A 58-year-old woman with upper abdominal bloating for 2 months was admitted to our hospital. Computed tomography (CT) of the abdomen showed multiple, round low-density shadows in the liver [Figure 1]. In addition, gastroscopy showed multiple bulges in the gastric fundus [Figure 2]. Moreover, endoscopic ultrasound showed multiple, anechoic round masses in the outer wall of the fundus and anterior wall of the stomach, with no internal blood flow signals. Partitions were visible between the masses, the sizes were approximately 75 mm × 47 mm, and the boundaries were consistent with liver imaging; hence, cysts were suspected [Figure 3, Video 1]. After obtaining patient consent, endoscopic ultrasound-fine needle aspiration (EUS-FNA) (Cook Echo-3-19, Wilson-Cook, Winston-Salem, NC) was performed. Three sites in the fundus and gastric body were selected for FNA [Figure 4], and 30 mL of 1% lauromacrogol was injected into the cavity [Figure 5]. The cystic cavity shrank significantly, the masses disappeared, and there was no bleeding at the puncture sites [Figure 6]. Then, 160 mL of pale yellow, clear cystic fluid was extracted [Figure 7]. No obvious discomfort was reported by the patient, and her bloating disappeared. Liquid-based cytology of the cystic fluid indicated the presence of epithelial cells and red blood cells but not tumor cells [Figure 8]. A repeat CT scan performed a week later showed that the sizes of the multiple low-density areas in the left lobe of the liver significantly reduced [Figure 9]. The patient was discharged and was followed up.
Figure 1.
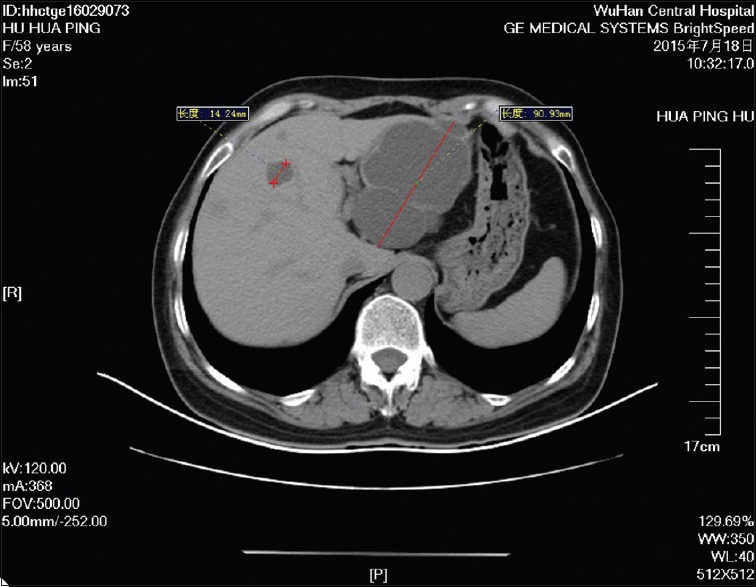
Computed tomography of the abdomen showed multiple, round low-density shadows in the liver
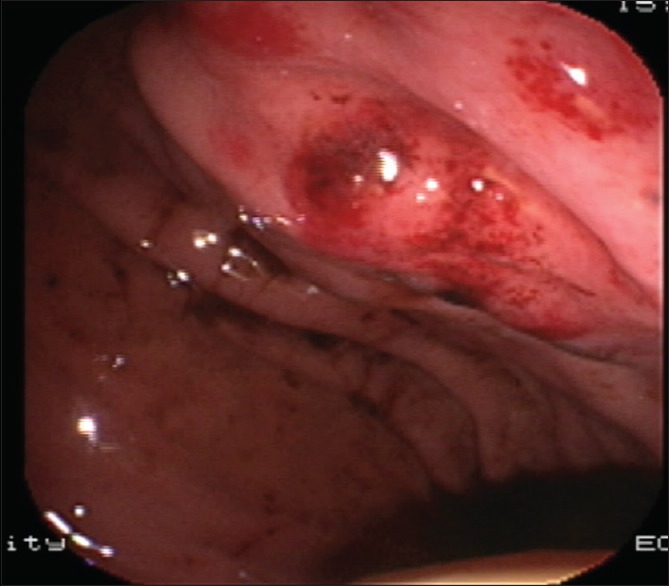
Figure 2.
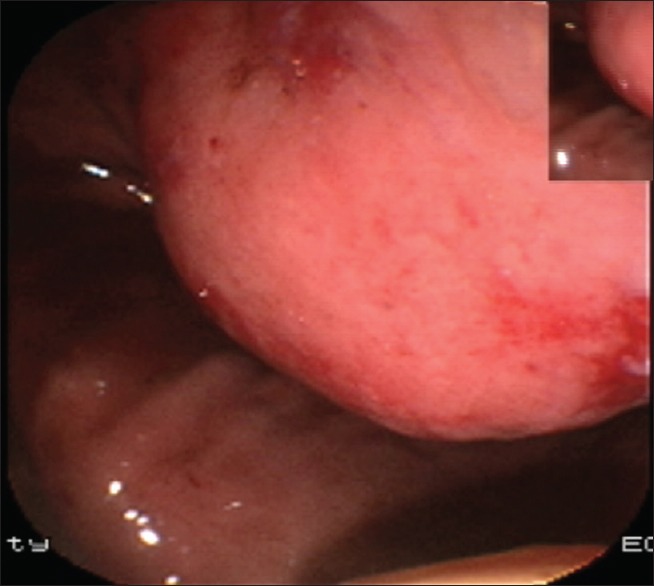
Gastroscopy showed multiple bulges in the gastric fundus
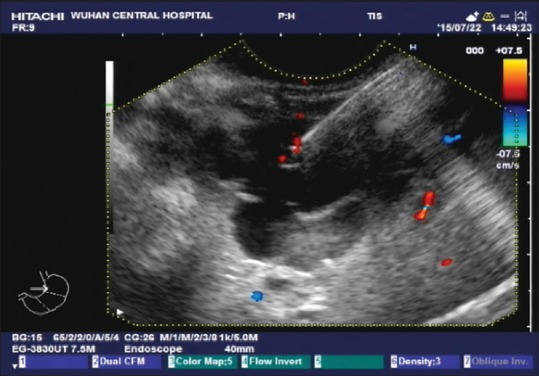
Figure 3.
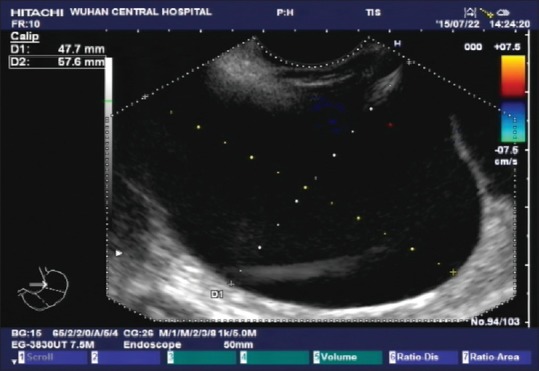
Endoscopic ultrasound showed multiple anechoic round masses in the outer wall of the fundus and anterior wall of the stomach, with no internal blood flow signals. Partitions were visible between the masses, the sizes were approximately 75 mm × 47 mm, and the boundaries were consistent with liver imaging; hence, cysts were suspected
Figure 4.
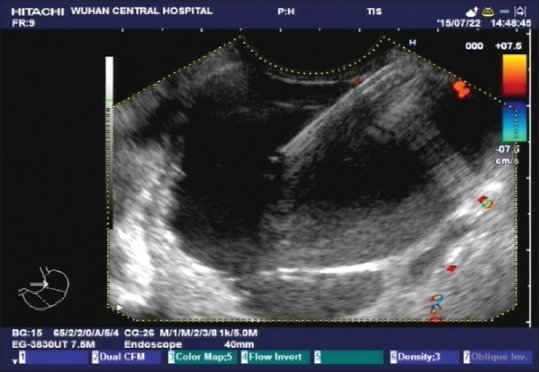
Three sites in the fundus and gastric body were selected for fine needle aspiration
Figure 5.
Thirty milliliters of 1% lauromacrogol was injected into the cavity
Figure 6.
The cystic cavity shrank significantly, the masses disappeared, and there was no bleeding at the puncture sites
Figure 7.

One hundred and sixty milliliters of pale yellow, clear cystic fluid was extracted
Figure 8.
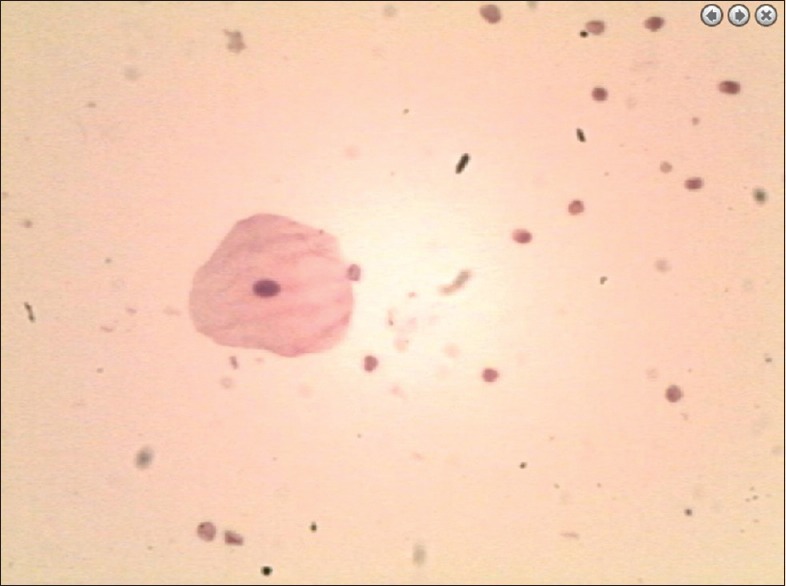
Liquid-based cytology of the cystic fluid indicated the presence of epithelial cells and red blood cells but not tumor cells
Figure 9.
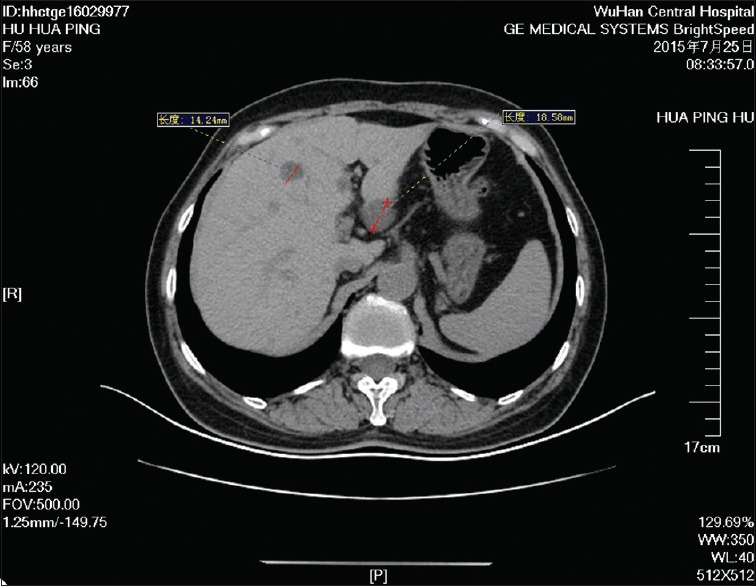
A repeat computed tomography scan performed a week later showed that the sizes of the multiple low-density areas in the left lobe of the liver significantly reduced
Small cysts without clinical symptoms or signs are easily overlooked by patients but are occasionally found during ultrasound, CT, and other such examinations.[1] Hepatic cysts are more common in elderly individuals than in young individuals, and their incidence is higher in women than in men. The population incidence has been reported to be 2.5%–4.25%.[2] Hepatic cysts are the most common benign lesions in the liver, and operative laparoscopy is the most common surgical treatment for hepatic cyst drainage.[3] However, some patients have underlying diseases and poor physical conditions and are thus unable to endure surgery and anesthesia. Furthermore, operative laparoscopy has some limitations. Previous studies have reported recurrence rates of 10%–25% within 1 year after laparoscopic treatment of hepatic cysts.[4,5] Since Bean and Rodan[6] first reported the successful treatment of hepatic cysts using percutaneous ultrasound-guided aspiration and ethanol sclerotherapy in 1985, cystic percutaneous drainage combined with sclerotherapy has gradually replaced surgical treatment and has become the mainstream treatment for hepatic cysts.[2]
With the development and popularization of EUS technology, we assessed whether EUS-FNA could be applied for the treatment of hepatic cysts. On review of the literature, we noted that relevant international reports on the use of EUS-FNA for hepatic cysts are rare and only one case report was found. In this previous case report,[7] EUS-FNA was used to successfully treat an infectious hepatic cyst in an elderly male patient in 2014. We recently started preliminary exploration of the use of EUS-FNA for the treatment of hepatic cysts. Considering anatomical relationships, we believe that hepatic cysts close to the gastric wall can be treated with EUS-FNA, and the most accessible lesion site is the left lobe of the liver. The presence of a lesion at the gastric wall with inward bulging can result in gastric stenosis, therefore causing bloating, early satiety, abdominal pain, postprandial vomiting, and other symptoms. After explaining the viable treatment options (operative laparoscopic hepatic cyst drainage, percutaneous ultrasound-guided hepatic cyst aspiration, and EUS-FNA) to the patient and her immediate family members, the patient chose and provided consent for EUS-FNA. The patient's hepatic cyst was located in the left lobe of the liver, and it was responsible for her bloating condition. The cystic wall was adjacent to the anterior wall of the stomach, and there was almost no liver tissue between the cystic and gastric walls. The needle path through the organ and tissue was very short. There was almost no bleeding at the aspiration sites during the procedure, suggesting that EUS-FNA is safe for such cysts.
In this preliminary stage, we believe that EUS-FNA is safe and effective for the treatment of cysts in the left lobe of the liver and that it can be used to supplement traditional surgery and percutaneous aspiration treatments. The new sclerosing agent used in EUS-FNA (1% lauromacrogol) has been shown to have fewer side effects than traditional anhydrous ethanol[8] and can thus be used as a replacement. However, EUS-FNA is a relatively new technique for the treatment of hepatic cysts, with few published studies and case reports. Owing to the lack of long-term follow-up data and the absence of statistical data from randomized controlled trials, further prospective controlled studies with a large sample size are needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent form. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The Youth Clinical Research Funded Projects of the Health and Family Planning Committee of Wuhan, Hubei Province, China(No. WX17Q08); Hubei Province Natural Science Foundation (No. 2015CFB730); The Clinical Research Funded Projects of the Health and Family Planning Committee of Wuhan, Hubei Province, China(No. WX13A07)funded the study.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.eusjournal.com
REFERENCES
- 1.Ker J. The liver and right atrium-hepatic cyst as a cause of arrhythmia. Clin Med Insights Cardiol. 2010;4:63–7. doi: 10.4137/cmc.s5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blonski WC, Campbell MS, Faust T, et al. Successful aspiration and ethanol sclerosis of a large, symptomatic, simple liver cyst: Case presentation and review of the literature. World J Gastroenterol. 2006;12:2949–54. doi: 10.3748/wjg.v12.i18.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macutkiewicz C, Plastow R, Chrispijn M, et al. Complications arising in simple and polycystic liver cysts. World J Hepatol. 2012;4:406–11. doi: 10.4254/wjh.v4.i12.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gloor B, Ly Q, Candinas D. Role of laparoscopy in hepatic cyst surgery. Dig Surg. 2002;19:494–9. doi: 10.1159/000067603. [DOI] [PubMed] [Google Scholar]
- 5.Miliadis L, Giannakopoulos T, Boutsikos G, et al. Spontaneous rupture of a large non-parasitic liver cyst: A case report. J Med Case Rep. 2010;4:2. doi: 10.1186/1752-1947-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean WJ, Rodan BA. Hepatic cysts: Treatment with alcohol. AJR Am J Roentgenol. 1985;144:237–41. doi: 10.2214/ajr.144.2.237. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi H, Tamai T, Numata M, et al. Endoscopic ultrasonography-guided transmural drainage of an infected hepatic cyst due to Edwardsiella tarda: A case report. Clin J Gastroenterol. 2014;7:422–8. doi: 10.1007/s12328-014-0520-4. [DOI] [PubMed] [Google Scholar]
- 8.Brunken C, Pfeiffer D, Tauber R. Long term outcome after percutaneous sclerotherapy of renal cysts with polidocanol. Urologe A. 2002;41:263–6. doi: 10.1007/s00120-001-0144-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


