Abstract
Myosins are a superfamily of actin-based molecular motor proteins, which hydrolyze ATP and generate various forms of eukaryotic motility and muscle contraction. Myosin light chain 20 (MLC20) is small ring around the neck region of heavy chain of myosins. Phosphorylation of MLC20 is thought to play a key role in regulation of smooth muscle contraction. Calcium- and calmodulin-dependent myosin light chain kinase (MLCK) is considered the primary regulator of MLC20 phosphorylation. However, several observations in smooth muscle contraction cannot be explained by the mode of phosphorylation. By performing a series of experiments in vitro and in vivo, we report here MLCK-independent MLC20 phosphorylation. Gene expression study reveals that expression of MLCK in smooth muscles is inconsistent with MLC20 phosphorylation at Ser19. None of inactivating calmodulin/MLCK, depriving of calcium and silencing MLCK expression by siRNA blocks effectively the phosphorylation of MLC20 at Ser19. In addition, by overexpressing active human MAP (mitogen-activated protein)-ERK kinase kinase-1 (MEKK1) and blocking its downstream messengers, we have demonstrated a new regulatory system of MLC phosphorylation via MEKK1, which downregulates Ser19 phosphorylation of MLC20 through its downstream molecules, p38, JNK, and ERK in human bladder smooth muscle cells.
Keywords: smooth muscle cell, myosin light chain kinase, myosin light chain 20, phosphorylation, MAP kinase pathway
Introduction
Cell motility or muscle contraction is a complex process that requires interaction of numerous reactions and coordinated regulation. One of two major contractile proteins is myosin. Myosins are a large superfamily of motor proteins, which bind and hydrolyze ATP, interact with actin filaments and ultimately produce force for cell migration and muscle contraction [Mermall et al., 1998]. Structurally, a myosin molecule contains two parts: (1) heavy chain(s) that consists of a highly conserved globular head domain, which includes ATP- and actin-binding sites, and a tail domain, which anchors and positions the globular head for actin interaction; (2) 20-kilodolton MLC20 that wraps around neck region of myosin heavy chain. Understanding how these contractile proteins are regulated is important not only for insight into muscle-related physiological and pathological processes, such as muscle contraction, blood pressure/flow, food propulsion, airway constriction, and uterine contraction, but also for comprehending cell-motility-related events such as cell migration [Jay et al., 1995], apoptosis [Fazal et al., 2005], embryogenesis [Royou et al., 2004], neurite outgrowth [Amano et al., 1998; Bridgman et al., 2001], wound healing, cancer metastasis [Betapudi et al., 2006], cytokinesis, phagocytosis, and secretion. Phosphorylation of MLC20 initiates the actomyosin interaction and is believed to play critical roles in regulating muscle contraction and cell motility [Perrie et al., 1973; Adelstein, 1983; Sweeney and Stull, 1990; Gallagher et al., 1997; Xia et al., 1998; Johnson and Lapadat, 2002; Szczesna et al., 2002]. MLCK is believed to be the primary regulator of phosphorylation of MLC20 [Sweeney et al., 1993; Somlyo and Somlyo, 1994; Smith et al., 1999; Hatch et al., 2001]. It is a calcium- and calmodulin-dependent enzyme and requires the calcium-calmodulin complex for its activity [Gallagher et al., 1997]. However, several studies suggest calcium- [Ozaki et al., 1987a,b; Suematsu et al., 1991; McFawn et al., 2003; Formigli et al., 2004] and MLCK-independent phosphorylation of MLC20 [Emmert et al., 2004]. Isometric contraction and MLC20 phosphorylation were also observed in embryo fibroblasts, which contain no detectable MLCK [Emmert et al., 2004]. The present study demonstrates that MLC20 may be phosphorylated in the absence of MLCK or active MLCK-calmodulin complex.
The biological process of cell migration is similar to that of muscle cell contraction. This similarity led us to hypothesize that MAP kinase pathway is involved in regulation of activation of contractile molecules. MAP kinase signaling pathways are evolutionally well conserved and regulate a large number of physiological processes, including cell proliferation, differentiation, migration, development, immune function, stress responses, and apoptosis [Chang and Karin, 2001; Pearson et al., 2001; Johnson and Lapadat, 2002; Zhang et al., 2003]. MAP kinases also respond to extracellular chemical and physical stresses, thereby controlling cell survival and adaptation to environments. In this article, we test our hypothesis and describe a role of MAP kinase pathway in the regulation of MLCK-independent phosphorylation of MLC20.
Materials and Methods
Tissue and Cell Sample Preparation
Substrain 129 mice were killed by cervical dislocation and samples of aorta, bladder, heart, large and small intestines, skeletal muscle, stomach, and uterus were immediately collected to ice-cold PBS. Connective tissues were removed. After washed twice with 1× PBS, about 0.2 g of tissues were homogenized in ice-cold lysis buffer by precooled glass homogenizers. The lysis buffer consists of 50 mM Tris, 300 mM NaCl, 3 mM EGTA, 0.1 mM sodium orthovanadate, 10% glycerol v/v, 1% NP-40 v/v, and 0.3% SDS w/v, pH 7.6. Protease and phosphatase inhibitor cocktails (Sigma Co., Germany) were added before use (except for special indication). For cell lysate preparation, culture dishes were put on ice, and cells were immediately washed once with cold 1× PBS. Ice-cold lysis buffer was added onto dishes. Then, cells were scraped into cold 1.5 ml centrifuge tubes with cell scrapers. The homogenized tissues or cultured cells were rotated at 4°C for 30 min, followed by 14,000 rpm spin in desktop centrifuge at 4°C for 15 min. All reagents and tools were precooled on ice and always kept on ice during the procedure. Only fresh tissue or cell lysates were used for experiments. Total protein concentration was measured by Pierce Protein Assay Reagents. All experiments conducted with these animals and cells are based on Chinese laws.
Adenovirus Preparation
To generate adenovirus to express MEKK1, a HA-tagged 4.9-kb DNA fragment containing the cDNA for human wild-type MEKK1 was cloned at downstream of cytomegalovirus (CMV) promoter of pAdtrack, which has a separate GFP expression cassette. To prepare MEKK1-containing viral DNA, the shuttle vector was recombined with adenoviral DNA in bacteria. Then, recombinant adenoviral plasmid was used to produce adenovirus in packaging cells as described previously [He et al., 1998]. Control adenovirus was prepared in the same procedure but human MEKK1 was replaced by LacZ (β-galactosidase). Following the similar procedure, we also prepared Rho A-(L63) and GFP-containing control adenoviruses. After amplification, viruses were titrated by agarose overlay plaque assay.
Human Smooth Muscle Cell Culture and Transient Transfection
Human bladder smooth muscle cells (hBSM) were isolated from peri-cancer tissue of a bladder cancer patient. The cells have all the biochemical characteristics of smooth muscle cells, including expression of h-caldesmon, smooth muscle-specific actin, and myosin (not shown). Cells that were passaged less than seven times were used for experiments. hBSM cells were maintained in M199 (Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal calf serum at 37°C in humidified atmosphere of 5% CO2-95% air. Cells were dissociated by brief trypsinization and subculture.
The cells were seeded in six-well plates at a density of about 0.3 × 105 cells for transient transfection of MLCK siRNA (Santa Cruz, CA). Then, transfection was performed with cells at 40 to 50% confluence (estimated by scanning the cultures using an inverted phase microscope) by Lipofectamine following the manufacturer’s protocol. Briefly, Lipofectamine 2000 was diluted in serum-free M199 medium (6 μl/250 μl) and incubated at room temperature for 5 min. The siRNA was diluted in serum-free M199 medium (250 μ) and added to the Lipofectamine 2000 mixture at indicated concentration. The incubation was continued for additional 20 min at room temperature. Medium in six-well plates was replaced by 1.1 ml of fresh serum-free M199 medium, and siRNA-Lipofectamine 2000 mixture was added to cultures. The cells were harvested for Western blotting 48 h after transfection.
Viral Infection and Chemical Treatments
When cell confluence reached about 80%, M199 medium was replaced by 1 ml for 3.5-cm plates or 4 ml for 10-cm plates with fresh medium. Adenoviruses were added onto cells directly and mixed. Dishes were kept in CO2 culture incubator and rocked once every 15 min. After 1 h, additional growth medium was supplied to normal level. Three days after infection (except for special indication), cells were harvested for Western blotting. Briefly, dishes were put on ice and medium was removed. Cells were washed with ice-cold 1× PBS and lysis buffer was added onto cells. Immediately, cells were scraped into precooled 1.5 ml centrifuge tubes by cell scrapers. The cells with lysis buffer were rotated at 4°C for 30 min. Cell pellets were spun down at 14,000 rpm at 4°C for 10 min. Total protein concentration in supernatant was measured with Pierce Protein Assay Reagents.
For chemical treatments, medium was replaced by M199 that contained same concentration of DMSO v/v as other chemical treatments in control plates. Chemicals solved in DMSO were added into dishes at indicated concentration and mixed well by rocking plates. Plates were put back into incubator for additional 40 min. Then, cells were harvested following the procedure above for Western blotting. All inhibitory peptides, MAPK inhibitors, and Y27632 were purchased from EMD Chemicals Inc, NJ. PKC inhibitor G1918 and activator PMA were obtained from Sigma Co, MO.
Cell Permeabilization
The hBSM cells were permeabilized as described [Vandermerwe et al., 1989; Riedinger et al., 2005] with slight modification. Briefly, hBSM cells were washed twice (once briefly, once for 10 min) with buffer I (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 8.3 mM glucose, 20 mM Hepes, and 0.1% BSA w/v; pH 7.4), and then once with Ca2+-free buffer I for 5 min at 37°C on a slowly rocking shaker. Cells were then permeabilized by incubation in permeabilization buffer (140 mM sodium propionate, 4 mM KCl; 25 mM sodium Pipes, 0.1% BSA w/v; 3 ug/ml alfa-toxin, 0.5 mM EGTA, 6 mM MgCl2, and 5 mM ATP, pH 6.6) for 10 min at 37°C on a slowly rocking shaker. Alfa toxin was purchased from Sigma Co, MO.
Western Blotting
Indicated certain amount of protein was loaded onto 10% PAGE-SDS gels, which was subjected to electrophoresis in running buffer (BioRad Co., Hercules, CA). Then, proteins were transferred overnight to Immobilon-P Transfer Membrane (Millipore Co., MA. Cat. IPVH00010) by semidry transfer machine. After being blocked in 5% milk in TBST buffer (25 mM Tris, 140 mM NaCl, 3 mM KCl, pH 7.4 and 0.1% Tween 20 v/v was added before use) for 1 h, membranes were incubated with primary antibodies plus actin antibody (as a loading control) for 1-h shake at room temperature or overnight at 4°C. Membranes were washed three times (5 min each) by TBST buffer, followed by 1-h incubation with secondary antibodies at room temperature. Then, membranes were developed by ECL Western Blotting Detection Reagents (GE Healthcare, NJ). The signal was detected by Fujifilm Luminescent Image Analyzer LAS-3000 (Fuji Corp., CT). Antibodies against p-MLC20, MLCK, p-p38, and actin were from Sigma Co, MO. Rho A antibody was purchased from Santa Cruz Co, CA. The antibody against p-MLC20 bind to the Ser19 site. Antibody against p-ERK was a product of EMD Chemicals Inc, NJ. Horseradish peroxidase-linked second antibodies were from GE Healthcare UK Limited. MLC20 (Ser-1) antibody was a gift from Dr. Xianggui Chen at Xinhua University.
The intensity of a blot band was evaluated from images using Adobe Photoshop as described previously [Ge S, 2004]. Briefly, a highest quality digital TIF image of each blot membrane was created using Fujifilm Luminescent Image Analyzer LAS-3000. To quantify relative amount of protein on images, the mean pixel intensity of individual bands was determined using Adobe Photoshop. To obtain a mean pixel intensity value corresponding solely to specific protein immunoreactivity, a background noise value of pixel size (equal to that used to outline protein bands and selected from an irrelevant area of the images) was subtracted from each protein value. Corrected mean pixel intensity values were used to calculate relative protein amount for each band.
Immunoprecipitation and Immune Complex Kinase Assay
Tissue lysate of 150 μg total protein was incubated with monoclonal antibody of 15 μg protein by rotating at 4°C for 2 h. Then, 150 μl Protein A agarose beads was added to the mix and continued to rotate at 4°C for additional 1 h. Beads were washed three times with ice-cold 1× PBS and one time with ice-cold 1× kinase assay buffer. Kinase buffer contained 20 mM Tris, 10 mM MgCl2, 200 μM ATP, and 1 mM DTT, pH 7.5. Immune complex kinase assay was performed as previously described [Ichijo et al., 1997; Cherkasova, 2006]. The complex beads were added into smooth muscle extracts. The reaction was performed in kinase buffer at 30°C for 1 h. Tubes were mixed once every 10 min by brief vortex. Then, reaction was stopped by 5× SDS-sample loading buffer for SDS-PAGE gel and Western blotting.
Results
MLC20 Phosphorylation Is Inconsistent With MLCK in Smooth Muscles
MLCK is widely expressed in variety of animals and tissues, such as bovine smooth muscle [Kobayashi et al., 1992], rat skeletal muscle [Roush et al., 1988], mouse cardiac muscle [Herring et al., 2000], human brain [Potier et al., 1995], and chicken embryo fibroblasts [Watterson et al., 1995]. By Western blotting, we examined MLCK expression and level of MLC20 phosphorylation in different smooth muscle tissues of mice. Notably, the level of MLCK in smooth muscles was not consistent with MLC20 phosphorylation (Fig. 1). In addition, Fig. 1 shows that MLC20 phosphorylation occurred in the absence of MLCK in aorta and large intestine smooth muscles from the first and third mice.
Fig. 1. Inconsistence between expression of MLCK and phosphorylation of MLC20 in mouse smooth muscles.
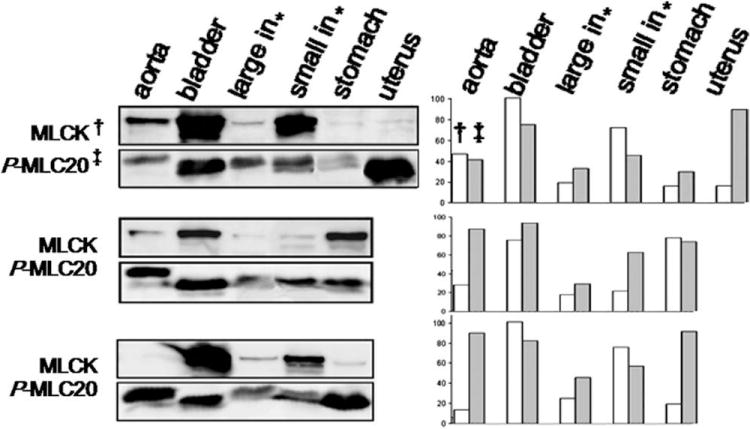
Western blotting shows inconsistency between MLCK level and phosphorylation of MLC20 in smooth muscles from one female and two male mice. *Large and small intestines; †MLCK; ‡phosphor-MLC20.
Immunoprecipitated MLCK Does Not Initiate Phosphorylation of MLC20 in Tissue Extracts
Using monoclonal MLCK antibody, we precipitated MLCK from fresh high-MLCK bladder smooth muscle. The antibody-MLCK immunocomplex was mixed with freshly prepared small intestine extracts containing low levels of MLCK and MLC20 phosphorylation (without phosphatase inhibitors) for a kinase assay. The results showed that the immunoprecipitated MLCK did not trigger phosphorylation of MLC20 (Fig. 2A).
Fig. 2. None of MLCK, inhibitory peptides of calmodulin, or MLCK and EGTA affects MLC20 phosphorylation.
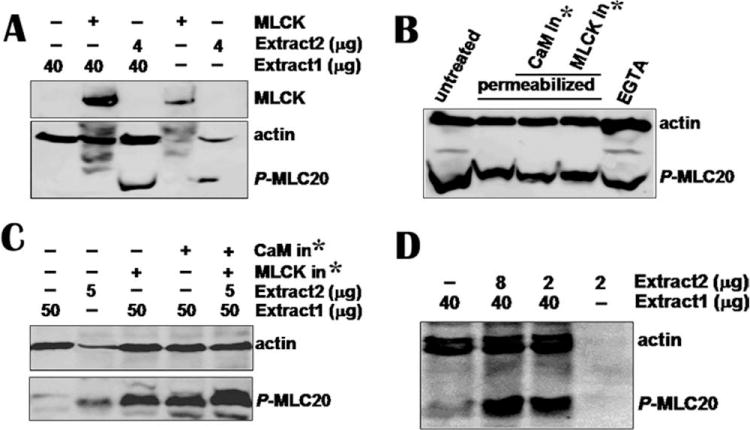
(A) Immunoprecipitated MLCK from 100 μg tissue was added to tissue extract 1 containing low −p-MLC20 and MLCK (Line 2). The immunocomplex beads containing MLCK from 50 μg tissue was loaded onto line 4. Reaction was stopped after 1-h incubation at 30°C by loading buffer and reaction mixture was loaded onto SDS-PAGE gel. Antibodies against p-MLC20 and actin were used to detect membranes. Western blotting shows that immunoprecipitated MLCK did not trigger phosphorylation of MLC20 in tissue extract 1 containing low phosphor-MLC20 (p-MLC20) and low MLCK (Line 2). However, 4 μg extract 2 containing high p-MLC20 and no detectable MLCK phosphorylated MLC20 (line 3). (B) Permeabilized hBSM cells were treated with calmodulin and MLCK inhibitory peptides (1000 nM each) for 3 h. EGTA (20 mM) was incubated with cells for 2 h. Cells were harvested for Western blotting and mixture of antibodies for p-MLC20 and actin was used to detected membranes. No significant inhibition on MLC20 phosphorylation was observed. (C) CaM and MLCK inhibitors did not inhibit MLC20 phosphorylation when they were incubated with smooth muscle extracts. (D) Small amount of high p-MLC20 without detectable MLCK tissue catalyzed MLC20 phosphorylation in low p-MLC20 and MLCK tissue extract. *CaM or MLCK inhibitor.
In contrast, the freshly isolated tissue extracts (Fig. 2A, extract 2) containing low levels of MLCK but high level of phosphor-MLC20 phosphorylation, when added to the extracts (Fig. 2A, line 3, extract 1) containing low-MLCK and low-phosphor-MLC20, displayed a synergistic MLC20 phosphorylation (Figs. 2A and 2D). These results suggest that additional factors rather than MLCK in low-MLCK tissue induced MLC20 phosphorylation.
Neither Calmodulin Nor MLCK Inhibitors Block MLC20 Phosphorylation
Calmodulin is a ubiquitous, calcium-binding protein that is widely believed to be necessary for MLCK catalysis activity. We used calmodulin [Torok et al., 1998] and MLCK [Lukas et al., 1999] inhibitory peptides to block calmodulin and MLCK in alfa-toxin-permeabilized cells. To leave enough time for inhibition, cells were incubated with inhibitory peptides in CO2 incubator for 3 h. We found that neither calmodulin nor MLCK inhibitors caused a significant decrease in MLC20 phosphorylation (Fig. 2B). The inhibitors also failed to block MLC20 phosphorylation catalyzed by tissue extract 2, which contained high basal levels of phosphor-MLC20 (Fig. 2C).
Phosphorylation of MLC20 Is Ca2+- and Calmodulin-Independent
To investigate further the role of MLCK in MLC20 phosphorylation, hBSM cells were washed with PBS containing 20 mM EGTA and incubated with medium 199 containing 20 mM EGTA for 2 h to deprive calcium of cells. Then, cells were harvested for Western blotting. The results show that calcium depriving did not block the phosphorylation of MLC20 (Fig. 2B).
siRNA-Mediated MLCK Knockdown Does Not Affect Phosphorylation of MLC20
To confirm the data above, we transfected siRNA (Santa Cruz, CA) against human MLCK into human bladder smooth muscle (hBSM) cells to silence MLCK expression. The Western blots show that phosphorylation level of MLC20 was not altered (Fig. 3A).
Fig. 3. siRNA-mediated knockdown of MLCK does not affect MLC20 phosphorylation but adenovirus-mediated MEKK1 over-expression activates downstream messengers and thereby inhibits MLC20 phosphorylation.
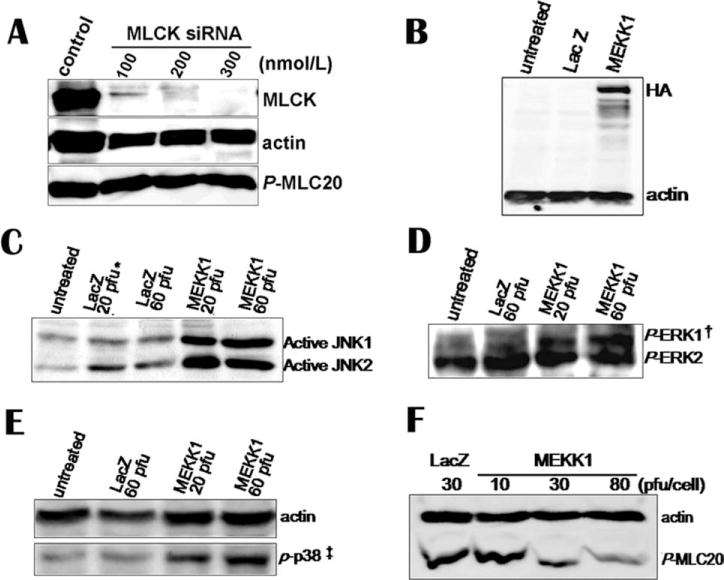
(A) siRNA against MLCK was introduced to hBSM cells by transfection. Cells were harvested for Western blotting. MLCK, actin and phosphorylated MLC20 were detected with antibodies. (B) Overexpression of HA-tagged MEKK1 in hBSM cells was detected by HA antibody. (C–E) Overexpressed MEKK1 activated its downstream molecules, JNK1/2, ERK1, and p38. (F) MEKK1 inhibited MLC20 phosphorylation in a dose-dependent manner in hBSM cells. *Plaque-forming units. Here, it stands for pfu per cell. † and ‡ phosphor-ERK1/2 and phosphor–p38.
Together, the data above suggest the existence of a MLCK-independent mechanism in the regulation of MLC20 phosphorylation in smooth muscle cells.
MLC20 Phosphorylation Is Regulated to a Great Extent by MAP Kinase Pathway
HA-tagged-human MEKK1 was overexpressed in hBSM cells by adenovirus bearing MEKK1. Overexpression of MEKK1 was confirmed by antibody against HA in Western blotting analysis (Fig. 3B). Active human MEKK1 induced the phosphorylation of its downstream effectors (i.e., JNK1/2, ERK1, and p38) (Figs. 3C–3E). Then, we examined whether MEKK1 was sufficient to regulate phosphorylation of MLC20. Three days after hBSM cells were infected with MEKK1-containing adenovirus, cells were harvested for cell lysate. To our surprise, MEKK1 overexpression caused a dose-dependent decrease in phosphorylation of MLC20 (Fig. 3F).
MAP Kinase Inhibitors Neutralize the Inhibition of MEKK1 on Phosphorylation of MLC20
To investigate further whether the downstream effectors of MEKK1 is involved in MLC20 phosphorylation, the hBSM cells infected by MEKK1-containing adenovirus were treated by the MAP kinase inhibitors, SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and ERK inhibitor for 40 min. Western blotting analysis shows that all the MAP kinase inhibitors prevented partly MEKK1-mediated decrease of MLC20 phosphorylation (Fig. 4A). This suggests the inhibitory effect of MEKK1 on MLC20 phosphorylation was mediated through its downstream MAPKs, p38, JNK, and ERK.
Fig. 4. MAP kinase inhibitors counteract the inhibition of MEKK1 on MLC20 phosphorylation.
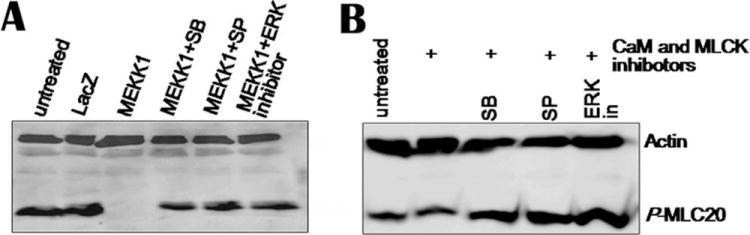
(A) Three days after adenovirus infection (90 pfu/cell), MEKK1-adenovirus-infected cells were incubated with SB (30 μM), SP (30 μM), and ERK inhibitor (60 μM) for 40 min. Cells were harvested for Western blotting. Membranes were detected by antibodies against p-MLC20 and actin. (B) Permeabilized cells were treated with 1000 nM of calmodulin and MLCK inhibitors for 3 h and then exposed to MAP kinase inhibitors for 40 min. Cell lysates were analyzed by Western blotting. Neither calmodulin or MLCK inhibitors affected the inducement of MAP kinase inhibitors on MLC20 phosphorylation.SB, SB 203580, a p38 inhibitor; SP, SP600125, a JNK inhibitor; ERK in, ERK inhibitory peptide.
Calmodulin and MLCK Inhibitors Do Not Affect the Inducement of MAP Kinase Inhibitors on Phosphorylation of MLC20
To test whether calmodulin and MCLK are involved in the phosphorylation MLC20 induced by MAP kinase inhibitors, we treated permeabilized cells with inhibitory peptides for 3 h to inactivate calmodulin and MLCK. Then, cells were exposed to MAP kinase inhibitors for 40 min. An increase of MLC20 phosphorylation was observed in calmodulin- and MLCK-inhibitor-treated cells. The results indicate that calmodulin and MLCK are not involved in the MLC20 phosphorylation induced by MAP kinase inhibitors (Fig. 4B).
MAP Kinase Inhibitors Induce Phosphorylation of MLC20
In addition, we tested the effects of MAP kinase inhibitors on MLC20 phosphorylation in hBSM cells. Forty minutes after exposed to MAP kinase inhibitors, cells were harvested for Western blotting. MAP kinase inhibitors increased phosphorylation of MLC20 in a dose-dependent manner (Figs. 5B–5D).
Fig. 5. MAP kinase inhibitors increase MLC20 phosphorylation in hBSM cells.
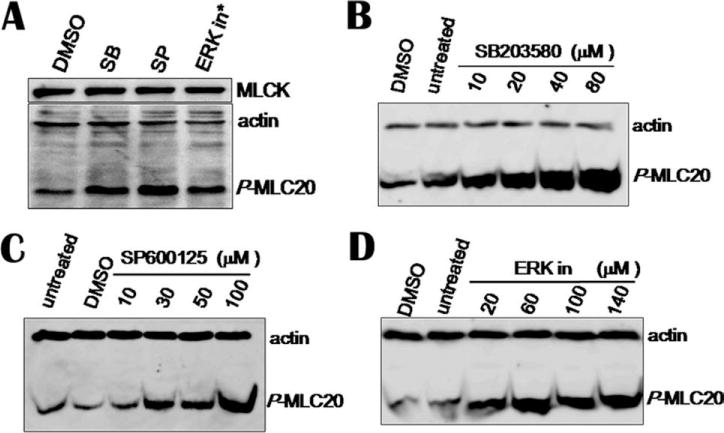
(A) Cells were incubated with 30 μM of SB and SP, and 60 μM ERK inhibitor for 40 min. Same volume of DMSO was used as vehicle control. (B–D) MAP inhibitors increased phosphorylation of MLC20 in dose-dependent manner. *ERK inhibitor.
MLC20 Phosphorylation Is Not Necessary for Cell Migration
To explore the relationship between the MLC20 phosphorylation and muscle contraction, we infected BSM cells with adenovirus that expresses both MEKK1 and GFP. In previous study, we have demonstrated MEKK1 overexpression inhibits phosphorylation of MLC20 (Figs. 3F and 4). GFP-fluorescent cells, which expressed MEKK1, still migrated cross the scratch wound by a pipette tip (Fig. 6).
Fig. 6. Migration of cells expressing MEKK1.
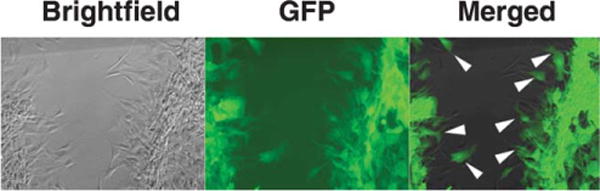
Two days after cells grown on 24-well plate were infected with MEKK- and GFP-containing adenovirus (30 pfu/cell), a scratch was made with a pipette tip in the middle of the well in a 24-well plate. Pictures were taken at 14 h after wounding. The figure shows that fluorescent cells, which express MEKK1 that inhibits MLC20 phosphorylation, were migrating across the wound.
Rho-Rho kinase has been reported to activator of MLC20 [Amano et al., 1996]. In previous experiments, we observed that overexpression of Rho A induced keratinocyte migration in serum- and cytokine-free medium (not shown). In this study, we examined the effects of inhibitors of Rho kinase, PKC and p38 on migration of cultured BSM cells by in vitro wound assay. Cells were planted to 24-well plates. Twenty four hours later, the cells were infected with adenovirus bearing Rho A (20 pfu/cell). Two days after viral infection, a scratch wound was created on cell surface in the middle of each well with a micropipette tip and cells were immediately incubated with 2% FBS M199 that containing Rho A, PKC and p38 inhibitors. Then, cell migration was photographed at 0, 6, and 20 h. We did not find stimulation of overexpressed Rho A for BSM cell migration because the untreated cells moved across the wound quickly as Rho A-overexpressing cells in serum-free M199. Inhibitor of Rho kinase did not block the BSM cell migration in three independent experiments but G1918 and PD 98059 (PKC and MEK1 inhibitors) block cell migration (Fig. 7).
Fig. 7. Effects of Rho A, PKC, and MAPK on BSM cell migration.
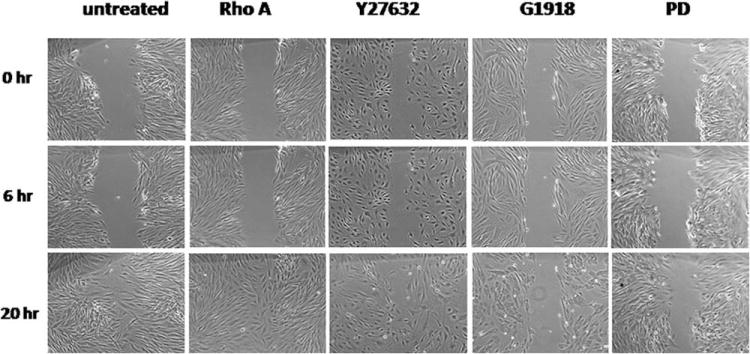
Two days after infected by adenovirus (30 pfu/cell), a wound was made with a pipette tip in the middle of each well. The cells were kept in inhibitor-containing media for 24 h. Cell migration was photographed at 0, 6, and 20 h. Rho A, infected by Rho A-containing adenovirus (30 pfu/cell); Y27632, a specific inhibitor of Rho kinase (20 μM); G1918, PKC inhibitor (150 nM); PD, PD 98059, a MEK1 inhibitor (50 μM).
Both PKC and Rho Kinase Induce MLC20 Phosphorylation
Rho A-bearing adenovirus and PMA were used to activate Rho kinase and PKC, respectively. In contrast, Y27632 and G1918 were used to inhibit their activity. Two days after viral infection, cells were exposed to the chemicals for 40 min and then harvested for Western blotting. Both inhibitors of Rho kinase inhibitor Y27632 and PKC G1918 inhibited phosphorylation level of MLC20 (Fig. 8).
Fig. 8. Effects of PKC and Rho A on MLC20 phosphorylation.
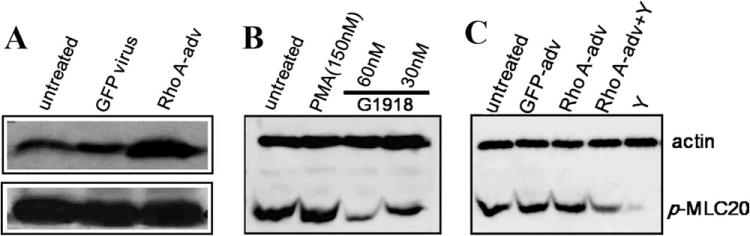
(A) Rho A-containing adenovirus was tested by Western blotting. Two days after infected with control and Rho A-containing adenoviruses (20 pfu/cell), cells were harvested for Western blotting. Rho A and actin were detected by corresponding antibodies separately. (B) G1918, a PKC inhibitor (150 nM) resulted in decrease of MLC20 phosphorylation in cultured BSM cells. (C) Rho kinase inhibitor Y27632 (20 μM) inhibited MLC20 phosphorylation at Ser19.
Discussion
The phosphorylation/dephosphorylation of MLC20 is thought to a key event in the regulation of smooth muscle contraction and other myosin-based cellular activities; hence, molecules that regulate MLC20 phosphorylation are considered the primary determinant of muscle contraction and cell migration. In addition, it is believed that Ca2+ binds to calmodulin, and that Ca2+-calmodulin complex with catalytic subunit of MLCK activates enzyme to phosphorylate serine at position of 19 on MLC20 [Hartshorne, 1987]. However, Ca2+ concentration does not always parallel the degree of MLC phosphorylation and contraction [Morgan and Morgan, 1984; Kanaide, 1999; Hirano et al., 2003]. When the extent of MLC phosphorylation or force of contraction induced by agonist stimulation is higher than that caused by a depolarization induced increase in the Ca2+ concentration, this is called Ca2+-sensitization [Somlyo and Somlyo, 1994]. Our data did not show any effect of EGTA on MLC20 phosphorylation. This suggests calcium-independent phosphorylation of BSM cells.
We initially focused on regulation of expression of MLCK by cell signaling to explore regulation of smooth muscle contraction. After we observed inconsistency between MLCK level and MLC20 phosphorylation, we extended our research to another—the relationship between MLCK and MLC20 phosphorylation. Several lines of evidences deriving from our experiments support the notion that MLC20 phosphorylation is regulated by a MLCK-independent manner. First, the level of MLC20 phosphorylation of smooth muscle tissues is inconsistent with MLCK level. Smooth muscle tissues that do not have detectable MLCK have a high level of MLC20 phosphorylation. To the contrary, some smooth muscles have high levels of MLCK but MLC20 is not phosphorylated (Fig. 1). Second, MLCK immunoprecipitated from smooth muscle does not activate MLC20 phosphorylation in low-MLCK and high-MLC20 tissue. Third, MLC20 phosphorylation is accepted as Ca2+ independent. In this study, EGTA, a Ca2+ chelator, does not block phosphorylation of MLC20 effectively. Fourth, calmodulin inhibitor (RRKWQKTGHAVRAIGRL) [Torok and Trentham, 1994; Torok et al., 1998] and MLCK inhibitor (RKKY-KYRRK) [Lukas et al., 1999] do not affect phosphorylation of MLC20. Fifth, siRNA-mediated MLCK knockdown does not decrease MLC20 phosphorylation. In addition, tissue extract without detectable MLCK induces phosphorylation of MLC20 from the smooth muscle, which has low phosphorylation level of MLC20.
MLC20 is expressed at a similar level in different smooth muscle tissues and hBSM cells. We noticed that MLC20 was phosphorylated completely after tissue lysates containing phosphatase inhibitor were stored at −80°C for several days and thawed several times. All these frozen lysates had similar levels of p-MLC20 after storage. In this study, although we did not measure the total MLC20 in tissues and cells using special antibody against total MLC20, we observed a similar expression level of MLC20 in these tissues (not shown).
MAP kinase (MAPK) cascades are composed of MAPKK kinases (MAPKKKs), MAPK kinases (MAPKKs), and MAP kinases (MAPKs). MAP kinases are regulated by phosphorylation cascades. This study shows for the first time that the phosphorylation of MLC20 is downregulated to a large extent by MAP kinase pathway. Several data support the conclusion above. First, adenovirus-mediated over-expression of MEKK1 inhibits phosphorylation of MLC20 in a dose-dependent manner. MEKK1, an important member of MAPKKK, is a large protein that activates substrates, such as MKKs and MEKs. MEKK1 has binding sites for multiple components of MAP kinase modules. For instance, MEKK1 can bind directly to JNK [Xu and Cobb, 1997; Xia et al., 1998] and ERK [Karandikar et al., 2000]. In addition, endogenous MEKK1 colocalizes with α-actinin in stress fibers and on microtubules [Christerson et al., 1999]. Second, SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and ERK inhibitors increase phosphorylation of MLC20 in hBSM cells by inactivating endogenous MAPKs (p38, JNK, and ERK). Third, SB203580, SP600125, and ERK inhibitor counteract the role of exogenous MEKK1 in inhibition of MLC20 phosphorylation. MEKK1 inhibits MLC20 phosphorylation through p38, JNK, and ERK.
One potential problem with MAPKs being regulators of MLC20 phosphorylation is how the messenger molecules, p38, JNK, and ERK result in the same biological effect—inhibition of MLC20 phosphorylation. Several mechanisms can be speculated for regulation of MLC phosphorylation by MAP kinase pathway. One possibility is that p38, JNK, and ERK or their downstream molecules have a common effector or substrate. The effector activated in series leads to inhibition of phosphorylation of MLC20. The assumption is based largely on that MAPKs (p38, JNK, and ERK) have common effectors, such as Elk-1. This remains to be established. Another possibility is that these three MAPKs have similar functions in regulating MLC20 phosphorylation. This speculation is based on their similar or related role in various physiological processes [Xia et al., 1995; Chuang et al., 2000; Zhan et al., 2003]. Fortunately, the level of MLC20 phosphorylation has been regulable under the control of MAP kinase pathway regardless of the mechanisms involved in signal transduction.
Researchers reported myosin phosphorylation-independent contraction of smooth muscle [Oishi et al., 1991; Sato et al., 1992; Kishi et al., 2000; Seasholtz, 2003]. Recently, a study in cultured smooth muscle cells shows that MLC20 phosphorylation is not obligatory for the smooth muscle to contract [Nakamura et al., 2008]. Our data support this notion that phosphorylation of MLC20 is not necessary for BSM cell migration.
In summary, we describe a MLCK-independent phosphorylation of MLC20 in smooth muscle. MLC20 phosphorylation is downregulated to a great extent by MAP kinase pathway. MEKK1 decreases phosphorylation of MLC20 through activating its downstream molecules, p38, JNK, and ERK.
Acknowledgments
This work was supported by JSCAF Sci-Tech Innovation Talent Grant 2009C. The authors thank Dr. Xianggui Chen (Xihua University) for MLC antibody (Ser1).
The corrected proof of this article was published online as an EarlyView Article on 18 August 2010. Several changes have been made subsequently. Specifically, Dr. Samuel Chacko and Mary John have withdrawn as coauthors; references to grant support provided by Dr. Chacko have been removed; institutional references to the University of Pennsylvania have been removed; and a limited amount of the data (an estimated 5%), which was performed while Dr. Deng was at the University of Pennsylvania, have been withdrawn. This work was repeated in Dr. Deng’s own laboratory under the same experimental conditions. This notice is included in the online and print versions of the article to indicate that these revisions have been implemented and are now published.
Footnotes
Monitoring Editor: Joseph Sanger
References
- Adelstein RS. Regulation of contractile proteins by phospherylation. J Clin Invest. 1983;72(6):1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3(3):177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Betapudi V, Licate LS, Egelhoff TT. Distinct roles of non-muscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66(9):4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21(16):6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LF, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cherkasova VA. Measuring MAP kinase activity in immune complex assays. Methods. 2006;40(3):234–242. doi: 10.1016/j.ymeth.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Christerson LB, Vanderbilt CA, Cobb MH. MEKK1 interacts with alpha-actinin and localizes to stress fibers and focal adhesions. Cell Motil Cytoskeleton. 1999;43(3):186–198. doi: 10.1002/(SICI)1097-0169(1999)43:3<186::AID-CM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 2000;21(7):1423–1432. [PubMed] [Google Scholar]
- Emmert DA, Fee JA, Goeckeler ZM, Grojean JM, Wakatsuki T, Elson EL, Herring BP, Gallagher PJ, Wysolmerski RB. Rhokinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am J Physiol Cell Physiol. 2004;286(1):C8–C21. doi: 10.1152/ajpcell.00428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal F, Gu LZ, Ihnatovych I, Han Y, Hu W, Antic N, Carreira F, Blomquist JF, Hope TJ, Ucker DS, de Lanerolle P. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol Cell Biol. 2005;25(14):6259–6266. doi: 10.1128/MCB.25.14.6259-6266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formigli L, Meacci E, Vassalli M, Nosi D, Quercioli F, Tiribilli B, Tani A, Squecco R, Francini F, Bruni P, Orlandini SZ. Sphingosine 1-phosphate induces cell contraction via calcium-independent/Rho-dependent pathways in undifferentiated skeletal muscle cells. J Cell Physiol. 2004;198(1):1–11. doi: 10.1002/jcp.10366. [DOI] [PubMed] [Google Scholar]
- Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18(1):1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- Ge S PJ. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine monocyte chemoattractant protein-1 by human astrocytes. J Biol Chem. 2004;279:6688–6695. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ. Biochemistry of the contractile process in smooth muscle. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 423–482. [Google Scholar]
- Hatch V, Zhi G, Smith L, Stull JT, Craig R, Lehman W. Myosin light chain kinase binding to a unique site on F-actin revealed by three-dimensional image reconstruction. J Cell Biol. 2001;154(3):611–617. doi: 10.1083/jcb.200105079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou SB, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol. 2000;279(5):C1656–C1664. doi: 10.1152/ajpcell.2000.279.5.C1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Derkach DN, Hirano M, Nishimura J, Kanaide H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem. 2003;248(1–2):105–114. doi: 10.1023/a:1024180101032. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, tenDijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Jay PY, Pham PA, Wong SA, Elson EL. A mechanical function of myosin-II in cell motility. J Cell Sci. 1995;108:387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kanaide H. Measurement of [Ca2+] in smooth muscle strips using frontsurface fluorometry. In: Lambert DG, editor. Methods in Molecular Biology. Totowa, NJ: Humana Press Inc; 1999. pp. 269–277. [DOI] [PubMed] [Google Scholar]
- Karandikar M, Xu SC, Cobb MH. MEKK1 binds Raf-1 and the ERK2 cascade components. J Biol Chem. 2000;275(51):40120–40127. doi: 10.1074/jbc.M005926200. [DOI] [PubMed] [Google Scholar]
- Kishi H, Mikawa T, Seto M, Sasaki Y, Kanayasu-Toyoda T, Yamaguchi T, Imamura M, Ito M, Karaki H, Bao J, Nakamura A, Ishikawa R, Kohama K. Stable transfectants of smooth muscle cell line lacking the expression of myosin light chain kinase and their characterization with respect to the actomyosin system. J Biol Chem. 2000;275(2):1414–1420. doi: 10.1074/jbc.275.2.1414. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Inoue A, Mikawa T, Kuwayama H, Hotta Y, Masaki T, Ebashi S. Isolation of cDNA for bovine stomach 155 kDa protein exhibiting myosin light chain kinase-activity. J Biochem. 1992;112(6):786–791. doi: 10.1093/oxfordjournals.jbchem.a123976. [DOI] [PubMed] [Google Scholar]
- Lukas TJ, Mirzoeva S, Slomczynska U, Watterson DM. Identification of novel classes of protein kinase inhibitors using combinatorial peptide chemistry based on functional genomics knowledge. J Med Chem. 1999;42(5):910–919. doi: 10.1021/jm980573a. [DOI] [PubMed] [Google Scholar]
- McFawn PK, Shen L, Vincent SG, Mak A, Van Eyk JE, Fisher JT. Calcium-independent contraction and sensitization of airway smooth muscle by p21-activated protein kinase. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L863–L870. doi: 10.1152/ajplung.00068.2002. [DOI] [PubMed] [Google Scholar]
- Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279(5350):527–533. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth-muscle of ferret portal-vein. J Physiol London. 1984;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Xie C, Zhang Y, Gao Y, Wang HH, Ye LH, Kishi H, Okagaki T, Yoshiyama S, Hayakawa K, Ishikawa R, Kohama K. Role of non-kinase activity of myosin light-chain kinase in regulating smooth muscle contraction, a review dedicated to Dr. Setsuro Ebashi. Biochem Biophys Res Commun. 2008;369(1):135–143. doi: 10.1016/j.bbrc.2007.11.096. [DOI] [PubMed] [Google Scholar]
- Oishi K, Takano-Ohmuro H, Minakawa-Matsuo N, Suga O, Karibe H, Kohama K, Uchida MK. Oxytocin contracts rat uterine smooth muscle in Ca2(+)-free medium without any phosphorylation of myosin light chain. Biochem Biophys Res Commun. 1991;176(1):122–128. doi: 10.1016/0006-291x(91)90898-h. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Ishihara H, Kohama K, Nonomura Y, Shibata S, Karaki H. Calcium-independent phosphorylation of smooth-muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria-Okadai) J Pharmacol Exp Ther. 1987a;243(3):1167–1173. [PubMed] [Google Scholar]
- Ozaki H, Kohama K, Nonomura Y, Shibata S, Karaki HG. Direct activation by okadaic acid of the contractile elements in the smooth-muscle of guinea-pig taenia-coli. Naunyn-Schmiedebergs Arch Pharmacol. 1987b;335(3):356–358. doi: 10.1007/BF00172811. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Perrie WT, Smillie LB, Perry SV. Phosphorylated light-chain component of myosin from skeletal-muscle. Biochem J. 1973;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier MC, Chelot E, Pekarsky Y, Gardiner K, Rossier J, Turnell WG. The human myosin light-chain kinase (mlck) from hippocampus—Cloning, sequencing, expression, and localization to 3qcen-q21. Genomics. 1995;29(3):562–570. doi: 10.1006/geno.1995.9965. [DOI] [PubMed] [Google Scholar]
- Riedinger HJ, Eger F, Trummler K, Probst H. Replication of simian virus 40 (SV40) DNA in virus-infected CV1 cells selectively permeabilized for small molecules by Staphylococcus aureus alpha-toxin: Involvement of mitochondria in the fast O-2-dependent regulation of SV40 DNA replication. Biochem J. 2005;386:557–566. doi: 10.1042/BJ20040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush CL, Kennelly PJ, Glaccum MB, Helfman DM, Scott JD, Krebs EGP. Isolation of the cdna-encoding rat skeletal-muscle myosin light chain kinase—Sequence and tissue distribution. J Biol Chem. 1988;263(21):10510–10516. [PubMed] [Google Scholar]
- Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15(2):838–850. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Hori M, Ozaki H, Takano-Ohmuro H, Tsuchiya T, Sugi H, Karaki H. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J Pharmacol Exp Ther. 1992;261(2):497–505. [PubMed] [Google Scholar]
- Seasholtz TM. The RHOad less traveled: The myosin phosphorylation-independent path from Rho kinase to cell contraction. Focus on “Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation”. Am J Physiol Cell Physiol. 2003;284(3):C596–C598. doi: 10.1152/ajpcell.00530.2002. [DOI] [PubMed] [Google Scholar]
- Smith L, Su XJ, Lin PJ, Zhi G, Stull JT. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J Biol Chem. 1999;274(41):29433–29438. doi: 10.1074/jbc.274.41.29433. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal-transduction and regulation in smooth-muscle. Nature. 1994;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E, Resnick M, Morgan KG. Ca2+-independent change in phosphorylation of the myosin light chain during relaxation of ferret aorta by vasodilators. J Physiol London. 1991;440:85–93. doi: 10.1113/jphysiol.1991.sp018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Stull JT. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal-muscle—Implications for regulation of actin myosin interaction. Proc Natl Acad Sci USA. 1990;87(1):414–418. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light-chain phosphorylation in vertebrate striated-muscle—Regulation and function. Am J Physiol. 1993;264(5):C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Zhao JJ, Jones M, Zhi G, Stull J, Potter JD. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol. 2002;92(4):1661–1670. doi: 10.1152/japplphysiol.00858.2001. [DOI] [PubMed] [Google Scholar]
- Torok K, Trentham DR. Mechanism of 2-chloro-(epsilon-aminolys(75)) -[6-[4-(n,n-diethylamino) phenyl]-1,3,5-t riazin-4-yl]calmodulin interactions with smooth-muscle myosin light-chain kinase and derived peptides. Biochemistry. 1994;33(43):12807–12820. doi: 10.1021/bi00209a012. [DOI] [PubMed] [Google Scholar]
- Torok K, Cowley DJ, Brandmeier BD, Howell S, Aitken A, Trentham DR. Inhibition of calmodulin-activated smooth-muscle myosin light-chain kinase by calmodulin-binding peptides and fluorescent (phosphodiesterase-activating) calmodulin derivatives. Biochemistry. 1998;37(17):6188–6198. doi: 10.1021/bi972773e. [DOI] [PubMed] [Google Scholar]
- Vandermerwe PA, Millar RP, Wakefield IK, Davidson JS. Mechanisms of luteinizing-hormone exocytosis in Staphylococcus-aureus-alpha-toxin-permeabilized sheep gonadotropes. Biochem J. 1989;264(3):901–908. doi: 10.1042/bj2640901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson DM, Collinge M, Lukas TJ, Vaneldik LJ, Birukov KG, Stepanova OV, Shirinsky VP. Multiple gene-products are produced from a novel protein-kinase transcription region. FEBS Lett. 1995;373(3):217–220. doi: 10.1016/0014-5793(95)01048-j. [DOI] [PubMed] [Google Scholar]
- Xia ZG, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNKp38 map kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wu ZG, Su B, Murray B, Karin M. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 1998;12(21):3369–3381. doi: 10.1101/gad.12.21.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SC, Cobb MH. MEKK1 binds directly to the c-Jun N-terminal kinases stress-activated protein kinases. J Biol Chem. 1997;272(51):32056–32060. doi: 10.1074/jbc.272.51.32056. [DOI] [PubMed] [Google Scholar]
- Zhan YM, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arteriosclerosis Thromb Vasc Biol. 2003;23(5):795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang W, Hayashi Y, Jester JV, Birk DE, Gao M, Liu CY, Kao WWY, Karin M, Xia Y. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. EMBO J. 2003;22(17):4443–4454. doi: 10.1093/emboj/cdg440. [DOI] [PMC free article] [PubMed] [Google Scholar]


