If the brain is the body’s central processing unit, then the blood–brain barrier is its firewall. A specialized network of cells that lines the brain’s vascular system, the blood–brain barrier selectively ushers in nutrients and other essential biomolecules while denying entry to most everything else. But the same system that protects the brain also stymies many therapeutics that could potentially treat disease.
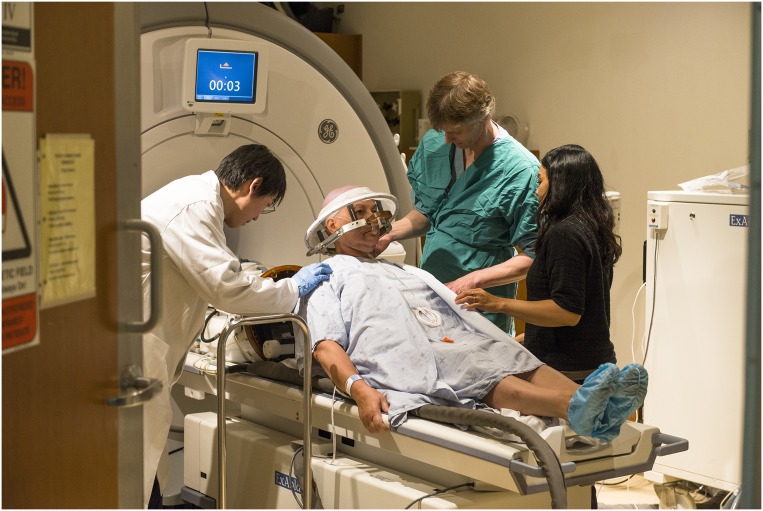
Fig. 1.
In November 2015, researchers at Sunnybrook Health Sciences Centre in Toronto began a clinical trial to noninvasively breach the blood–brain barrier with focused ultrasound in an attempt to deliver chemotherapy to brain tumors. Image courtesy of Doug Nicholson (Sunnybrook, Toronto).
A handful of drugs slip into the brain by passive diffusion—among them, antidepressants and medications for schizophrenia and epilepsy, along with caffeine, alcohol, and nicotine. These molecules are exceedingly small. They also can readily dissolve into the lipid membranes that encase blood–brain barrier cells. But new drug leads of this type are growing more elusive. “Most of the small molecule combinations have been explored,” says biomedical engineer Peter Searson at Johns Hopkins University in Baltimore, MD.
Given these challenges, researchers are looking for other ways to slip past the barrier. Brain-penetrating antibodies and viruses could, for example, ferry therapeutic cargo across the border. A few groups are using ultrasound to temporarily open parts of the blood–brain barrier. “Over the last 20 years, blood–brain barrier research went from just a little small cottage industry to people really bringing the newest tools and approaches to bear on problem,” says bioengineer Eric Shusta at the University of Wisconsin–Madison. Finding ways to stealthily shuttle drugs across the blood–brain barrier could have big therapeutic implications.
Barriers to Discovery
Although attempts to penetrate the barrier have only recently gained steam, the blood–brain barrier concept has been around for more than 100 years. The origins of the idea are the subject of some debate, but many accounts start in 1885 with an accidental finding by German researcher Paul Ehrlich. While studying oxygen consumption in different organs, Ehrlich injected mice with an indicator dye that flowed throughout the body, staining all organs but one: the brain. At the time, Ehrlich believed that neural tissue simply didn’t bind well to dye. But Ehrlich’s student Edwin Goldmann later managed to stain the brain by injecting dye directly into the cerebrospinal fluid. Together, the findings suggested the brain was somehow protected from general circulation (1, 2).
German neurologist Max Lewandowsky is generally credited with coining the term “blood–brain barrier” to describe this concept in 1900, after his own animal studies showed that certain neurotoxins worked only when injected into the brain and not when introduced into the bloodstream. Direct evidence for such a barrier, however, would await the rise of the electron microscope in the 1950s and 60s, which ultimately revealed that strands of proteins joined together the endothelial cells lining the brain’s vasculature, walling off passing molecules. These so-called “tight junctions” are also found elsewhere in the circulatory system, but they are especially tight in the brain, restricting flow down to 10–15 angstroms (1, 2).
To control passage, an intricate system of transport proteins—surface receptors on endothelial cells—pluck specific molecules from the bloodstream, such as insulin, and expel them on the opposite end of the endothelium into the brain. At the same time, efflux pumps police endothelial cells for unwanted molecules (including many pharmaceutical products) and eject them into the blood. A growing body of research suggests that endothelial cells work closely with several cell types, including neighboring neurons and glial cells in the brain that together regulate permeability of the blood–brain barrier (3).
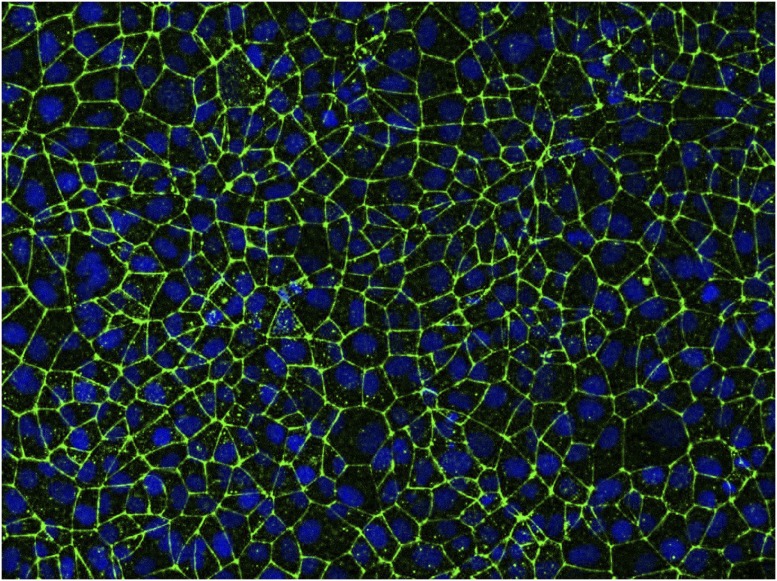
Fig. 2.
In an in vitro model of the blood–brain barrier, endothelial cells pack tightly together, with their nuclei (blue) surrounded by a key tight junction protein known as claudin-5 (green). Image courtesy of Eric Shusta and Tongcheng Qian (University of Wisconsin–Madison, Madison, WI).
Trojan Horses
Given what researchers know about the barrier, how might they safely penetrate its defenses? Some have turned to the “Trojan horse” approach, hijacking existing transport systems to deliver disease-fighting agents across the border. The strategy generally involves decorating therapeutics with antibodies or antibody fragments that bind to specific receptors on the blood-side surface of endothelial cells. Once activated, these receptors enter the cell, where they often shuttle to the brain side of the border and unload their cargo into the brain.
Among the best-studied targets are the insulin receptor and the receptor for transferrin, an iron-binding protein in the blood; in recent years, both have attracted interest from biotechnology companies. In an effort to combat Alzheimer’s disease, researchers at Genentech in South San Francisco, CA, have created a two-sided antibody that crosses the barrier by grabbing the transferrin receptor with one arm. Inside the brain, the antibody releases the receptor, and, with the other arm, binds and disrupts its main target: the enzyme beta-secretase, which produces the amyloid-beta protein that accumulates in the brain in Alzheimer’s disease. In a proof-of-concept study in 2014, the team showed that intravenous injections of the antibody lowered amyloid-beta levels in the brains of monkeys (4).
Some companies have already begun clinical trials using the Trojan horse strategy. ArmaGen, in Calabasas, CA, is tackling a pair of enzyme-deficiency disorders that cause a harmful buildup of complex sugars throughout the body, including in the brain. Although conventional enzyme replacement therapies can mitigate damage to other organs, they cannot breach the blood–brain barrier. For each condition, the company has developed a therapeutic that fuses the missing enzyme with antibodies against the insulin receptor. ArmaGen says the initial clinical trials have had promising results; the company and its partners are now planning follow-up studies.
For many neurological disorders, the insulin and transferrin receptors may not be ideal targets: the receptors are found throughout the body and hence can potentially take up therapeutics meant for the brain. “The holy grail is a highly abundant, efficient transporter present only at the blood–brain barrier,” Shusta says. Using an in vitro blood–brain barrier model they developed, Shusta’s group is scouring vast libraries of antibodies for those that can get through, potentially via receptors that are yet unknown.
Other brain-penetrating strategies involve viruses, which are naturally built to deliver genetic material into host cells. In 2009, researchers led by Brian Kaspar at Nationwide Children’s Hospital in Columbus, Ohio, showed that one strain of harmless adeno-associated virus, AAV9, could cross the blood–brain barrier in mice (5). Biotech firm AveXis in Bannockburn, Illinois, (where Kaspar is chief scientific officer) is currently running clinical trials of an AAV9-based gene therapy for spinal muscular atrophy, a disease that kills motor neurons.
Others are looking for additional viruses to carry therapeutics into the brain. At the California Institute of Technology in Pasadena, Viviana Gradinaru’s group developed a high-throughput system for generating mutations in the shell of AAV9 (which helps the virus target different tissues and cells) and testing millions of these variations (6). In June, her team discovered one highly efficient version that, at relatively low intravenous doses, can deliver genes to about 70% of neurons in the cerebral cortex of adult mice (7). Gradinaru plans to use the virus as a basic research tool but says the finding could lay the groundwork for potential gene therapies in the future.
Making Waves
Taking a different tack altogether, other researchers are testing the use of tiny bubbles and ultrasound to temporarily open portions of the blood–brain barrier. When used for medical imaging, ultrasonic waves enter the body, where the pattern of absorption by and reflection against tissues paints a picture. But at certain frequencies and intensities, ultrasound can also cause vibration, friction, or even heat in tissues. In recent years, researchers have learned to fine-tune these parameters to briefly open the blood–brain barrier.
The microbubble technique, which was first demonstrated in 2001 in rabbits (8), is the subject of several ongoing clinical trials. Patients are first injected with microscopic bubbles that spread throughout the circulatory system. Then, with pulses of ultrasound, researchers vibrate the bubbles in a portion of the brain’s vasculature. Through a mechanism that is not yet fully understood, this causes the blood–brain barrier to open for a few hours before sealing itself again. Ultimately, the microbubbles dissolve in the blood.
At Sunnybrook Health Sciences Centre in Toronto, researchers are using a special ultrasound-transducing helmet that holds more than a thousand beams. When carefully coordinated and directed with the help of MRI, these beams focus on a small stretch of the blood–brain barrier. One clinical trial, led by neurosurgeon Todd Mainprize, aims to deliver chemotherapy drugs into the brains of patients with glioblastoma, a fast-growing, hard-to-treat brain tumor. The team’s initial study examines whether opening the blood–brain barrier with ultrasound is safe and effective, and whether chemotherapy drugs can get through. In another trial, other Sunnybrook researchers are testing whether the technique can be used safely in Alzheimer’s patients.
Meanwhile, at Sorbonne University in Paris, neurosurgeon Alexandre Carpentier is leading clinical trials of an ultrasound-delivering brain implant to help administer chemotherapy to glioblastoma patients (9). The device requires invasive surgery, but once lodged in the skull over the target area, it does not require further imaging (which would necessitate additional time and cost) to aim and operate; at the time of treatment, researchers insert a needle through the skin that connects the implant to an external controller.
Despite a flurry of interest, many researchers are still wary of this general strategy. “The blood–brain barrier is designed to keep harmful molecules out,” says neuroscientist Choi-Fong Cho at Brigham and Women’s Hospital in Boston: “Even transiently opening the blood–brain barrier could allow all sorts of things to get across.” Nathan McDannold at Brigham and Women’s Hospital, who helped pioneer the microbubble technique in animals, says his data suggest that focused ultrasound causes relatively little damage and could be appropriate for treating grave conditions, such as intractable brain tumors. “If we start talking about pain medicines or psychiatric drugs,” he says, “the bar is going to be much higher.” McDannold is currently working on new technologies to monitor the microbubbles in real time, to help guide the ultrasound beams or tweak their intensity to maximize safety and efficacy of the helmet technique.
With an increasing number of tools to choose from, researchers are finally beginning to chip away at the formidable blood–brain barrier. But even as entryways present themselves, researchers will have to find ways to make drugs pinpoint specific regions or cell types once inside the brain. “It’s just essential that we develop drugs that are highly efficacious and can cross the blood–brain barrier,” says Cho. “But we also need to find the balance where they’re not too toxic and getting everywhere in the brain.”
Supplementary Material
References
- 1.Liddelow SA. Fluids and barriers of the CNS: A historical viewpoint. Fluids Barriers CNS. 2011;8:2. doi: 10.1186/2045-8118-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders NR, et al. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front Neurosci. 2014;8:404. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuwelt EA, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu YJ, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 5.Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KY, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]