Abstract
Both rumination and attentional biases have been proposed as key components of the RDoC Negative Valence Systems construct of Loss. Although theorists have proposed that rumination, particularly brooding rumination, should be associated with increased sustained attention to depression-relevant information, it is not clear whether this link would be observed in a non-depressed sample or whether it is specific to brooding versus reflective rumination. To address these questions, the current study examined the link between brooding rumination and attentional biases in a sample of non-depressed individuals (n = 105). Attentional biases were assessed using eye tracking during a passive viewing task in which participants were presented with 2 × 2 arrays of angry, happy, sad, and neutral faces. In line with predictions, higher levels of brooding rumination were associated with greater sustained attention to sad faces and less sustained attention to happy faces. These results remained significant after controlling for participants’ prior history of major depression and current nonclinical level of depressive symptoms, suggesting that the link between brooding rumination and attentional biases is at least partially independent of current or past depression.
Keywords: brooding rumination, depression, attention biases, eye tracking, sustained attention
The goal of the NIMH Research Domain Criteria (RDoC) initiative is to develop “new ways of classifying mental disorders based on behavioral dimensions and neurobiological measures” (NIMH, 2008). A central part of this initiative is to identify key constructs linked to disruptions in well-established neural circuits that should be tied to specific influences across multiple units of analysis. Within the Negative Valence Systems construct of Loss, disruption in cortico-limbic circuitry has been highlighted, particularly hyperactivity in limbic regions that is not effectively down-regulated by prefrontal regions. Disruption in this same circuit is thought to underlie many of the information-processing biases implicated in risk for depression, including rumination and attentional biases for affective stimuli (for reviews, see Disner, Beevers, Haigh, & Beck, 2011; Gibb, McGeary, & Beevers, 2016; Woody & Gibb, 2015), both of which are also highlighted in the RDoC matrix for the Loss construct.
Rumination is typically defined as a repetitive, and often uncontrolled, process developing from a narrowed attentional focus on the causes and consequences of feelings of depression (Nolen-Hoeksema, Wisco & Lyubomirsky, 2008; Koster et al., 2011; Whitmer & Gotlib, 2012). A number of studies have now shown that rumination increases risk for depression in children, adolescents, and adults (Abela & Hankin, 2011; Gibb, Grassia, Stone, & Uhrlass, 2012; Nolen-Hoeksema et al., 2008). Consistent with the RDoC matrix, there is evidence that rumination is associated with heightened amygdala activity as well as altered activity in prefrontal regions (Disner et al., 2011). Researchers have identified two distinct forms of depressive rumination, termed brooding and reflection (Treynor, Gonzalez, and Nolen-Hoeksema, 2003). Brooding is characterized by a passive comparison of one’s current state with an unachieved standard (e.g. “Think about a recent situation, wishing it had gone better”) whereas reflection typically consists of purposefully turning inward to engage in cognitive problem solving (e.g. “Write down what you are thinking and analyze it”). There is consistent evidence that brooding is more strongly related to depression (e.g., suicide attempts) than is reflection (e.g., Nolen-Hoeksema et al., 2008; Schoofs, Hermans, & Raes, 2010; Treynor et al., 2003).
As noted above, there is evidence that attentional biases are also driven by disruption in corticolimbic circuitry (for reviews, see Disner et al., 2009; Gibb et al., 2016). Although early research produced mixed results for the relation between attentional biases and depression, more recent research, which has focused on longer stimulus presentation times (≥ 1000ms) and facial rather than word stimuli, has produced more consistent support for the hypothesis that depression is associated with increased sustained attention to depression-relevant stimuli (for reviews, see Armstrong & Olatunji, 2012; Peckham, McHugh, & Otto, 2010). There is also preliminary evidence from eye-tracking studies that currently depressed adults exhibit less sustained attention to positive stimuli than non-depressed individuals (e.g. Kellough et al., 2008). Importantly, there is evidence that attentional biases are not merely a correlate of depression, but also predict prospective changes in depressive symptoms (for a review, see Gibb et al., 2016).
We have argued that rumination represents an ideal starting point for researchers seeking to link influences across multiple units of analysis within the RDoC construct of Loss (Woody & Gibb, 2015). Indeed, independent of RDoC, theorists had suggested that rumination should be linked to attentional biases, specifically increased sustained attention to negative information (e.g., Koster et al., 2011; Whitmer & Gotlib, 2013). Supporting the link between rumination and attentional biases, there is initial evidence that levels of brooding, but not reflection, are associated with increased attention to sad faces in currently depressed adults (Joormann, Dkane, & Gotlib, 2006) and dysphoric undergraduate students (Duque, Sanchez & Vazquez, 2014). There is also evidence that brooding rumination is associated with greater difficulty disengaging attention from negative relative to positive word stimuli, though this relation was reduced to a non-significant trend when the impact of depressive symptoms was statistically controlled (Southworth et al., in press). In addition, in a sample of non-dysphoric undergraduate students, reflection, but not brooding, was associated with greater attention to sad and angry faces and less attention to happy faces (Duque et al., 2014), suggesting that the link between brooding rumination and attentional biases may differ based on current mood state.
The goal of the current study was to address this question and determine (i) whether brooding rumination is associated with attentional biases in a nondepressed sample and (ii) whether this relation is specific to brooding rumination or whether it would also be observed for reflective rumination. In doing so, we used a standardized passive viewing task in which participants are presented with a series of trials in which four facial stimuli of different emotions (angry, happy, sad, neutral) from the same actor are presented simultaneously on the computer screen for an extended period of time (20 seconds) while their gaze is assessed using an eye tracker. The extended presentation duration allows one to determine whether patterns of attentional allocation change over time in a free viewing situation. Based on prior theory and research, we predicted that higher levels of brooding rumination would be associated with greater attention toward sad facial stimuli and less attention toward happy facial stimuli across the entire trial. To determine if these relations are specific to brooding rumination, we also included an assessment of reflection. Finally, although we focused on individuals with no current depressive diagnosis, to provide a more stringent test of our hypotheses, we also assessed participants’ history of major depression and current depressive symptom levels. We predicted that the relation between brooding rumination on attention biases would be maintained even after statistically controlling for the impact of past histories of major depression and participants’ current levels of depressive symptoms.
Method
Participants
Participants were 105 parents of children recruited from the community who were participating in a larger RDoC study of attentional biases in children. This sub-study was designed specifically to examine the relation between rumination and attentional biases in adults within an RDoC framework. Although data collection for the RDoC study is ongoing, the current sample size provides adequate power (.80) to detect small to medium sized effects (r2 ≥ .07). Exclusion criteria for the adults in this study were the presence of current major depressive disorder, current substance dependence, or history of bipolar disorder or psychotic symptoms. The average age of the participants was 36.70 (SD = 7.74), 89% were women, and 85% were Caucasian. The median annual family income was $45,001–50,000.
Measures
Depression Diagnoses and symptoms
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002) was used to assess for lifetime histories of DSM-IV Axis I disorders. The SCID-I is a widely used diagnostic interview with well-established psychometric properties (Zanarini & Frankenburg, 2001).
The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) was used to assess participants’ current levels of depressive symptoms. Participants’ levels of depressive symptoms were in the minimal to mild range (M = 4.81, SD = 4.80; range = 0–18). A number of studies have supported the psychometric properties of the BDI-II (e.g., Beck et al., 1996) and it exhibited good internal consistency in this sample (α = .81).
Rumination
The Ruminative Response Scale (RRS; Treynor et al., 2003) was used to assess levels of rumination. The RRS is a 22-item self-report questionnaire assessing the frequency that ruminative thoughts or behaviors occur when the person is feeling sad, down, or depressed. For the current study we used the two five-item subscales that assess brooding and reflective rumination and both subscales exhibited good internal consistency in this sample (brooding: α = .76; reflection: α = .72).
Attentional biases
A passive viewing task was used to assess participants’ attentional biases to emotion. In this task, four adult faces exhibiting either an angry, happy, sad, or neutral emotion are arrayed in a 2 × 2 grid on screen (i.e., upper left, upper right, lower left, lower right). Each face was 13 cm high (17° visual angle) × 12 cm wide (8° visual angle). There was 20 cm (19° visual angle) between the center of each stimulus horizontally and 16 cm vertically (25° visual angle). Facial stimuli for the task were drawn from the Karolinska Directed Emotional Faces (KDEF; Lundqvist, Flykt, & Ohman, 1998) stimulus set. Mean arousal ratings for the images used in the study were obtained from a recent validation study of the KDEF stimulus set (Goeleven, De Raedt, Leyman, Verschuere, 2008) and were as follows: angry (M = 3.51, SD = .43), happy (M = 3.74, SD = .33), neutral (M = 2.54, SD = 2.54) and sad (M = 3.40, SD = .38). A one-way ANOVA with emotion as the independent variable and arousal rating as the dependent variable was significant, F(3, 60) = 34.47, p < .001, ηp2 = .63. Post hoc comparisons revealed that all emotional stimuli used in the study (i.e. angry, happy and sad faces) had significantly higher mean arousal ratings than neutral faces, all ps < .001. Additionally, happy faces had higher arousal ratings than sad faces, p = .008. There were no other significant differences in mean arousal ratings between emotion types.
Stimuli were presented on a 24-inch Tobii T60XL eye tracker (60-Hz data rate, 1920 × 1200 pixels). Eye movements were recorded using infrared Pupil Centre Corneal Reflection, which illuminates the cornea and calculates gaze direction in relation to the monitor location. Participants sat 65 cm from the monitor. Gaze data is reported by the manufacturer to be accurate to 0.58° with an error (drift) of 0.18°. Prior to the task, participants completed a 9-point calibration of the eye tracker where they were asked to look at specific points at the center and corners of the monitor. Accuracy was confirmed by visual inspection of fixations recorded during the calibration procedure.
Stimuli were presented in their original color and were cropped of non-facial features (e.g., hair, neck and shoulders) using an oval mask. Each trial started with a fixation cross presented for 1s, followed by the appearance of four faces from the same actor representing angry, happy, sad, and neutral faces that were presented for 20s. In each trial, faces depicting each emotion were presented randomly in each of the four different quadrants and each emotion occurred with equal frequency in all four quadrants. The task consisted of a total of 16 trials (8 with female actors, 8 with male actors). Participants were asked to simply view the images as they normally would a photo album and individuals’ attention to each face was estimated from the duration of participants’ gaze position. Attentional allocation during each trial was measured as gaze durations lasting greater than or equal to 100ms within each of the face locations. Relative differences in gaze durations between different facial expressions of emotion are typically interpreted as indicating an attentional bias for the emotion represented (see Kellough, et al., 2008, for similar methods). In line with previous studies (e.g. Kellough et al., 2008) each trial was divided into 4-s epochs (5 total), which allowed us to test for potential changes in attentional allocation across the duration of each trial. In each epoch, the proportion of gaze duration (i.e., sustained attention) to each emotion was calculated by dividing the total duration of gaze to that emotion by the total duration of gaze to all of the faces within that epoch. Gaze proportions were initially calculated for each of the trials and then averaged across trials.1
Procedure
Parent-child dyads were recruited from the community through a variety of means (e.g., television, newspaper, flyers). As part of the larger study, parents were administered the SCID-I, BDI-II, and RRS. For this sub-study, parents were asked to complete the passive viewing task. All study procedures were approved by the university’s Institutional Review Board.
Results
Correlations and descriptive statistics are presented in Table 1.2 To test our hypotheses, we used general linear models with Emotion (angry, happy, sad, neutral) and Epoch (1, 2, 3, 4, 5) as the within subject variables and continuous levels of either brooding or reflective rumination as a between subjects variable. The dependent variable for these analyses was participant’s proportion of gaze duration to each of the four emotions during each epoch. Thus, similar to a repeated-measures ANOVA, we used Emotion and Epoch as repeated measures. The key difference from a repeated measures ANOVA is that general linear models allow the inclusion of continuous between subjects predictor variables, which allowed us to keep the brooding and reflection scales as continuous rather than artificially dichotomizing them, which can lead to Type I or Type II error (MacCallum, Zhang, Preacher, & Rucker, 2002).
Table 1.
Correlations and descriptive statistics for study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | M | SD | |
|---|---|---|---|---|---|---|---|---|
| 1. Brooding | – | 7.93 | 2.39 | |||||
| 2. Reflection | .52*** | – | 7.45 | 2.64 | ||||
| 3. Proportion Attention Angry | .16 | .15 | – | 0.20 | 0.07 | |||
| 4. Proportion Attention Happy | −.20* | −.15 | −.76*** | – | 0.36 | 0.15 | ||
| 5. Proportion Attention Sad | .21* | .16 | .58*** | −.77*** | – | 0.19 | 0.06 | |
| 6. Proportion Attention Neutral | .05 | .02 | .04 | −.57*** | .10 | – | 0.24 | 0.08 |
| 7. BDI-II | .42** | .18 | .15 | −.04 | .004 | −.06 | 4.82 | 4.80 |
BDI-II = Beck Depression Inventory-II
p < .05.
p < .01.
p < .001.
Focusing first on brooding rumination, although the main effect of Brooding was not significant, F(1, 103) = 0.75, p = .39, ηp2 = .007, there was a significant Brooding × Emotion interaction, F(3, 309) = 3.48, p = .01, ηp2 = .03. There was also a significant main effect of Emotion, F(3, 309) = 14.86, p < .001, ηp2 = .12, which was qualified by a significant Emotion × Epoch interaction, F(3, 1236) = 3.72, p < .001, ηp2 = .03. Finally, the Brooding × Emotion × Epoch interaction was not significant, F(12, 1236) = 1.51, p = .11, ηp2 = .01. Probing the significant Emotion × Epoch, we examined the main effect of Epoch within each emotion separately, with significant tests followed by posthoc comparisons. The mean gaze duration for each emotion and epoch is presented in Table 2, with significant differences across epochs for each emotion denoted by different superscripts. We found that whereas attention to happy faces generally increased across the course of each trial, attention to angry and sad faces decreased and attention to neutral faces remained stable. Next, examining the form of the Brooding × Emotion interaction we examined the correlation between brooding and proportion of gaze duration each emotion, collapsing across the 5 epochs (scatter plots for these relations are presented in Figure 1). Consistent with our predictions, high levels of brooding rumination were associated with greater sustained attention to sad faces, r = .21, p = .03, 95% CI [.01, .37], and less attention to happy faces, r = −.20, p = .04, 95% CI [−.37, −.01]. In contrast, levels of brooding rumination were not significantly associated with sustained attention to angry, r = .16, p = .10, 95% CI [−.03, .33] or neutral, r = .05, p = .59, 95% CI [−.14, .23] faces. To determine if these effects were simply due to the influence of current or past depression, we examined the relations between brooding and attention biases while statistically controlling for the influence of participants’ lifetime history of MDD (yes, no) as well as their current BDI-II scores. In these analyses, brooding rumination remained significantly associated with attention to sad, r = .22, p = .02, 95% CI [.03, .39] and happy, r = −.19, p = .04, 95% CI [−36, −.001] faces suggesting that the relations were not due simply to the presence of depression history or subclinical depression in our sample.
Table 2.
Means (standard deviations) for participant’s proportion of attention to each emotional face type in each epoch.
| Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 | |
|---|---|---|---|---|---|
| Angry | .23a (.06) | .21b (.08) | .20b (.10) | .18c (.10) | .18c (.10) |
| Happy | .32a (.08) | .34b (.15) | .37c (.19) | .38c,d (.20) | .40d (.20) |
| Sad | .22a (.06) | .20a (.08) | .18b (.09) | .18b (.10) | .18b (.09) |
| Neutral | .23a (.06) | .24a (.09) | .24a (.10) | .25a (.11) | .24a (.10) |
Note. Means in the same row with different superscripts differ significantly.
Figure 1.
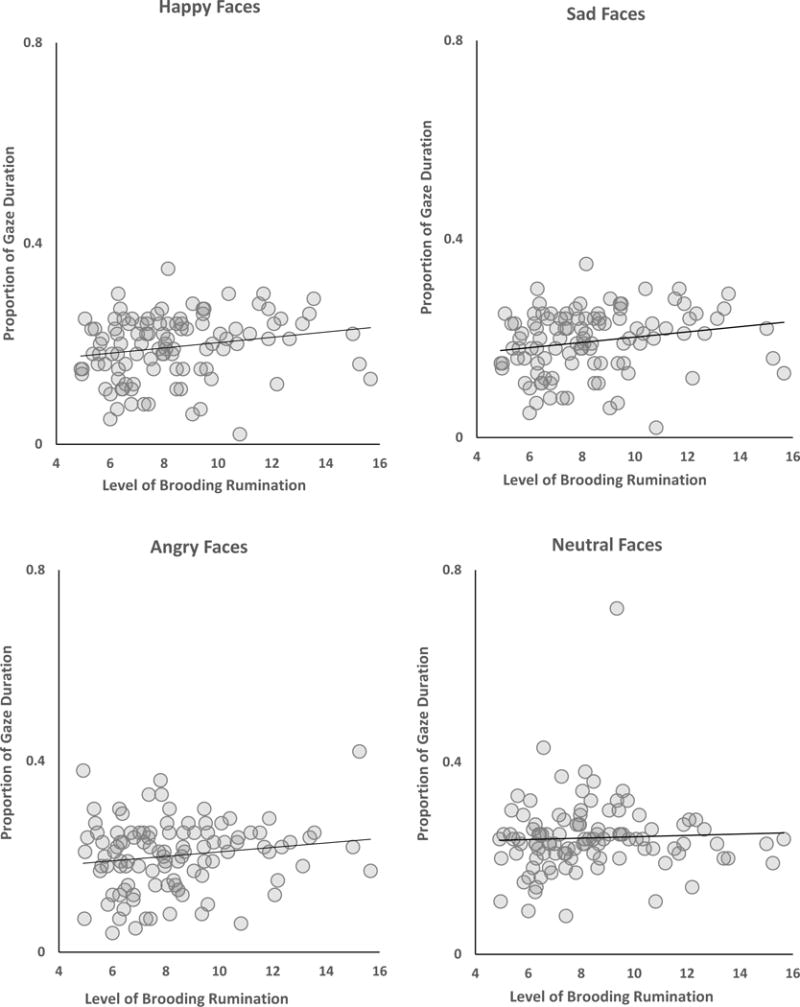
Scatterplots for the correlation between level of trait brooding rumination the proportion of gaze duration to each and facial expression with linear regression line and r-square value.
Next, we examined the relation between reflective rumination and participants’ attentional biases. In these analyses, the significant main effect of Emotion, F(1, 103) = 14.56, p < .001, ηp2 = .12 and the significant Emotion × Epoch interaction were maintained, F(1, 103) = 1.84, p = .03, ηp2 = .01. However, the main effect of Reflection, F(1, 103) = .76, p = .38, ηp2 = .007, as well as the Reflection × Emotion, F(3, 309) = 2.24, p = .08, ηp2 = .02, the Reflection × Epoch, F(4, 412) = .10, p = .98, ηp2 = .001, and the Reflection × Emotion × Epoch, F(12, 1236) = 0.52, p = .90, ηp2 = .005, interactions were all nonsignificant.3,4
Discussion
The developers of the RDoC matrix have proposed influences across multiple units of analysis that are thought to reflect the same underlying construct. Rumination and attentional biases have been proposed as key components of the RDoC construct of Loss and both are thought to reflect disruption in the same corticolimbic circuitry (Disner et al., 2011; Woody & Gibb, 2015). Indeed, a number of theorists have proposed models linking rumination and attentional biases (Koster et al., 2011; Whitmer & Gotlib, 2013). Although there is preliminary support for the link between higher levels of brooding rumination and increased attention to sad facial stimuli (Duque et al., 2014; Joormann et al., 2006; Southworth et al., in press), there is some question about whether this relation would be observed in the absence of current depression and whether it is specific to brooding versus reflective rumination. This line of research is important not only for the construct validation of the RDoC Loss construct, but also for the development of preventive interventions designed to target cognitive mechanisms of risk prior to the emergence of depression.
Supporting the RDoC framework and consistent with prior theory (Koster et al., 2011; Whitmer & Gotlib, 2013), we found that higher levels of brooding rumination were associated with increased attention to sad faces and less attention to happy faces in a nondepressed sample of adults. The relation between brooding and attentional biases was not moderated by epoch, suggesting that brooding is related to biases in sustained attention to both stimulus types across the full 20-second trials. These relations were also specific to brooding rumination and were not observed for reflective rumination. In addition, they were maintained when we statistically controlled for participants’ prior history of MDD as well as their current nonclinical levels of depressive symptoms, suggesting that the results are at least partially independent of current or past depression. These results are in line with the hypothesis that brooding rumination is related to a narrowed focus of attention on congruent information, with a corresponding attentional avoidance of information that is maximally incongruent. It is this pattern of attentional deployment that may contribute to increased risk for the onset and maintenance of depression (cf. Joormann & Vanderlind, 2014; Nolen-Hoeksema et al., 2008).
A key strength of this study was the use of eye tracking to directly assess patterns of attentional allocation within the context of a passive viewing task, which allowed for the relative salience of each emotion to compete for attention over a long stimulus presentation. Importantly, therefore, we were able to show that although individuals generally tended to look more at happy faces and less at the negative (angry, sad) faces over the course of each 20 second trial, individuals with high levels of brooding rumination exhibited a different pattern of attentional allocation, looking more at sad faces and less at happy faces across the entire duration of the trial. Another key strength was the focus on a nondepressed sample, which allowed us to demonstrate that the relation between brooding rumination and attentional biases was not due simply to the presence depression as a third variable. Despite these strengths, there were limitations as well, which highlight paths for future research. First, given the cross-sectional design of this study, we are unable to draw any conclusions about the temporal or bidirectional relation between brooding rumination and attentional biases. An important area of research, therefore, is determining whether interventions designed to directly target the process of rumination (e.g. Wells, 1990) or attentional biases (e.g. Wells & Beevers, 2010) also help to improve the other factor. Importantly, there is initial evidence that modification of attentional biases may reduce state rumination (Cohen, Daches, Mor & Henik, 2014). Second, the majority of participants in this study were women, which precluded any examination of potential gender moderation of our findings. Women tend to ruminate more than men, and brooding, but not reflective rumination, has been found to mediate the sex difference in depression (Treynor et al., 2003). Therefore, it will be important for future research to determine whether the magnitude of the link between rumination and attentional biases is similar for women and men.
In conclusion, the current results support the link between brooding rumination and attentional biases for depression-relevant stimuli – increased attention to sad faces and decreased attention to happy faces – and suggest that these links are not due simply to the presence of current depression. As such they add to a growing body of research highlighting links across units of analysis within the RDoC Negative Valence Systems construct of Loss and are an important step in developing a more fine-grained understanding of the inter-relations of factors thought to increase risk for depression. If the links between these constructs can also be demonstrated experimentally, it will provide important information for the development of more targeted intervention efforts designed to treat or prevent depression.
Acknowledgments
This project was supported by National Institute of Mental Health grant MH098060 awarded to B. E. Gibb.
Footnotes
The pattern of significant results were identical when we used raw gaze durations rather than proportion of gaze.
Because none of the relations between rumination and attention biases was moderated by epoch, we present proportion of attention for each emotion type averaged across all epochs in the Table.
Although not a primary focus of the paper, our eye-tracking data allowed us to examine biases in initial orienting of attention (proportion of first fixations to certain emotional stimuli over others), which we would not expect to be linked to rumination. To examine this, we conducted general linear models with Emotion (angry, happy, sad, neutral) as the within subject variable and brooding or reflection as a continuous independent variable. The proportion of first fixations to each emotion type was the dependent variable in each analysis. As expected, the brooding and reflection main effects, as well as the brooding × Emotion and reflection × Emotion, interactions were all non-significant (lowest p = .26).
Exploratory analyses were also conducted to determine whether participants’ histories of MDD moderated any of the relations examined. None of these analyses was significant (lowest p = .14).
References
- Abela JRZ, Hankin BL. Rumination as a vulnerability to depression during the transition from earl to middle adolescence: A multiwave longitudinal study. Journal of Abnormal Psychology. 2011;120:259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 2012;32:704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II) Manual for Beck Depression Inventory-II 1996 [Google Scholar]
- Cohen N, Daches S, Mor N, Henik A. Inhibition of negative content—a shared process in rumination and reappraisal. Frontiers in Psychology. 2014;5:622. doi: 10.3389/fpsyg.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Duque A, Sanchez A, Vazquez C. Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition and Emotion. 2014;28:1347–1366. doi: 10.1080/02699931.2014.881327. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/PW/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. BW (2002) [Google Scholar]
- Gibb BE, Grassia M, Stone LB, Uhrlass DJ. Brooding rumination and risk for depressive disorders in children of depressed mothers. Journal of Abnormal Child Psychology. 2012;40:317–326. doi: 10.1007/s10802-011-9554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2016;171:65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska directed emotional faces: A validation study. Cognition and Emotion. 2008;22:1094–1118. [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Joormann J, Vanderlind WM. Emotion regulation in depression: The role of biased cognition and reduced cognitive control. Clinical Psychological Science. 2014;2:402–421. [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. 2008;46:1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska directed emotional faces (KDEF) CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; 1998. pp. 91–630. [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. The National Institute of Mental Health Strategic Plan. Bethesda, MD: National Institute of Mental Health; 2008. [ http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. Meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Schoofs H, Hermas D, Raes F. Brooding and reflection as subtypes of rumination: Evidence from confirmatory factor analysis in nonclinical samples using the Dutch Ruminative Response Scale. Journal of Psychopathology and Behavioral Assessment. 2010;32:609–617. [Google Scholar]
- Southworth F, Grafton B, MacLeod C, Watkins E. Heightened ruminative disposition is associated with impaired disengagement from negative relative to positive information: Support for the impaired disengagement” hypothesis. Cognition and Emotion. doi: 10.1080/02699931.2015.1124843. (in press) [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Wells A. Panic disorder in association with relaxation induced anxiety: An attentional training approach to treatment. Behavior Therapy. 1990;21:273–280. [Google Scholar]
- Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychological Bulletin. 2013;139:1036–1061. doi: 10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into depression research. Current Opinion in Psychology. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. Attainment and maintenance of reliability of Axis I and Axis II disorders over the course of a longitudinal study. Comprehensive Psychiatry. 2001;42:369–374. doi: 10.1053/comp.2001.24556. [DOI] [PubMed] [Google Scholar]


