Abstract
The Hokkaido Study on Environment and Children’s Health is an ongoing study consisting of two birth cohorts of different population sizes: the Sapporo cohort and the Hokkaido cohort. Our primary study goals are (1) to examine the effects of low-level environmental chemical exposures on birth outcomes, including birth defects and growth retardation; (2) to follow the development of allergies, infectious diseases, and neurobehavioral developmental disorders and perform a longitudinal observation of child development; (3) to identify high-risk groups based on genetic susceptibility to environmental chemicals; and (4) to identify the additive effects of various chemicals, including tobacco smoking. The purpose of this report is to update the progress of the Hokkaido Study, to summarize the recent results, and to suggest future directions. In particular, this report provides the basic characteristics of the cohort populations, discusses the population remaining in the cohorts and those who were lost to follow-up at birth, and introduces the newly added follow-up studies and case-cohort study design. In the Sapporo cohort of 514 enrolled pregnant women, various specimens, including maternal and cord blood, maternal hair, and breast milk, were collected for the assessment of exposures to dioxins, polychlorinated biphenyls, organochlorine pesticides, perfluoroalkyl substances, phthalates, bisphenol A, and methylmercury. As follow-ups, face-to-face neurobehavioral developmental tests were conducted at several different ages. In the Hokkaido cohort of 20,926 enrolled pregnant women, the prevalence of complicated pregnancies and birth outcomes, such as miscarriage, stillbirth, low birth weight, preterm birth, and small for gestational age were examined. The levels of exposure to environmental chemicals were relatively low in these study populations compared to those reported previously. We also studied environmental chemical exposure in association with health outcomes, including birth size, neonatal hormone levels, neurobehavioral development, asthma, allergies, and infectious diseases. In addition, genetic and epigenetic analyses were conducted. The results of this study demonstrate the effects of environmental chemical exposures on genetically susceptible populations and on DNA methylation. Further study and continuous follow-up are necessary to elucidate the combined effects of chemical exposure on health outcomes.
Keywords: Birth cohort study; Environmental chemicals; Exposure measurement; Pregnancy outcomes; Birth size; Thyroid, reproductive, and steroid hormones; Neurobehavioral development; Allergies and infectious diseases; Genetic susceptibility; Epigenetics
Background
In 1997, Colborn et al. warned of the dangers of environmental chemicals as endocrine disruptors, which could lead to impaired reproductive capacity [1]. Since that warning, a myriad of animal and epidemiological studies have evaluated the adverse health effects of these endocrine-disrupting chemicals (EDCs) [2–4]. Although the use of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), polychlorinated biphenyls (PCBs), perfluorooctanoic sulfonate (PFOS), and perfluorooctanoic acid (PFOA) is regulated, these chemicals are still detected in the environment and in the human body. Concentrations of PFOS and PFOA, the conventional perfluoroalkyl substances (PFASs), have been decreasing; however, the concentrations of newly developed PFASs such as perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) have been increasing [5]. Accumulating evidence has shown the various adverse health effects of exposure to environmental chemicals; however, few studies have focused on vulnerable populations such as fetuses and children. Thus, together with the Developmental Origins of Health and Disease (DOHaD) theory, which proposes that the early life environment has widespread consequences for later health, this prospective birth cohort study was established.
The Hokkaido Study on Environment and Children’s Health: Malformation, Development and Allergy is an ongoing birth cohort study launched in 2002. The Hokkaido Study is one of the oldest birth cohort studies not only in Japan but also in Asia [6]. The Hokkaido Study consists of two cohorts of different population sizes; the Sapporo cohort mainly focuses on the deep investigation and understanding of child neurobehavioral development by taking advantage of the relatively small number of participants and study area, while the Hokkaido cohort focuses on rare diseases such as birth defects and developmental disorders (e.g., autism spectrum disorder and attention deficit hyperactivity disorder) which are not commonly found in small cohorts. These two cohorts of different sizes allow us to examine various outcomes including child growth, development, allergy, and infectious diseases.
Our primary study goals include (1) examining the possible negative effects of perinatal environmental chemical exposure on birth outcomes, including birth defects and growth retardation; (2) following the development of allergies, infectious diseases, and neurobehavioral developmental disorders and performing a longitudinal observation of child development; (3) identifying high-risk groups with genetic susceptibilities to environmental chemical exposures; and (4) identifying the additive effects of lifestyle including tobacco smoking and folic acid intake encountered in the daily environment.
Our previous cohort profile update in 2013 [7] introduced our findings since the beginning of the study. We reported that higher concentrations of toxic equivalents (TEQ) of dioxin and other specific congeners in the maternal blood increased the adverse effects on infant birth weight, neurobehavioral development, and immune function. However, we were only able to produce limited reports on the effects of gene-environment interactions between environmental chemicals and maternal/infant genes on adverse health outcomes.
Over the 14 years of follow-ups, new research questions have arisen and the study focus has expanded beyond its primary goals. Studies have reported an increasing prevalence of children with developmental disabilities and behavioral problems [8, 9]. The prevalence of childhood allergic conditions is also increasing [10, 11]. These childhood health issues are of global public health concern; thus, the longitudinal observations of our cohort study will provide evidence on the associations between the in utero environment and child health outcomes.
Emerging evidence has indicated that epigenetic modifications including DNA methylation may link the intrauterine environment to lifelong health trajectories [12]. The effects of in utero environmental chemical exposure on DNA methylation in the developing fetus are gaining increasing attention. The wide range of data collected in this cohort study allows us to further investigate new research questions including DNA methylation and its health outcomes.
The purpose of this report is to update the progress of the Hokkaido Study, to summarize the recent results, and to suggest its future directions. In particular, this report focuses on (1) providing the basic characteristics of the whole cohort population as the fixed data are now available after the closing of recruitment in 2012, and presenting the comparisons of the population remaining in the cohort with those who were lost to follow-up at birth; (2) introducing the nested case-cohort study design, in which a sub-cohort is used as a comparison group for all cases that occur in the cohort; and (3) introducing the details of the follow-up studies, including child neurobehavioral problems at preschool ages. This design is effective when a cohort is followed for health outcomes by avoiding the costs of collecting and processing covariate information and exposure assessment in the selected sub-cohort population. This strategy is applied to the Hokkaido cohort, which contains more than 20,000 participants.
Methods
Study areas, participants, and baseline questionnaire
The details of the Hokkaido Study have been described in previous reports [7, 13]. Briefly, the Hokkaido Study consists of two birth cohorts: the Sapporo cohort and the Hokkaido (large-scale) cohort. Two cohorts differed in terms of the strategies. The Sapporo cohort enrolled 514 pregnant women at 23–35 weeks of gestation who planned to deliver at Toho Hospital in Sapporo city from July 2002 to October 2005. Among 1796 potentially eligible women, the women had incomplete partner’s information, the women had decided to enroll in the Japanese cord blood bank (22% of those of approached), or the women decided to deliver the baby at another hospital (3% of those of approached) was excluded. Ultimately, 514 pregnant women agreed to participate. The Hokkaido cohort enrolled pregnant women before 13 weeks of gestational age who visited one of the associated hospitals or clinics of three university hospitals in the Hokkaido prefecture from February 2003 to March 2012. The participation rate was 55%. Thirty-seven associated hospitals or clinics, including three university hospitals, were evenly distributed throughout the Hokkaido prefecture, accounting for approximately 40% of the institutes with delivery units in this prefecture [14]. Parent and infant medical records were obtained from physicians at the participating hospitals or clinics. Both the Sapporo and Hokkaido cohorts were conducted after obtaining written informed consent from all participants. The institutional ethical board for human gene and genome studies at Hokkaido University Center for Environmental and Health Sciences and Hokkaido University Graduate School of Medicine approved the study protocol.
We also established a sub-cohort of 4869 participants, which corresponded to 23.3% of all participants in the Hokkaido Study. In this sub-cohort, we included 500 participants who were randomly selected from each enrollment year between 2003 and 2011, and all 369 participants from the enrollment year 2012.
Follow-up and outcome measures: the Sapporo cohort
Details of the information collected at the time of enrollment and birth have been described previously [7, 13]. A self-administered questionnaire was completed at the time of enrollment to obtain parental baseline information. Maternal medical and infant birth records were obtained from the hospital. Follow-up studies were conducted at 6 and 18 months and 3.5 and 7 years of age, which collected information regarding childhood neurological and behavioral development and medical histories of asthma, allergies, and infectious diseases. Measurements of the second and fourth digits were made from photocopies of the ventral surface of the right and left hands at school age; the ratio of the second finger to fourth finger lengths (2D/4D) was calculated in order to evaluate the effects of prenatal Leydig cell function [15].
The details of the follow-up questionnaires and tests are shown in Table 1.
Table 1.
Follow-ups performed at different ages
| Neurobehavioral development | Allergy | Anthropometric measurements/puberty | |
|---|---|---|---|
| Sapporo cohort | |||
| 6–7 months | BSID-II, FTII, EES | ||
| 1.5 years | BSID-II, DDST, EES | ISAAC | Health checkup data |
| 3.5 years | K-ABC, CBCL, WAIS-R, EES | ISAAC | Health checkup data |
| 7 years | WISC-III, WCST-KFS, CBCL, J-PSAI, 2D/4D | ISAAC | Health checkup data |
| 12 years | Tanner staging, onset of puberty | ||
| Hokkaido cohort | |||
| 1 year | ISAAC, ATS-DLD | Health checkup data | |
| 1.5 years | M-CHAT | ||
| 2 years | ISAAC | Health checkup data | |
| 3 years | KIDS, SDQ | ||
| 4 years | ISAAC | Health checkup data | |
| 5 years | SDQ, DCDQ | ||
| 6 years | ADHD-RS, ASQ | ||
| 7 years | ISAAC, home visit | Health checkup data | |
| 8 years | ADHD-RS, Conners 3P, ASQ, CBCL, J-PSAI, WISC-IV | ||
| 9 years | Tanner staging, onset of puberty | ||
ADHD-RS Attention Deficit Hyperactivity Disorder-Rating Scale, ASQ Autism Screening Questionnaire, ATS-DLD American Thoracic Society-Division of Lung Disease, M-CHAT Modified Checklist for Autism in Toddlers, BSID-II Bayley Scales of Infant Development second edition, CBCL Child Behavior Checklist, Conners 3P Conner’s 3rd Edition for Parents, DCDQ Developmental Coordination Disorder Questionnaire, DDST Denver Developmental Screening Tests, EES Evaluation of Environmental Stimulation, FTII Fagan Test of Infant Intelligence, ISAAC International Study of Asthma and Allergies in Childhood, J-PSAI Japanese Pre-School Activities Inventory, K-ABC Kaufman Assessment Battery for Children, KIDS Kinder Infant Development Scale, SCQ Social Communication Questionnaire, SDQ Strengths and Difficulties Questionnaire, WAIS-R Wechsler Adult Intelligence Scale-Revised, WISC-III Wechsler Intelligence Scale for Children third edition, WCST-KFS Wisconsin Card Sorting Test (Keio Version), WISC-IV Wechsler Intelligence Scale for Children fourth edition
Follow-up and outcome measures: the Hokkaido cohort
Observations were made to assess the prevalence of birth defects, asthma and allergies, and neurobehavioral developmental disorders [7]. The definitions of birth outcomes were as follows: Very low birth weight (VLBW) was birth weight <1500 g, extremely low birth weight (ELBW) was birth weight <1000 g, and small for gestational age (SGA) was defined as birth weights lower than the 10th percentile of the normative reference birth weight, per gestational age, sex, and parity, among live-born infants. Additionally, new follow-ups particularly focusing on the socioeconomic status (SES) and child developmental and behavioral problems have begun since the last cohort profile update. In these follow-ups, we also obtained information on childcare environment and maternal mental health and stress. The 1.5- and 3-year-old follow-ups started in October 2013 and January 2015, using widely used questionnaires, namely the Modified Checklist for Autism in Toddlers (M-CHAT) at 1.5 years old and Kinder Infant Development Scale (KIDS) and Strengths and Difficulties Questionnaire (SDQ) at 3 years old, respectively. The follow-ups of 5- and 6-year-old participants started in October 2014. Table 1 contains a detailed description of the follow-up questionnaires and tests.
Specimen collection and measurements
The details of specimen collection are described in our previous cohort profile [7]. In the Sapporo cohort, maternal non-fasting blood samples were collected at the time of the hospital examination following recruitment, at the 23rd–35th week of gestation. Samples of cord blood, placenta, maternal hair, and breast milk from nursing mothers were collected after delivery. In the Hokkaido cohort, maternal non-fasting blood samples were collected during the first and the third trimester and at delivery. Cord blood samples were collected immediately after the birth. Table 2 summarizes the samples measured for exposures and biochemical markers along with the measurement methods in the Hokkaido Study.
Table 2.
Items measured in The Hokkaido Study on Environment and Children’s Health
| Measurement | Method | Specimen |
|---|---|---|
| Exposure measurements | ||
| Dioxins, PCBs, OH-PCBs (congener level) | HRGC/HRMS | Maternal blood, milk |
| PFOS, PFOA, and other PFASs | UPLC-MS/MS | Maternal blood |
| Phthalate metabolites | GC-MS, LC-MS/MS | Maternal blood, child urine |
| Chlorinated pesticides | GC-NCIMS, GC-HRMS | Maternal blood |
| Cotinine | ELISA | Maternal and cord blood |
| Bisphenol A | ID-LC-MS/MS | Maternal and cord blood |
| Me-Hg | Oxygen combustion-gold amalgamation method | Maternal hair |
| Phthalate esters and organophosphate flame retardants | GC-MS | House dust |
| Mite allergens | ELISA | House dust |
| Biological measurements | ||
| Thyroid hormones (TSH, FT3, FT) | ELISA | Maternal and cord blood |
| Folic acid | CLEIA | Maternal blood |
| Fatty acids (palmitic, stearic, palmitoleic, oleic, linoleic, arachidonic, α-linolenic, EPA, DHA) | GC-MS | Maternal blood |
| TG | TC E-Test Wako | Maternal blood |
| IgE, IgA | ELISA | Cord blood |
| Adipokines (adiponectin, leptin) | ELISA and RIA | Cord blood |
| Steroid hormones (estradiol, testosterone, progesterone, cortisol, cortisone, DHEA, androstenedione) | LC-MS/MS | Cord blood |
| Reproductive hormones (inhibin B, INSL-3, SHBG, FSH, LH) | ELISA, RIA, EIA | Cord blood |
DHA docosahexaenoic acid, DHEA dehydroepiandrosterone, EPA eicosapentaenoic acid, FSH follicle-stimulating hormone, FT4 free thyroxine, IgA immunoglobulin A, IgE immunoglobulin E, INSL-3 insulin-like factor 3, LH luteinizing hormone, Me-Hg methylmercury, OH-PCB hydroxylated polychlorinated biphenyl, PCB polychlorinated biphenyl, PFASs perfluorinated alkyl substances, PFOA perfluorooctanoic acid, PFOS perfluorooctanoic sulfonate, SHBG sex hormone-binding globulin, TG triglyceride, TSH thyroid-stimulating hormone
In the Sapporo cohort, the levels of 29 dioxin and dioxin-like PCB (DL-PCB) congeners, 58 other PCB congeners, and 5 hydroxylated PCB (OH-PCB) congeners in maternal blood and breast milk were measured [16–18]. The PFOS and PFOA levels in maternal blood, cord blood, and breast milk were analyzed at Hoshi University [19]. The levels of 29 persistent organochlorine pesticides, mono(2-ethylhexyl) phthalate (MEHP) (a primary metabolite of di(2-ethylhexyl) phthalate (DEHP)), and bisphenol A in maternal blood were measured. Bisphenol A levels in cord blood were also analyzed. Finally, total mercury (Hg) levels in maternal hair samples were measured [20].
In the Hokkaido cohort, simultaneous analysis of 11 PFASs in maternal plasma collected during the third trimester of pregnancy was conducted [5]. In addition, cotinine concentrations in maternal plasma were measured. Based on self-reports and plasma cotinine levels using receiver operating characteristic curve analysis, cutoff values of 0.21 and 11.48 ng/mL were established to separate smokers and exposed nonsmokers from unexposed nonsmokers, respectively [21]. Household dust and child urine samples were collected at 7 years [7].
As outcomes, thyroid, steroid, and reproductive hormone levels were measured using cord blood samples. Adipokine levels were also measured in cord blood samples. In addition to hormone levels, IgE and IgA levels were measured.
Genetic and epigenetic analysis
Genotyping of single nucleotide polymorphisms (SNPs) was performed using allelic discrimination assays, with fluorogenic probes and 5′ nuclease (TaqMan) (Applied Biosystems, Foster, CA, USA) [22] or a nanofluidic integrated fluidic circuit-based genotyping system for the medium-throughput multiplexing of 96 individual human DNA samples and 96 individual SNP assays (Dynamic Array, Fluidigm Corporation, South San Francisco, CA, USA) [23–25]. In the Sapporo cohort, offspring patterns of insulin-like growth factor 2 (IGF2), H19, and long interspersed element 1 (LINE1) DNA methylation in cord blood were analyzed by pyrosequencing using a PyroMark Q24 system (Qiagen, Hilden, Germany) and assessed using the Qiagen Pyro Q-CpG Software [26]. Cord blood DNA methylation at more than 450,000 CpG sites was quantified using an Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol [27, 28].
Results
Participant characteristics and birth outcomes
The fixed dataset as of the end of 2015 included 20,926 women who had initially registered. However, 121 of the participants requested that their data not be included in any analysis; thus, we included data from 20,805 participants. The basic characteristics of the Sapporo (n = 514) and Hokkaido (n = 20,805) cohorts are shown in Tables 3 and 4, respectively. In the Sapporo cohort, 10 participants dropped out before delivery. There are 7 pairs of twins in the Sapporo cohort. Among 497 singleton neonates, the prevalence of preterm delivery, low birth weight, and small for gestational age (SGA) was 5.6, 5.8, and 6.2%, respectively. As shown in Table 4, the follow-up rate of the Hokkaido cohort is 94.1%. In the Hokkaido cohort, we defined those whose data was not available in the medical records at delivery as lost to follow-ups. The participants who were lost to follow-up showed younger maternal age, higher prevalence of nulliparity and drinking, and lower prevalence of passive smoking. The proportions of missing values in the questionnaire were comparable between the two groups (Table 4). Characteristics of participants in follow-ups and in sub-cohort were comparable. We considered sub-cohort population reasonably represented whole cohort population, and thus, the sub-cohort populations can be used for future nested case-cohort study.
Table 3.
Basic characteristics of the Sapporo cohort participants (n = 514)
| Number | Mean (±SD), Med (25–75%), or % | |
|---|---|---|
| Maternal characteristics | ||
| Age at entry (years) | 510 | 30.4 ± 4.9 |
| Maternal body mass index | 506 | 21.2 ± 3.2 |
| Nulliparous (%) | 240 | 47.7 |
| Education (years) | ||
| ≤9 | 14 | 2.8 |
| 10–12 | 211 | 41.5 |
| 13–16 | 274 | 53.9 |
| ≥17 | 9 | 1.8 |
| Household income (million yen/year) | ||
| <3 | 95 | 18.8 |
| 3 to <5 | 250 | 49.5 |
| 5 to <7 | 105 | 20.8 |
| 7 to 10 | 48 | 9.5 |
| ≥10 | 7 | 1.4 |
| Drinking habit during pregnancy (%) | 157 | 30.9 |
| Smoking habit during pregnancy (%) | 103 | 20.3 |
| Caffeine intake during pregnancy (mg/day) | 508 | 124 (76–188) |
| Paternal characteristics | ||
| Age at entry (years) | 503 | 32.3 ± 5.6 |
| Education (years) | ||
| ≤9 | 31 | 6.1 |
| 10–12 | 190 | 37.5 |
| 13–16 | 242 | 47.8 |
| ≥17 | 43 | 8.5 |
| Smoking habit during pregnancy (%) | 354 | 69.8 |
| Child characteristics | ||
| Sex, boys (%) | 246 | 48.1 |
| Gestational age (weeks) | 504 | 38.9 ± 1.5 |
| Birth weight (g) | 511 | 3025.6 ± 420.7 |
| Birth length (cm) | 511 | 47.9 ± 2.2 |
| Types of delivery, vaginal (%) | 397 | 78.8 |
Missing data were excluded from the calculation
SGA small for gestational age
Table 4.
Comparison of the characteristics of the Hokkaido cohort participants included and lost to follow-up
| Follow-ups (n = 19,579) | Lost to follow-up (n = 1226) | Sub-cohort (n = 4869) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean or % | 95% CI | n | Mean or % | 95% CI | n | Mean or % | 95% CI | |
| Maternal characteristics | |||||||||
| Age at entry (years) | 19,549 | 29.9 | 29.8–29.9 | 1166 | 29.4 | 29.1–29.7 | 4831 | 29.9 | 29.8–30.0 |
| Maternal body mass index | 18,428 | 21.2 | 21.1–21.2 | 1141 | 20.9 | 20.7–21.0 | 4608 | 21.2 | 21.1–21.3 |
| Nulliparous (%) | 7226 | 42.1 | 41.4–42.9 | 595 | 54.5 | 51.5–57.4 | 1726 | 40.9 | 39.4–42.3 |
| History of reproductive therapy (%) | 925 | 4.9 | 4.6–5.3 | 73 | 6.2 | 5.0–7.8 | 259 | 5.5 | 4.9–6.2 |
| Regular use of any medicine (%) | 1956 | 10.3 | 9.8–10.7 | 139 | 11.3 | 9.7–13.2 | 519 | 11.0 | 10.1–11.9 |
| Regular use of any supplement (%) | 5852 | 30.7 | 30.0–31.3 | 378 | 30.8 | 28.3–33.5 | 1565 | 33.1 | 31.8–34.5 |
| Drinking habit at 1st trimester (%) | 2180 | 11.7 | 11.3–12.2 | 141 | 12.1 | 10.4–14.1 | 531 | 11.4 | 10.5–12.4 |
| Smoking habit 1st trimester (%) | 2136 | 12.0 | 11.6–12.5 | 135 | 12.2 | 10.5–14.3 | 487 | 11.6 | 10.7–12.6 |
| Passive smoking in house (%) | 11,752 | 65.9 | 65.2–66.6 | 687 | 61.2 | 58.3–64.0 | 2852 | 63.6 | 62.2–65.0 |
| Education, compulsory education only (%) | 1011 | 5.4 | 5.1–5.7 | 74 | 6.4 | 5.1–7.9 | 247 | 5.3 | 4.7–6.0 |
| Occupation, no job | 8137 | 41.6 | 40.9–42.3 | 499 | 40.7 | 38.0–43.5 | 2012 | 41.5 | 40.1–42.9 |
| Income, <3,000,000 yen/year (%) | 3662 | 22.8 | 22.2–23.5 | 240 | 23.8 | 21.2–26.5 | 916 | 22.7 | 21.4–24.0 |
| Paternal characteristics | |||||||||
| Age at entry (years) | 18,311 | 31.7 | 31.6–31.8 | 1135 | 31.1 | 30.8–31.4 | 4595 | 31.7 | 31.5–31.9 |
| Drinking habit (%) | 13,375 | 72.1 | 71.5–72.8 | 863 | 75.2 | 72.6–77.6 | 3319 | 71.5 | 70.2–72.8 |
| Smoking habit 1st trimester (%) | 10,855 | 66.4 | 65.7–67.1 | 650 | 64.3 | 61.3–67.2 | 2644 | 65.0 | 63.5–66.4 |
| Education, compulsory education only (%) | 1431 | 7.8 | 7.4–8.2 | 78 | 6.9 | 5.6–8.5 | 347 | 7.5 | 6.8–8.3 |
| Occupation, no job | 417 | 2.2 | 2.0–2.4 | 41 | 3.4 | 2.6–4.7 | 117 | 2.5 | 2.0–3.0 |
“Lost to follow-ups” included participants who dropped out before delivery or whose data at birth had not been recorded. Missing data were excluded from the calculation
CI confidence interval
The prevalence of pregnancy complications was also examined. The most common complication was pregnancy hypertension, observed in 491 women (2.5%). Other complications included gestational diabetes mellitus (159, 0.8%), pregnancy with preexisting diabetes (34, 0.2%), oligoamnios (205, 1.1%), thyroid dysfunction (133, 0.7%), placenta previa (18, 0.1%), and placental abruption (16, 0.1%). Table 5 shows the outcomes of singleton births. The incidences of spontaneous abortion, stillbirths, and preterm birth of singleton fetuses were 1.1, 0.32, and 4.9%, respectively. We observed low birth weight (LBW) and macrosomia in 9.0 and 1.0% of births, respectively. The incidences of VLBW and ELBW among singleton deliveries were 1.4 and 1.1%, respectively. Based on the Japan Pediatric Society database of birth weights [29], the incidences of SGA and term SGA in this study were 7.0 and 6.5%, respectively. All birth defects after 12 weeks of gestation, including 55 marker anomalies, were recorded. The prevalence of all birth defects was 18.9/1000 births. Approximately 10% of all patients with birth defects were delivered between 12 and 21 weeks of gestation. Among births with congenital malformations of the nervous system, 39% were delivered before 22 weeks of gestation. All patients with anencephaly and encephalocele were delivered before 22 weeks of gestation [30].
Table 5.
Outcomes of singleton fetuses (n = 18,882) at birth in the Hokkaido cohort
| Outcomes | Number | Percent | 95% CI |
|---|---|---|---|
| Miscarriagea | 200 | 1.1 | 0.9–1.2 |
| Artificial abortionb | 52 | 0.28 | 0.21–0.36 |
| Stillbirthc | 60 | 0.32 | 0.24–0.41 |
| Live birth | 18,570 | 98.3 | 98.2–98.5 |
| Preterm birthd | 923 | 4.9 | 4.6–5.2 |
| Moderate preterm birth | 795 | 4.2 | 3.9–4.5 |
| Very preterm birth | 112 | 0.59 | 0.49–0.71 |
| Extremely preterm birth | 47 | 0.24 | 0.18–0.33 |
| Low birth weighte | 1693 | 9.0 | 8.6–9.4 |
| Very low birth weight | 268 | 1.4 | 1.2–1.6 |
| Extremely low birth weight | 204 | 1.1 | 0.9–1.2 |
| Macrosomiaf | 190 | 1.0 | 0.9–1.2 |
| Small for gestational ageg | 1308 | 7.0 | 6.7–7.4 |
| Term small for gestational ageh | 1211 | 6.5 | 6.2–6.9 |
| Small for reference fetal weighti | 814 | 4.8 | 4.5–5.2 |
Missing data were excluded from the calculation
CI confidence interval
aMiscarriage was defined as the loss of a pregnancy at <22 completed gestational weeks
bAn abortion brought about intentionally at <22 completed gestational weeks
cStillbirth was defined as the birth of a dead fetus at ≥22 completed gestational weeks
dPreterm birth was defined as birth at 22–36 completed gestational weeks. Preterm birth was subdivided into three categories of prematurity: moderately preterm (32–36 completed weeks), very preterm (28–31 completed weeks), and extremely preterm (22–27 completed weeks)
eLow birth weight, very low birth weight, and extremely low birth weight were birth weights <2500, <1500, and <1000 g, respectively
fMacrosomia is birth weight ≥4000 g
gSmall for gestational age infants had birth weights less than the 10th percentile of the reference birth weight estimated by using gestational age, sex, and parity
hTerm small for gestational age infants were the small for gestational age infants among the term birth neonates
iSmall for reference fetal weight infants had birth weights <1.5 standard deviations of the reference ultrasound-based fetal weight estimated from gestational age, sex, and parity
Exposure levels of environmental chemicals
The exposure levels of environmental chemicals in the Sapporo cohort are shown in Table 6. The median levels of total TEQ and total PCBs in maternal blood were 13.9 pg/g lipid and 107 ng/g lipid, respectively. The median levels of PFOS and PFOA were 5.20 and 1.30 ng/mL, respectively. Among 29 organochlorine pesticides, the highest concentration detected was 651.99 pg/g wet mass of p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE). The detailed concentrations of organochlorine pesticides can be found in our previous literature [31]. MEHP was detected in all 493 maternal blood samples, with a median concentration of 9.95 ng/mL. Concentrations (detection percentages above the detection limit) of bisphenol A in maternal and cord blood were 0.057 ng/mL (68.8%) and 0.051 ng/mL (76.3%), respectively. Finally, total Hg concentration in maternal hair was 1.40 μg/g.
Table 6.
Exposure levels of environmental chemicals in the Sapporo cohort
| Percentile | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number | DL | >DL (%) | Min | 25th | 50th | 75th | Max | |
| Maternal blood | ||||||||
| Total dioxins (TEQ pg/g lipid) | 426 | n/a | n/a | 3.17 | 9.95 | 13.9 | 18.2 | 43.4 |
| Total PCBs (ng/g lipid) | 426 | n/a | n/a | 17.8 | 73.0 | 107 | 148 | 41,460 |
| p,p′-DDE | 379 | 0.60 | 100 | 99.52 | 401.53 | 650.99 | 1011.48 | 4575.67 |
| PFOS (ng/mL) | 447 | 0.5 | 100 | 1.30 | 3.40 | 5.20 | 7.00 | 16.2 |
| PFOA (ng/mL) | 447 | 0.5 | 92.8 | 0.25 | 0.80 | 1.30 | 1.80 | 5.30 |
| MEHP (ng/mL) | 493 | 0.278 | 100 | 1.94 | 5.82 | 9.95 | 16.3 | 101.7 |
| Bisphenol A (ng/mL) | 59 | 0.04 | 76.3 | <DL | 0.040 | 0.057 | 0.072 | 0.419 |
| Cord blood | ||||||||
| Bisphenol A (ng/mL) | 285 | 0.04 | 68.8 | <DL | <DL | 0.051 | 0.076 | 0.217 |
| Maternal hair | ||||||||
| Me-Hg (μg/g) | 430 | n/a | 100 | 0.24 | 0.96 | 1.40 | 1.89 | 7.55 |
p,p′-DDE p,p′-dichlorodiphenyldichloroethylene, DL detection limit, Me-Hg methylmercury, MEHP mono(2-ethylhexyl) phthalate, n/a not applicable, PCBs polychlorinated biphenyls, PFOA perfluorooctanoic acid, PFOS perfluorooctanoic sulfonate, TEQ toxicity equivalency quantity
In the Hokkaido cohort, the concentrations of 11 PFASs in the third trimester maternal plasma were measured (Table 6). PFOS and PFOA were detected in all samples, and the detection percentages of PFNA, PFDA, perfluorodecanoic acid, perfluorotridecanoic acid (PFTrDA), perfluorododecanoic acid (PFDoDA), and perfluorohexane sulfonate (PFHxS) were above 80%, whereas the detection percentages of perfluorohexanoic acid, perfluoroheptanoic acid, and perfluorotetradecanoic acid were less than 50% [32]. The measurements of dioxins, PCBs, phthalates, and BPA are currently ongoing in the Hokkaido cohort.
Health effects
Birth size
The effects of PCDD/PCDF and DL-PCB, PFOS, and PFOA exposure on birth size were presented in our 2012 cohort profile [33, 34]. Since the last cohort profile update [7], prenatal exposure to other chemicals including PCBs, total Hg, and DEHP have been investigated in association with birth size. The results are shown in Table 7. We observed that PCDD/PCDFs and PFASs were associated with decreased birth weight [33, 34]; however, DEHP, PCBs, and total Hg were not associated with birth weight [35, 36]. Our investigation not only was on conventional birth weight but also included cord adipokines considered to be biomarkers of metabolic function [35, 37].
Table 7.
Findings from the Sapporo cohort on the associations between exposures and birth size
| Exposures | Outcome | Number | Findings | Reference |
|---|---|---|---|---|
| PCDD/PCDFs | Birth weight | 398 | Significant decrease. Individual congener assessment found 2,3,4,7,8-PeCDF had a significant negative influence (per log10 unit: β = −24.5 g, 95% CI −387.4 to −61.5). | [33] |
| PCBs | Birth weight | 367 | No association. | [36] |
| PFASs | Birth weight | 428 | PFOS was negatively correlated (per log10 unit: β = −269.4 g, 95% CI −465.7 to −73.0). | [34] |
| Ponderal index | 177 | PFOA was negatively associated (per log10 unit: β = −0.73 kg/m3, 95% CI −1.44 to −0.02). | [26] | |
| Cord adipokines | 168 | PFOS was positively associated with adiponectin (per log10 unit: β = 0.12, 95% CI 0.01 to 0.22). | [37] | |
| DEHP | Ponderal index | 167 | Significant decrease (per log10 unit: β = −1.28, 95% CI −2.43 to −0.13). | [35] |
| Cord adipokines | 167 | Significant decrease in leptin among girls (per log10 unit: β = −0.31, 95% CI −0.52 to −0.10). Significant increase in adiponectin among boys (per log10 unit: β = 4.63, 95% CI 0.77 to 8.49). | ||
| Me-Hg | Birth weight/SGA | 367 | No association with birth weight. The risk of SGA by weight decreased with increasing Me-Hg. | [36] |
PCDD polychlorinated dibenzo-p-dioxin, PCDF polychlorinated dibenzofuran, PCB polychlorinated biphenyls, PFASs perfluorinated alkyl substances, PFOS perfluorooctanoic sulfonate, PFOA perfluorooctanoic acid, DEHP di(2-ethylhexyl) phthalate, Me-Hg methylmercury, SGA small for gestational age
Thyroid, reproductive, and steroid hormone levels
Maternal and infant thyroid hormone levels were investigated in association with prenatal chemical exposure [38–41]. The findings suggested that PFOS possibly disrupted both maternal and infant thyroid hormone levels and that Σdioxin levels were possibly associated with increased infant free thyroxin levels (Table 8).
Table 8.
Findings from the Sapporo cohort on the association between exposures and hormone levels at birth
| Exposures | Outcome | Number | Findings | Reference |
|---|---|---|---|---|
| PFAS | Maternal/infant TSH, FT4 | 392 | Maternal PFOS levels were inversely correlated with maternal serum TSH and positively associated with infant serum TSH levels, whereas maternal PFOA showed no significant relationship with TSH or FT4 levels among mothers and infants. | [38] |
| DEHP | Infant TSH, FT4 | 328 | No association. | [39] |
| Bisphenol A | Infant TSH, FT4 | 285 | No association. | [40] |
| PCB | Infant TSH, FT4 | 358 | Log10 Σdioxin (TEQ) was associated with increased FT4 (β = 0.224 ng/dL, 95% CI 0.016 to 0.433) overall, and the association was more significant among boys (β = 0.299 ng/dL, 95% CI 0.011 to 0.587). | [41] |
| PFASs | Cord reproductive hormones | 189 | PFOS levels were positively associated with E2 and T/E2 and inversely with P4; inhibin B and PFOA levels were positively associated with inhibin B levels in boys. Significant inverse associations were observed between PFOS levels and P4 and PRL levels in girls. | [45] |
| Cord steroid hormones | 185 | Cortisol and cortisone reduced in association with PFOS level (Q4 vs. Q1: β = −23.98 ng/mL, 95% CI −47.12 to −11.99; β = −63.21 ng/mL, 95% CI −132.56 to −26.72). DEHA increased (Q4 vs. Q1: β = 1.33 ng/mL, 95% CI 0.17 to 1.82). DHEA decreased (Q4 vs. Q1: β = −1.23 ng/mL, 95% CI −1.72 to −0.25) in association with PFOA. | [44] | |
| DEHP | Cord reproductive hormones | 202 | MEHP was associated with reduced levels of T/E2, P4, and inhibin B. Inverse associations between maternal MEHP levels T/E2, P4, inhibin B, and INSL3 for males. | [42] |
| Cord steroid hormones | 202 | MEHP was associated with reduced cortisol and cortisone levels and glucocorticoid/adrenal androgen ratio whereas increased DHEA levels and DHEA/androstenedione ratio. | [43] | |
| Bisphenol A | Cord reproductive hormones | 285 | Negatively associated with PRL (β = −0.38). | [40] |
DEHP di(2-ethylhexyl) phthalate, DHEA dehydroepiandrostenedione, E2 estradiol, INSL3 insulin-like factor 3, Me-Hg methylmercury, MEHP mono(2-ethylhexyl) phthalate, P4 progesterone, PCDD polychlorinated dibenzo-p-dioxin, PCDF polychlorinated dibenzofuran, PCB polychlorinated biphenyls, PFASs perfluorinated alkyl substances, PFOS perfluorooctanoic sulfonate, PFOA perfluorooctanoic acid, PRL prolactin, T testosterone, TSH thyroid-stimulating hormone, FT4 free thyroxine
Steroid and reproductive hormone levels were also investigated in the Sapporo cohort (Table 8). We examined the relationship between prenatal exposure to PFOS, PFOA, DEHP, and bisphenol A and the levels of steroid and reproductive hormones in cord blood [40, 42–45]. Prenatal exposure to PFOS, but not PFOA, showed an inverse dose-response relationship with cortisol and cortisone concentrations. The DHEA level was positively associated with PFOS concentration and negatively associated with PFOA concentration. We observed no significant associations between PFASs and androstenedione levels. PFOS was associated with decreased cortisol/DHEA and glucocorticoid/androgenic hormone ratios, indicating that PFOS may shift steroidogenesis to androgenic hormones [44]. PFOS and PFOA showed both positive and inverse associations with the levels of several reproductive and steroid hormones [44, 45]. Similarly, MEHP was associated with reduced levels of several reproductive and steroid hormones, especially in boys [42, 43]. Bisphenol A was associated with reduced prolactin levels [40]. Some of the associations observed in this study between environmental chemicals, including PFASs, DEHP, and bisphenol A, and cord hormone levels were sex-specific.
Neurobehavioral development
In addition to the effects of PCDD/PCDF on the Bayley Scales of Infant Development second edition scores at 6 months reported in our previous update [7, 46], the associations between PFASs, DEHP, and bisphenol A exposure and childhood neurobehavioral development were investigated using the same assessment tool [39, 47, 48]. The results are shown in Table 9. PFOA, but not PFOS, adversely affected mental development in girls at 6 months of age [47]. DEHP and bisphenol A exposures were not associated with child mental or psychomotor development [39, 40]. Stronger adverse effects of prenatal exposure to dioxins on neurodevelopmental have recently been reported in male children [49]. Prenatal environments such as SES are reportedly associated with child behavioral problems [50] and intellectual ability [51].
Table 9.
Findings from the Sapporo and Hokkaido cohorts on the relationship between exposures and neurodevelopment
| Exposures | Outcome | Age at testing | Number | Findings | Reference |
|---|---|---|---|---|---|
| Sapporo cohort | |||||
| PCDD/PCDFs | BSID-II | 6 months | 134 | Several dioxin isomers showed adverse effects on motor development in 6-month-old male infants. | [46] |
| PCDD/PCDFs, PCBs | BSID-II | 18 months | 190 | At 18 months of age, the associations observed at 6 months disappeared. The levels of six dioxin isomers were significantly positively associated with mental development in 18-month-old girls. | [49] |
| PFASs | BSID-II | 6 and 18 months | 173 | PFOA was negatively associated with mental development in 6-month-old girls (per log10 unit: β = −0.296, 95% CI −11.96 to −0.682). | [47] |
| DEHP | BSID-II | 6 and 18 months | 328 | Not associated. | [39] |
| Bisphenol A | BSID-II, CBCL, K-ABC | 6 and 18 months 3.5 years 3.5 years |
285 | Not associated with mental and psychomotor development, but associated with internalizing problems at 42 months (per log10 unit: β = 4.37, 95% CI 0.11 to 8.64). | [48] |
| SES | K-ABC | 3.5 years | 145 | Family income is an optimum indicator of SES in the association with intellectual ability in Japanese children aged 42 months. | [51] |
| Hokkaido cohort | |||||
| SES | SDQ | 5 years | 2553 | Maternal prepregnancy BMI ≥30 kg/m2, primipara, maternal education ≤high school, family income during pregnancy <3 million yen/year, and boy gender were the factors associated with increased odds ratio of the likelihood of child behavioral problems. | [50] |
BSID-II Bayley Scales of Infant Development second edition, CBCL Child Behavior Checklist, DEHP di(2-ethylhexyl) phthalate, K-ABC Kaufman Assessment Battery for Children, Me-Hg methylmercury, PCB polychlorinated biphenyls, PCDD polychlorinated dibenzo-p-dioxin, PCDF polychlorinated dibenzofuran, PFASs perfluorinated alkyl substances, PFOS perfluorooctanoic sulfonate, PFOA perfluorooctanoic acid, SDQ Strengths and Difficulties Questionnaire, SES socioeconomic status, BMI body mass index
Allergies and infectious diseases
The prevalence rates of wheezing, eczema, food allergy, and otitis media at 1.5 years of age in the Sapporo cohort were 11.3, 11.3, 17.0, and 18.7%, respectively. In the Hokkaido cohort, the prevalence rates of wheezing, eczema, and allergic rhino-conjunctivitis symptoms at 2 years of age were 19.3, 17.8, and 4.4%, respectively, and at 4 years of age were 18.7, 19.0, and 5.4%, respectively. The prevalence rates observed in the Hokkaido Study are slightly higher than those reported in the Japanese population [52].
Since our last cohort update [7, 53, 54], the associations between prenatal exposure to PFASs, including long-chain compounds, and allergic diseases at 1, 2, and 4 years of age have been investigated. The main findings are shown in Table 10. Overall, newly emerged PFASs such as PFDoDA and PFTrDA were associated with reduced infant allergies, whereas PFOS increased the risk of infectious diseases [32, 55, 56].
Table 10.
Findings from the Sapporo and Hokkaido cohorts on the relationships between exposures and allergies and infectious diseases
| Exposures | Outcome | Number | Findings | Reference |
|---|---|---|---|---|
| Sapporo cohort | ||||
| Dioxins | Otitis media | 364 | Polychlorinated dibenzofuran was associated with increased risk among male infants (OR 2.5, 95% CI 1.1–5.9). 2,3,4,7,8-pentachlorodibenzo-furan was associated with increased risk of otitis media (OR 5.3, 95% CI 1.5–19). |
[53] |
| PFASs | Cord IgE/infectious disease | 343 | Cord IgE levels decreased with high maternal PFOA concentration among females. No associations among maternal PFOS and PFOA levels and food allergy, eczema, wheezing, or otitis media in the 18-month-old infants. |
[54] |
| Hokkaido cohort | ||||
| PFASs | Eczema | 2063 | At 24 months, the risk in association with higher maternal PFTrDA levels decreased (OR 0.62, 95% CI 0.45–0.86). | [32] |
| Total allergic diseases/eczema/wheezing | 1558 | ORs in the Q4 vs. Q1 for total allergic diseases decreased for PFDoDA (OR 0.621, 95% CI 0.454–0.847) and PFTrDA (OR 0.712, 95% CI 0.524–0.966). OR (Q4 vs. Q1) for wheezing in relation to higher maternal PFHxS levels was 0.728 (95% CI 0.497–1.06). | [56] | |
| Infectious diseases | 1558 | PFHxS was associated with higher risk of total infections disease among girls (Q1 vs. Q4: OR 1.55, 95% CI 0.976–2.45). | [55] | |
IgE immunoglobulin E, Q quartile, OR odds ratio, PFASs perfluorinated alkyl substances, PFDoDA perfluorododecanoic acid, PFHxS perfluorohexane sulfonate, PFOA perfluorooctanoic acid, PFOS perfluorooctanoic sulfonate, PFTrDA perfluorotridecanoic acid, CI confidence interval
Maternal fatty acid concentrations during pregnancy
Maternal fatty acids (FAs) are essential for fetal growth. In our study, nine types of FAs and triglycerides (TGs) were investigated in association with PFAS and DEHP exposure. Our study found that PFOS but not PFOA was negatively associated with the levels of palmitic, palmitoleic, oleic, linoleic, α-linolenic, and arachidonic acids and TG. PFOS was associated with reduced essential FAs, including omega-3 and omega-6 FAs [57]. We also found that a tenfold increase in blood MEHP levels was correlated with a decrease in TG levels as well as similar relationships in palmitic, oleic, linoleic, and α-linolenic acids [58]. These were the first studies to address the association between PFASs, DEHP, and FAs in pregnant women. The results provided important evidence regarding the association of relatively low levels of PFOS or DEHP with the concentrations of essential and long-chain polyunsaturated FAs.
Effects of exposure and gene polymorphisms
The effects of maternal smoking during pregnancy and maternal genetic polymorphisms on infant birth weight were examined in the Sapporo and Hokkaido cohorts [22, 59–63]. The results are shown in Table 11. In both cohorts, birth weight was significantly lower among infants born to smoking mothers having certain genetic variations, e.g., in the genes aromatic hydrocarbon receptor (AhR), cytochrome P450 1A1 (CYP1A1), glutathione S-transferase mu 1, nicotinamide adenine dinucleotide phosphate, cytochrome P450 2EI, 5,10-methylenetetrahydrofolate reductase, and X-ray cross-complementing gene 1.
Table 11.
Adverse effects of infant birth weight in relation to maternal environmental exposure and genetic polymorphisms
| Environmental exposure | Maternal genetic polymorphism | Maternal risk genotypes | Birth weight reduction (g) | Reference |
|---|---|---|---|---|
| Active smoking | AhR (G>A, Arg554Lys) | Arg/Arg | −211 | [22] |
| CYP1A1 (m1/m2) | m1/m2 + m2/m2 | −170 | ||
| GSTM1 (non-null/null) | Null | −171 | ||
| Combination with AhR (G>A, Arg554Lys) and CYP1A1 (m1/m2) | Arg/Arg (AhR) and m1/m2 + m2/m2 (CYP1A1) | −315 | ||
| Combination with CYP1A1 (m1/m2) and GSTM1 (non-null/null) | m1/m2 + m2/m2 (CYP1A1) and null (GSTM1) | −237 | ||
| NQO1 (C>T, Pro187Ser) | Pro/Pro | −159 | [62] | |
| CYP2E1 (c1/c2) | c1/c1 | −195 | ||
| MTHFR (A1298C) | AA | −106 | [63] | |
| CYP1A1 (A>G, Ile462Val) | AG/GG | −62 | [61] | |
| XRCC1 (C>T, Arg194Trp) | CT/TT | −59 | ||
| Combination with AhR (G>A, Arg554Lys), CYP1A1 (A>G, Ile462Val), and XRCC1 (C>T, Arg194Trp) | GG (AhR), AG/GG (CYP1A1), and CT/TT (XRCC1) | −145 | ||
| Dioxin (total TEQ) | GSTM1 (non-null/null) | Null | −345 | [59] |
AhR, aromatic hydrocarbon receptor, CYP1A1 cytochrome P450 1A1, CYP2E1 cytochrome P450 2E1, GSTM1 glutathione S-transferase mu 1, MTHFR, methylenetetrahydrofolate reductase, NQO1 nicotinamide adenine dinucleotide phosphate quinone oxidase 1, XRCC1 X-ray cross-complementing gene 1, TEQ toxicity equivalency quantity
The associations between maternal exposure to dioxins during pregnancy and maternal genetic polymorphisms were also examined in the Sapporo cohort. Maternal polymorphisms in the AhR (G>A, Arg554Lys) and CYP1A1 (T>C, MspI) genes were associated with maternal dioxin concentration [60]. Moreover, among 29 dioxin congeners, reduced infant birth weight was associated with increased levels of certain dioxin congeners [59] (Fig. 1).
Fig. 1.
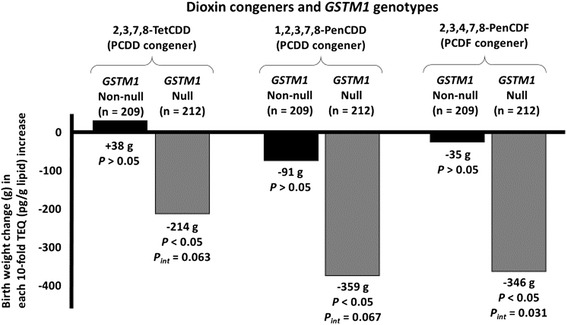
Association of maternal PCDD and PCDF congeners and maternal GSTM1 (non-null/null) genotypes with reduction of infant birth weight in the multiple linear regression model (N = 421) [59]
Effects of epigenetic modification
In multiple linear regression models, IGF2 methylation was significantly inversely associated with log-unit increases in PFOA levels but had no significant effects on H19 or LINE1 methylation after full adjustment [26]. IGF2 methylation was significantly positively associated with ponderal index values at birth but was not associated with birth weight or birth length. Mediation analysis [64] showed a significant indirect effect of PFOA exposure on ponderal index via IGF2 methylation. Remarkably, around one fifth of the effects of prenatal PFOA exposure on reduced ponderal index can be explained by methylation at only the IGF2 gene. These results indicated that the effects of prenatal PFOA exposure could be mediated through DNA methylation. Further study is required to determine the potential for long-term adverse health effects of DNA methylation changes induced by PFOA exposure.
Discussion
Exposure levels
The mean total dioxin TEQ level of maternal blood in this study was 13.9 TEQ pg/g lipid, which was lower than the levels reported in other studies in Taiwan, Europe, the USA, and even in Japan [33]. Concentrations of PCB 153 in maternal serum have been reported in several different birth cohorts, with median concentrations ranging from 10.7 ng/g lipid weight in Poland to 450 ng/g lipid in the Faroe Islands [36]. In the present study, the maternal PCB 153 concentration of 21.0 ng/g was considerably lower than that among populations in Europe and similar to that of the eastern coast of the USA, South Korea, and Taiwan [36].
Similar to those of dioxins and PCBs, the levels of PFOS and PFOA measured in the Sapporo cohort were lower than those reported in studies in Europe, the USA, and other Asian countries [65–70]. In the Hokkaido cohort, a temporal trend analysis of PFAS levels from 2003 to 2011 revealed that PFOS and PFOA concentrations declined, whereas PFNA and PFDA concentrations increased [5]. There are limited data regarding long-chain PFAS concentrations in different populations worldwide. Thus, it is important to evaluate the long-term trends in the levels of these compounds in humans. Among non-DL-PCBs, PCDDs/PCDFs and DL-PCBs, PFOS, PFOA, and methylmercury (Me-Hg), almost all chemicals were significantly correlated [20]. p,p′-DDE exposure levels in this study were comparable to those of two previous studies in Japan, indicating that exposure levels are low in Japan [31].
The levels of MEHP detected in this study were slightly higher than those in American adults, elderly Swedish, and pregnant Australian women, although the exposure levels were not exactly comparable to those of the other studies due to the differences in measurement methods [42]. The slightly higher MEHP levels in this study can be acceptable as the levels of DEHP in household dust in Sapporo were higher than those found in studies from other countries, and DEHP intake in the Sapporo population was higher than that of most other studies [71, 72]. The relatively lower detection limit in this study made it possible to evaluate the lower levels of total bisphenol A observed in the Sapporo cohort. Although the correlation was not significant, bisphenol A levels in maternal and cord blood were similar; thus, the placenta may not reduce or protect the transfer of bisphenol A from the mother to the fetus [73].
Birth outcomes
In Japan, spontaneous abortion is defined as the death of the fetus between 12 and 21 weeks of gestational age [74]. The proportion of stillbirths among all births was 3.3 per 1000 in the Hokkaido area in 2012 [75]. We observed a comparable proportion (3.2 per 1000) in this study. The proportion of preterm births among singleton deliveries was 4.8% in 2012 according to the National Vital Statistics of Japan [75], comparable to the proportion (4.9%) observed in the present study.
Birth weight is a commonly used variable in perinatal research. In the Hokkaido area, the mean birth weight in 2012 was 3010 g [75]. According to the National Vital Statistics, the average birth weight has recently decreased [74]. Increased mortality has been historically observed in neonates with low weight. Birth weight is believed to reflect neonatal health in the perinatal period. The pathological considerations of the placenta provide biological evidence for the significance of birth weight [76]. Numerous studies have identified the risk factors for LBW. Therefore, LBW seems to be preventable. Moreover, birth weight may be associated with future morbidity, in addition to neurologic deficits and behavioral problems [77, 78].
Health effects
This updated cohort profile provides evidence that exposure to environmental chemicals may be associated with not only fetus/infant but also maternal levels of hormones and FAs. Disruption of maternal hormone levels and FAs may consequently influence fetal and child health. Pregnant women are vulnerable to environmental exposure; thus, further investigation of their influence on maternal health is warranted. Regarding birth weight and birth size, we have added evidence of the lack of association between prenatal exposures to PCBs, Me-Hg, and DEHP, and birth weight. In addition to birth weight, the most common indicator of birth size, we also investigated the association of prenatal exposures with SGA, ponderal index values, and cord blood metabolic biomarkers such as adiponectin and leptin. Although no association was observed with birth weight, the risk of SGA by weight decreased with increased Me-Hg concentration [36]. However, no studies have suggested a reasonable assumption about the direct protective role of low Me-Hg exposure on in utero fetal growth. To our knowledge, the present study is the first to investigate the effect of prenatal DEHP exposure on both birth size and fetal metabolic biomarkers. The decreased ponderal index values observed in girls may be explained by decreased leptin levels.
Our findings on thyroid hormone levels imply that PFOS is more influential than PFOA on human thyroid hormones. The extended elimination time of PFOS could explain our finding. Our results indicate that prenatal exposure to PFASs and DEHP are associated with reproductive and steroid hormone levels. Our findings suggest that glucocorticoid and DEHA dyshomeostasis at birth are associated with in utero PFAS exposure, which may adversely affect the HPA axis and steroid hormone homeostasis in later life. Both PFOS and DEHP are known ligands of nuclear, estrogen, and androgen receptors, which are essential regulators of steroidogenesis [79–81]. The mechanism behind our observation could be explained by the aforementioned ligand properties of PFOS and DEHP.
We observed inverse and positive associations between bisphenol A and prolactin levels among boys and girls, respectively, yet studies on the association between bisphenol and prolactin are limited. Further studies are needed to identify the effect of prenatal exposure to bisphenol A on fetus reproductive hormones.
We found that prenatal PFOA exposure was negatively associated with mental development in girls at 6 months of age; however, no association was found between prenatal DEHP exposure and infant neurodevelopment at an early age. In our future work, postnatal environmental factors such as nutrition, daycare attendance, and access to educational resources should be taken into account, as these factors are known to influence child development. Continuous neurobehavioral development assessment throughout childhood will allow us to project the trajectory of child neurobehavioral development in our cohort studies. Inconsistent findings have been reported on the association between environmental chemical exposures and behavioral problems. The related symptoms at certain ages and evidence from prospective studies remain insufficient; thus, further studies are required.
The immunomodulatory [82] and immune-toxic [83, 84] effects of PFASs may impair immune function in humans, resulting in increased infectious disease prevalence and decreased allergic reactions. Future work should carefully investigate the trajectory of allergy and infectious diseases in association with prenatal exposure to environmental chemicals other than PFASs.
Genetic susceptibility to environmental exposure
Our findings on gene polymorphisms suggest that the presence of maternal smoking [22, 61–63] and dioxin exposure [59] during pregnancy affects not only the genes encoding chemical metabolizing enzymes but also the genes encoding DNA repair possibly resulting in adverse fetal health effects, such as infant birth size. Our findings suggest that there are populations genetically susceptible to the effects of exposure to environmental chemicals. Policy on the regulation of environmental chemicals should be made in consideration of these susceptible populations.
Epigenetic effects
We demonstrated that prenatal PFOA exposure resulted in reduced IGF2 methylation in cord blood, which in turn was associated with reduced ponderal index values at birth [26], suggesting that exposure to environmental chemicals in utero may contribute to ponderal index via effects on DNA methylation. There is now compelling evidence that environmental factors may influence DNA methylation, suggesting that fetal exposure may contribute to lifelong health problems via these effects [12]. A number of epigenome-wide association studies (EWAS) have been conducted in humans to estimate the effect of maternal smoking. Recent meta-analyses of EWAS have reported that prenatal exposure to maternal smoking [85] and NO2 air pollution [86] influences offspring DNA methylation at birth. However, few studies have linked prenatal exposure to long-term response via DNA methylation.
Future study directions
Firstly, investigations on the effects of multiple chemical exposures are warranted. Humans are constantly exposed to multiple environmental chemicals, and estimation of the combined risks of the mixture effects is required.
Secondly, the results of our study suggest that in utero exposure to environmental chemicals may contribute to infant health by affecting DNA methylation. To determine the potential long-term health effects of prenatal exposure via epigenetic mechanisms, a genome-wide array technology is valuable; however, few studies have used these approaches to assess the effects of prenatal exposure to environmental chemicals on child DNA methylation. We are conducting a genome-wide study to elucidate the association between prenatal chemical exposure and DNA methylation.
Thirdly, long-term follow-up of the participants is needed. The importance of the intrauterine and early childhood environment and later disease risk led to the establishment of the DOHaD theory. Children in the Hokkaido Study are now reaching the pubertal stage. The impact of fetal and early childhood exposures to environmental chemicals on neurobehavioral development, asthma and allergies, growth, and reproductive functions will be observed continuously and will require confirmation.
Lastly, the strengthening of the collaborations and integration with other birth cohort studies are important. The Birth Cohort Consortium of Asia (BiCCA) was launched in 2012, and 24 cohorts from 11 countries are currently participating [6]. Environmental risks or disease burdens vary from region to region. International cooperation among Asian cohort studies could help in exploring regional hazards, understanding the health impact of environmental toxicants, and developing effective prevention strategies. Although there are many challenges in facilitating different cohort studies, cooperative relationships will accelerate the establishment of new research within the BiCCA.
Conclusions
The results of the Hokkaido Study suggest that even relatively low levels of exposure to environmental chemicals may have adverse effects on child health. When implementing policies and guidelines, it is important to consider individual genetic susceptibility to these effects. Further studies on the combined effects of chemical exposure, contribution to infant health via effects on DNA methylation, and long-term follow-up of the participants are essential.
Acknowledgements
We would like to express our appreciation to all of the study participants of the Hokkaido Study on Environment and Children’ Health. We also express our profound gratitude to all personnel in the hospitals and clinics that collaborated with the study, including Sapporo Toho Hospital, Keiai Hospital, Endo Kikyo Maternity Clinic, Shiroishi Hospital, Memuro Municipal Hospital, Aoba Ladies Clinic, Obihiro-Kyokai Hospital, Akiyama Memorial Hospital, Sapporo Medical University Hospital, Hokkaido University Hospital, Kitami Red Cross Hospital, Hoyukai Sapporo Hospital, Gorinbashi Hospital, Hashimoto Clinic, Asahikawa Medical College Hospital, Hakodate Central General Hospital, Ohji General Hospital, Nakashibetsu Municipal Hospital, Sapporo Tokushukai Hospital, Asahikawa Red Cross Hospital, Wakkanai City Hospital, Kushiro Rosai Hospital, Sapporo-Kosei General Hospital, Shibetsu City General Hospital, Nikko Memorial Hospital, Sapporo City General Hospital, Kohnan Hospital, Hakodate City Hospital, Hokkaido Monbetsu Hospital, Tenshi Hospital, Hakodate Goryoukaku Hospital, Nakamura Hospital, Kin-ikyo Sapporo Hospital, Kitami Lady’s Clinic, Engaru-Kosei General Hospital, Kushiro Red Cross Hospital, Nayoro City General Hospital, and Obihiro-Kosei General Hospital. We also deeply express our gratitude to the staff of The Hokkaido Study on Environment and Children’s Health for their considerable efforts to support our study, including M. Hoshiba, N. Kanda, M. Kihara, R. Nakanishi, A. Kondo, and H. Sato.
The Members of The Hokkaido Study on Environment and Children’s Health
S. Sasaki, T. Ikeno, E. Okada, S. Nishihara, R. M. Ketema, T. Kita, I. Kashino, T. Baba, T. S. Braimoh, S. Minakami, K. Cho, N. Shinohara, K. Moriya, T. Mitsui, T. Saito, S. Suyama, T. Nomura, S. Konno, H. Matsuura, M. Ishizuka (Hokkaido University, Sapporo), T. Endo, T. Baba (Sapporo Medical University, Sapporo), F. Sata (Chuo University, Tokyo), K. Sengoku, Y. Saijo, E. Yoshioka, T. Miyamoto (Asahikawa Medical University, Asahikawa), M. Yuasa (Juntendo University, Tokyo), J. Kajiwara, T. Hori (Fukuoka Prefectural Institute of Health and Environmental Sciences, Fukuoka), Y. Chisaki, T. Matsumura, F. Mizutani, J. Yamamoto, Y. Onoda (IDEA Consultants, Inc., Shizuoka), T. Kawai, T. Tsuboi (Japan Industrial Safety and Health Association).
Funding
The Hokkaido Study is supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Health, Labour and Welfare; the Japan Society for the Promotion of Science; the Ministry of Education, Culture, Sports, Science and Technology; and an Environment Research and Technology Development fund.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because the study involves human participants with a nondisclosure provision of individual data stated in the written informed consent in order to prevent compromise of study participants’ privacy, but are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RK designed the study and developed the methodology. AA, MM, CM, SI, SK, YAB, KY, RM, NT, KI, and HG collected the data and performed the analyses. AA, MM, CM, SI, SK, YAB, RM, NT, and TH drafted the manuscript. RK and TH provided critical revision of the manuscript. All authors take full responsibility for the content of this paper. All authors read and approved the final manuscript.
Consent for publication
Not applicable
Ethics approval and consent to participate
The institutional ethical board for human gene and genome studies at Hokkaido University Center for Environmental and Health Sciences (reference no. 14, March 22, 2012) and Hokkaido University Graduate School of Medicine (May 31, 2003) approved the study protocol.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ADHD-RS
Attention Deficit Hyperactivity Disorder-Rating Scale
- AhR
Aromatic hydrocarbon receptor
- ASQ
Autism Screening Questionnaire
- ATS-DLD
American Thoracic Society-Division of Lung Disease
- BiCCA
Birth Cohort Consortium of Asia
- BMI
Body mass index
- BSID-II
Bayley Scales of Infant Development second edition
- CBCL
Child Behavior Checklist
- CI
Confidence interval
- Conners 3P
Conner’s 3rd Edition for Parents
- CYP1A1
Cytochrome P450 1A1
- CYP2E1
Cytochrome P450 2E1
- DCDQ
Developmental Coordination Disorder Questionnaire
- DDST
Denver Developmental Screening Tests
- DEHP
di(2-Ethylhexyl) phthalate
- DHA
Docosahexaenoic acid
- DHEA
Dehydroepiandrosterone
- DL
Detection limit
- DL-PCB
Dioxin-like PCB
- DOHaD
Developmental Origins of Health and Disease
- EDCs
Endocrine-disrupting chemicals
- EES
Evaluation of Environmental Stimulation
- ELBW
Extremely low birth weight
- EPA
Eicosapentaenoic acid
- EWAS
Epigenome-wide association studies
- FA
Fatty acid
- FSH
Follicle-stimulating hormone
- FT4
Free thyroxine
- FTII
Fagan Test of Infant Intelligence
- GSTM1
Glutathione S-transferase mu 1
- IgA
Immunoglobulin A
- IgE
Immunoglobulin E
- IGF2
Insulin-like growth factor 2
- INSL-3
Insulin-like factor 3
- ISAAC
International Study of Asthma and Allergies in Childhood
- J-PSAI
Japanese Pre-School Activities Inventory
- K-ABC
Kaufman Assessment Battery for Children
- KIDS
Kinder Infant Development Scale
- LBW
Low birth weight
- LH
Luteinizing hormone
- LINE1
Long interspersed element 1
- M-CHAT
Modified Checklist for Autism in Toddlers
- Me-Hg
Methylmercury
- MEHP
Mono(2-ethylhexyl) phthalate
- MTHFR
Methylenetetrahydrofolate reductase
- NQO1
Nicotinamide adenine dinucleotide phosphate quinone oxidase 1
- OH-PCB
Hydroxylated PCB
- ORs
Odds ratios
- p,p′,
dichlorodiphenyldichloroethylene
- PCB
Polychlorinated biphenyl
- PCDD
Polychlorinated dibenzo-p-dioxin
- PCDF
Polychlorinated dibenzofuran
- PFASs
Perfluoroalkyl substances
- PFDA
Perfluorodecanoic acid
- PFDoDA
Perfluorododecanoic acid
- PFHxS
Perfluorohexane sulfonate
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctanoic sulfonate
- PFTrDA
Perfluorotridecanoic acid
- SCQ
Social Communication Questionnaire
- SDQ
Strengths and Difficulties Questionnaire
- SES
Socioeconomic status
- SGA
Small for gestational age
- SHBG
Sex hormone-binding globulin
- SNP
Single nucleotide polymorphism
- TEQ
Toxicity equivalency quantity
- TG
Triglyceride
- TSH
Thyroid-stimulating hormone
- VLBW
Very low birth weight
- WAIS-R
Wechsler Adult Intelligence Scale-Revised
- WCST-KFS
Wisconsin Card Sorting Test (Keio Version)
- WISC-III
Wechsler Intelligence Scale for Children third edition
- WISC-IV
Wechsler Intelligence Scale for Children fourth edition
- XRCC1
X-ray cross-complementing gene 1
Contributor Information
Reiko Kishi, Phone: +81-11-706-4746, Email: rkishi@med.hokudai.ac.jp.
the members of The Hokkaido Study on Environment and Children’s Health:
M. Hoshiba, N. Kanda, M. Kihara, R. Nakanishi, A. Kondo, and H. Sato
References
- 1.Colborn T, Dumanoski D, Myers J. Our stolen future: are we threatening our fertility, intelligence, and survival? A scientific detective story. New York: Plume; 1997. [Google Scholar]
- 2.Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166:E1–E7. doi: 10.1001/archpediatrics.2012.241. [DOI] [PubMed] [Google Scholar]
- 3.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 5.Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003-2011. Environ Int. 2013;60:89–96. doi: 10.1016/j.envint.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Kishi R, Zhang J, Ha E, Chen P, Tian Y, Xia Y, et al. Birth Cohort Consortium of Asia (BiCCA)—current and future perspectives. Epidemiology. 2016. In press. [DOI] [PubMed]
- 7.Kishi R, Kobayashi S, Ikeno T, Araki A, Miyashita C, Itoh S, et al. Ten years of progress in the Hokkaido Birth Cohort Study on Environment and Children’s Health: cohort profile—updated 2013. Environ Health Prev Med. 2013;18:429–450. doi: 10.1007/s12199-013-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in us children, 1997-2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 9.Pastor PN, Reuben CA, Duran CR. Identifying emotional and behavioral problems in children aged 4-17 years: United States, 2001-2007. Natl Health Stat Report. 2012;1-17 [PubMed]
- 10.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief. 2013;1-8 [PubMed]
- 11.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993-2014. Respir Med. 2015;109:982–990. doi: 10.1016/j.rmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Leenen FA, Muller CP, Turner JD. DNA methylation: conducting the orchestra from exposure to phenotype? Clin Epigenetics. 2016;8:92. doi: 10.1186/s13148-016-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishi R, Sasaki S, Yoshioka E, Yuasa M, Sata F, Saijo Y, et al. Cohort profile: the Hokkaido Study on Environment and Children’s Health in Japan. Int J Epidemiol. 2011;40:611–618. doi: 10.1093/ije/dyq071. [DOI] [PubMed] [Google Scholar]
- 14.Survey of obstetrics medical institution, 2008. General Affairs Division, Health Policy Bureau, Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/topics/bukyoku/isei/kinkyu/dl/01d.pdf (in Japanese). Accessed 21 Mar 2017.
- 15.Mitsui T, Araki A, Imai A, Sato S, Miyashita C, Ito S, et al. Effects of prenatal Leydig cell function on the ratio of the second to fourth digit lengths in school-aged children. PLoS One. 2015;10:e0120636. doi: 10.1371/journal.pone.0120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todaka T, Hirakawa H, Kajiwara J, Hori T, Tobiishi K, Onozuka D, et al. Concentrations of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in blood collected from 195 pregnant women in Sapporo city, Japan. Chemosphere. 2007;69:1228–1237. doi: 10.1016/j.chemosphere.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 17.Todaka T, Hirakawa H, Kajiwara J, Hori T, Tobiishi K, Onozuka D, et al. Concentrations of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in blood and breast milk collected from 60 mothers in Sapporo city, Japan. Chemosphere. 2008;72:1152–1158. doi: 10.1016/j.chemosphere.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Todaka T, Hori T, Hirakawa H, Kajiwara J, Yasutake D, Onozuka D, et al. Congener-specific analysis of non-dioxin-like polychlorinated biphenyls in blood collected from 195 pregnant women in Sapporo city, Japan. Chemosphere. 2008;73:923–931. doi: 10.1016/j.chemosphere.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyashita C, Sasaki S, Saijo Y, Okada E, Kobayashi S, Baba T, et al. Demographic, behavioral, dietary, and socioeconomic characteristics related to persistent organic pollutants and mercury levels in pregnant women in Japan. Chemosphere. 2015;133:13–21. doi: 10.1016/j.chemosphere.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Braimoh TS, Yila TA, Yoshioka E, Kishi R. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy—a validation study in northern Japan. Sci Total Environ. 2011;412:114–118. doi: 10.1016/j.scitotenv.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki S, Kondo T, Sata F, Saijo Y, Katoh S, Nakajima S, et al. Maternal smoking during pregnancy and genetic polymorphisms in the Ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol Hum Reprod. 2006;12:77–83. doi: 10.1093/molehr/gal013. [DOI] [PubMed] [Google Scholar]
- 23.Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real-time PCR in a microfluidic dynamic array. PLoS One. 2008;3:e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Tan HW, Fang W, Meinhardt LW, Mischke S, Matsumoto T, et al. Developing single nucleotide polymorphism (SNP) markers from transcriptome sequences for identification of longan (Dimocarpus longan) germplasm. Hortic Res. 2015;2:14065. doi: 10.1038/hortres.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Lin M, Crenshaw A, Hutchinson A, Hicks B, Yeager M, et al. High-throughput single nucleotide polymorphism genotyping using nanofluidic dynamic arrays. BMC Genomics. 2009;10:561. doi: 10.1186/1471-2164-10-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi S, Azumi K, Goudarzi H, Araki A, Miyashita C, Kobayashi S, et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido Study. J Expo Sci Environ Epidemiol. 2017;27:251–259. doi: 10.1038/jes.2016.50. [DOI] [PubMed] [Google Scholar]
- 27.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval J, Heyn H, Moran S, Serra-Musach J, Pujana MA, Bibikova M, et al. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics. 2011;6:692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 29.Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014;56(5):702–708. doi: 10.1111/ped.12331. [DOI] [PubMed] [Google Scholar]
- 30.Hanaoka T, Tamura N, Ito K, Sasaki S, Araki A, Ikeno T, et al. Prevalence and risk of birth defects observed in a prospective cohort study; the Hokkaido Study on Environment and Children’s Health. J Epidemiol. 2017. In press. [DOI] [PMC free article] [PubMed]
- 31.Kanazawa A, Miyasita C, Okada E, Kobayashi S, Washino N, Sasaki S, et al. Blood persistent organochlorine pesticides in pregnant women in relation to physical and environmental variables in the Hokkaido Study on Environment and Children’s Health. Sci Total Environ. 2012;426:73–82. doi: 10.1016/j.scitotenv.2012.02.073. [DOI] [PubMed] [Google Scholar]
- 32.Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Int. 2014;65:127–134. doi: 10.1016/j.envint.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Konishi K, Sasaki S, Kato S, Ban S, Washino N, Kajiwara J, et al. Prenatal exposure to PCDDs/PCDFs and dioxin-like PCBs in relation to birth weight. Environ Res. 2009;109:906–913. doi: 10.1016/j.envres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect. 2009;117:660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minatoya M, Araki A, Miyashita C, Sasaki S, Goto Y, Nakajima T, et al. Prenatal di-2-ethylhexyl phthalate exposure and cord blood adipokine levels and birth size: The Hokkaido Study on Environment and Children’s Health. Sci Total Environ. 2017;579:606–611. doi: 10.1016/j.scitotenv.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 36.Miyashita C, Sasaki S, Ikeno T, Araki A, Ito S, Kajiwara J, et al. Effects of in utero exposure to polychlorinated biphenyls, methylmercury, and polyunsaturated fatty acids on birth size. Sci Total Environ. 2015;533:256–265. doi: 10.1016/j.scitotenv.2015.06.108. [DOI] [PubMed] [Google Scholar]
- 37.Minatoya M, Itoh S, Miyashita C, Araki A, Sasaki S, Iwasaki Y, et al. Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines: The Hokkaido Study on Environment and Children’s Health. Environ Res. 2017;156:175–182. doi: 10.1016/j.envres.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Kato S, Itoh S, Yuasa M, Baba T, Miyashita C, Sasaki S, et al. Association of perfluorinated chemical exposure in utero with maternal and infant thyroid hormone levels in the Sapporo cohort of Hokkaido Study on the Environment and Children’s Health. Environ Health Prev Med. 2016;21(5):334–344. doi: 10.1007/s12199-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minatoya M, Nakajima S, Sasaki S, Araki A, Miyashita C, Ikeno T, et al. Effects of prenatal phthalate exposure on thyroid hormone levels, mental and psychomotor development of infants: The Hokkaido Study on Environment and Children’s Health. Sci Total Environ. 2016;565:1037–1043. doi: 10.1016/j.scitotenv.2016.05.098. [DOI] [PubMed] [Google Scholar]
- 40.Minatoya M, Sasaki S, Araki A, Miyashita C, Itoh S, Yamamoto J, et al. Cord blood bisphenol A levels and reproductive and thyroid hormone levels of neonates: The Hokkaido Study on Environment and Children’s Health. Epidemiology. 2017; Special Issue. In press. [DOI] [PubMed]
- 41.Baba T, Yoshioka E, Sasaki S, Miyashita C, Yuasa M, Itoh S, Kajiwara J, Todaka T, Kato S, Kobatasi S, Okada E, Kashino I, Yila T.A, Braimoh T, Kishi R. Levels of PCB congeners in maternal blood and their effect on maternal and neonatal thyroid hormones—result from Hokkaido Study on Environment and Children’s Health. The 6th International Congress of Asian Society of Toxicology (ASIATOX-VI). Sendai, Miyagi, Japan. 2012.
- 42.Araki A, Mitsui T, Miyashita C, Nakajima T, Naito H, Ito S, et al. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: The Hokkaido Study on Environment and Children’s Health. PLoS One. 2014;9:e109039. doi: 10.1371/journal.pone.0109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki A, Mitsui T, Goudarzi H, Nakajima T, Miyashita C, Itoh S, et al. Prenatal di(2-ethylhexyl) phthalate exposure and disruption of adrenal androgens and glucocorticoids levels in cord blood: The Hokkaido Study. Sci Total Environ. 2017;581-582:297–304. doi: 10.1016/j.scitotenv.2016.12.124. [DOI] [PubMed] [Google Scholar]
- 44.Goudarzi H, Araki A, Itoh S, Sasaki S, Miyashita C, Mitsui T, et al. The association of prenatal exposure to perfluorinated chemicals with glucocorticoid and androgenic hormones in cord blood samples: The Hokkaido Study. Environ Health Perspect. 2017;125:111–118. doi: 10.1289/EHP142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh S, Araki A, Mitsui T, Miyashita C, Goudarzi H, Sasaki S, et al. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ Int. 2016;94:51–59. doi: 10.1016/j.envint.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima S, Saijo Y, Kato S, Sasaki S, Uno A, Kanagami N, et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ Health Perspect. 2006;114:773–778. doi: 10.1289/ehp.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, et al. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Sci Total Environ. 2016;541:1002–1010. doi: 10.1016/j.scitotenv.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Minatoya M, Sasaki S, Nakajima S, Yamamoto J, Araki A, Itoh S, et al. Prenatal bisphenol A exposure and birth outcomes: The Hokkaido Study. In 2014 Conference of International Society for Environmental Epidemiology Asia Chapter. Shanghai, China; 2014.
- 49.Nakajima S, Sasaki S, Kato S, Nakamura Y, Sengoku Y, Kajiwara J, et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 and 18 month. In Annual Conference of the Japanese Society for Hygiene Kanazwa, Japan; 24-26 March, 2014.
- 50.Minatoya M, Itoh S, Araki A, Tamura N, Yamazaki K, Nishihara S, et al. Associated factors of behavioural problems in children at preschool age: The Hokkaido Study on Environment and Children’s Health. Child Care Health Dev. 2016. [DOI] [PubMed]
- 51.Kita T, Nakajima S, Ikeno T, Kishi R. The association between parental socioeconomic status and intellectual ability in Japanese infants aged 42 month: The Hokkaido Study. In International Epidemiological Association 2014, World Congress of Epidemiology; Anchorage, Alaska, USA. 17-24 August 2014
- 52.Third council for promotion of measures against allergies [http://www.mhlw.go.jp/stf/shingi2/0000121262.html]. Accessed 21 Mar 2017.
- 53.Miyashita C, Sasaki S, Saijo Y, Washino N, Okada E, Kobayashi S, et al. Effects of prenatal exposure to dioxin-like compounds on allergies and infections during infancy. Environmen Res. 2011;111:551–558. doi: 10.1016/j.envres.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 54.Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res. 2012;112:118–125. doi: 10.1016/j.envres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Goudarzi H, Miyashita C, Okada E, Kashino I, Chen C-J, Ito S, et al. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environ Int. 2017, in press. [DOI] [PubMed]
- 56.Goudarzi H, Miyashita C, Okada E, Kashino I, Kobayashi S, Chen CJ, et al. Effects of prenatal exposure to perfluoroalkyl acids on prevalence of allergic diseases among 4-year-old children. Environ Int. 2016;94:124–132. doi: 10.1016/j.envint.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 57.Kishi R, Nakajima T, Goudarzi H, Kobayashi S, Sasaki S, Okada E, et al. The association of prenatal exposure to perfluorinated chemicals with maternal essential and long-chain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: The Hokkaido Study. Environ Health Perspect. 2015;123:1038–1045. doi: 10.1289/ehp.1408834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia X, Harada Y, Tagawa M, Naito H, Hayashi Y, Yetti H, et al. Prenatal maternal blood triglyceride and fatty acid levels in relation to exposure to di(2-ethylhexyl)phthalate: a cross-sectional study. Environ Health Preven Med. 2015;20:168–178. doi: 10.1007/s12199-014-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi S, Sata F, Miyashita C, Sasaki S, Ban S, Araki A, et al. Dioxin-metabolizing genes in relation to effects of prenatal dioxin levels and reduced birth size: The Hokkaido Study. Reprod Toxicol. 2016;67:111–116. doi: 10.1016/j.reprotox.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi S, Sata F, Sasaki S, Ban S, Miyashita C, Okada E, et al. Genetic association of aromatic hydrocarbon receptor (AhR) and cytochrome p450, family 1, subfamily a, polypeptide 1 (CYP1A1) polymorphisms with dioxin blood concentrations among pregnant Japanese women. Toxicol Lett. 2013;219:269–278. doi: 10.1016/j.toxlet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi S, Sata F, Sasaki S, Braimoh TS, Araki A, Miyashita C, et al. Combined effects of AhR, CYP1A1, and XRCC1 genotypes and prenatal maternal smoking on infant birth size: biomarker assessment in the Hokkaido Study. Reprod Toxicol. 2016;65:295–306. doi: 10.1016/j.reprotox.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki S, Sata F, Katoh S, Saijo Y, Nakajima S, Washino N, et al. Adverse birth outcomes associated with maternal smoking and polymorphisms in the n-nitrosamine-metabolizing enzyme genes NQO1 and CYP2E1. Am J Epidemiol. 2008;167:719–726. doi: 10.1093/aje/kwm360. [DOI] [PubMed] [Google Scholar]
- 63.Yila TA, Sasaki S, Miyashita C, Braimoh TS, Kashino I, Kobayashi S, et al. Effects of maternal 5,10-methylenetetrahydrofolate reductase c677t and a1298c polymorphisms and tobacco smoking on infant birth weight in a Japanese population. J Epidemiol. 2012;22:91–102. doi: 10.2188/jea.JE20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York, NY, USA: Guilford Press; 2013. [Google Scholar]
- 65.Ashley-Martin J, Dodds L, Arbuckle TE, Bouchard MF, Fisher M, Morriset AS, et al. Maternal concentrations of perfluoroalkyl substances and fetal markers of metabolic function and birth weight. Am J Epidemiol. 2017;185:185–193. doi: 10.1093/aje/kww213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YJ, Kim MK, Bae J, Yang JH. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere. 2013;90:1603–1609. doi: 10.1016/j.chemosphere.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 68.Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014;62:104–112. doi: 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein CR, Wolff MS, Calafat AM, Kato K, Engel SM. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: a pilot study. Reprod Toxicol. 2012;34:312–316. doi: 10.1016/j.reprotox.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, et al. Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese dwellings. Sci Total Environ. 2014;468-469:147–157. doi: 10.1016/j.scitotenv.2013.07.107. [DOI] [PubMed] [Google Scholar]
- 72.Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Yoshioka E, Kanazawa A, et al. Comparisons of urinary phthalate metabolites and daily phthalate intakes among Japanese families. Int J Hyg Environ Health. 2015;218:461–470. doi: 10.1016/j.ijheh.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto J, Minatoya M, Sasaki S, Araki A, Miyashita C, Matsumura T, et al. Quantifying bisphenol A in maternal and cord whole blood using isotope dilution liquid chromatography/tandem mass spectrometry and maternal characteristics associated with bisphenol A. Chemosphere. 2016;164:25–31. doi: 10.1016/j.chemosphere.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Gynecology JSoOa: Definition of miscarriage and preterm birth (in Japanese). Acta Obstet Gynaecol Jap. 1992;44
- 75.Statistics and Information Department Ministry of Health Labour and Welfare . Vital statistics of Japan, 2012. Tokyo: Health Labour and Welfare Statistics Association; 2012. [Google Scholar]
- 76.Zhang J, Savitz DA. Duration of gestation and timing of birth. In: Buck Louis GM, Platt RW, editors. Reproductive and perinatal epidemiology. NY: Oxford University Press; 2011. pp. 152–167. [Google Scholar]
- 77.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 78.Tanabe K, Tamakoshi K, Kikuchi S, Murotsuki J. Learning disability in 10- to 16-year-old adolescents with very low birth weight in Japan. Tohoku J Exp Med. 2014;232:27–33. doi: 10.1620/tjem.232.27. [DOI] [PubMed] [Google Scholar]
- 79.Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20:8031–8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- 80.Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201:133–141. doi: 10.1016/S0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 81.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver x receptor-β, and retinoid x receptor-α. Toxicol Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 82.Smialowicz RJ, DeVito MJ, Williams WC, Birnbaum LS. Relative potency based on hepatic enzyme induction predicts immunosuppressive effects of a mixture of PCDDs/PCDFs and PCBs. Toxicol Appl Pharmacol. 2008;227:477–484. doi: 10.1016/j.taap.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 83.Grandjean PEW, Andersen E, Budtz-Jorgensen F, Nielsen K, Molbak P, Weihe C, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci. 2008;103:77–85. doi: 10.1093/toxsci/kfn015. [DOI] [PubMed] [Google Scholar]
- 85.Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98:680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gruzieva O, Xu CJ, Breton CV, Annesi-Maesano I, Anto JM, Auffray C, et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125:104–110. doi: 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because the study involves human participants with a nondisclosure provision of individual data stated in the written informed consent in order to prevent compromise of study participants’ privacy, but are available from the corresponding author upon reasonable request.


