Abstract
Background and Aims:
Thoracic paravertebral block (TPB) is one of the effective methods for management of post-operative pain in thoracic surgeries. The aim of the study was to evaluate effectiveness of addition of dexmedetomidine to paravertebral block with bupivacaine in improving the postoperative pain relief and pulmonary functions in patients undergoing thoracic surgeries.
Methods:
A prospective randomized double-blinded study was performed on forty patients scheduled for thoracic surgery. A paravertebral catheter was secured under ultrasound guidance preoperatively for all patients. Group B (n = 20) received a bolus dose of 0.25% bupivacaine at 0.3 mL/kg followed by continuous infusion of 0.125% bupivacaine 0.1 mL/kg/h. Group (BD) received a bolus dose of 0.25% bupivacaine + dexmedetomidine 1 μg/kg at 0.3 mL/kg followed by continuous infusion of dexmedetomidine 0.2 μg/kg/h + 0.125% bupivacaine 0.1 mL/kg/h. Anaesthesia technique was standardized for all patients. Postoperatively, all patients were assessed during first 24 hours for intraoperative fentanyl and post-operative morphine requirements, Visual Analogue Scores (VAS) scores at rest and during cough, and postoperative pulmonary functions.
Results:
Post-operative morphine consumption in the first 24 hours and intraoperative fentanyl requirement were significantly less in group BD (2.95 ± 1.986 mg, 80.75 ± 31.551μg respectively) compared to group B (9.85 ± 3.468 mg, 186 ± 39.683 μg respectively). Group BD showed less VAS scores during cough and better postoperative pulmonary functions (P < 0.05).
Conclusion:
Addition of dexmedetomidine to paravertebral bupivacaine in patients undergoing thoracic surgeries provides more effective analgesia with improvement in post-operative pulmonary functions.
Key words: Analgesia, dexmedetomidine, paravertebral, pulmonary functions
INTRODUCTION
Adequate management of post-operative pain is very crucial after thoracic surgery to avoid increase in post-operative morbidity and mortality. Serious pulmonary complications have been found with inadequate post-operative pain management. Adequate analgesia allows effective cough with consequently effective clearance of secretions. Effective cough prevents formation of mucus plugs and atelectasis.[1] Thoracic paravertebral block (TPB) is one of the effective methods that have been used for management of post-operative pain after unilateral thoracic, breast and upper abdominal surgeries.[2] The use of ultrasound guided technique in TPB allows better localization of the paravertebral space with less incidence of undesirable complications.[3] TPB can provide analgesia with more haemodynamic stability due to unilateral sympathetic block, in contrast to thoracic epidural block.[2] Many adjuvants have been used to improve the analgesic efficacy of paravertebral block as magnesium, dexmedetomidine, fentanyl, clonidine.[2,4,5]
Dexmedetomidine is a selective α-2 agonist that can provide analgesia by decreasing the availability of epinephrine and norepinephrine on post-synaptic α-2 receptors. This is done by a negative feedback mechanism produced by its central action on presynaptic α-2 receptors.[6] Dexmedetomidine has been used extensively as an adjuvant to local anaesthesia in intrathecal, epidural, paravertebral and peripheral nerve block.[4]
We hypothesised that adding dexmedetomidine to continuous bupivacaine infusion via paravertebral catheter in thoracic surgeries can improve the analgesic profile of paravertebral bupivacaine.
METHODS
This prospective randomised double-blinded study was conducted from January 24, 2017, to March 24, 2017, after obtaining approval from the local Institutional Ethics Committee. Signed written informed consent was obtained from 40 patients belonging to American Society of Anesthesiologists' (ASA) physical status 1 or 2 scheduled for elective open thoracic surgery (e.g., metastatectomy or lobectomy). Patients with significant respiratory disorders (forced vital capacity (FVC) or forced expiratory volume in first second (FEV1) <50% of the predicted values), cardiac diseases, coagulation disorders, infection at the site of the block, body mass index >35 kg/m2, allergy to local anaesthetics or dexmedetomidine, pre-existing neurological disorders, psychiatric disorder, inability to use the patient-controlled analgesia (PCA) device and communication difficulties were excluded.
During the pre-anaesthetic assessment, echocardiography was done and baseline pulmonary function tests and arterial blood gases were obtained. All patients were educated how to report pain on the 11-point visual analogue scale (VAS), where 0 = no pain and 10 = worst imaginable pain, and were also educated how to use the patient-controlled analgesia device. Patients also received instructions about the use of the hand-held spirometer (Vitalograph) and preoperative baseline peak expiratory flow rate (PEFR), FEV1 and forced vital capacity (FVC) were recorded.
Randomisation of the patients was done using a computer-generated randomization programme (permuted block technique) with concealment of the random allocation numbers in opaque sealed closed envelopes that were opened the day before surgery after obtaining the trial consent. Patients were allocated into two groups of 20 each. Patients in group B received a bolus dose of 0.25% bupivacaine at 0.3 mL/kg in the paravertebral space over 5 minutes, followed by continuous catheter infusion of 0.125% bupivacaine at a rate of 0.1 mL/kg/h. This infusion was continued postoperatively for 24 hours. Group (BD): received a bolus dose of dexmedetomidine 1 μg/kg + 0.25% bupivacaine at 0.3 mL/kg in the paravertebral space over 5 minutes, followed by continuous catheter infusion of dexmedetomidine at a rate of 0.2 μg/kg/h + 0.125% bupivacaine at a rate of 0.1 mL/kg/h mixed in a single syringe pump.[7] This infusion was continued postoperatively for 24 hours.
The patients and all staff involved in patient management and data collection were blinded to the group assignment. The drugs administered in the paravertebral region was prepared according to group randomization by a staff anaesthesiologist not related to the patient management and not involved in the data collection. A similar total volume of the paravertebral injectate for the initial bolus and the following infusion doses were given to all patients of the study to ensure assessor blindness.
Continuous monitoring using non-invasive blood pressure measurements, electrocardiography and pulse oximetry was initiated on arrival of the patients to the operating room. Midazolam 2 mg IV was given to relieve anxiety. Ultrasound- guided (US) thoracic paravertebral block (TPVB) was performed in all patients before administration of general anaesthesia. A linear multi-frequency 13–16 MHz probe (Fujifilm Sonosite.inc Bothell, WA 98021, USA) was used for scanning. The block was performed on the side where thoracotomy was scheduled. Under all aseptic precautions, the paravertebral space was located by applying the probe 2.5 cm lateral to the tip of the fifth spinous process. Local anaesthesia was given with is 5% lignocaine used for local infiltration. Then, an 18-gauge Tuohy needle (Epidural kit; Portex, Smiths Group, London, UK) was inserted perpendicularly till it hit the sixth vertebral transverse process. Then, the needle was redirected cephalic at 15 degrees towards the desired paravertebral space. The linear ultrasound probe was used to guide the direction of the needle by an out-of-plane approach till puncture of the superior costo-transverse ligament. After negative aspiration, the medication prepared for each group of the study was injected according to group assignment. The correct placement of the injected drug was confirmed by visualization of anterior displacement of the parietal pleura and expansion of the paravertebral space between the pleura and the superior costo-transverse ligament under ultrasound.[8] After injection of the drug, a 20-gauge epidural catheter (Epidural kit; Portex, Smiths Group, London, UK) was introduced through the needle up to 4 cm from the needle tip, then the needle was removed and the catheter was secured at the skin.
Thirty minutes after injection of the study medications, the pinprick test was used to assess the success of the block. Pinprick sensation in dermatomes (T3–T7) of the blocked side was assessed in comparison to the normal contralateral side. Evident loss of pinprick sensation in the target dermatomes indicated successful block. The data of patients with failed block was not considered for statistical analysis.
All patients received the same anaesthetic protocol, surgical techniques and same team of surgeons performed the procedure. General anaesthesia was induced with propofol 2 mg/kg IV and fentanyl 2 μg/kg IV. Rocuronium 0.6 mg/kg IV was used to facilitate tracheal intubation. A left-sided double-lumen endobronchial tube (Mallinckrodt's 37 or 39 Fr) was passed and its position confirmed by a fiberoptic bronchoscope. After induction of general anaesthesia a central venous catheter was introduced in the internal jugular vein using a ultrasound guidance. An arterial line was introduced in the radial artery to obtain continuous invasive blood pressure monitoring and for obtaining samples of serial arterial blood gases and serial haematocrit when needed. Sevoflurane with Minimum Alveolar Concentration (MAC) 2-2.5% and oxygen was used for maintenance of anaesthesia and a peripheral nerve stimulator was used to guide supplemental doses of rocuronium 0.1mg/kg IV. Patients were ventilated with setting of tidal volume at 7-10 mL/kg and adjustment of respiratory rate to keep the end-tidal CO2 between 30 and 35 mmHg. Monitoring of the patients included pulse oximetry, capnography, continuous electrocardiogram, central venous pressure, invasive arterial blood pressure and peak airway pressure. Additional doses of fentanyl (0.5 μg/kg) were given to keep heart rate and mean arterial blood pressure within 20% of baseline values. Blood transfusions and fluids were given as required to keep haemoglobin level ≥10 g/dL, central venous pressure ≥7 cm H2O and urine output ≥1 mL/kg/h. Ondansetron 4 mg IV was given 10 min before the wound closure. At the end of surgery, neostigmine 0.05 mg/kg and atropine 0.02 mg/kg were used to reverse neuromuscular blockade and extubation was performed after full return of consciousness.
After recovery from anaesthesia, all patients were transferred to the ICU where a PCA (patient- controlled analgesia) device with a morphine solution (1 mg/mL) was connected to the IV route of the patients. All patients received patient-controlled analgesia with the same setting, which was a demand dose of 1 mL and a lockout interval of 10 min and without a continuous background infusion.[9] Subsequently, total amount of PCA morphine consumed during the first postoperative 24 h was assessed as the primary outcome. The following parameters were assessed as the secondary outcomes: total amount of intra-operative fentanyl requirement after induction of general anaesthesia (after the initial fentanyl dose during induction); visual analogue scale (VAS) scores to assess the intensity of pain in patients after 30 min and subsequently 2, 4, 8, 12, and 24 h after the recovery from anaesthesia, both at rest and on coughing; peak expiratory flow rate (PEFR), forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1), expressed as a percentage from the predicted values, measured pre-operatively and after 24 hours from recovery. Any complication from the technique or from the drugs used in the study (pneumothorax, bradycardia (heart rate (HR) <50 beat/minute), hypotension (mean arterial blood pressure (MAP) <65 mm hg), hypoxia (oxygen saturation <90% in room air), and post-operative nausea and vomiting) were properly managed and recorded.
The primary outcome measure was total morphine consumption in the first post-operative 24 hours. G Power 3.1 program was used for calculating sample size. Sample size estimation was done based on the previous study done by Mohta et al.,[4] who found that the mean total morphine consumption in group (B) was 18.3 ± 13.5 mg compared to 2.4 ± 2.8 mg in group (BD). The expected reduction in total morphine consumption was 15.9 mg. Taking into consideration that power (1-β) was 80% and significance (α) was 0.05, the calculated minimum sample size was 15 patients per group or total of 30 patients. We increased the number of patients to 40 patients to compensate for possible drop-outs.
SPSS (statistical package for social sciences: Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp) was used for statistical analysis. Mean and standard deviation were used for description of quantitative continuous data. Median and range were used for ordinal data (VAS scores). Student t-test was used to compare means of 2 independent groups. Mann Whitney test was used to compare medians of 2 independent groups. Bonferoni correction was applied for multiple comparisons. Chi-square test was used to assess proportion independence. P values ≤0.05 were considered statistically significant
RESULTS
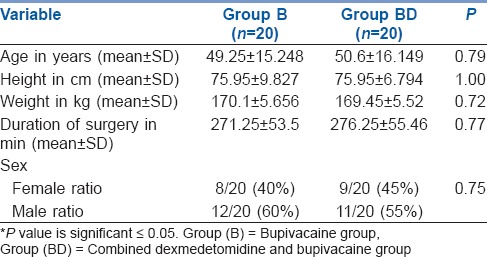
After screening 48 patients for eligibility, 8 patients were excluded from the study. Six patients were excluded by exclusion criteria and two patients refused to participate in the study. All these 40 patients completed the study, without any dropouts or failed blocks [Figure 1]. The present study revealed no significant difference in demographic data between the groups [Table 1].
Figure 1.
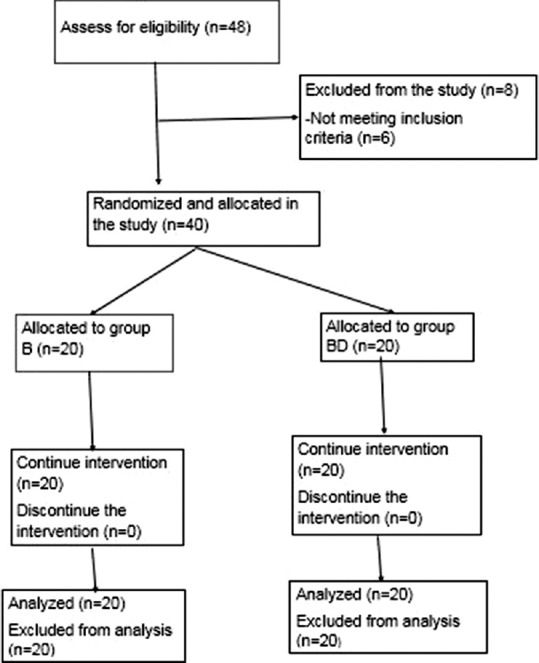
Consort chart for allocation of cases in the study
Table 1.
Demographic data
Patients who received paravertebral dexmedetomidine and bupivacaine infusion significantly required less intraoperative fentanyl (80.7 ± 31.5 μg) than patients who received paravertebral bupivacaine infusion alone (186 ± 39.6 μg) with a P value <0.001. Post-operative morphine consumption in the first 24 hours was less in group BD (2.9 ± 1.9 mg) compared with that of group B (9.8 ± 3.4 mg) and P value was <0.001.
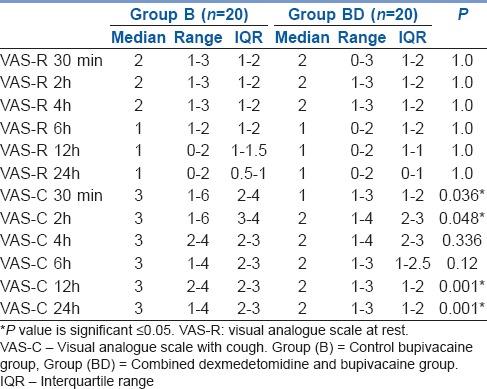
Although adding dexmedetomidine to bupivacaine infusion in the paravertebral space did not achieve a significant change in post-operative VAS scores at rest at all time points, patients in group BD had lower VAS scores during cough after 30 minutes, 2, 12 and 24 post-operative hours (P < 0.05, Table 2).
Table 2.
Visual analogue scores at rest and with cough
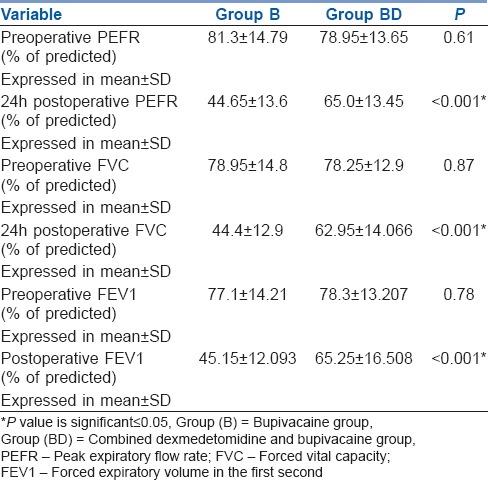
Better post-operative pulmonary functions (PEFR, FVC, FEV1) were observed in Group BD. [Table 3].
Table 3.
Pulmonary functions
Eight patients from group B suffered from postoperative nausea and vomiting (PONV), while only three patients in group BD had (PONV). In group BD, six patients developed hypotension and two patients developed bradycardia, while only two patients developed hypotension and one patient developed bradycardia in group B. Oxygen saturation did not decrease below 90% throughout the study periods in all patients. No other complications related to the paravertebral technique, were observed in this study.
DISCUSSION
This study revealed that adding dexmedetomidine to bupivacaine infusion in a paravertebral catheter introduced with an ultrasound guided technique can improve the analgesic efficacy of paravertebral block in patients undergoing thoracic surgeries. Improving analgesic efficacy is evident by lower post-operative morphine consumption. The combination of paravertebral dexmedetomidine and bupivacaine can decrease intraoperative fentanyl requirements and can produce a better values of VAS scores during cough at most post-operative time points. Most probably, as a sequence of improving the analgesic efficacy, the paravertebral infusion of bupivacaine-dexmedetomidine combination can improve post-operative pulmonary functions.
The difference in the incidence of PONV and hypotension was not statistically significant. This may be due to the sample size which was not large enough to detect a difference; however these were not outcomes of interest in our study.
Improvement of the analgesic profile, shown in our study, can be explained by additive effects produced by different mechanisms of action. Local anaesthetic produces analgesia by sodium channel blockade, while dexmedetomidine produces analgesia as a selective α-2 agonist. The analgesic effect of α-2 agonists is mediated peripherally by inhibition of post-synaptic α-2 adrenoreceptors. This is done by decreasing the release of epinephrine and norepinephrine from presynaptic α-2 adrenoreceptors. Dexmedetomidine has also a central action in the form of activation of α-2 adrenoreceptors present in locus coeruleus, and decreasing the release of substance P at the dorsal horn neurons, resulting in inhibition of the nociceptive pathway.[4,10] The α-2 agonist effect of dexmedetomidine is 8 times more specific than that of clonidine, consequently dexmedetomidine has less undesirable actions related to α-1 receptors.[4,11]
Previous studies showed that improvement of duration and quality of analgesia by using multiple injections was associated with increased incidence of procedural complications.[12,13] Addition of fentanyl or clonidine to paravertebral bupivacaine has been found to provide better analgesia and reduce postoperative morphine consumption in patients undergoing breast surgeries.[5] But this improvement was associated with more incidence of post-operative nausea and vomiting if fentanyl was added and hypotension if clonidine was added.[5]
In a recent study, a single paravertebral bolus of dexmedetomidine 1μg/kg was added to 0.5% bupivacaine to provide a better analgesia for patients undergoing major breast surgeries.[4] A decrease in post-operative morphine consumption was found in patients receiving paravertebral combination of dexmedetomidine and bupivacaine.[4] Our study was in accordance with this finding. But, in this study, it was found that combination of drugs in the paravertebral region did not change intraoperative fentanyl requirement compared to paravertebral bupivacaine alone. This finding was different from that found in our study. We used a lower paravertebral bupivacaine concentration (0.25%), thus requiring more intraoperative amount of fentanyl. Whereas paravertebral bupivacaine concentration of 0.5% was used in the other study. In addition, the scope of our study was on thoracic surgeries with a higher pain intensity.
Continuous infusion of dexmedetomidine when added to local anaesthetic can decrease the requirement of both intraoperative and post-operative opioids in elective thoracotomy. This has been demonstrated in a recent study.[7] Our observation also supports this view.
One of the favorable points in our study is that the improvement in analgesic quality produced by adding paravertebral dexmedetomidine was evaluated in objective variables (post-operative pulmonary functions) in addition to the standard subjective variables (opioid requirements). Pain is the most important cause of post-operative pulmonary dysfunction, which may lead to post-operative pulmonary complications.[2,14]
One of the limitations in this study is inability to assess the incidence of post-thoracotomy pain syndrome, due to short duration of post-operative assessment (24 hours). However, it was previously proved that adding paravertebral dexmedetomidine to bupivacaine did not affect the incidence of post-thoracotomy pain syndrome.[7]
Another limitation is inability to detect a significant difference in side effects from the drugs used in the study. This may be due to inadequate sample size. A third limitation was not evaluating the degree of post-operative sedation, which may result as a side effect from dexmedetomidine or as a reflection from increased use of narcotics.
CONCLUSION
We therefore conclude that combination of paravertebral dexmedetomidine and bupivacaine in patients undergoing thoracic surgeries can provide a better analgesia, thus decreasing post-operative morphine requirements.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Esme H, Apiliogullari B, Duran FM, Yoldas B, Bekci TT. Comparison between intermittent intravenous analgesia and intermittent paravertebral subpleural analgesia for pain relief after thoracotomy. Eur J Cardiothorac Surg. 2012;41:10–3. doi: 10.1016/j.ejcts.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammar AS, Mahmoud KM. Does the addition of magnesium to bupivacaine improve postoperative analgesia of ultrasound-guided thoracic paravertebral block in patients undergoing thoracic surgery? J Anesth. 2014;28:58–63. doi: 10.1007/s00540-013-1659-8. [DOI] [PubMed] [Google Scholar]
- 3.O Riain SC, Donnell BO, Cuffe T, Harmon DC, Fraher JP, Shorten G. Thoracic paravertebral block using real-time ultrasound guidance. Anesth Analg. 2010;110:248–51. doi: 10.1213/ANE.0b013e3181c35906. [DOI] [PubMed] [Google Scholar]
- 4.Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30:252–60. doi: 10.1007/s00540-015-2123-8. [DOI] [PubMed] [Google Scholar]
- 5.Barlacu CL, Frizelle HP, Moriaty DC, Buggy DJ. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–7. doi: 10.1111/j.1365-2044.2006.04793.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 7.Dutta V, Kumar B, Jayant A, Mishra AK. Effect of continuous paravertebral dexmedetomidine administration on intraoperative anesthetic drug requirement and post-thoracotomy pain syndrome after thoracotomy: A randomized controlled trial. J Cardiothorac Vasc Anesth. 2017;31:159–65. doi: 10.1053/j.jvca.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Sakura S, Nomura T, Saito Y. Ultrasound guided thoracic paravertebral block in breast surgery. Anaesthesia. 2009;64:223–5. doi: 10.1111/j.1365-2044.2008.05843.x. [DOI] [PubMed] [Google Scholar]
- 9.Howell PR, Gambling DR, Pavy T, McMorland G, Douglas MJ. Patient-controlled analgesia following caesarean section under general anaesthesia: A comparison of fentanyl with morphine. Can J Anaesth. 1995;42:41. doi: 10.1007/BF03010570. [DOI] [PubMed] [Google Scholar]
- 10.Biswas S, Das RK, Mukherjee G, Ghose T. Dexmedetomidine as an adjuvant to levobupivacaine in supraclavicular brachial plexus block: A randomized double blind prospective study. Ethiop J Health Sci. 2014;24:203–8. doi: 10.4314/ejhs.v24i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed SA, Fares KM, Mohamed AA, Alieldin NH. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician. 2014;17:E589–98. [PubMed] [Google Scholar]
- 13.Moller JF, Nikolajsen I, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: A randomized double-blind study. Anesth Analg. 2007;105:1848–51. doi: 10.1213/01.ane.0000286135.21333.fd. [DOI] [PubMed] [Google Scholar]
- 14.Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005;95:816–21. doi: 10.1093/bja/aei250. [DOI] [PubMed] [Google Scholar]


