Abstract
Di-2-ethylhexyl terephthalate (DEHTP), a structural isomer of di-2-ethylhexyl phthalate (DEHP), is a plasticizer used in a variety of commercial applications, but data on Americans’ exposure to DEHTP do not exist. We investigated the exposure to DEHTP in a convenience group of U.S. adults by analyzing urine collected anonymously in 2000 (N = 44), 2009 (N = 61), 2011 (N = 81), 2013 (N = 92), and 2016 (N = 149) for two major DEHTP oxidative metabolites: mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP) and mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP). For comparison, we also quantified the analogous DEHP metabolites mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) and mono-2-ethyl-5-carboxypentyl phthalate (MECPP). We detected MECPTP, MEHHP, and MECPP in all samples collected in 2016 with geometric means of 13.1, 4.1, and 6.7 ng/mL, respectively; we detected MEHHTP in 91% of the samples (geometric mean = 3.1 ng/mL). Concentrations of MECPTP correlated well with those of MEHHTP (R2= 0.8, p < 0.001), but did not significantly correlate with those of MEHHP (p > 0.05) suggesting different sources of exposure to DEHP and DEHTP. We also evaluated the fraction of the metabolites eliminated in their free (i.e., unconjugated) form. The median percent of unconjugated species was lower for the DEHP metabolites (MECPP [45.5%], MEHHP [1.9%]) compared to the DEHTP metabolites (MECPTP [98.8%], MEHHTP [21.2%]). Contrary to the downward trend from 2000 to 2016 in urinary concentrations of MEHHP and MECPP, we observed an upward trend for MEHHTP and MECPTP. These preliminary data suggest that exposure to DEHTP may be on the rise. Nevertheless, general population exposure data using MEHHTP and MECPTP as exposure biomarkers would increase our understanding of exposure to DEHTP, one of the known DEHP alternatives.
Keywords: Di-2-ethylhexyl terephthalate, Di-2-ethylhexyl phthalate, DEHTP, DEHP, Plasticizers, Exposure, Oxidative metabolites
Introduction
Di-2-ethylhexyl terephthalate (DEHTP) is used as a plasticizer in food and drink contact materials, medical devices, children’s toys, and childcare articles, among other applications (Beeler 1976). DEHTP is a structural isomer of another plasticizer: di-2-ethylhexyl phthalate (DEHP). In the past decade, use of DEHP has been restricted in the United States and some European countries because of concerns regarding DEHP potential human toxicity (CPSC 2014; European Union 2005). In contrast, DEHTP has Food Contact Notification clearance from the US Food & Drug Administration (Eastman chemical company 2014); DEHTP also complies with the European Commission regulation for use in food contact applications (EFSA 2008). Therefore, DEHTP has been used as an alternative to DEHP, and both production volume and application of DEHTP are expected to increase (Eastman chemical company 2014). As a result, human exposure to DEHTP might also increase.
DEHTP is used as an alternative to DEHP (Eastman chemical company 2014), but some animal and human studies suggest potential adverse effects upon exposure to high doses of DEHTP. For example, chronic dietary exposure caused general toxicity in rats (Ball et al. 2012). Female Sprague–Dawley rats administered DEHTP in the diet for 90 days showed no major organ or systemic toxicity at 0.5% (v/v) dose levels, but some effects on hematology parameters and increase in relative liver weights were observed at the 1.0% dose (Barber and Topping 1995). In a study conducted with 203 human volunteers for evidence of sensitization to DEHTP (0.5%, v/v) following 3 weeks of dermal application three times a week, two subjects had slight erythema to DEHTP (David et al. 2003).
In order to better understand the extent of human exposure to DEHTP and its potential impact on health using biomonitoring, identifying and quantifying exposure biomarkers is a necessary step. Oxidative metabolites of DEHTP, mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP), mono-2-ethyl-5-oxohexyl terephthalate (MEOHTP), and mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), have been identified in vitro (Silva et al. 2015) and in vivo (Lessmann et al. 2016a, b). In humans, MECPTP was identified as the major metabolite of DEHTP, followed by MEHHTP (Lessmann et al. 2016b). Biomonitoring data are limited to one study in which both compounds were detected in the urine of 34 German adults (Lessmann et al. 2016b), but information about the extent of Americans’ exposure to DEHTP does not exist. To fill in this gap, we quantified MECPTP and MEHHTP in urine samples collected in a period of 16 years [2000 (N= 44), 2009 (N= 61), 2011 (N= 81), 2013 (N = 92), and 2016 (N = 149)] from U.S. adults with no documented DEHTP exposure. We also compared the concentrations of these biomarkers to mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) and mono-2-ethyl-5-carboxypentyl phthalate (MECPP), two metabolites of DEHP.
Materials and methods
Chemicals
We purchased MEHHTP, MECPTP, d4-MEHHTP, d4-MECPTP, and 13C6-MECPP from CanSyn (Ontario, Canada), and MEHHP, MECPP, and d4 -MEHHP from ADM (Germany). Acetonitrile (HPLC grade), water (HPLC grade), and methanol (99.8%, HPLC grade) were purchased from Honeywell Burdick & Jackson (Muskegon, MI). β-glucuronidase (Escherichia coli-K12) was purchased from Roche Biomedical (Mannheim, Germany). All chemicals and reagents were used without further purification.
Analytical method
The analytical method for measuring phthalate oxidative metabolites in urine is adapted from previously published methods (Silva et al. 2007). Briefly, urine (0.1 mL) and calibration standards were spiked with an internal standard solution containing deuterium- or 13C-labeled analogs of the target metabolites and a buffered solution of β-glucuronidase (Escherichia coli-K12; 25 μL, pH 6.5/1 M ammonium acetate), and incubated at 37 °C for a minimum of 120 min. The target analytes in the spiked urine were extracted using on-line solid phase extraction, chromatographically resolved by high-performance liquid chromatography (Figure S1) and quantified using negative ion electrospray-ionization tandem mass spectrometry (Table 1). Analysis of urine samples collected in 2016 was repeated with addition of 1M ammonium acetate buffer solution without β-glucuronidase to quantify unconjugated metabolites (Silva et al. 2007); we did not investigate for other potential conjugates (e.g., sulfates). The limits of detection (LODs), calculated as 3S0, where S0 is the standard deviation as the concentration approaches zero (Taylor 1987), were 0.4 ng/mL for MEHHTP and MECPTP and 0.2 ng/mL for MECPP and MEHHP. For concentrations <LOD, we imputed a value equal to the LOD divided by the √2. Statistical significance was as set to p < 0.05. Trend analysis for MECPP and MECPTP using regression analysis was performed by SAS software.
Table 1.
Analytical parameters for the quantification of DEHP and DEHTP metabolites
| Parent chemical | Urinary metabolite | Internal standard | MS/MS scan, native (ISDa) | Collision energy (eV)b |
|---|---|---|---|---|
| DEHP | MEHHP | d4-MEHHP | 293/145 (297/145) | 17 |
| MECPP | 13C6-MECPP | 307/159 (313/159) | 17 | |
| DEHTP | MEHHTP | d4-MEHHTP | 293/121 (297/125) | 22 |
| MECPTP | d4-MECPTP | 307/165 (311/169) | 20 |
ISD-internal standard
Collision energy applied in ThermoScientific Vantage mass spectrometer
Subjects
We collected urine anonymously in 2000, 2009, 2011, 2013, and 2016 from demographically diverse groups of U.S. male and female adults. No personal information from the subjects was available. Samples were collected between 8:00 AM and 5:00 PM and were not necessarily first-morning voids. We cannot rule out the possibility that some donors contributed urine in different collection years and/or multiple urine specimens, albeit collected during different days or time of day, during any given collection year. After collection, samples were stored at −70 °C until analysis. The Centers for Disease Control and Prevention (CDC) Internal Review Board approved the urine collection and analysis. A waiver of informed consent was requested under 45 CFR 46.116(d).
Results and discussion
In humans, DEHTP metabolizes extensively before elimination in urine (Lessmann et al. 2016b) and its oxidative metabolites (e.g., MEHHTP, MECPTP) can be used as biomarkers of exposure to DEHTP (Lessmann et al. 2016a, b). Interestingly, MEHHTP and MECPTP are structural isomers of MEHHP and MECPP, respectively, which have been used extensively as DEHP exposure biomarkers (Koch et al. 2004, 2005, 2006; Silva et al. 2006).
In this study, we quantified the concentrations of MEHHTP, MECPTP, MEHHP, and MECPP in urine collected in 2000, 2009, 2011, 2013, and 2016 from convenience samples of U.S. adults. The geometric mean and select percentile concentrations, and detection frequencies are listed in Table 2. We observed an increase in detection frequencies of MEHHTP (from 7 to 91%) and MECPTP (18–100%) from 2000 to 2016 (Table 2). Similarly, we observed an upward trend in the geometric mean concentrations of MEHHTP (<LOD to 3.1 ng/mL from 2011 to 2016) and MECPTP (<LOD to 13.1 ng/mL from 2009 to 2016, p < 0.001-trend analysis 2011–2016) (Table 2). By contrast, during the same time period, geometric means of the two DEHP metabolites decreased from 8.7 to 4.1 ng/mL (MEHHP) and 12.1 to 6.7 ng/mL (MECPP, p = 0.005-trend analysis 2000–2016) (Table 2). Together, these findings suggest that DEHTP exposures may be on the rise perhaps because DEHTP can be used as a replacement of DEHP in consumer products (Eastman chemical company 2014).
Table 2.
Urinary concentrations of DEHP and DEHTP metabolites in a convenience sample of U.S. adults
| Parent chemical | Urinary metabolite | Collection year | N | Select percentilesb (ng/mL) | Frequency of detection (%) | Geometric Meanb (ng/mL) | Median unconjugated metabolitec,d (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 25th | Median | 75th | 90th | |||||||
| DEHP | MEHHP | 2016 | 149 | 1.8 | 4.3 | 10.2 | 19.0 | 100 | 4.1 | 1.9 |
| 2013 | 92 | 2.2 | 4.9 | 9.4 | 16.2 | 100 | 4.3 | |||
| 2011 | 81 | 17 | 4.2 | 10.2 | 22.0 | 100 | 4.1 | |||
| 2009 | 61 | 2.7 | 5.1 | 13.5 | 21.5 | 100 | 5.9 | |||
| 2000 | 44 | 4.8 | 11.2 | 22.5 | 30.0 | 100 | 8.7 | |||
| MECPP | 2016 | 149 | 2.6 | 6.6 | 15.3 | 30.9 | 100 | 6.7 | 45.5 | |
| 2013 | 92 | 4.6 | 7.9 | 15.9 | 29.6 | 100 | 7.8 | |||
| 2011 | 81 | 4.0 | 7.3 | 17.9 | 41.5 | 100 | 9.3 | |||
| 2009 | 61 | 5.9 | 10.9 | 11.6 | 32.6 | 100 | 10.1 | |||
| 2000 | 44 | 6.7 | 16.6 | 28.0 | 37.9 | 100 | 12.1 | |||
| DEHTP | MEHHTP | 2016 | 149 | 1.1 | 3.0 | 7.6 | 22.5 | 91 | 3.1 | 21.2 |
| 2013 | 92 | 0.6 | 1.1 | 3.0 | 7.1 | 77 | 1.3 | |||
| 2011 | 81 | <LODa | <LOD | 1.1 | 4.5 | 44 | <LOD | |||
| 2009 | 61 | <LOD | <LOD | 0.6 | 1.8 | 34 | <LOD | |||
| 2000 | 44 | <LOD | <LOD | <LOD | 1.2 | 7 | <LOD | |||
| MECPTP | 2016 | 149 | 4.5 | 15.7 | 37.1 | 92.2 | 100 | 13.1 | 98.8 | |
| 2013 | 92 | 2.1 | 4.8 | 10.8 | 44.6 | 95 | 4.6 | |||
| 2011 | 81 | 1.5 | 2.0 | 4.8 | 14.1 | 86 | 2.0 | |||
| 2009 | 61 | <LOD | <LOD | 0.6 | 1.1 | 38 | <LOD | |||
| 2000 | 44 | <LOD | <LOD | <LOD | 1.3 | 18 | <LOD | |||
LOD-limit of detection; LOD-0.4 ng/mL for both MEHHTP and MECPTP
<LOD was treated as LOD√2
Only total concentrations >LOD were used in the calculation
Only urine samples collected in 2016 were analyzed without β-glucuronidase for unconjugated metabolites
We observed higher median concentrations of MEHHTP (3.0 ng/mL) and MECPTP (15.7 ng/mL) in 2016 samples than concentrations reported in a German pilot study (<0.3 and 0.9 ng/mL for MEHHTP and MECPTP, respectively) conducted around the same time period (Lessmann et al. 2016b). These differences may reflect potentially higher exposure to DEHTP in the United States than in Germany. However, because of the pilot nature of the two studies and non-representative nature of the samples analyzed, these results must be interpreted with caution until representative data from both countries become available.
Both DEHP and DEHTP produced a carboxylic acid (MECPP and MECPTP) as their major metabolite (Table 2), but we observed some differences in their concentration patterns. In the 2016 samples tested, the geometric mean concentration of MECPTP was higher than MECPP (13.1 vs. 6.7 ng/mL); however, the geometric mean concentration of MEHHTP was lower than of MEHHP (3.1 vs. 4.1 ng/mL) (Table 2). These results may suggest potential differences in toxicokinetics (i.e., adsorption, distribution, metabolism, elimination) between DEHP and DEHTP.
Glucuronidation facilitates urinary elimination of phthalate metabolites (Silva et al. 2003). Despite structural similarities between DEHP and DEHTP, interestingly, we observed that glucuronidation of their analogous metabolites differed. Specifically, we found that 98.8% of MECPTP but only 45.5% of MECPP eliminated in their free (i.e., unconjugated) form (Table 2). In contrast, MEHHP and MEHHTP eliminated mostly conjugated (median free metabolites were 1.9 and 21.2%, respectively). Our MECPTP results are in agreement with previous findings where humans administered with DEHTP eliminated 91.2% of MECPTP in its free form (Lessmann et al. 2016b). However, in our study population, only 21.2% of MEHHTP eliminated as the free metabolite, compared to >70% of unconjugated MEHHTP in males administered DEHTP (Lessmann et al. 2016b). The reasons for the differences in the percentage of MEHHTP eliminated as free species in these two studies remain, at present, unknown, but may relate to inter-individual differences because the human metabolism study relied on data from three male adults (Lessmann et al. 2016b).
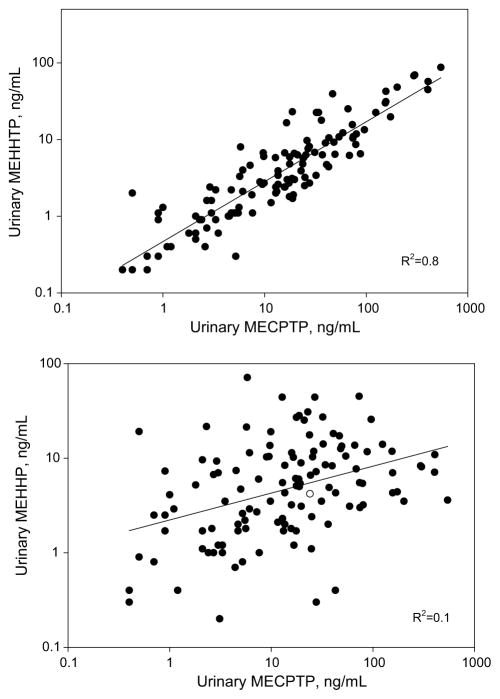
As expected, because MEHHTP and MECPTP share a common precursor, their concentrations correlated well (R2= 0.8, Fig. 1). However, urinary MECPTP did not correlate well with MEHHP (p > 0.05, Fig. 1) suggesting that the sources of DEHTP and DEHP exposure likely differ as expected if DEHTP is indeed replacing DEHP in certain commercial applications.
Fig. 1.
Correlation analyses of urinary concentrations of MECPTP and MEHHTP (top); and MECPTP and MEHHP (bottom)
In conclusion, the frequent detection of DEHTP metabolites among a diverse group of U.S. male and female adults suggests widespread exposure to DEHTP. Further, the apparent increase in detection frequency and magnitude of the concentrations with time suggests that DEHTP exposure in the United States may be on the rise. Last, these pilot data suggest that the DEHTP metabolites, MEHHTP and MECPTP can serve as biomarkers of exposure to DEHTP in large-scale biomonitoring studies such as the National Health and Nutrition Examination Survey.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00204-017-1956-3) contains supplementary material, which is available to authorized users.
Disclaimer The use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Ball GL, McLellan CJ, Bhat VS. Toxicological review and oral risk assessment of terephthalic acid (TPA) and its esters: a category approach. Crit Rev Toxicol. 2012;42:28–67. doi: 10.3109/10408444.2011.623149. [DOI] [PubMed] [Google Scholar]
- Barber ED, Topping DC. Subchronic 90-day oral toxicology of Di(2-ethylhexyl) terephthalate in the rat. Food Chem Toxicol. 1995;33:971–978. doi: 10.1016/0278-6915(95)00060-f. [DOI] [PubMed] [Google Scholar]
- Beeler AD. New terephthalate plasticizers for PVC. Plast Eng. 1976;32:40–41. [Google Scholar]
- CPSC. Chronic hazard advisory panel on phthalates and phthalates alternatives. Bethesda, MD 20814: U.S. Consumer Product Safety Commission. Directorate for Health Sciences; 2014. [Google Scholar]
- David RM, Lockhart LK, Ruble KM. Lack of sensitization for trimellitate, phthalate, terephthalate and isobutyrate plasticizers in a human repeated insult patch test. Food Chem Toxicol. 2003;41:589–593. doi: 10.1016/s0278-6915(02)00282-x. [DOI] [PubMed] [Google Scholar]
- Eastman chemical company. [Accessed 15 March 2017];Why Eastman 168 is a non phthalate plasticizer. 2014 http://www.eastman.com/Literature_Center/Internal/L243.pdf.
- EFSA. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with foold (AFC) on a request related to a 12th list of substances for food control materials. T EFSA J. 2008;628–633:1–19. [Google Scholar]
- European Union. Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles) Council, European Parliament 2005 [Google Scholar]
- Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004;78:123–130. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—An update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- Lessmann F, Schutze A, Weiss T, Brüning T, Koch HM. Determination of metabolites of di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line cleanup. J Chromatogr B. 2016a;1011:196–203. doi: 10.1016/j.jchromb.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Lessmann F, Schutze A, Weiss T, Langsch A, Otter R, Brüning T, et al. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch Toxicol. 2016b;90:1659–1667. doi: 10.1007/s00204-016-1715-x. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol. 2003;77:561–567. doi: 10.1007/s00204-003-0486-3. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Needham LL, Calafat AM. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology. 2006;219:22–32. doi: 10.1016/j.tox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Calafat AM, Ye X. Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol In Vitro. 2015;29:716–721. doi: 10.1016/j.tiv.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JK. Quality assurance of chemical measurements. Lewis Publishers; Chelsea, MI: 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.