Significance
The intestine is the major entrance site for nutrients, electrolytes, and water while constituting an effective barrier against toxins, antigens, and enteric flora. Hence, the intestinal mucosa harbors one of the largest populations of lymphocytes in the body. Nutrients such as vitamin A have been recognized to influence gut homing and differentiation of these cells. We identified the ISX protein as a critical molecular mediator of this cross talk between diet and immunity. This transcription factor regulates vitamin A production from dietary precursor molecules. Loss of this control in mice disrupts vitamin A homeostasis and impairs immunity. Reported genetic polymorphisms in the Isx gene have been associated with inflammatory disorders in humans, indicating that our findings in mice have clinical implications.
Keywords: carotenoids, retinoids, lymphocytes, intestine, BCO1
Abstract
The intestinal epithelium is a major site for the conversion of dietary β-carotene to retinaldehyde by the enzyme BCO1. The majority of retinaldehyde is further metabolized to retinol (vitamin A), esterified and packaged into triacylglycerol-rich chylomicrons for bodily distribution. Some serve on-site for the synthesis of retinoic acid, a hormone-like compound, which exerts pleiotropic and dominant effects on gastrointestinal immunity. We report here that the intestine-specific homeobox protein ISX is critical to control the metabolic flow of β-carotene through this important branching point of vitamin A metabolism. This transcription factor represses Bco1 gene expression in response to retinoic acid signaling. In ISX-deficient mice, uncontrolled Bco1 gene expression led to increased retinoid production in the intestine. Systemically, this production resulted in highly elevated hepatic retinoid stores. In the intestine, it increased the expression of retinoic acid-inducible target genes such as Aldh1a2, Dhrs3, and Ccr9. The β-carotene–inducible disruption of retinoid homeostasis affected gut-homing and differentiation of lymphocytes and displayed morphologically in large lymphoid follicles along the intestine. Furthermore, it was associated with an infiltration of the pancreas by gut-derived lymphocytes that manifested as a pancreatic insulitis with β-islet cell destruction and systemic glucose intolerance. Thus, our study identifies an important molecular interlink between diet and immunity and indicates that vitamin A homeostasis must be tightly controlled by ISX to maintain immunity and tolerance at the intestinal barrier.
Dietary lipids impact many aspects of mammalian biology (1). As a classic example, the vitamin A metabolite all-trans-retinoic acid (RA) is critical for organogenesis, cell differentiation, and immunity (2, 3). Because animals cannot synthesize this lipid from endogenous metabolites, precursor molecules must be acquired by the intestinal epithelium from the diet. These precursors exist in the diet either as preformed vitamin A, mainly retinyl esters (RE), or provitamin A carotenoids, mainly β-carotene (BC). Ultimately, all naturally occurring retinoids in the food chain are metabolically derived from carotenoids, and BC is a major source for these compounds in the human diet (4, 5).
The content of provitamin A in natural food sources is variable and is subject to seasonal fluctuations (4). Recently, we described an intrinsic regulatory mechanism, which helps mammals cope with this fluctuation. Central to this regulation is the RA-inducible transcription factor ISX that is expressed in epithelial cells of the intestine (6, 7). ISX suppresses gene expression of the scavenger receptor class B type 1 (Scarb1) and the β-carotene-15,15′-dioxygenase (Bco1), which encode proteins that respectively mediate the uptake of carotenoids and their conversion into retinoids (8–10). This negative feedback regulation controls the utilization of dietary BC for retinoid production in mice depending on demand and availability (9, 10).
Common single-nucleotide polymorphisms in ISX, BCO1, or SCARB1 genes affect serum BC levels in humans, suggesting that the role of ISX in the control of intestinal BCO1 activity is well conserved (11). However, several studies indicate that the ISX protein may serve additional functions (6). For instance, this transcription factor has been implicated in cell proliferation and inflammatory responses (12, 13). Moreover, genetic polymorphisms in the ISX gene have been associated with inflammatory bowel disease in genome-wide association studies (14). Therefore, a common denominator for the transcription factor’s putative dual role in vitamin A metabolism and immunity remains to be defined.
In the gastrointestinal tract, absorbed dietary BC is metabolized to RE for transport and bodily distribution, but also can be metabolized to RA, which via nuclear receptor binding can elicit a large spectrum of on-site immune responses (15). Previously, it has been shown that unbalanced supplies of dietary vitamin A can increase susceptibility to infectious diseases (16, 17), promote inflammation (18, 19), and can result in loss of tolerance against food antigens (20). Thus, we speculated that ISX lies at an important intersection of vitamin A metabolism and immunity and plays a critical role in the homeostatic control of these processes. To explore this putative role of the transcription factor, we examined ISX-deficient mice under various dietary conditions. We observed several indicators that retinoid metabolism and gastrointestinal immunity was compromised in these animals. The pathology was exacerbated with BC supplementation and associated with inflammatory processes.
Results
Isx Genotype Affects Intestinal Immune Homeostasis.
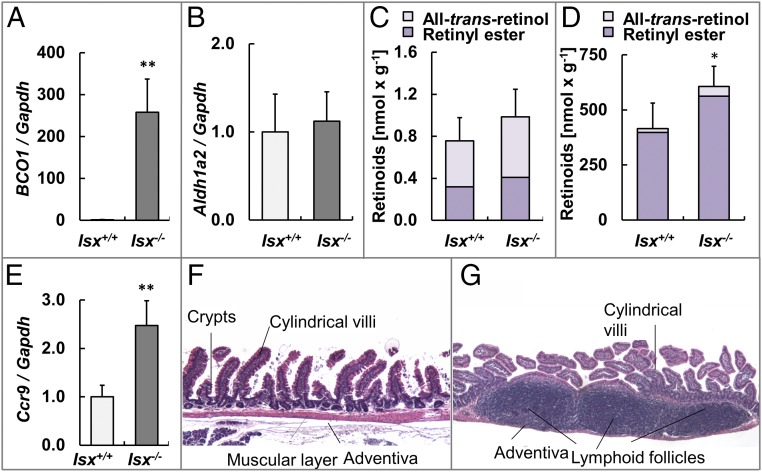
To define the role of ISX in gastrointestinal immunity, we analyzed the intestines of 7-mo-old Isx−/− and Isx+/+ (wild-type) mice. We first measured mRNA expression levels of key enzymes of intestinal vitamin A metabolism such as Bco1 and the retinal dehydrogenase 2 (Aldh1a2). In Isx−/− mice, we observed a greater than 200-fold increase in mRNA levels of Bco1 in the jejunum compared with controls (Fig. 1A). Expression levels of the RA-forming enzyme Aldh1a2 were not significantly altered between genotypes (Fig. 1B). To directly assess biochemical consequences of ISX deficiency, we performed HPLC analyses for retinoid composition in lipid extracts from the jejunum. These analyses revealed that all-trans-retinol (ROL) and RE levels increased in ISX-deficient animals compared with controls (Fig. 1C). Alterations in retinoid metabolism of ISX-deficient animals also were mirrored in significantly higher levels of RE in hepatic stores of Isx−/− mice compared with wild-type mice (Fig. 1D).
Fig. 1.
ISX-deficient mice display enlarged lymphoid follicles in the small intestine. (A and B) Quantitative RT-PCR analysis of jejunal Bco1 and Aldh1a2 mRNA levels normalized to Gapdh. (C and D) Levels of nonpolar retinoids (all-trans-retinol and retinyl ester) in jejunal (C) and hepatic (D) lipid extracts. (E) Quantitative RT-PCR analysis of jejunal Ccr9 mRNA levels normalized to Gapdh. (F and G) Representative H&E-stained cross sections through the small intestinal wall of a Isx+/+ (F) and a Isx−/− mouse (G). Values indicate mean ± SD of results from at least five animals per genotype. Threshold of significance was set at *P < 0.05 and **P < 0.01. (Magnification: 20×.)
To examine whether the Isx genotype affected immune cell differentiation, we determined the mRNA expression levels of the chemokine receptor Ccr9 in a total RNA preparation of the jejunum. This chemokine receptor is expressed in gut-homing T and B cells in intestinal lymph follicles in response to RA signaling (21). We observed a significant increase in Ccr9 mRNA levels in the jejunum in Isx−/− mice compared with Isx+/+ mice (Fig. 1E). To discern if changes in Ccr9 gene expression were accompanied by cellular alterations, we performed histology. ISX-deficient mice displayed an overall normal stratification of the intestinal cell layers (Fig. 1F). However, several regions in the duodenum and jejunum of these animals displayed large follicles. Microscopic inspection revealed that these structures were filled with mononuclear cells which morphologically resembled lymphocytes (Fig. 1G).
ISX Is Critical to Control Intestinal β-Carotene Absorption and Conversion to Retinoids.
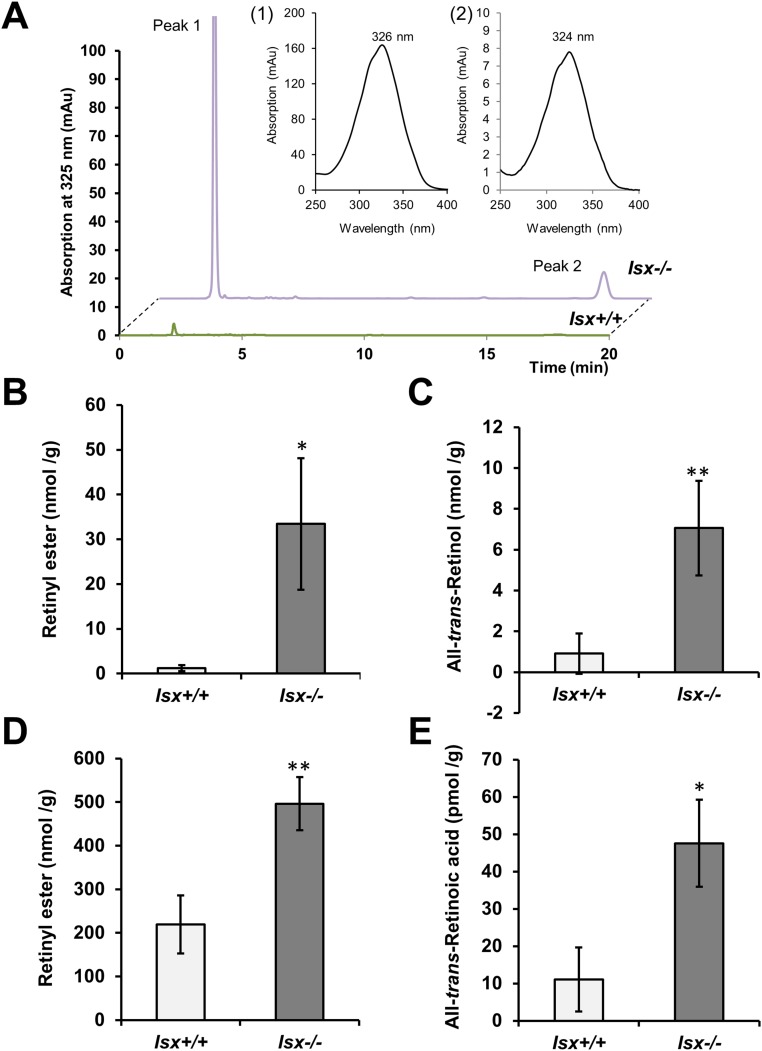
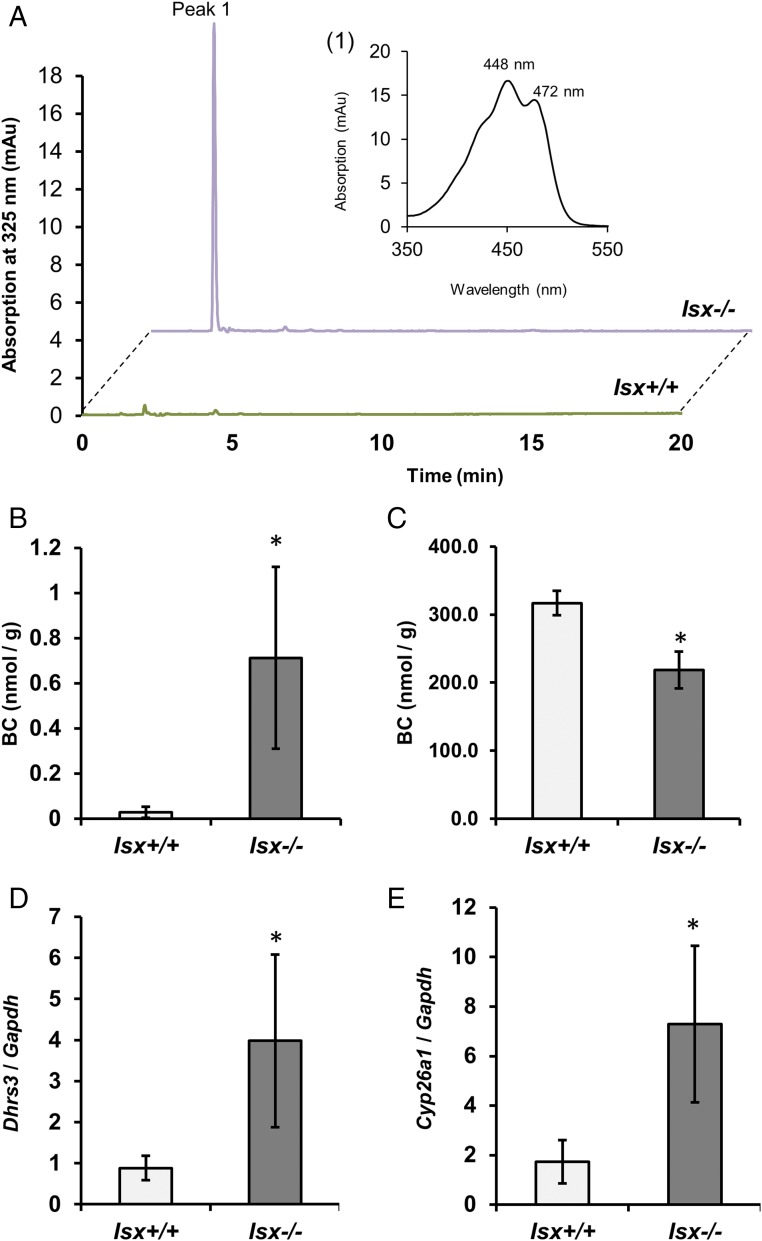
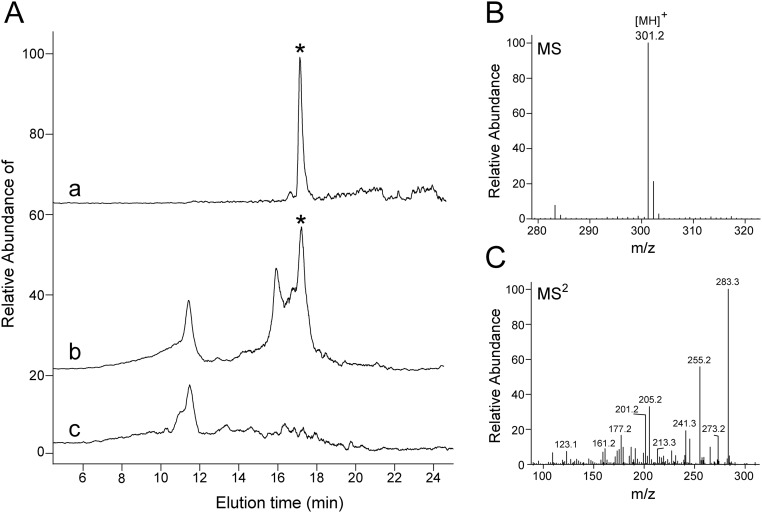
We next determined whether phenotype of ISX-deficient mice was associated with altered intestinal retinoid metabolism. Thus, we challenged Isx−/− and Isx+/+ mice with BC supplementation. Upon a 6-wk dietary intervention, Isx−/− mice showed a 27-fold higher level of RE in the jejunum than Isx+/+mice (Fig. S1 A and B). Similarly, jejunal ROL levels were significantly increased in these mice (Fig. S1 A–C). The increased utilization of the provitamin for retinoid production was echoed in high levels of BC in the jejunal lipid extracts of Isx−/− mice (Fig. S2 A and B). Conversely, Isx+/+ displayed a significantly higher amount of nonabsorbed BC in the feces compared with Isx−/− mice (Fig. S2C). Increased vitamin A production was also observed in highly elevated body stores of the vitamin in liver (Fig. S1D). LC-MS analysis of jejunal lipid extracts revealed that RA levels were fourfold higher in Isx−/− mice compared with Isx+/+ mice (Figs. S1E and S3). Accordingly, expression levels of Dhrs3 mRNA were increased in the jejunums of Isx−/− mice (Fig. S2D). This short-chain retinal dehydrogenase converts retinaldehyde to ROL, and its expression is induced by retinoid signaling (22). Moreover, we observed an increase of the expression of the RA-catabolizing enzyme Cyp26a1 in liver of Isx−/− mice compared with Isx+/+ mice (Fig. S2E). Thus, we concluded that ISX is required to maintain vitamin A homeostasis in response to dietary BC.
Fig. S1.
Retinoid levels in Isx+/+ and Isx−/− mice upon β-carotene supplementation. Four-week-old mice of both genotypes were subjected to dietary intervention for 6 wk with a diet containing β-carotene (50 mg/kg). Retinoids were determined in different tissues by HPLC analysis and LC-MS analysis, respectively. (A) Characteristic HPLC traces of jejunal lipid extracts of Isx+/+ and Isx−/− mice detected two major peaks at 325 nm. (Insets) The spectral characteristics of peak 1 (retinyl esters) and peak 2 (all-trans-retinol). (B and C) Quantification of retinyl ester and all-trans-retinol content of the jejunum of mice. (D) Retinyl ester content of the liver. (E) All-trans-retinoic acid content of the jejunum. Values in B–D indicate mean ± SD of results from five animals per genotype. Values in E indicate mean ± SD of results from three animals per genotype. Statistical significance was assessed by two-tailed t test. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Fig. S2.
β-carotene levels and expression of retinoid-responsive genes in Isx+/+ and Isx−/− mice. Four-week-old mice of both genotypes were subjected to dietary intervention for 6 wk with a diet containing β-carotene (50 mg/kg). β-carotene was determined in jejunum and feces by HPLC analysis. (A) Characteristic HPLC traces of jejunal lipid extracts of Isx+/+ and Isx−/− mice detected one major peak at 460 nm. Insets) The spectral characteristics of peak 1 (β-carotene). (B and C) Quantification of β-carotene content of the jejunum and feces of mice. (D and E) Quantitative RT-PCR analysis of (D) jejunal Dhrs3 and (E) hepatic Cyp26a1 mRNA levels. Values in B–E indicate mean ± SD of results from four animals per genotype. Statistical significance was assessed by two-tailed t test. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Fig. S3.
Detection and quantification of retinoic acid in mouse jejunum. (A) Selected LC-MS extracted ion chromatogram of m/z = 301.2 [MH]+ from the tissue extract indicating the presence of retinoic acid. The chromatogram “a” corresponds to 2 pmol of synthetic all-trans-retinoic acid standard, whereas traces “b” and “c” relate to samples from an Isx−/− and Isx+/+ mouse, respectively. Position of a peak that corresponds to all-trans-retinoic acid is indicated by the asterisks. (B) MS spectrum averaged between 17 and 18 min of elution for a sample from the tissue extract. (C) Characteristic fragmentation pattern of the dominant ion of m/z = 301.2 [MH]+ confirms the chemical identity of the detected retinoic acid molecule.
Dietary β-Carotene Affects Intestinal Immune Cell Differentiation During Adolescence.
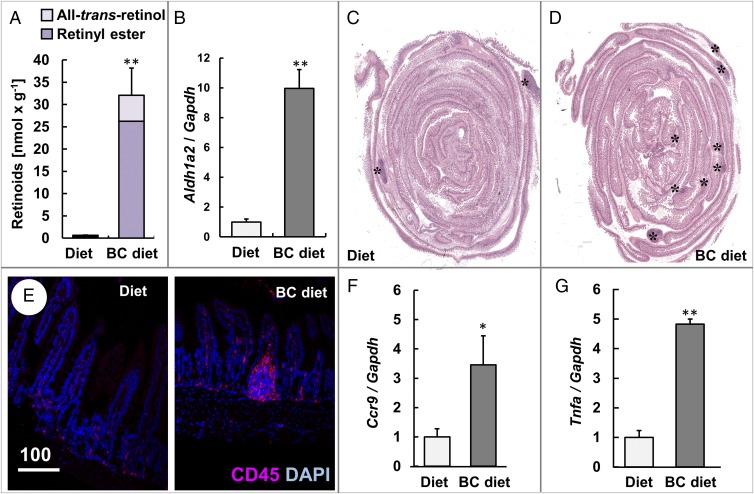
We next analyzed whether dietary BC can exacerbate the immune phenotype of Isx−/− mice. For this purpose, we randomized isogeneic Isx−/− littermates. One group received a diet supplemented with BC, while the other group received the same diet without BC supplement. After 10 wk of dietary intervention, supplemented mice had highly increased intestinal retinoid levels, whereas levels in nonsupplemented littermates were comparable to wild-type mice (Fig. 2A and Fig. S1). We also observed an increase of the expression levels of Aldh1a2 (Fig. 2B). The expression of this gene is controlled by feed-forward regulation via RA in the intestinal mucosa (23). Histological analyses revealed that large lymphoid follicles were distributed along the entire small intestine in supplemented Isx−/− mice (Fig. 2 C and D), while in nonsupplemented Isx−/− mice, few large lymphoid follicles were detectable in the ileum (Fig. 2C). Immunofluorescence microscopy demonstrated the accumulation CD45+ cells in the lymphocyte follicles of Isx−/− mice supplemented with BC (Fig. 2E). The stimulatory effects of BC supplementation on gastrointestinal immunity of ISX-deficient mice were further confirmed by a fourfold increase of Ccr9 mRNA expression levels in jejunal RNA preparations (Fig. 2F). Because ISX has been associated with inflammation (12, 13), we also evaluated the expression levels of the tumor necrosis factor α (TNFα) gene which was fivefold higher in BC-supplemented Isx−/− mice compared with nonsupplemented littermates (Fig. 2G).
Fig. 2.
β-Carotene supplementation increases the number of lymphocyte follicles, lymphocyte marker protein, and the proinflammatory cytokine. Four-week-old Isx−/− mice were fed with either diet or β-carotene (BC) supplemented diet. (A) Retinoid content of the intestines. (B) Quantitative RT-PCR analysis of jejunal Aldh1a2 mRNA levels. (C and D) Representative H&E-stained cross section through the intestine. The asterisks (*) indicate the location of lymphoid follicles. (Magnification: 20×.) (E) Intestinal cross section immunostained for CD45 (red). Nuclei are stained with DAPI (blue). (Scale bar, 100 µm.) (F and G) Quantitative RT-PCR analysis of jejunal (F) Ccr9 and (G) Tnfa mRNA levels. Values in A, B, F, and G indicate mean ± SD of results from five animals per supplementation group. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Maternal β-Carotene Supplementation Increases the Number and Size of Intestinal Lymphoid Follicles in ISX-Deficient Offspring.
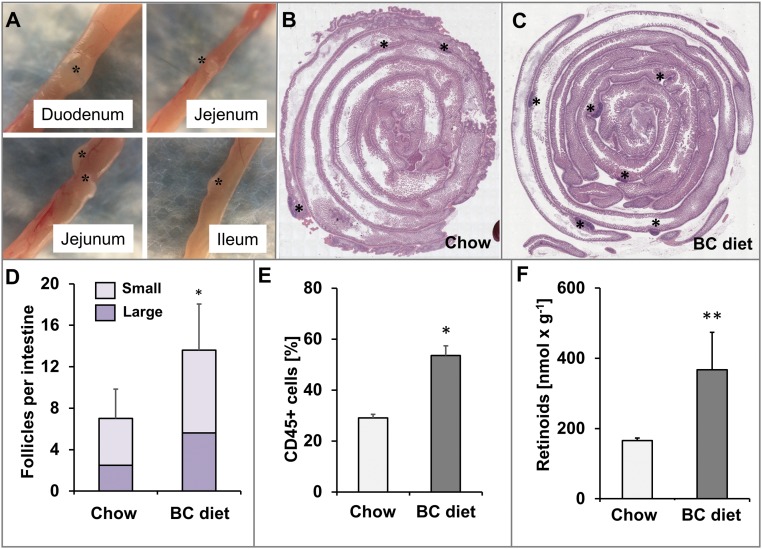
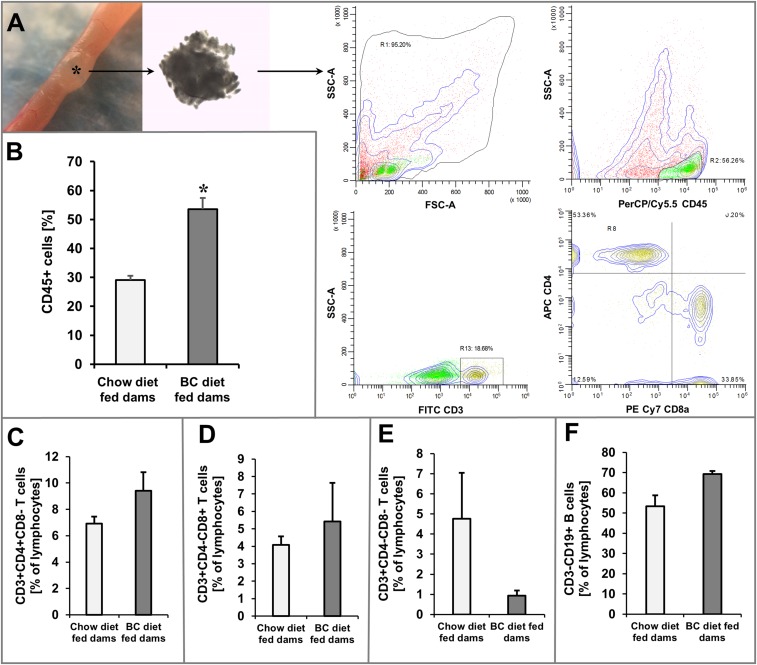
Dietary vitamin A is delivered via the maternal blood stream to the embryo (24) and affects lymphocyte differentiation in the developing gastrointestinal tract (25). Therefore, we wondered whether ISX might be a molecular linker between maternal vitamin A and immunity of the offspring. Thus, we subjected ISX-deficient dams to BC supplementation. As controls, we employed Isx−/− dams, which received regular mouse breeder chow rich in preformed retinoids. We obtained viable Isx−/− offspring both from BC-supplemented and nonsupplemented dams. At the age of weaning, we killed the offspring and analyzed their intestines. Large lymphoid follicles became detectable by macroscopic inspection in all parts of the intestine of offspring (Fig. 3A). Histological analyses confirmed the macroscopic observation and revealed an increase in the size and number of follicles in BC-supplemented offspring compared with nonsupplemented offspring (Fig. 3 B–D). We dissected the follicles from offspring of both supplementation groups and used flow cytometry to compare their lymphocyte composition. This analysis revealed significant overall increase of CD45+ cells in BC-supplemented offspring (Fig. 3E and Fig. S4). Analyses of the T-cell (CD3+) populations suggested alteration in the composition of CD4+ and CD8+ single-positive T cells and CD4− CD8− double-negative T cells in offspring of supplemented versus offspring of nonsupplemented dams (Fig. S4). To demonstrate that the immune phenotype was associated with altered vitamin A homeostasis, we performed HPLC studies for hepatic retinoids. Analyses of the two dietary groups revealed that liver stores of offspring of BC-supplemented dams displayed approximately double the amount of RE compared with offspring of nonsupplemented dams (Fig. 3F).
Fig. 3.
β-Carotene supplementation of ISX-deficient dams increases the number and size of lymphoid follicles in the intestine of the offspring. Pregnant Isx−/− mice were fed with either chow or β-carotene (BC) supplemented diet, and 4 wk-old offspring were analyzed. (A) Macroscopic view of lymphoid follicles (asterisks) in different parts of the intestine of offspring. (Magnification: 12×.) (B and C) Representative H&E-stained cross sections through the entire intestine of offspring (duodenum is in the center). Lymphoid follicles are marked by asterisks. (Magnification: 20×.) (D) Quantification of the number of small and large lymphoid follicles in the intestine of offspring. (E) The percentage of CD45+ cells in dissected intestinal lymphoid follicles of offspring. (F) Retinoid content of the liver of offspring. Values in D and F indicate mean ± SD of results from five animals, and values in E of four lymphoid follicles from two animals per supplementation group. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Fig. S4.
β-carotene supplementation of dams affects lymphoid immunity in the offspring. (A) Intestinal lymphoid follicles were dissected, and isolated cells were subjected to analysis by flow cytometry. T-cell gating strategy and representative analyses of CD45+, CD3+, CD4+, and CD8+ cells. SSC-A, Side-scatter area; FSC-A, forward-scatter area. (Magnification: 12×.) (B) Percentage of CD45+ cells of isolated cells in offspring from chow diet and BC diet-fed dams. Percentage of (C) CD3+CD4+CD8− T cells, (D) CD3+CD4−CD8+ T cells, (E) CD3+CD4−CD8− T cells, and (F) CD3−CD19+ B cells in isolated cells of offspring from chow diet and BC diet-fed dams. Values indicate mean ± SD of results from four lymphoid follicles from two animals per supplementation group. Threshold of significance was set at *P < 0.05.
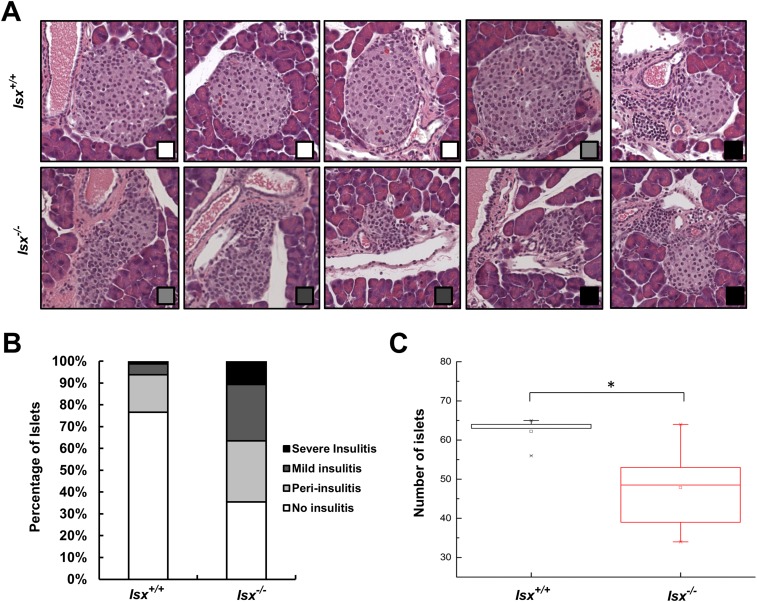
ISX-Deficient Mice Develop a Severe Insulitis.
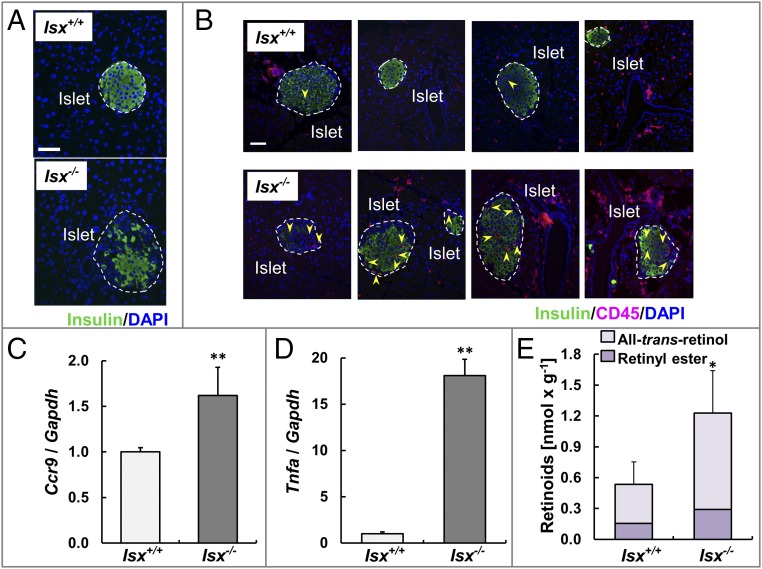
Recent work has indicated that diet is able to influence the immune system and thus can affect the development of inflammatory disease (26, 27). For instance, activated T helper cells expressing Ccr9 have been detected in pancreatic islet lesions of the nonobese diabetic mouse model (28), and excess dietary vitamin A supplementation has been associated with loss of tolerance against common food antigens (20). We observed that immunity was altered in the intestines of ISX-deficient mice and that inflammatory markers were increased (Fig. 3). To analyze whether these mice developed any inflammation in the adjacent pancreas, we serially sectioned the organ. Pancreatic islets of wild-type mice displayed a circular shape with distinct borders to the surrounding cells as indicated by staining for insulin. In contrast, the islets of Isx−/− mice were irregularly shaped (Fig. 4A). To quantify the extent of the pancreatic pathology of ISX-deficient animals, we blindly scored the degree and occurrence of insulitis in serial sections of the pancreas. Two different cross sections of the whole pancreas (head to the tail) from 7-mo-old Isx−/− and Isx+/+ mice (n = 6 each) were analyzed, and the degree of insulitis was scored according to the scale displayed in Fig. S5. From a total number of 282 islets that were examined in Isx−/− mice, 65% displayed the characteristic morphology of insulitis, with infiltrated immune cells (Fig. S5B). From these islets, 43% were identified as periinsulitis, 40% as mild insulitis, and 17% as severe insulitis. In contrast, only a few morphologically altered islets were detectable in pancreases of control mice. Out of 306 islets analyzed from Isx+/+ mice, 23% displayed periinsulitis from which 84% were scored as an early stage of periinsulitis without altered eyelet morphology (Fig. 4B, third panel from the left). To further characterize the pancreas, we counted the total number of islets per pancreas. In this respect, Isx−/− mice had a significantly reduced average number of islets per pancreas compared with control mice (Fig. S5C).
Fig. 4.
ISX-deficient mice develop a pancreatic insulitis. (A) Immunostaining for insulin (green) of β-islets of 7-mo-old Isx+/+ (Top) and Isx−/− (Bottom) mice maintained on standard diet. DAPI staining of nuclei is shown in blue. (Scale bar, 50 μm.) (B) Immunostaining of insulin (green) and CD45 (red) of representative pancreatic sections of these mice. DAPI staining of nuclei is shown in blue. The yellow arrows indicate CD45+ cells. (Scale bar, 50 μm.) (C and D) Quantitative RT-PCR analysis of Ccr9 (C) and Tnfa (D) mRNA levels of these mice. (E) Retinoid content of the pancreas of these mice. Values in C–E indicate mean ± SD of results from five animals per genotype. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Fig. S5.
Pancreas morphology of Isx−/− and Isx+/+ mice. (A) H&E-stained cross sections of representative islets of Isx−/− and Isx+/+ mice. The degree of insulitis is indicated by the squares (bottom right corner) and refers to the legend displayed in B. (Magnification: 20×.) (B) Quantification of the histological appearance of islets of Isx−/− and Isx+/+ mice. Insulitis-scoring criteria were used as follows, and representative histology is shown in A: No insulitis (white); periinsulitis with infiltration of lymphocytes restricted to the periphery of the islet (light gray); mild insulitis with less than 50% of the islet area infiltrated by lymphocytes (dark gray); and severe insulitis with more than 50% of the islet area infiltrated with lymphocytes (black). (C) Total numbers of β-islets in pancreatic cross sections of Isx−/− and Isx+/+ mice. Values indicate mean ± SD of results from six animals per genotype. Statistical significance compared with the control group was assessed by ANOVA followed by Scheffé tests using software origin 9. Threshold of significance was set at *P < 0.05.
Staining for CD45 confirmed that pancreatic islets of ISX-deficient animals were infiltrated with lymphocytes (Fig. 4B, arrows). Infiltration of the pancreas by gut-derived lymphocytes was further indicated by an increase of Ccr9 mRNA expression in the total RNA preparations of the pancreas (Fig. 4C). Analysis for Tnfa mRNA expression showed that this maker of inflammation was 18-fold elevated in the pancreas of Isx−/− mice compared with controls (Fig. 4D). Since retinoids play a significant role in pancreas development and maintenance (29), we also determined their levels in the pancreas. We observed a slight increase in ROL and RE level in the pancreas of Isx−/− mice (Fig. 4E).
ISX-Deficient Mice Display Impaired Glucose Metabolism.
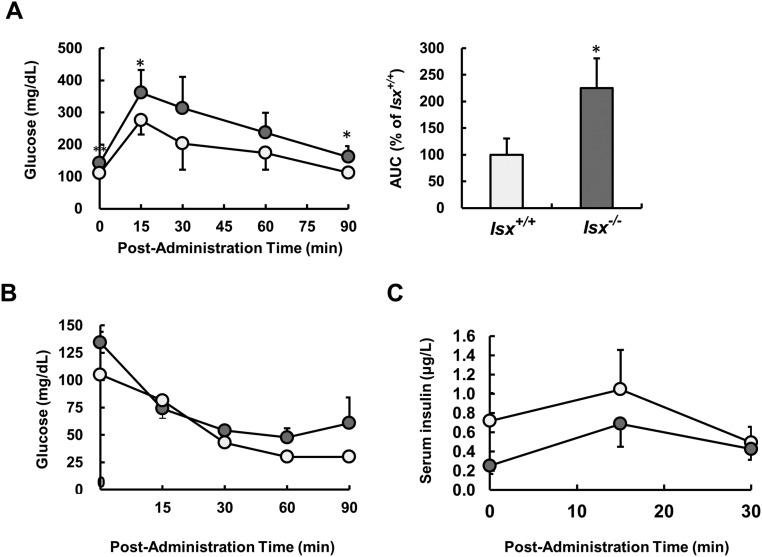
To examine whether endocrine function of the pancreas was impaired in Isx−/− mice, we subjected animals to oral glucose tolerance tests (GTT). These tests revealed that Isx−/− mice displayed a reduced clearance rate of blood glucose after a bolus dose of glucose compared with sex- and age-matched control mice (Fig. S6A). Because vitamin A metabolism has been associated with insulin resistance of peripheral tissues (30), we also performed insulin tolerance tests. However, Isx−/− mice showed no difference in the level of blood glucose at each time point compared with control mice (Fig. S6B). This observation suggested that the Isx−/− genotype was not causing resistance to insulin in the periphery. To directly demonstrate that the endocrine pancreas function was impaired in ISX deficiency, we determined serum insulin after glucose administration. In this test, Isx−/− mice secreted lower amounts of insulin in the serum in response to glucose injection compared with respective Isx+/+ control animals (Fig. S6C).
Fig. S6.
Glucose metabolism is impaired in Isx−/− mice upon β-carotene supplementation. (A) Blood glucose levels from glucose tolerance tests of 7-mo-old Isx−/− (dark gray circles) and Isx+/+ (light gray circles) mice raised on standard diet. (Right) The area under the curve (AUC) as percentage of wild type. (B) Blood glucose levels after insulin injection of Isx−/− (dark gray circles) and Isx+/+ (light gray circles) mice. (C) Serum insulin levels after glucose administration of Isx−/− (open circles) and Isx+/+ (filled circles) mice. Values indicate mean ± SD of results from five animals per genotype. Statistical significance compared with the control group was assessed by ANOVA followed by Scheffé tests using software origin 9. Threshold of significance was set at *P < 0.05.
Dietary β-Carotene Induces Insulin Resistance in ISX-Deficient Mice.
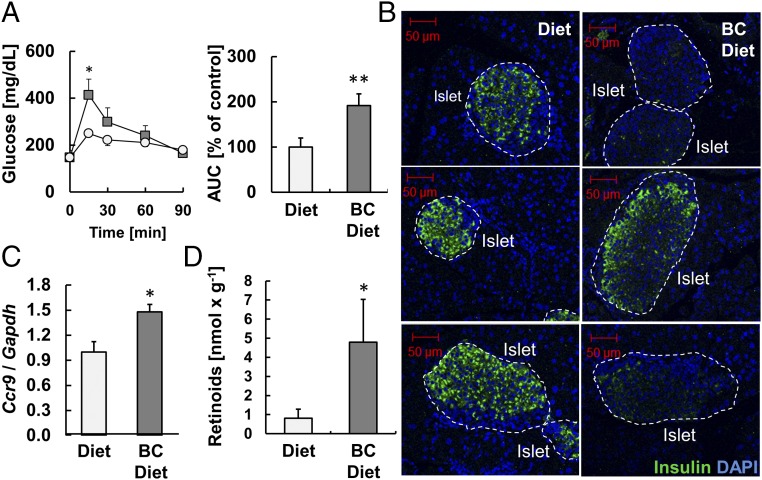
We showed that ISX-deficient mice developed a destructive pancreatic insulitis. If this pathology relates to intestinal vitamin A metabolism, one would expect that BC supplementation would expedite its occurrence. Thus, we performed GTTs with Isx−/− littermates raised on a diet supplemented with BC or the same diet supplemented with preformed retinoids as major sources of vitamin A. This analysis revealed that Isx−/− mice fed with BC for 10 wk had a reduced glucose clearance compared with siblings maintained on the retinoid-supplemented diet (Fig. 5A). Consistently, pancreatic sections of BC-supplemented mice displayed morphological alterations of β-islets in immunohistochemistry for insulin (Fig. 5B). Moreover, Ccr9 mRNA expression levels were increased in BC-supplemented Isx−/− mice compared with littermates maintained on a retinoid-supplemented diet (Fig. 5C). Biochemical analyses of pancreas lipid extracts also unfolded increased retinoid levels in BC-supplemented animals compared with retinoid-supplemented controls (Fig. 5D).
Fig. 5.
β-Carotene supplementation impairs glucose metabolism in ISX-deficient mice. (A) Blood glucose levels from glucose tolerance tests of nonsupplemented (circles) and β-carotene (BC) supplemented (squares) Isx−/− mice. (Right) The area under the curve (AUC) as percentage of control group. (B) Representative β-islets immunostained for insulin (green). DAPI staining of nuclei is shown in blue. (Scale bar, 50 μm.) (C) Quantitative RT-PCR analysis of Ccr9 mRNA levels. (D) Retinoid (retinol and retinyl esters) levels of the pancreas of these mice. Values indicate mean ± SD of results from five animals per supplementation group. Threshold of significance was set at *P < 0.05 or **P < 0.01.
Discussion
In the intestine, absorbed BC has a dual metabolic fate as a precursor for either RE or RA synthesis. We here provide evidence that a coordinated flux of dietary BC into these pathways evidently depends on regulation by the transcription factor ISX and is essential to maintain immunity and tolerance at this important barrier. Loss of ISX in mice affected retinoid metabolism in the intestine and also systemically. In the intestine, retinoids, including its transcriptionally active form RA, were significantly increased upon BC supplementation of ISX-deficient mice. This accumulation was largely prevented by ISX in the wild-type mice, which showed lower absorption and conversion rates of the provitamin. The enhanced absorption and conversion of dietary BC induced the expression of RA-inducible target genes such as Aldh1a2, Dhrs3, Cyp26a1, and the chemokine receptor Ccr9. This biochemical phenotype was associated with an increase in the number of intestinal lymphocytes, which accumulated in large lymphoid follicles. This phenotype was observed in aged ISX-deficient mice raised on diets with trace amounts of BC and was exacerbated by BC supplementation of the animals. The consequences of the ISX deficiency for immunity are consistent with previous studies which show that RA acts as an autacoid, a short-ranging hormonal signal (31), which can induce the expression of gut-homing receptors on T and B cells, promotes conversion of Foxp3+ regulatory T cells, and provokes T-cell–independent IgA switches in naϊve B cells (21, 32, 33).
Our data also indicate that ISX plays a more general role for systemic retinoid homeostasis as related to immunity. In this context, it is important to note that RE from chylomicrons, the major transport mode of dietary vitamin A, can be transferred from the maternal circulation to the embryo (24). Additionally, existing evidence indicates that the maternal vitamin A status can imprint the size of secondary lymphoid organs and the efficiency of immune responses in the offspring (25). In keeping with a deterministic role of maternal vitamin A for the developing immune system, BC-supplemented ISX-deficient dams gave birth to pups with elevated hepatic retinoid stores and an increased number and size of secondary lymphoid organs along the intestine. These lymphoid follicles contained a larger number of CD45+ lymphocytes and displayed alterations in T-cell composition compared with offspring of dams which received RE instead of BC supplementation. Thus, our data indicate that ISX is an important molecular link between the diet and gastrointestinal immunity throughout the mouse life cycle.
An important question is why the utilization of dietary BC for retinoid production is restricted by ISX in mammals. At first glance, this issue is surprising because vitamin A is a limited nutrient, and its deficiency is associated with disease and mortality. Accordingly, supplementation with preformed retinoids has been proven to be beneficial in malnourished populations (34). However, there is also evidence that excessive amounts of dietary vitamin A can trigger inflammatory responses (31, 35). Our studies in ISX-deficient mice indicate that dietary vitamin A has a narrow window of benefit for immunity. Thus, the utilization of BC, which exists in quite significant amounts in many fruits and vegetables (50–100 mg/kg), must be controlled to prevent the development of inflammatory diseases. As we report here, ISX-deficient mice developed a pancreatic insulitis that was associated with an infiltration of the pancreas by gut-derived lymphocytes. Though we did not clarify all molecular details, our data suggested that the loss of the ISX-dependent control of provitamin A metabolism triggered this pathology. This conclusion conforms to results from other studies, which demonstrate that excessive amounts of performed retinoids can promote inflammation and can trigger loss of tolerance at the intestinal barrier (20, 36). Such failure to establish tolerance against commensal flora and food antigens has been implicated in the autoimmunity that underlies type 1 diabetes (26). In keeping with this proposal, an invasion of activated T helper cells expressing Ccr9 has been observed in pancreatic islet lesions of the nonobese diabetic mouse model (28). Similarly, we observed a pancreatic infiltration of CD45+ cells and an elevation of Ccr9 mRNA levels in total pancreatic RNA preparations of ISX-deficient mice. This phenotype was accompanied by highly elevated expression levels of Tnfa mRNA encoding an inflammatory marker of lymphocytes.
In conclusion, our study provided evidence that ISX plays an important role in maintenance of immunity and tolerance in response to diet-derived signals at the intestinal barrier. Our findings indicate that retinoid production from dietary BC must be tightly controlled to avoid disturbances in lymphoid immunity, which can trigger inflammatory responses in the gastrointestinal tract. In future studies, the ISX-deficient mouse will be a versatile model to study the molecular details of this intriguing interplay between diet and gastrointestinal immunity. A better understanding of the molecular factors which control mucosal immunity and tolerance will aid in the development of nutritional intervention strategies to improve health in neonatal and adult life.
Materials and Methods
Female Isx−/− and Isx+/+ mice were generated as described (6, 9). All mice were on a C57/BL6;129Sv mixed genetic background. Mouse colonies were routinely maintained on breeder chow (29,000 IU vitamin A/kg diet). In supplementation experiments, mice were fed a diet based on AIN93G formulation (with 4,000 IU vitamin A/kg) without and with BC supplementation (50 mg/kg). HPLC analysis for BC and nonpolar retinoids was performed as previously described (10, 37). All-trans-retinoic acid was extracted, and LC-MS analysis was performed by a protocol adapted from Kane et al. (38). For qRT-PCR analysis an ABI Step-One Plus RT-qPCR instrument (Applied BioSystems) was used as previously described (10). For immunohistochemistry, protocols were applied as previously described (9). Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Scheffé tests using software origin 9 (OriginLab Corporation), with threshold of significance set as indicated in the figure legends. A detailed description of the materials and methods is provided in SI Materials and Methods.
SI Materials and Methods
Animals, Husbandry, and Experimental Diets.
Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee. Experiments were carried out with female Isx−/− and Isx+/+ littermates which have been previously established (6, 9, 10). All mice were on a C57/BL6;129Sv mixed genetic background. During the breeding and weaning periods (up to 4 wk of age), mice were maintained on breeder chow containing ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000; LabDiet). In supplementation experiments of dams, Isx−/− dams were fed a diet based on AIN93G formulation (with 4,000 IU vitamin A/kg) with BC supplementation (50 mg/kg) or a standard breeder chow diet. In BC supplementation experiments the diet based on an AIN93G formulation (with 4,000 IU vitamin A/kg) was supplemented with BC (50 mg/kg). BC was incorporated in the form of beadlets into the diet, and in the control diet, placebo beadlets were added (DSM Ltd.). All experimental diets were prepared by Research Diets, Inc. Experimental animals were maintained at 24 °C in a 12–12-h light–dark cycle and had free access to food and water. At the end of the dietary interventions, animals were killed in the fed state, blood was drawn, and tissues were dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until further analyses were conducted.
HPLC Analyses of Retinoids.
Nonpolar retinoids were extracted from serum, small intestine, pancreas, and liver homogenates as previously described (10, 37). HPLC analysis was performed on a normal-phase Zorbax Sil (5 μm, 4.6 × 150 mm) column (Agilent). Chromatographic separation was achieved by isocratic flow of 10% ethyl acetate per 90% hexane at a flow rate of 1.4 mL/min and quantification of retinoids as previously described (10, 37).
LC-MS Analysis of Retinoids.
Retinoic acid was extracted from dissected mouse jejuna as described by Kane et al. (38). MS-based detection of RA was performed with an LXQ linear ion trap mass spectrometer (Thermo Fisher Scientific) equipped with an electrospray ionization (ESI) interface and coupled to an Agilent 1100 HPLC (Agilent Technologies). Chromatographic separation of RA extracted from mouse tissues was achieved on a reverse-phase X-Bridge C18 HPLC column (100 × 2.1 mm; 3.5 μm) (Waters) by a linear gradient of acetonitrile from 50 to 100% in water developed within 15 min at a flow rate of 0.5 mL/min. All solvents contained 0.1% formic acid (vol/vol). The HPLC eluate was directed into the mass spectrometer via an ESI probe operated in the positive ionization mode. Parameters of ionization and detection were tuned with synthetic all-trans-RA standard to ensure the highest sensitivity. The amount of RA in the analyzed samples was estimated based on the calibration plots of the peak area of the authentic standard for the ion intensity at m/z = 301.2 against the known amount of RA.
RT-qPCR Analyses.
Total RNA isolation was carried out with TRIzol reagent (Invitrogen) or RNeasy Mini Kit (Qiagen GmbH) according to manufacturer’s instructions. RNA concentration and purity was measured with a NanoDrop spectrophotometer (ND-1000; Thermo Fisher Scientific). The Applied Biosystems reverse transcription kit (4368814; Applied Biosystems) was used. RT-qPCR was carried out with TaqMan probes (Applied BioSystems) for Bco1 (Mm01251350_m1), Aldh1a2 (Mm00501306_m1), Ccr9 (Mm02528165_s1), Dhrs3 (Mm00488080_m1), Cyp26a1 (Mm00514486_m1), and Tnfa (Mm00443258_m1). As endogenous control, Gapdh (Mm99999915_g1) was used. All real-time PCR experiments were performed with an ABI Step-One Plus RT-qPCR instrument (Applied BioSystems) as previously described (9, 10).
Histology and Immunohistochemistry.
Mouse small intestine and pancreas were dissected and fixed overnight by immersion in 4% paraformaldehyde in PBS at 4 °C and embedded in paraffin as previously described (9). For immunostaining, paraffin-slide sections were deparaffinized and rehydrated. Tissue sections were then incubated overnight at 4 °C with an appropriate primary antibody: either the PE Rat Anti-Mouse CD45 (553081 from BD Biosciences), PE Rat IgG2a isotype control (553930 from BD Biosciences), or guinea pig polyclonal anti-insulin (ab7842 from Abcam PLC) antibody at a 1:200 dilution. For tissue morphology, sections were stained with conventional Haematoxylin and Eosin (H&E) method. Fluorescent images were acquired with a Zess LSM 510 META laser-scanning confocal microscope (Carl Zeiss MicroImaging) by using a multiline argon laser (excitation 488 and 555 nm) or a 405-diode laser (excitation 405) with a 20× C-Apochromat, NA 1.2-O objective. Metamorph Imaging software was employed for image acquisition.
Flow Cytometry.
Small intestines were harvested and immediately placed in PBS on ice. Lymphoid follicles of duodenal and/or jejunal parts were excised with scissors and mechanically dissociated and filtered with a 70-µm Nylon Cell Strainer (Falcon #352350; Corning Science). Cells were collected by centrifugation (500 × g at 4 °C). The cell pellet was resuspended in 50 μL PBS and transferred into a 5-mL polystyrene round-bottom tube (Falcon #352058). The cell suspension was incubated for 15 min in the dark at room temperature with conjugated antibodies according to the manufacturer’s instructions: PerCP/Cy5.5 anti-mouse CD45 (#103131), FITC anti-mouse CD3 (#100203), APC anti-mouse CD4 (#100411), PE/Cy7 anti-mouse CD8a (#100721), and PE anti-mouse CD19 (#152407) from BioLegend. Samples were centrifuged at 4 °C for 20 min, discharged, washed with 4 mL cold PBS, and precipitated by centrifugation (1,000 g) at 4 °C for 20 min. Samples were resuspended in 200 µL cold PBS and analyzed with an Attune NxT Flow Cytometer equipped with WinList 3D 8.0 software.
Blood Glucose and Insulin Measurements.
For glucose tolerance tests, Isx−/− and Isx+/+- mice were fasted for 6 h, and a 20% (wt/vol) glucose solution was administered orally (2 mg/g body weight). Blood samples were taken from the tail vein before and after 15, 30, 60, and 90 min of glucose administration. Glucose levels were determined using a blood glucose level monitor (EasyMax; EPS Bio Technology Corp.). Serum insulin levels were determined using an ELISA kit (Mercodia Mouse Insulin). For insulin tolerance tests, 4-h–fasted mice were injected in the i.p. cavity with human insulin (0.1 units/mL) at a dose of 0.5 units/kg body weight. Blood samples were taken from the mouse tail vein before and after 15 and 30 min of insulin injection. Glucose levels in the blood were determined using a blood glucose level monitor as described above.
Statistical Analyses.
Results are presented as mean ± SD, and the number of experiments is indicated in the figure legends. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Scheffé tests using software origin 9 (OriginLab Corporation), with threshold of significance set as indicated in the figure legends.
Acknowledgments
The research was supported in part by US National Institute of Health Grants EY020551 (to J.v.L.), EY023948 (to M.G.), and the visual science training program T32-EY07157. E.A.K. was supported through the summer undergraduate research program of the pharmacology department. We thank Scott Howell and Catherine Doller from the Case Western Reserve University visual science core facility (Core Grant P30-EY11373) for expert help with histology, microscopy, and imaging.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714963114/-/DCSupplemental.
References
- 1.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 2.Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr. 2012;96(Suppl):1166S–1172S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick TB, et al. Vitamin deficiencies in humans: Can plant science help? Plant Cell. 2012;24:395–414. doi: 10.1105/tpc.111.093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grune T, et al. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140(Suppl):2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi MY, et al. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- 7.Seino Y, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- 8.Lobo GP, et al. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo GP, et al. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem. 2013;288:9017–9027. doi: 10.1074/jbc.M112.444240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widjaja-Adhi MA, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet. 2015;24:3206–3219. doi: 10.1093/hmg/ddv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borel P, Desmarchelier C. Genetic variations associated with vitamin A status and vitamin A bioavailability. Nutrients. 2017;9:E246. doi: 10.3390/nu9030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu SH, et al. Proinflammatory homeobox gene, ISX, regulates tumor growth and survival in hepatocellular carcinoma. Cancer Res. 2013;73:508–518. doi: 10.1158/0008-5472.CAN-12-2795. [DOI] [PubMed] [Google Scholar]
- 13.Sue S, et al. Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis. J Gastroenterol. 2016;51:949–960. doi: 10.1007/s00535-016-1176-2. [DOI] [PubMed] [Google Scholar]
- 14.Dinu I, et al. SNP-SNP interactions discovered by logic regression explain Crohn’s disease genetics. PLoS One. 2012;7:e43035. doi: 10.1371/journal.pone.0043035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Brown C, Ortiz C, Noelle RJ. Leukocyte homing, fate, and function are controlled by retinoic acid. Physiol Rev. 2015;95:125–148. doi: 10.1152/physrev.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- 17.Spencer SP, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crockett SD, Porter CQ, Martin CF, Sandler RS, Kappelman MD. Isotretinoin use and the risk of inflammatory bowel disease: A case-control study. Am J Gastroenterol. 2010;105:1986–1993. doi: 10.1038/ajg.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy D, Siegel CA, Sands BE, Kane S. Possible association between isotretinoin and inflammatory bowel disease. Am J Gastroenterol. 2006;101:1569–1573. doi: 10.1111/j.1572-0241.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 20.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerschmidt SI, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zolfaghari R, Chen Q, Ross AC. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am J Physiol Gastrointest Liver Physiol. 2012;303:G578–G588. doi: 10.1152/ajpgi.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molenaar R, et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186:1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 24.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:88–98. doi: 10.1016/j.bbalip.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhoen M, Ferreira C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat Med. 2015;21:709–718. doi: 10.1038/nm.3894. [DOI] [PubMed] [Google Scholar]
- 28.McGuire HM, et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Brun PJ, Wongsiriroj N, Blaner WS. Retinoids in the pancreas. Hepatobiliary Surg Nutr. 2016;5:1–14. doi: 10.3978/j.issn.2304-3881.2015.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muenzner M, et al. RBP4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and RARalpha activity. Mol Cell Biol. 2013;33:4068–4082. doi: 10.1128/MCB.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 33.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 34.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96(Suppl):1204S–1206S. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 35.Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao X, et al. Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model. PLoS One. 2015;10:e0118176. doi: 10.1371/journal.pone.0118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amengual J, et al. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem. 2013;288:34081–34096. doi: 10.1074/jbc.M113.501049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]