Abstract
Background/Objectives
Paroxysmal nocturnal haemoglobinuria (PNH) is characterized by intravascular haemolysis with a negative direct antiglobulin test (DAT). Eculizumab is a humanized monoclonal antibody that inhibits complement component C5 and is approved for PNH treatment. Recent publications demonstrated that some patients with PNH develop a positive DAT during eculizumab treatment. These published clinical trials investigated a highly selected patient population. Therefore, it seems important to study this topic in a general PNH patient population with a longer follow-up.
Materials and Methods
We analysed haemolytic activity, RBC transfusion requirement, effect on DAT and ferritin levels in 41 patients with PNH before and during eculizumab therapy with a median follow-up of 24 months (range 1–63 months).
Results
During eculizumab therapy, median LDH decreased (1657–258 U/ l; P < 0·0001), while median haemoglobin increased (9·2–10·3 g/ dl). Eighteen of 32 pts (56%) who previously required regular transfusions became transfusion independent. DAT was positive for C3d in 72·4% of 21 eculizumab-treated pts with available DAT. Ferritin levels increased (69–348 ng / ml, P < 0·0001). This increase was more pronounced in pts with ongoing transfusion dependency during eculizumab therapy.
Conclusion
Eculizumab therapy for PNH should be added to the list of possible causes for a positive DAT. Intravascular haemolysis was inhibited by eculizumab, but signs of extravascular haemolysis should be monitored. Because renal iron loss was stopped, eculizumab-treated pts can be prone to iron overload and therefore ferritin concentrations should be monitored closely.
Keywords: direct antiglobulin test positive by C3d, eculizumab, paroxysmal nocturnal haemoglobinuria
Introduction
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare haematopoietic stem cell disorder [1, 2]. It is caused by acquired somatic mutations of the X-linked phosphatidyl-inositol-glycan (PIG-A) gene [3, 4], which result in partial or complete deficiency of GPI-linked proteins on the surface of affected cells. The lack of GPI-linked proteins leads to an increased susceptibility of RBC to activated complement and destruction by the membrane attack complex leading to the clinical features of PNH: chronic intravascular haemolytic anaemia, thromboembolism and bone marrow failure [5]. The International PNH Interest Group (IPIG) classifies PNH into three categories: classical PNH, PNH in the setting of another specified bone marrow disorder and subclinical PNH [6].
Eculizumab is a humanized monoclonal antibody that inhibits the cleavage of complement component C5 and thereby prevents activation of the terminal complement cascade [7]. Eculizumab therapy in PNH leads to a highly significant reduction in complement-mediated intravascular haemolysis and its related morbidities [8–11]. However, not all eculizumab-treated patients with PNH achieve transfusion independence, despite efficient complement inhibition [8–10, 12–14]. It has been demonstrated that some pts develop a positive direct antiglobulin test (DAT) during eculizumab treatment due to deposition of C3 fragments on PNH red cells [12–14]. The published clinical trials included a highly selected patient population and preferentially included pts with classical PNH [8–11]. Therefore, it seems important to study a long-term follow-up in a general PNH patient population including a substantial proportion of pts with PNH in the context of other bone marrow disorders.
We report a series of 41 eculizumab-treated patients with PNH. Almost half of our pts had PNH in the setting of another specified bone marrow disorder. We found a high proportion of pts who became DAT positive. However, in contrast to other reports [13], we could not confirm a correlation of DAT positivity and poor response.
Materials and methods
Patient characteristics
We report a retrospective analysis of 41 pts (18 men, 23 women), with a median age of 31 years (11–79 years) at the time of diagnosis. The median age at the initiation of eculizumab therapy was 40 years (18–80 years). According to the IPIG classification, 22 pts had classical PNH and 19 pts had PNH in the context of a bone marrow disorder [6]. Two of these pts had a chronic myeloproliferative syndrome, and 17 pts had aplastic anaemia (AA). Four pts had been enrolled to the TRIUMPH trial [9] and five pts to the SHEPHERD trial [8] (Table 1). Here, we report a median follow-up of 48 months of these pts after the end of TRIUMPH or SHEPHERD trial. The other 32 pts started treatment after approval of eculizumab and have not been reported so far. The time frame of data collection comprises the last visit before and the last follow-up during eculizumab therapy. Seven pts died during the study period due to pulmonary failure (n = 1), PNH-related complications (n = 2), progression of associated bone marrow failure syndrome and / or stem cell transplantation (n = 4).
Table 1.
PNH patient characteristics
| Patient no. | PNH class | Previous studies | LDH pre (U / l) | LDH last FU (U / l) | Total bilirubin pre (μM) | Total bilirubin last FU (μM) | Hb pre (g / dl) | Hb last FU (g / dl) | RBC pre | RBC last FU | Ferritin pre (ng / ml) | Ferritin last FU (ng / ml) | C3c / C3d+ pre | C3c / C3d+ last FU | Therapy duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 2a | S | 2944 | 226 | 140·0 | 77·0 | 7·9 | 10·7 | Yes | No | 28 | 213 | Neg | C3d+ | 60 |
| 02 | 1 | S | 2703 | 770 | 12·8 | 17· 1 | 10·7 | 12·5 | Yes | No | 27 | 441 | Neg | C3d+ | 60 |
| 03 | 1 | S | 3048 | 223 | n.a | n.a | n.a | 10·3 | Yes | Yes (reg) | 2245 | 4196 | Neg | C3d+ | 59 |
| 04 | 2a | T | 2788 | 223 | 44·0 | 60·5 | 7·8 | 11· 1 | Yes | No | 115 | 888 | Neg | C3d+ | 60 |
| 05 | 1 | N | 456 | 479 | 18·8 | 27·4 | 14·4 | 14·9 | No | No | 38 | 138 | Neg | n.a. | 19 |
| 06 | 2a | T | 3057 | 222 | 30·6 | 30·0 | 8·9 | 8·5 | Yes | Yes (int) | 477 | 1935 | Neg | C3c+, C3d+ | 61 |
| 07 | 1 | S | 3569 | 235 | 44·5 | 30·8 | 9·7 | 11·6 | Yes | Yes (int) | 124 | 606 | Neg | C3d+ | 60 |
| 08 | 2a | S | 1657 | 115 | 30·0 | 29·1 | 11·0 | 10·7 | Yes | No | 63 | 1001 | Neg | C3d+ | 55 |
| 09 | 2a | N | 3014 | 212 | 45·0 | 18·3 | 10·0 | 11·0 | Yes | No | 91 | 1300 | Neg | n.a. | 30 |
| 10 | 2a | N | 360 | 174 | 87·2 | 34·0 | 12·0 | 13·5 | Yes | No | 17 | 318 | n.a. | Neg | 30 |
| 11 | 1 | N | 2248 | 333 | 25·0 | 33·0 | 8·1 | 9·7 | Yes | No | 27 | n.a | Neg | n.a. | 3 |
| 12 | 2a | T | 1585 | 225 | 18·0 | 29·1 | 10·5 | 8·7 | Yes | Yes (int) | 18 | 168 | n.a. | C3d+ | 52 |
| 13 | 1 | N | 5191 | 307 | 50·0 | 30·8 | 7·5 | 10·8 | Yes | Yes (int) | 56 | 774 | Neg | C3d+ | 29 |
| 14 | 1 | N | 2274 | 246 | 33·3 | 35·6 | 13·0 | 9·5 | Yes | No | 75 | 378 | Neg | C3d+ | 23 |
| 15 | 1 | N | 1557 | 341 | 5·1 | 14·2 | 12·1 | 12·7 | Yes | No | 91 | 129 | Neg | n.a. | 26 |
| 16 | 2a | N | 660 | 93 | 34·0 | 26·0 | 9·1 | 8·3 | Yes | Yes (reg) | 127 | 698 | Neg | Neg | 12 |
| 17 | 1 | N | 2464 | 246 | 30·8 | 30·8 | 9·4 | 8·8 | Yes | Yes (int) | 104 | n.a | Neg | Pos | 25 |
| 18 | 1 | N | 2456 | 292 | 77·0 | 11·3 | 5·3 | 10·2 | Yes | No | 14 | n.a | Neg | Pos | 24 |
| 19 | 1 | N | 2724 | 366 | 32·5 | 56·4 | 9·1 | 10·8 | Yes | No | 163 | 145 | Neg | C3d+ | 31 |
| 20 | 2a | N | 910 | 275 | n.a | 12·0 | 5·8 | 7·6 | No | Yes | 135 | N.a | n.a. | n.a. | 5 |
| 21 | 2b | N | 1883 | 1829 | n.a | 42·8 | 7·0 | 8·1 | Yes | Yes (reg) | 15 | 68 | Neg | Neg | 2 |
| 22 | 1 | N | 551 | 136 | 10·3 | 5·1 | 10·1 | 12·0 | No | No | 17 | 46 | Neg | Neg | 17 |
| 23 | 2a | N | 975 | 207 | 31·3 | 35·7 | 12·8 | 12·5 | No | No | 41 | 39 | Neg | n.a. | 2 |
| 24 | 1 | N | 1012 | 258 | 26·0 | 49·0 | 8·9 | 12·5 | Yes | No | 11 | 166 | Neg | C3d+ | 26 |
| 25 | 2a | N | 1544 | 292 | 20·0 | 47·9 | 10·0 | 9·9 | Yes | No | 100 | 49 | C3d+ | Neg | 25 |
| 26 | 2a | N | 840 | 350 | 9·7 | 7·5 | 7·0 | 8·5 | Yes | Yes (reg) | n.a | 1883 | n.a. | Neg | 7 |
| 27 | 1 | N | 2814 | 168 | 40·0 | 27·0 | 5·0 | 11·3 | Yes | No | 18 | 182 | n.a. | Pos | 30 |
| 28 | 2a | T | 3770 | 293 | 54·7 | 20·5 | 7·8 | 9·2 | Yes | Yes (reg) | 100 | 986 | Neg | C3d+ | 63 |
| 29 | 1 | N | 723 | 201 | 29·8 | 22·8 | 6·0 | 9·3 | Yes | No | 76 | 1798 | n.a. | n.a. | 4 |
| 30 | 2a | N | 436 | 216 | 22·2 | 22·2 | 9·5 | 10·5 | Yes | Yes (reg) | 3821 | 3700 | Neg | n.a. | 1 |
| 31 | 2a | N | 787 | 228 | 38·0 | n.a | 12·3 | 13·3 | Yes | No | 172 | 116 | Neg | n.a. | 19 |
| 32 | 1 | N | 2283 | 339 | n.a | 17· 1 | 9·2 | 9·1 | Yes | No | 14 | 244 | Neg | C3d+ | 24 |
| 33 | 1 | N | 609 | 256 | 28·0 | 24·0 | 12·8 | 12·1 | No | No | 53 | 76 | Neg | C3d+ | 34 |
| 34 | 1 | N | 2014 | 268 | 29·1 | 34·2 | 8·7 | 12·0 | No | No | 30 | 67 | Neg | C3d+ | 8 |
| 35 | 1 | N | 472 | 205 | 14·0 | 18·8 | 13·9 | 13·6 | No | No | 41 | n.a | Neg | n.a. | 8 |
| 36 | 1 | N | 3793 | 309 | 30·8 | 25·7 | 11·3 | 9·9 | Yes | No | 245 | 309 | Neg | C3d+ | 7 |
| 37 | 1 | N | 1331 | 266 | 15·4 | 12·0 | 7·2 | 6·9 | No | Yes | 22 | 572 | Neg | Neg | 4 |
| 38 | 1 | N | 344 | 309 | 6·8 | 35·9 | 9·2 | 8·8 | Yes | Yes (reg) | 688 | N.a | Neg | C3d+ | 6 |
| 39 | 2a | N | 1186 | 277 | 10·0 | 30·0 | 9·1 | 10·1 | No | No | 32 | 872 | Neg | n.a. | 15 |
| 40 | 2a | N | 1070 | 298 | 28·4 | 22·6 | 9·5 | 9·5 | Yes | Yes (reg) | 1493 | 3347 | Neg | n.a. | 7 |
| 41 | 2b | N | 2722 | 1770 | 12·0 | 10·3 | 6·4 | 5·9 | Yes | Yes (reg) | 1692 | n.a | C3c+ | Neg | 2 |
Pre-eculizumab = last follow-up before eculizumab start, n. a., not available; LDH, lactate dehydrogenase; Hb, haemoglobin; RBC, red-blood-cell; int, intermittent (RBC transfusions with a time interval > every 12 weeks); reg, regular (RBC transfusions with a time interval > every 12 weeks); FU, last follow-up during eculizumab; Neg, negative; Pos, positive.
PNH classification: 1 = classical PNH; 2 = PNH in the context of another defined bone marrow disorder, either AA (2a) or myeloproliferative syndrome (2b).
Previous enrolled in the TRIUMPH (=T) or the SHEPHERD (=S) trial. N = have not been reported before.
Two patients had additional anti-erythrocytic alloantibodies or warm autoantibodies.
Data acquisition was standardized and performed by one data collector. Data collection and analysis were approved by the Ethical Committee of the University of Ulm. Informed consent in accordance with the declaration of Helsinki was obtained from all pts.
Eculizumab treatment
Eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT, USA) was administered according to the approved dose regimen (600 mg weekly for 4 weeks followed by maintenance treatment with 900 mg every 14 ± 2 days). Indication for eculizumab treatment was based on the transfusion requirement and the presence of PNH-related clinical symptoms and complications [15]. Thus, 22% of the pts were not RBC transfusion dependent before the initiation of eculizumab therapy. In none of the pts, eculizumab treatment was discontinued due to adverse events. Median duration of eculizumab therapy up to the end of treatment or the last follow-up was 24 months (range 1–63 months).
Transfusion therapy
Patients with paroxysmal nocturnal haemoglobinuria were transfused with leucoreduced packed RBC units. RBC transfusions in a time interval of every 12 weeks or shorter were defined as regular transfusion requirement and an interval longer than 12 weeks as intermittent transfusion requirement.
Immunohaematology
We performed a direct antiglobulin test (DAT) with poly-specific and monospecific reagents (anti-IgA, anti-IgG, anti-C3c, anti-C3d) by gel microcolumns from DiaMed (Product identification numbers 50560 and 50830; Dia-Med-ID, Cressier-sur-Morat, Switzerland). Initially, tests were also performed with gel microcolumns from BioRad (Bio-Rad, Marnes-la-Coquette, France). Identical results were obtained with both reagents. Ferritin, LDH, total bilirubin, haemoglobin (Hb) and RBC requirement were determined before start and at last follow-up during eculizumab therapy.
Statistical analysis
Statistical analyses were carried out in Microsoft Office Access 2003 and GraphPad PRISM, version 4.00, using linear regression, including correlations, coefficient of correlation and confidence interval. P-values <0·05 were considered statistically significant. Comparisons of continuous parameters between groups were performed by the Mann–Whitney U-test. P-values are given for two-tailed comparison. Comparison of transfusion independence between groups was performed with the Fisher’s exact test.
Results
The analyses focus on some aspects of eculizumab from the perspective of transfusion medicine, in particular the effect of eculizumab on RBC transfusions, serological findings and iron stores.
Haemolytic activity and transfusion requirement during eculizumab treatment
Patients were classified into groups (1–4) according to their RBC transfusion requirement (median durations of eculizumab therapy are parenthesized): (1) pts without RBC requirement before and during eculizumab therapy (15 months); (2) pts with RBC requirement starting during eculizumab therapy (5 months); (3) pts with RBC transfusion dependency before and ongoing RBC transfusions during eculizumab (19 months); and (4) pts with RBC transfusion dependency before eculizumab therapy and no further RBC requirement during eculizumab therapy (26 months).
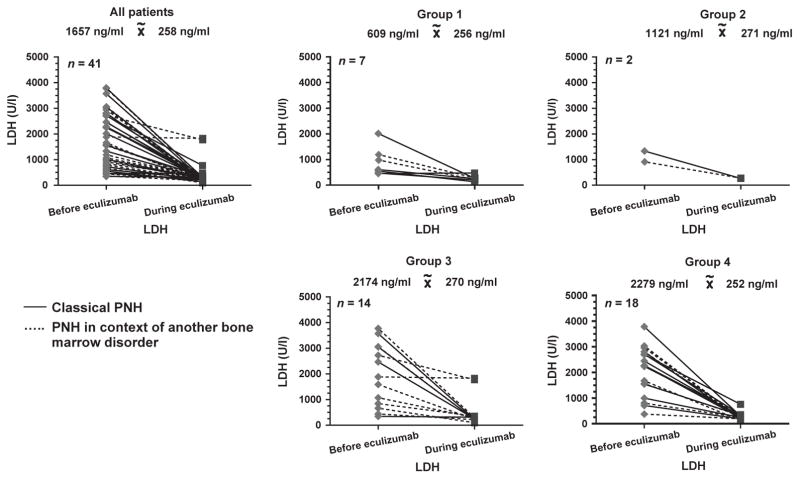
Lactate dehydrogenase decreased statistically significant from a median of 1657 U/ l (range 344–5191 U/ l) to 258 U/ l (93–1829 U/ l) (P < 0·0001) in all pts and in the subgroups (Fig. 1). This decline was observed both in pts with classical PNH (from a median of 2261 to 267 U/ l; P < 0·0001) and in pts with concomitant bone marrow disorders (from a median of 1544 to 226 U/ l; P < 0·0001) (Fig. 1). Median total bilirubin before eculizumab was 29·8 μM (5·1–140·0 μM) and decreased during eculizumab therapy to 27·4 μM (5·1–77·0 μM) (P = ns). Median haemoglobin before eculizumab was 9·2 g/ dl (5·0–14·4 g/ dl) and increased during eculizumab therapy to 10·3 g/ dl (5·9–14·9 g/ dl).
Fig. 1.
Lactate dehydrogenase (U / l) for all patients with paroxysmal nocturnal haemoglobinuria (PNH) and the four subgroups due to RBC requirement before and at the last follow-up during eculizumab therapy. group 1: no RBC requirement before and during eculizumab therapy; group 2: RBC requirement starting during eculizumab therapy; group 3: RBC transfusion dependency before and ongoing during eculizumab; group 4: RBC transfusion dependency before eculizumab therapy and no further RBC requirement during eculizumab therapy. The solid lines show pts with classical PNH; the dashed lines PNH in the context of another bone marrow disorder.
Despite this substantial suppression of intravascular haemolysis, not all pts achieved RBC transfusion independence. Seven pts (17%) did not require RBC transfusions before and during eculizumab therapy; five of these seven pts had classical PNH. Two pts (no. 20 and 37, Table 1) without transfusion requirement prior to eculizumab required RBC transfusions during eculizumab therapy (5%).
Fourteen pts (34%) required RBC transfusions before and during eculizumab therapy. Eighteen pts (44%) were transfusion dependent before and became transfusion independent during eculizumab therapy. The proportion of pts who achieved transfusion independence during eculizumab therapy did not differ significantly between classical PNH and PNH in the context of another bone marrow disorder (69% vs. 44%; P = 0·29). In 11 of the 16 pts with RBC requirements during eculizumab therapy, transfusions were required on a regular basis and in five pts intermittently. Ten of the 16 pts with ongoing RBC requirements were diagnosed with PNH in the context of another specified bone marrow disorder.
Development of positive antiglobulin test during eculizumab therapy
Before eculizumab therapy, DAT was positive for C3d in one of the 35 pts (2·8%) (Table 1). Two patients with PNH had additional warm autoantibodies and anti-erythrocyte alloantibodies (anti-D, anti-C, anti-E, anti-Lua and anti-Kpa) before initiation of eculizumab therapy.
Direct antiglobulin test was positive for C3d in 21 of 29 pts (72·4%) with available DAT during eculizumab therapy. One patient was also positive for C3c (Table 1). In 25 pts, DAT determinations are available before and during eculizumab therapy. In 19 of these 25 pts (76·0%), a change from negative C3c / C3d to positive C3d or positive C3c and C3d was observed. Fourteen of these 19 pts had classical PNH and five of these pts PNH in context of AA. Three of eight pts with a negative DAT during eculizumab therapy were transfusion independent (38%) compared with 13 / 21 (62%) with a positive DAT (P = 0·41). The mean LDH levels on eculizumab for DAT-positive and DAT-negative pts were 256 and 279 U/l (P = 0·81), respectively. No significant difference was observed in median follow-up between DAT-positive and DAT-negative pts (P = 0·126). Two DAT-positive pts in whom eculizumab was discontinued showed a negative DAT during follow-up.
Iron stores during eculizumab therapy
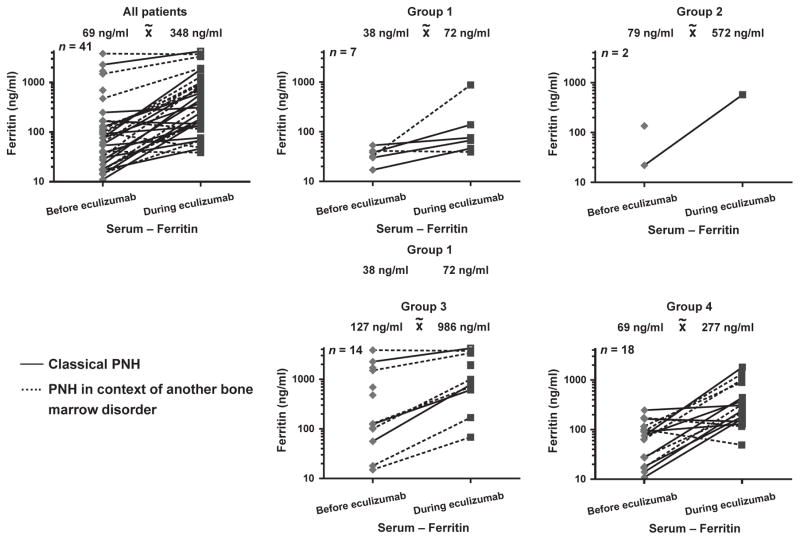
Ferritin levels were measured in all pts as a parameter of iron stores and increased significantly from a median of 69 ng / ml (range 11–3821 ng / ml) to a median of 348 ng / ml (range 39–4196 ng / ml) during eculizumab therapy (P < 0·001). The increase was more pronounced in pts with concomitant bone marrow disorders (increase from a median of 100–872 ng / ml) as compared to pts with classical PNH (increase from a median of 47 to 244 ng / ml) (Fig. 2). There was no significant correlation between ferritin levels and follow-up time (r2 = 0·00067; P = 0·8845). We grouped the pts according to their RBC transfusion requirement before and during eculizumab therapy. Ferritin increased in all these subgroups (Fig. 2). The greatest increase was observed in the subgroups with ongoing RBC transfusion requirement.
Fig. 2.
Ferritin (ng / ml) for all patients with paroxysmal nocturnal haemoglobinuria (PNH) and the four subgroups due to RBC requirement before and at the last follow-up during eculizumab therapy. group 1: no RBC requirement before and during eculizumab therapy; group 2: RBC requirement starting during eculizumab therapy; group 3: RBC transfusion dependency before and ongoing during eculizumab; group 4: RBC transfusion dependency before eculizumab therapy and no further RBC requirement during eculizumab therapy. The solid lines show pts with classical PNH; the dashed lines PNH in the context of another bone marrow disorder.
Discussion
In the pivotal clinical trials of anti-C5 antibody eculizumab for PNH (TRIUMPH and SHEPHERD) [9, 10], selected patient populations have been enrolled. In contrast to these trials, we analysed a more heterogeneous PNH patient population. Almost half (46%) of our pts had PNH in the setting of another specified bone marrow disorder [6]. In contrast, this group comprised only 23% and 30% of patients in the clinical trials TRIUMPH and SHEPHERD. While these clinical trials were confined to 6- and 12-month treatment periods, respectively, in our series, the median follow-up period of eculizumab therapy was 24 months.
Our data confirm that in the vast majority of pts, the intravascular haemolysis is substantially reduced as indicated by LDH levels [8–10]. But we observed two pts (no. 21 and 41, Table 1) who achieved just a small but not a clinically relevant decrease in LDH and still required regular RBC transfusions despite eculizumab treatment. Consequently, eculizumab was stopped in these pts. Notably, both of these pts had PNH in the setting of a myeloproliferative disorder with massive splenomegaly.
The proportion of pts who achieved transfusion independence in our series (56%) was similar to that in previous studies (49% in TRIUMPH; 51% in SHEPHERD). However, despite the highly effective reduction in intravascular haemolysis, ongoing transfusion requirement was observed in 44% of pts and only 32% of transfusion-independent pts achieved haemoglobin values ≥12·5 g/ dl.
The failure to achieve transfusion independence and normalization of haemoglobin might be a consequence of concomitant bone marrow failure in some of the pts, but it might also be caused due to ongoing extravascular haemolysis [12–16]. As eculizumab does not block the early stages of complement activation, C3 cleavage products may occur and bind to CD55 / CD59-deficient RBC. The inhibition of the terminal membrane attack complex formation by eculizumab may allow the survival of cells, which otherwise undergo a rapid intravascular haemolysis due to C5b-9 formation and thus unmask an otherwise undetectable extravascular haemolysis in PNH [14–16]. Our data show that loading of PNH RBC with C3d is a frequent event during eculizumab therapy in patients with PNH. Whereas Risitano et al. [14] found C3d coating of PNH cells in all eculizumab-treated pts, the proportion of C3d-positive pts was 72% in our series, which is almost identical to the proportion (68%) reported by Hill et al. [13] who investigated the phenomenon by flow cytometry. Interestingly, the two DAT-positive pts in whom eculizumab was discontinued showed a negative DAT during follow-up. Therefore, DAT positivity during eculizumab therapy in patients with PNH seems to be reversible.
The prognostic impact of a positive DAT during eculizumab is unclear. Risitano et al. [14] reported that the percentage of C3d-positive PNH RBC was lower in optimal responders to eculizumab than in the other groups. Hill et al. [13] reported a correlation between transfusion independence and a negative DAT. We could not confirm this observation. Three of eight pts with a negative DAT during eculizumab were transfusion independent (38%) compared with 13 / 21 (62%) with a positive DAT. The conflicting results may be due to different sensitivity of C3d detection or due to heterogeneity of patient populations. In our series, the proportion of pts with a positive DAT was significantly higher in classical PNH (17 / 19 pts; 89·5%) as compared to PNH in the setting of another bone marrow disorder (6 / 12 pts; 50·0%) (P = 0·03). A larger patient number is needed to confirm an association between a positive DAT and the classification of PNH or response to eculizumab.
Irrespective of the pathophysiological mechanism and the clinical consequences, this phenomenon is of relevance for correct interpretation of a positive DAT in pre-transfusion testing of eculizumab-treated patients with PNH. A newly positive DAT may be considered as the first indication of a developing immune response and might prompt the suspicion of a delayed haemolytic transfusion reaction. However, as shown here, a positive DAT with anti-C3d is a frequent phenomenon in eculizumab-treated pts. Information on eculizumab treatment must be reported to the serological laboratory to ensure correct interpretation of results in patients with PNH.
Many PNH pts with overt intravascular haemolysis who are not treated with targeted therapy develop iron deficiency due to haemoglobinuria or haemosiderinuria or both and even require iron replacement [15]. We showed an increase in ferritin levels due to efficient inhibition of intravascular haemolysis with a reduced iron loss during eculizumab treatment. Ongoing RBC transfusion requirement and inefficient haematopoiesis can accelerate this increase. So far, studies on the use of iron chelators in pts with iron overload during eculizumab therapy are missing. Especially in context of allogeneic stem cell transplantation as a therapy option in PNH [6, 15] and the known negative influence of iron overload on outcome [17], iron metabolism needs to be monitored closely during eculizumab therapy. Whereas iron replacement was commonly used in haemolytic PNH in the pre-eculizumab era [6], prophylactic iron administration should be stopped early in pts who are candidates for eculizumab treatment.
In conclusion, our data show that a positive DAT, essentially due to RBC-bound C3d, occurs in the majority of patients with PNH during eculizumab therapy. Ongoing eculizumab therapy in patients with PNH needs to be added to the list of causes for a positive DAT. Additionally, efficient suppression of intravascular haemolysis by eculizumab treatment can turn PNH into an iron-loading anaemia. Careful monitoring of iron stores is mandatory, and potentially treatment of iron overload may become necessary, in particular in pts with ongoing RBC transfusion requirement.
Acknowledgments
Funding
There is no source(s) of support in the form of grants, equipment or drugs to declare.
Footnotes
Authorship
BH and HS took primary responsibility for the paper; they designed research. BH, HS and WAF analysed the data and wrote the paper. SK and IvZ performed immunhaematology. RL performed data management and statistical work.
Conflict of interests
B. Höchsmann and H. Schrezenmeier were advisors for and received honoraria from Alexion. R. Leichtle, I. von Zabern, S. Kaiser and W.A. Flegel reported no potential conflicts of interest.
References
- 1.Dacie JV. Paroxysmal nocturnal haemoglobinuria. Proc R Soc Med. 1963;56:587–596. [PMC free article] [PubMed] [Google Scholar]
- 2.Oni SB, Osunkoya BO, Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970;36:145–152. [PubMed] [Google Scholar]
- 3.Miyata T, Takeda J, Iida Y, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259:1318–1320. doi: 10.1126/science.7680492. [DOI] [PubMed] [Google Scholar]
- 4.Mortazavi Y, Merk B, McIntosh J, et al. The spectrum of PIG-A gene mutations in aplastic anemia / paroxysmal nocturnal hemoglobinuria (AA / PNH): a high incidence of multiple mutations and evidence of a mutational hot spot. Blood. 2003;101:2833–2841. doi: 10.1182/blood-2002-07-2095. [DOI] [PubMed] [Google Scholar]
- 5.Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 6.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 10.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 11.Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 12.Berzuini A, Montanelli F, Prati D. Hemolytic anemia after eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2010;363:993–994. doi: 10.1056/NEJMc1005108. [DOI] [PubMed] [Google Scholar]
- 13.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95:567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113(17):4094–4100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 15.Schrezenmeier H, Höchsmann B. Eculizumab opens a new era of treatment for paroxysmal nocturnal hemoglobinuria. Expert Rev Hematol. 2009;2:7–16. doi: 10.1586/17474086.2.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Luzzatto L, Risitano AM, Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica. 2010;95:523–526. doi: 10.3324/haematol.2009.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]