibition in the retina: Correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodge FA, Jr, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei Z, et al. Conditional knock-out of vesicular GABA transporter gene from starburst amacrine cells reveals the contributions of multiple synaptic mechanisms underlying direction selectivity in the retina. J Neurosci. 2015;35:13219–13232. doi: 10.1523/JNEUROSCI.0933-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron. 2006;51:787–799. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlasits AL, et al. A role for synaptic input distribution in a dendritic computation of motion direction in the retina. Neuron. 2016;89:1317–1330. doi: 10.1016/j.neuron.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Lee S, Park SJ, Looger LL, Zhou ZJ. Receptive field properties of bipolar cell axon terminals in direction-selective sublaminas of the mouse retina. J Neurophysiol. 2014;112:1950–1962. doi: 10.1152/jn.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J Neurosci. 2001;21:7447–7454. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodieck RW. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965;5:583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.