Abstract
Background and Aims:
Post-operative nausea and vomiting (PONV) is a common and distressing complication after laparoscopic cholecystectomy (LC). The aim of this study was to evaluate the effect of intravenous (IV) dextrose administration for the prophylaxis of PONV after LC.
Methods:
In a double-blind, randomised controlled trial, a total of 150 female patients who were scheduled for elective LC were randomly assigned into two groups (A and B). Thirty minutes before induction of anaesthesia, patients received an infusion of 500 cc lactated Ringer's solution (Group A) and 5% dextrose in lactated Ringer's solution (Group B) and over a period of 30 min. All patients rated their nausea and vomiting intensity using the verbal rating scale immediately at post-anaesthesia care unit (PACU) arrival; 30, 60, 90 and 120 min after arriving at the PACU and 6, 12 and 24 h after surgery.
Results:
There was a statistically significant time trend and group effect along with significant differences in time/group interaction effect in both groups for nausea and vomiting scores (P < 0.05). A low negative correlation coefficient was found (r = −0.394, P < 0.001) between blood glucose levels and nausea scores upon PACU arrival. Dextrose administration reduced the odds of vomiting events compared to placebo (estimate: −0.87, odds ratio = 0.42, 95% confidence interval: 0.28–0.64).
Conclusion:
Administration of IV dextrose before anaesthesia induction may be recommended as an effective, and safe method for the prophylaxis of PONV after LC.
Key words: Cholecystectomy, dextrose, glucose, laparoscopic cholecystectomy, post-operative nausea and vomiting
INTRODUCTION
Post-operative nausea and vomiting (PONV) is a common and distressing complication after anaesthesia and surgery, with an incidence of approximately 30%.[1,2] Although PONV is usually self-limiting and non-fatal, it is unpleasant and leads to considerable post-operative discomfort and dissatisfaction.[1,3] For most surgical patients, PONV is a greater concern than is post-operative pain, and patients continue to rank PONV as the most unfavourable complication.[4,5] Moreover, PONV can cause dehydration, electrolyte imbalance, wound dehiscence, pulmonary aspiration and acid-base disturbances and can increase healthcare costs by increasing patients' stay in the post-anaesthesia care unit (PACU) and hospital. Hence, identifying effective strategies for PONV prophylaxis is crucial.[1,3] While several factors conferring increased risk of developing PONV have been identified, new guidelines state that cholecystectomy and laparoscopic surgery are associated with higher PONV incidence.[6] Likewise, research has demonstrated that PONV incidence is higher after laparoscopic cholecystectomy (LC) than after other types of surgery. In these types of cases, the reported incidence rate of PONV has ranged from 40% to 75%. Considering that patients undergoing LC are at higher risk for developing PONV, increasing attention has been directed towards prophylaxis for PONV in this population.[7,8]
Different pharmacological and non-pharmacological approaches have been used for preventing PONV. Nonetheless, the most effective prophylactic regimen has not been determined, particularly for high-risk patients, and the search for the optimal therapy continues.[3,9,10] Conflicting results have been presented for perioperative fluid therapy and dextrose administration, two proposed strategies for preventing PONV.[11,12,13,14,15] In two recently published studies investigating the effect of post-anaesthesia intravenous (IV) dextrose 5% on PONV, one reported a positive preventive effect;[16] however, the other study did not confirm this finding.[17] Furthermore, to the best of our knowledge, no study has evaluated the effectiveness of IV dextrose administration for the prevention of PONV in the high-risk group of patients undergoing LC.
Due to the importance of PONV prevention and to the very limited and controversial evidence that support the effectiveness of IV dextrose 5% administration in the prevention of PONV, the aim of this study was to evaluate the effect of IV dextrose administration for the prophylaxis of PONV after LC.
METHODS
Approval from the Mazandaran University of Medical Sciences Ethics Committee was obtained and informed consent was obtained from all patients. A total of 150 female patients (all non-smokers; grade I or II [American Society of Anesthesiologists' grade (ASA)], aged 18–65 years, who were scheduled for elective LC under general anaesthesia) were enrolled in this prospective, double-blind, randomised, placebo-controlled study. The study was carried out between June 2014 and October 2015 and registered in the Iranian Registry of Clinical Trials Database (IRCT201504086803N9).
Exclusions from the study included the following: patients with a previous history of PONV, motion sickness, coagulopathy, diabetes mellitus, or severe hypertension; patients with cardiac, renal or hepatic dysfunction, or who were pregnant or menstruating; patients whose operations were prolonged (more than 2 h), who had abnormal blood glucose on the morning of surgery, or who were unable to understand and use the verbal rating scale (VRS); patients receiving an antiemetic agent within 24 h before surgery or cases where complications occurred during the surgery. Patients who fulfilled the inclusion criteria were randomly allocated into two equally sized Groups A and B (each group, n = 75) by a nurse anaesthetist who was blinded to the study groups using a sealed-envelope technique and computer-generated random numbers.
All patients were instructed the day before surgery on how to rate the intensity of their nausea using the VRS (where 0 denotes the lowest possible nausea intensity and 10 denotes the worst imaginable intensity). Patients' demographic data were also collected at this time. All patients were fasted 8 h before surgery.
In the operating room, after venous access was established in the forearm of the non-dominant hand, patients received an infusion of either 500 mL lactated Ringer's solution (Group A) and 5% dextrose in lactated Ringer's solution (Group B) over a period of 30 min. Infusion of study fluids was started 30 min before induction of anaesthesia. All patients were monitored by electrocardiogram (ECG), capnography, non-invasive blood pressure (NIBP), and pulse oximetry (SPO2) during and after the anaesthesia, until the patient has recovered from anaesthesia.
All the patients received general anaesthesia using the same protocol. As for pre-medication, all patients received IV midazolam (0.02 mg/kg) and fentanyl (2 μg/kg) before anaesthesia induction. We induced general anaesthesia with propofol (2–2.5 mg/kg) and cisatracurium (0.15 mg/kg) and maintained it with 50% oxygen, 50% nitrous oxide (N2O), isoflurane (1–1.5 minimum alveolar concentration), morphine (0.1 mg/kg) and intermittent doses of cisatracurium (0.02 mg/kg). All operations were performed by the same surgeon using the traditional approach of four skin incisions. Depth of anaesthesia was ensured in all patients using the bispectral index, with a score of 40–60 indicating adequate depth of anaesthesia. Intraoperatively, all patients received IV fluid at the rate of 3 ml/kg/h throughout surgery. If a patient's systolic blood pressure decreased to <20% of baseline, additional crystalloid solution was administered and they were excluded from theanalysis. Post-operatively, maintenance fluid consisted of 0.9% normal saline infused at a continuous rate of 1.5 ml/kg/h.
Blood glucose levels were measured immediately before study fluid infusion, 30 min after the start of surgery and immediately after PACU arrival using a point-of-care device (Bionime Rightest GM110, England). All patients rated their nausea and vomiting intensity using VRS immediately; 30, 60, 90 and 120 min after PACU arrival and 6, 12 and 24 h after surgery. An anaesthesiology resident who was blinded to the study groups assessed the blood glucose level and PONV intensity for each patient. Post-operatively, those patients who had vomiting received 4 mg IV ondansetron, and their need for antiemetic medication was recorded.
For post-operative pain control, all patients received the same analgesic regimen, which included 1 g IV paracetamol three times a day after surgery and a diclofenac sodium suppository (100 mg) immediately after arrival at the PACU. In addition, all patients were ambulated within 6 h after completion of surgery.
The primary outcome measured by this study was the PONV incidence and intensity immediately at PACU arrival; 30, 60, 90 and 120 min after PACU arrival and 6, 12 and 24 h after surgery in the group A in comparison to the group B. Secondary outcomes included comparisons of antiemetic medication consumption as well as of blood glucose changes between groups.
The minimally clinically significant difference was assumed to be 2 points on the 10-point scale. Standard deviation (SD) was anticipated to reach 3.3 points. For alpha and beta equal to 0.05 and 0.2, respectively, with two-sided testing, sample size required was 75 patients per group.
We used the Shapiro–Wilk test to determine whether data were normally distributed. Descriptive baseline characteristics for the two groups (A and B) were tabulated as mean (SD), median (interquartile range) or percentages. Comparisons between the two groups for categorical data were statistically analysed using Chi-square or Fisher's exact test; the t-test or Mann–Whitney U-test was used for continuous data. The primary efficacy data on nausea and vomiting were examined using intention-to-treat analysis. The general linear model scores of nausea and vomiting between the two groups were compared by a repeated measurement analysis of variance test and post-hoc analysis. The time of evaluation was considered a within-subject factor and intervention state (A and B), a between-subject factor. The time group (interaction term) was considered as group differences (between groups A and B) in their response over time. Mauchly's sphericity test was used to verify the compound symmetry assumption. In addition, a generalised estimating equation (GEE) model was used to estimate the differences in values for the vomiting state (binary variable) at each time point between the two groups and also the time trend after treatment. This model (GEE) does not require the outcome variable to have a particular distribution. P ≤0.05 was considered statistically significant. Data were analysed using IBM SPSS Version 16 and STATA version 12 (SPSS/IBM; Armonk, New York, USA).
RESULTS
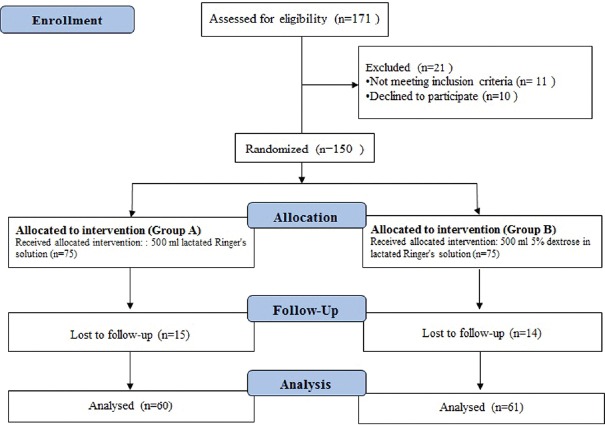
A total of 171 patients referred to our hospital for LC surgery were screened during the study period. Of these, 11 patients did not meet the inclusion criteria and 10 patients declined to participate in the study. Of the 150 patients equally allocated to one of the two groups, 15 (Group A, the control group) and 14 (Group B, the intervention group) were lost to follow up during the study period. Data were analysed for all 121 patients who completed the study [Figure 1].
Figure 1.
Flow chart of the study
Demographic and clinical characteristics of the final group of patients enrolled in the study (n = 121) are presented in Table 1. None of the differences in the demographic categories were statistically significant (P > 0.05).
Table 1.
Demographic and clinical characteristics of patients in both groups
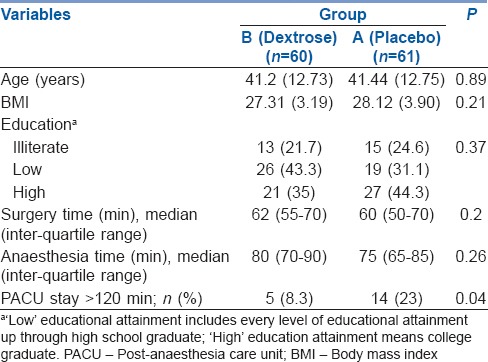
Table 2 summarises the mean and SD values of the pre- and post-operation nausea scores of each group. As shown in Table 2 and Figure 2, a statistically significant time trend (within-subject differences or time effect) existed regardless of group of study (P < 0.001). Regardless of time of the study, nausea scores in the group B were statistically significantly lower than those for the group A (between-subject differences or group effect) (P < 0.001). Contrary to the low and stable trend for nausea in the group B, the group A showed a significant downward trend (group time interaction or interaction effect) (P < 0.001). We found a low negative correlation coefficient (r = −0.394, P < 0.001) between blood glucose level and nausea score at PACU arrival.
Table 2.
Mean (standard deviation) of nausea scores at T1–T8 follow-up times in both groups
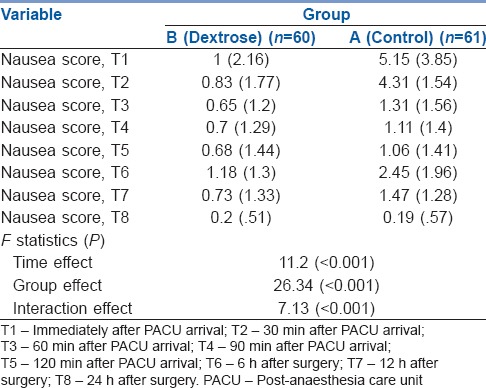
Figure 2.
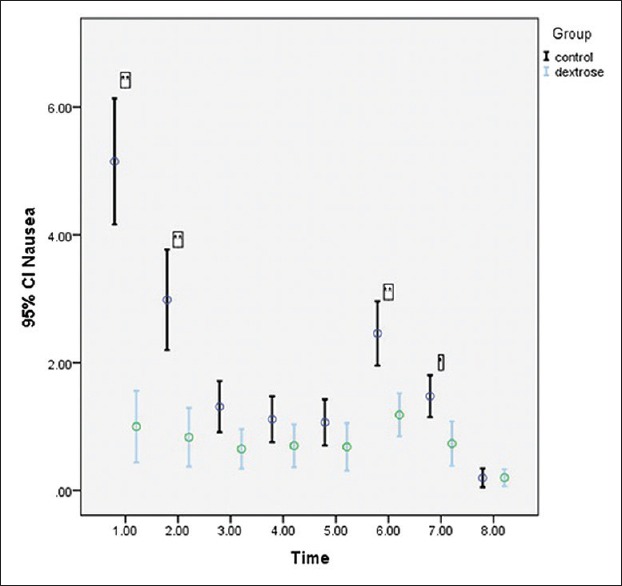
Trend of nausea score (according to VAS) was indicated before and after of interventions in laparoscopic cholecystectomy patients. There was statistically significant time (P < 0.001), group (P < 0.001), and interaction (P < 0.001) effects. In post-hoc analysis: * and ** indicate significant difference in nausea score (P < 0.05 and P < 0.001 respectively) between dextrose and control group. (1: Immediately after post-anaesthesia care unit arrival; 2:30 min after post-anaesthesia care unit arrival; 3:60 min after post-anaesthesia care unit arrival; 4:90 min after post-anaesthesia care unit arrival; 5:120 min after post-anaesthesia care unit arrival; 6:6 h after surgery; 7:12 h after surgery; 8:24 h after surgery)
Table 3 summarises the mean and SD values of the pre- and post-operation vomiting scores of each group. As shown in Table 3, there was a statistically significant time trend (within-subject differences or time effect) regardless of the group (P < 0.001). Vomiting scores were lower in the group B rather than in the group A irrespective of time point measured, and this difference was statistically significant (between-subject differences or group effect) (P < 0.001). The nausea score trend in the group B was different from that of the group A with respect to time/group interaction or interaction effect (P = 0.01), indicating that the magnitude of the differences between the two groups was not constant over time [Figure 3].
Table 3.
Mean (standard deviation) of vomiting scores at T1–T8 follow-up times in both groups
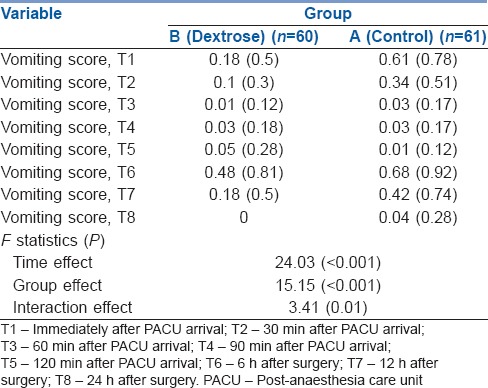
Figure 3.
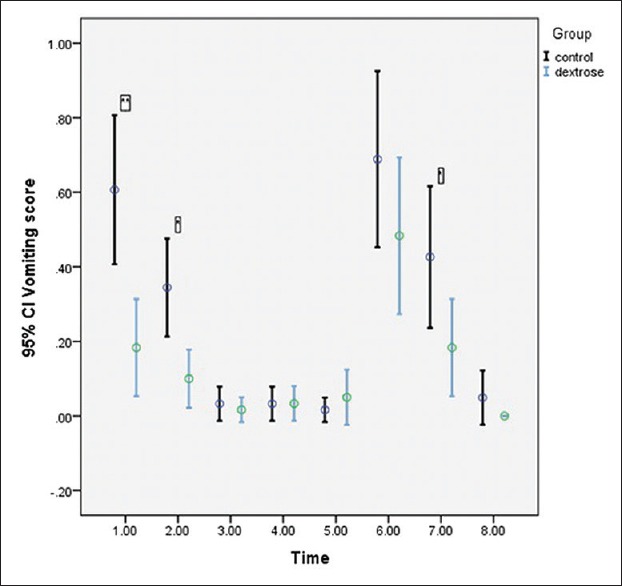
Trend of vomiting score (according to VAS) was indicated before and after of interventions in laparoscopic cholecystectomy patients. There was statistically significant time (P < 0.001), group (P < 0.001), and interaction (P < 0.001) effects. In post-hoc analysis: * and ** indicate significant difference in nausea score (P < 0.05 and P < 0.001 respectively) between dextrose and control group. (1: Immediately after post-anaesthesia care unit arrival; 2:30 min after post-anaesthesia care unit arrival; 3:60 min after post-anaesthesia care unit arrival; 4:90 min after post-anaesthesia care unit arrival; 5:120 min after post-anaesthesia care unit arrival; 6:6 h after surgery; 7:12 h after surgery; 8:24 h after surgery)
We found a low negative correlation coefficient (r = −0.41, P < 0.001) between blood glucose level and vomiting score at PACU arrival. The GEE model revealed that dextrose reduced the odds of vomiting compared to placebo (estimate: −0.87, odds ratio = 0.42, 95% confidence interval: 0.28–0.64).
While ondansetron was prescribed for 41.7% (n = 25) patients in the group B, this rate in the group A was 82% (n = 50) (P < 0.001). The median (interquartile range) ondansetron dose was 4 (4–8) mg and 0 (0–4) mg in the groups A and B, respectively (P < 0.001).
The blood glucose level upon arrival at the PACU in patients with and without vomiting was 121.09 (21.77) and 131.36 (24.82) mg/dl, respectively; this difference was statistically significant (P = 0.04).
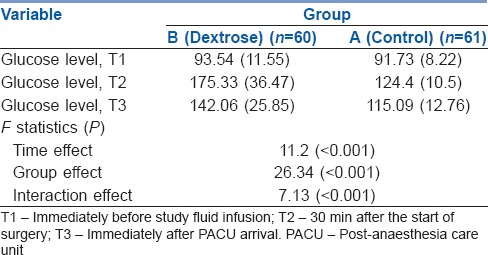
Table 4 summarises the mean and SD values for each group's mean pre- and post-operative glucose levels. Table 4 summarises a statistically significant time trend (within-subject differences or time effect) regardless of group of study (P < 0.001). Regardless of time of the study, the blood glucose level in the group B was higher than that of the group A; this difference was statistically significant (between-subject differences or group effect) (P < 0.001). The blood glucose level trend was different in the group B versus the group A for time/group interaction or interaction effect (P < 0.001), indicating that the magnitude of the differences between the two groups was not constant over time.
Table 4.
Mean (standard deviation) of glucose level at T1–T3 follow-up times in both groups
In this study, no adverse effects were observed in the 2 study groups
DISCUSSION
We evaluated the effectiveness of IV dextrose administration for PONV prophylaxis after LC. A major finding of this study was that patients receiving IV dextrose before induction of anaesthesia (group B) had significantly lower incidence and severity of nausea and vomiting and less frequently needed antiemetic medication postoperatively in comparison to the control group (Group A). Another main finding was that the patients in the dextrose group had significantly higher post-operative blood glucose levels compared to the control group though the blood glucose level in all participants was within the normal range.
As a common substitute for traditional open cholecystectomy, LC has been introduced in cholelithiasis management; however, the evidence shows excessive episodes of PONV after LC.[18,19] Many studies have been conducted to evaluate the efficacy of different antiemetic medications in preventing PONV after LC, with a variable success rate. Such medication may also be problematic in terms of side effects including hypotension, dysphoria, excessive sedation, hallucination and dry mouth.[20] The evidence regarding the effect of perioperative fluid therapy and glucose administration on PONV is controversial and somewhat scarce. It has been shown that pre-operative administration of oral carbohydrate-rich liquid may significantly reduce the incidence of PONV after LC and open cholecystectomy.[14,21] However, some other studies conducted on patients undergoing LC, coronary artery bypass surgery and thyroidectomy did not confirm this result.[22,23]
Consistent with our findings, it has shown a positive effect with administration of post-anaesthesia dextrose-containing IV fluids on the need for subsequent antiemetic use and length of PACU stay in healthy women undergoing outpatient gynaecologic surgery.[16] However, another study indicated that dextrose supplementation of IV fluids was not effective in preventing PONV as compared to IV fluid without supplemented dextrose after elective gynaecological laparoscopy.[24] In a study by Patel et al. conducted in patients undergoing gynaecologic, urologic or breast surgery, neither the time of onset nor the severity of PONV was improved after administration of IV dextrose during emergence from anaesthesia.[17] The different types of surgeries reviewed in that study, as well as its longer mean surgical time (mean: 174 min vs. 60 min in our study), may explain these inconsistencies. Sada et al. reported a significant improvement of post-operative nausea by pre-operative oral administration of carbohydrates in patients who had undergone open cholecystectomy as compared to those undergoing colorectal operations.[21]
It has been previously revealed that perioperative hyperglycaemia is associated with the increased risk of complications such as dehydration, electrolyte disturbances, fluid shifts, ketoacidosis, hyperosmolar states and also the increased risk of mortality and length of hospital stay during the post-operative period after general surgeries.[6,25,26] In our study, although the post-operative blood glucose level of the patients in the intervention group was within the accepted normal range in our study, patients without vomiting after surgery had significantly higher blood glucose concentrations as compared to those with vomiting. Moreover, nausea score and blood glucose level in patients after PACU arrival were negatively correlated. The mechanism by which PONV is improved by IV dextrose could possibly be related to the reduction of gastric acid secretion due to hyperglycaemia. Gastric contraction followed by nausea may be induced by increased gastric acid secretion.[10] It has been stated that blood glucose may have a regulating effect on gastric acid secretion. Hyperglycaemia can decrease gastric acid secretion by inhibiting the vagal cholinergic pathways.[27,28] Moreover, it has been shown that pre-operative glucose infusion could reduce the post-operative incidence of insulin resistance, which may contribute to PONV.[29,30,31,32] Insulin resistance is considered crucial in the post-operative outcomes of patients who undergo elective surgery.[32,33] In addition, increasing blood glucose levels may raise plasma cholecystokinin, which can modulate anxiety and pain through its functions within the brain, in turn decreasing pain and PONV;[34,35,36] post-operative pain is a known risk factor for PONV.[6] Future studies could focus on these potential relationships. Although gastric emptying may be delayed by hyperglycaemia, causing gastric fullness and nausea sensation,[24] it has been demonstrated that perioperative gastric emptying in patients undergoing LC is not a predictor of early PONV.[19]
The results of our study showed that the number of patients with length of stay in PACU ≥ 120 min were significantly lower in dextrose group. PONV is a major contributor to the direct and indirect costs for the institution. These costs are those that related to the patients' treatment and extended PACU and hospital stays.[37] It has been shown that occurrence of PONV can lead to an additional 47–61 min of patients' PACU stay.[38] Considering the efficacy and safety of ondansetron, most anaesthesiologists considered it as a gold standard of antiemetic therapy in clinical practice.[3,6,37] Our study showed that the ondansetron prescription rate was significantly higher in control group compared with the dextrose group. At our institution, one 4 mg IV dose of ondansetron costs $ 5.7 compared with $ 2.1 for a single IV solution dextrose 5%. We believe that using IV solution dextrose 5%, as low-price antiemetic prophylaxis in patients undergoing LC, provides opportunities for considerable cost savings while preserving efficacy. However, in the following situations, administration of 5% dextrose in lactated Ringer's solution is contraindicated: severe metabolic acidosis or alkalosis; conditions that could potentially affect lactate metabolism such as liver failure or anoxic states; hypersensitivity to corn products and in conditions that administration of calcium, sodium, potassium, lactate or chloride could be clinically harmful.[39]
This study has several limitations. First, we evaluated the effect of IV dextrose administration for prevention of PONV in healthy, non-smoking female patients who had undergone LC (which constitutes a high-risk group). Our findings may not be generalisable to other populations including patients who undergo surgeries of different duration or different types of surgery (as well as use different types of anaesthesia). Patients could also have received different formulations of dextrose-containing fluids. Undoubtedly, using this preventive method for PONV in diabetic patients is, while tentatively promising, debatable and demands further research. Moreover, we did not evaluate post-operative pain as a risk factor for PONV in our study; all patients received the same analgesic regimen after surgery. In addition, even though two groups received IV fluids with the same protocol, the dosages per kilogram body weight may be unequally distributed between groups, and thus the total volume of IV fluids may be a confounding variable. Finally, the results might be influenced by unknown variables; however, we tried to match known confounding factors such as female sex, history of PONV or motion sickness, non-smoking, younger age, general anaesthesia, using N2O, duration of anaesthesia, perioperative fasting and ASA physical status.
CONCLUSION
Administration of IV dextrose before anaesthesia induction may be recommended as an effective, safe and inexpensive method for the prophylaxis of PONV after LC. However, further well-designed randomised clinical trials are warranted to confirm the efficacy of this method and test the generalizability of our findings to other surgical populations as well as determine the optimal dose and timing of dextrose administration for prevention of PONV.
Financial support and sponsorship
This study was financially funded by Research Deputy of Mazandaran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the financial support provided by Research Deputy of Mazandaran University of Medical Sciences. In addition, the authors wish to thank all the study participants for their kind cooperation and support.
REFERENCES
- 1.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–98. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 2.Acalovschi I. Postoperative nausea and vomiting. Curr Anaesth Crit Care. 2002;13:37–43. [Google Scholar]
- 3.Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011;2011:748031. doi: 10.1155/2011/748031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–8. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107(Suppl 1):i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 7.Arslan M, Ciçek R, Kalender HÜ, Yilmaz H. Preventing postoperative nausea and vomiting after laparoscopic cholecystectomy: A prospective, randomized, double-blind study. Curr Ther Res Clin Exp. 2011;72:1–12. doi: 10.1016/j.curtheres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii Y. Management of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2011;25:691–5. doi: 10.1007/s00464-010-1193-9. [DOI] [PubMed] [Google Scholar]
- 9.Kovac AL. Update on the management of postoperative nausea and vomiting. Drugs. 2013;73:1525–47. doi: 10.1007/s40265-013-0110-7. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Dhiraaj S, Tandon M, Singh PK, Singh U, Pawar S, et al. Evaluation of capsaicin ointment at the Korean hand acupressure point K-D2 for prevention of postoperative nausea and vomiting. Anaesthesia. 2005;60:1185–8. doi: 10.1111/j.1365-2044.2005.04402.x. [DOI] [PubMed] [Google Scholar]
- 11.Dagher CF, Abboud B, Richa F, Abouzeid H, El-Khoury C, Doumit C, et al. Effect of intravenous crystalloid infusion on postoperative nausea and vomiting after thyroidectomy: A prospective, randomized, controlled study. Eur J Anaesthesiol. 2009;26:188–91. doi: 10.1097/EJA.0b013e32831c8793. [DOI] [PubMed] [Google Scholar]
- 12.Maharaj CH, Kallam SR, Malik A, Hassett P, Grady D, Laffey JG, et al. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. 2005;100:675–82. doi: 10.1213/01.ANE.0000148684.64286.36. [DOI] [PubMed] [Google Scholar]
- 13.Haentjens LL, Ghoundiwal D, Touhiri K, Renard M, Engelman E, Anaf V, et al. Does infusion of colloid influence the occurrence of postoperative nausea and vomiting after elective surgery in women? Anesth Analg. 2009;108:1788–93. doi: 10.1213/ane.0b013e3181a1968c. [DOI] [PubMed] [Google Scholar]
- 14.Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2005;92:415–21. doi: 10.1002/bjs.4901. [DOI] [PubMed] [Google Scholar]
- 15.Lauwick SM, Kaba A, Maweja S, Hamoir EE, Joris JL. Effects of oral preoperative carbohydrate on early postoperative outcome after thyroidectomy. Acta Anaesthesiol Belg. 2009;60:67–73. [PubMed] [Google Scholar]
- 16.Dabu-Bondoc S, Vadivelu N, Shimono C, English A, Kosarussavadi B, Dai F, et al. Intravenous dextrose administration reduces postoperative antiemetic rescue treatment requirements and postanesthesia care unit length of stay. Anesth Analg. 2013;117:591–6. doi: 10.1213/ANE.0b013e3182458f9e. [DOI] [PubMed] [Google Scholar]
- 17.Patel P, Meineke MN, Rasmussen T, Anderson DL, Brown J, Siddighi S, et al. The relationship of intravenous dextrose administration during emergence from anesthesia to postoperative nausea and vomiting: A randomized controlled trial. Anesth Analg. 2013;117:34–42. doi: 10.1213/ANE.0b013e318292ed5f. [DOI] [PubMed] [Google Scholar]
- 18.Gauchan S, Thapa C, Shakya P, Bhattarai R, Shakya S. Ondansetron and Granisetron for prevention of postoperative nausea and vomiting following laparoscopic cholecystectomy. JNMA J Nepal Med Assoc. 2014;52:682–6. [PubMed] [Google Scholar]
- 19.Wattwil M, Thörn SE, Lövqvist A, Wattwil L, Klockhoff H, Larsson LG, et al. Perioperative gastric emptying is not a predictor of early postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Anesth Analg. 2002;95:476–9. doi: 10.1097/00000539-200208000-00045. [DOI] [PubMed] [Google Scholar]
- 20.Ryu J, So YM, Hwang J, Do SH. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2010;24:812–7. doi: 10.1007/s00464-009-0670-5. [DOI] [PubMed] [Google Scholar]
- 21.Sada F, Krasniqi A, Hamza A, Gecaj-Gashi A, Bicaj B, Kavaja F, et al. A randomized trial of preoperative oral carbohydrates in abdominal surgery. BMC Anesthesiol. 2014;14:93. doi: 10.1186/1471-2253-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Järvelä K, Maaranen P, Sisto T. Pre-operative oral carbohydrate treatment before coronary artery bypass surgery. Acta Anaesthesiol Scand. 2008;52:793–7. doi: 10.1111/j.1399-6576.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 23.Bisgaard T, Kristiansen VB, Hjortsø NC, Jacobsen LS, Rosenberg J, Kehlet H, et al. Randomized clinical trial comparing an oral carbohydrate beverage with placebo before laparoscopic cholecystectomy. Br J Surg. 2004;91:151–8. doi: 10.1002/bjs.4412. [DOI] [PubMed] [Google Scholar]
- 24.McCaul C, Moran C, O'Cronin D, Naughton F, Geary M, Carton E, et al. Intravenous fluid loading with or without supplementary dextrose does not prevent nausea, vomiting and pain after laparoscopy. Can J Anaesth. 2003;50:440–4. doi: 10.1007/BF03021053. [DOI] [PubMed] [Google Scholar]
- 25.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783–8. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichardo-Lowden A, Gabbay RA. Management of hyperglycemia during the perioperative period. Curr Diab Rep. 2012;12:108–18. doi: 10.1007/s11892-011-0239-2. [DOI] [PubMed] [Google Scholar]
- 27.Loud FB, Holst JJ, Rehfeld JF, Christiansen J. Inhibition of gastric acid secretion in humans by glucagon during euglycemia, hyperglycemia, and hypoglycemia. Dig Dis Sci. 1988;33:530–4. doi: 10.1007/BF01798352. [DOI] [PubMed] [Google Scholar]
- 28.Lam WF, Masclee AA, De Boer SY, Lamers CB. Hyperglycaemia reduces gastrin-stimulated gastric acid secretion in humans. Eur J Clin Invest. 1998;28:826–30. doi: 10.1046/j.1365-2362.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Ljungqvist O, Thorell A, Gutniak M, Häggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J Am Coll Surg. 1994;178:329–36. [PubMed] [Google Scholar]
- 30.Ljungqvist O, Nygren J, Thorell A. Modulation of post-operative insulin resistance by pre-operative carbohydrate loading. Proc Nutr Soc. 2002;61:329–36. doi: 10.1079/PNS2002168. [DOI] [PubMed] [Google Scholar]
- 31.Ohara S, Sakuma S, Higuchi H, Shunichi T, Tanno M. Effects of preoperative oral rehydration therapy on postoperative nausea and vomiting after mastectomy: 1AP1-5. [Last accessed on 2017 Aug 02];Eur J Anaesthesiol. 2013 30:8. Available from: http://www.journals.lww.com/ejanaesthesiology/Fulltext/2013/06001/Effects_of_preoperative_oral_rehydration_therapy.23.aspx . [Google Scholar]
- 32.Ljungqvist O, Nygren J, Thorell A. Insulin resistance and elective surgery. Surgery. 2000;128:757–60. doi: 10.1067/msy.2000.107166. [DOI] [PubMed] [Google Scholar]
- 33.Abebe WA, Rukewe A, Bekele NA, Stoffel M, Dichabeng MN, Shifa JZ, et al. Preoperative fasting times in elective surgical patients at a referral hospital in Botswana. Pan Afr Med J. 2016;23:102. doi: 10.11604/pamj.2016.23.102.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa H, Shirohara H, Okabayashi Y, Nakamura T, Fujii M, Koide M, et al. Oral glucose ingestion stimulates cholecystokinin release in normal subjects and patients with non-insulin-dependent diabetes mellitus. Metabolism. 1996;45:196–202. doi: 10.1016/s0026-0495(96)90053-0. [DOI] [PubMed] [Google Scholar]
- 35.Kissin I, Bright CA, Bradley EL., Jr Acute tolerance to continuously infused alfentanil: The role of cholecystokinin and N-methyl-D-aspartate-nitric oxide systems. Anesth Analg. 2000;91:110–6. doi: 10.1097/00000539-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell VA, Jeong HJ, Drew GM, Vaughan CW. Cholecystokinin exerts an effect via the endocannabinoid system to inhibit GABAergic transmission in midbrain periaqueductal gray. Neuropsychopharmacology. 2011;36:1801–10. doi: 10.1038/npp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winston AW, Rinehart RS, Riley GP, Vacchiano CA, Pellegrini JE. Comparison of inhaled isopropyl alcohol and intravenous ondansetron for treatment of postoperative nausea. AANA J. 2003;71:127–32. [PubMed] [Google Scholar]
- 38.Metter SE, Kitz DS, Young ML, Baldeck AM, Apfelbaum JL, Lecky JH. Nausea and vomiting after outpatient laparoscopy: Incidence, impact on recovery room stay and cost. Anesth Analg. 1987;66:S116. [Google Scholar]
- 39.5% Dextrose in Lactated Ringer's Injection. [Last accessed on 2017 Aug 02]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019634s033lbl.pdf .