Abstract
Introduction
The Ro ribonucleoprotein particle, targeted in systemic lupus erythematosus (SLE) and Sjögren’s syndrome (SS), includes Ro60 (SSA) and La (SSA) autoantigen. Anti-Ro60 occurs in SLE and SS. The importance of α-fodrin and spectrin as well as anti-Ro and anti-fodrin/spectrin antibodies in SS and SLE, led us to hypothesize that rabbit immunization with Ro60 or 4-hydroxy-2-nonenal-modified Ro60 would induce anti-spectrin. In addition, we hypothesized that antibodies to Ro60 and La will develop in animals immunized with spectrin.
Materials and Methods
Two NZW rabbits each were immunized with 4-hydroxy-2-nonenal-modified Ro60 or unmodified Ro60. Methods used included ELISA, including an inside-out RBC membrane ELISA, and Crithidia lucilae assays.
Results
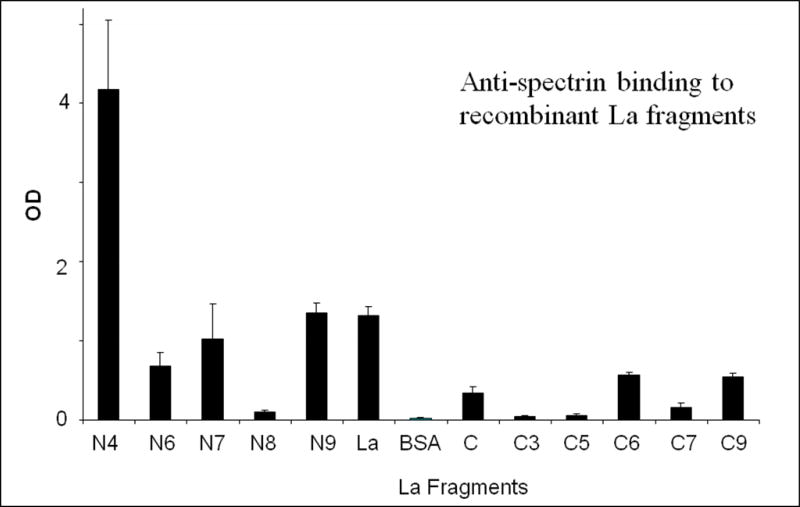
Commercial anti-spectrin sera bound significantly to Ro60 (OD 2.6 ± 0.1), Ro60 multiple antigenic peptides (MAPs) (3 out of 21 Ro60 MAPs), La (OD 4.4±0.5), and La fragments as well as to double stranded DNA but not to BSA (OD 0.6 ± 0.1). Anti-spectrin binding to purified spectrin could be inhibited by spectrin (>95%), and Ro60 or La (70%). When the binding of anti-spectrin was tested against a nested set of La fragments we found that a N4 fragment representing the C-terminal 256 aa (aa 159 to 408) bound the strongest (OD= 4.12) followed by a N9 fragment (the C-terminal 46aa; aa373 to 408 (OD=1.36). Also, significant anti-spectrin antibodies levels were induced by Ro60 and HNE-modified Ro60 immunization.
Discussion
We found intermolecular epitope spreading from Ro60/La to spectrin and vice versa, and this may have pathological significance in these animal models of autoimmunity.
Keywords: Spectrin, Fodrin, Ro60 (SS-A), La (SS-B), SLE, SS
Introduction
Systemic lupus erythematosus, a chronic autoimmune disorder, affects the skin, joints and several organ systems. Autoantibodies directed against self-antigens are commonly seen in this condition, including against Ro ribonucleoprotein. Anti-Ro60 occurs in up to 50% of patients with SLE and anti-La in substantially fewer patients (1,2). The presence of anti-Ro 60 is associated with photosensitive skin rash, subacute cutaneous lupus, deficiency of early complement components, renal disease, neonatal lupus, lymphopenia and neutropenia (3–5).
Anti-Ro60 occurs in up to 90% of patients with Sjögren’s syndrome (SS) (6,7). SS is characterized by lacrimal and salivary gland inflammation leading to keratoconjunctivitis sicca (dry eyes) and xerostomia (dry mouth). SS patient can have other systemic manifestations, like kidney, lung, skin, muscle, bone marrow, joints and vascular involvement. SS is considered primary when it occurs alone and secondary when SS occurs together with another inflammatory autoimmune disease such as rheumatoid arthritis, primary biliary cirrhosis, polymyositis, scleroderma or systemic lupus erythematosus (6). Severe fatigue is a common complaint in primary SS (8,9).
α –fodrin, the non-erythroid homolog of spectrin, is an autoantigen in SS. Antibodies to α-fodrin occur in SS. Immunization with α-fodrin induces SS in an animal model (10). Mucosal administration of α-fodrin has been demonstrated to inhibit experimentally induced SS in mice (11).
Spectrin consists of 2 non-identical subunits, α (MW 240,000) and β (MW 220,000 and constitutes a major component of the red blood cell (RBC) membrane skeleton. Spectrin localizes on the cytoplasmic side of the membrane and interacts with a number of proteins, forming an intracellular network. The RBC shape as well as elasticity of the lipid bilayer are controlled by such interactions (10,11).
Spectrin forms a tetramer by head-to-head association of αβ dimer pairs. An actin binding domain is located at either end of the tetramer in the N-terminal region of β spectrin. Protein 4.1 promotes the interaction of actin with spectrin. Actin filaments bring about the clustering of spectrin-4.1 complexes (spectrin/4.1/actin junctions). Tropomyosin, tropomodulin, adducin and dematin (4.9) are other proteins found in these junctions. Numerous membrane proteins are bound by protein 4.1, thus making these junctions to act as scaffolds for the assembly of protein complexes (10,12).
Free radical-mediated damage has been shown to be actively involved in the pathogenesis of SLE and other diseases (13–15). Reactive lipid peroxidation products can form adducts with lysine, histidine cysteine targets (16). One of the most common and reactive lipid oxidation products is 4-hydroxy-2-nonenal (HNE) (16). Higher levels of HNE-modified proteins have been found in with autoimmune diseases (13). HNE-protein adducts are potential neoantigens, and so could be involved in the pathogenesis of autoimmune diseases (17,18).
We have reported oxidatively modified proteins in the red cell membrane of SLE patients. Specifically, we found that catalase bound to red cell membrane is a possible protein target for 4-hydoxy-2-nonenal (HNE) (a by-product of oxidative damage to lipids) modification. We have also previously shown that immunization with HNE modified Ro60 induced accelerated autoimmunity by bringing about rapid intra and intermolecular epitope spreading.
We were interested to see whether animals immunized with Ro60 or HNE Ro60 would break tolerance to the spectrin autoantigen. We also hypothesized that the reverse would be true as well. That is, immunization of animals with human spectrin will bring about anti-spectrin antibodies and autoimmunity.
Materials and Methods
Materials
4-hydroxy-2-nonenal was purchased from Cayman Scientific, Ann Arbor, MI. Crithidia lucillae immunofluorescent anti-nDNA test kits were from Inova Diagnostics, San Diego, CA. Polylysine coated ELISA plates were from Fisher Scientific, Dallas, TX. Purified bovine Ro 60 was from Immunovision (Springdale, AK). Purified human spectrin, and anti-human spectrin were from Sigma Chemical Co., St. Louis, MO. Anti-rabbit IgG fluoroisothiocyanate was from Jackson Laboratories, Bar Harbor, ME. All other chemicals were of reagent grade.
Animals
The rabbits were fed standard rabbit feed and water ad libitum. The animals protocols were approved by the Institutional Animal Care and Use Committee according to established guidelines.
Ro 60 multiple antigenic peptides (MAPs)
Twenty-one MAPs were synthesized from the sequence of the Ro60 (19–21) at the Molecular Biology Resource Facility, University of Oklahoma Health Sciences Center, Oklahoma City by a manual stepwise solid phase procedure. An unrelated MAP with the sequence PPPGMRPP (22) from the Sm autoantigen was also synthesized.
Preparation of Ro 60 and HNE Ro 60 for immunization
Purified Ro60 was modified by the addition of 10 mM 4-hydroxy-2-nonenal (HNE) at room temperature for 24 hour in the presence of 10 mM sodium cyanoborohydride and then dialyzed against 0.1 N NaCl using a 10K molecular weight cut-off membrane. Ro60 treated with sodium cyanoborohydride and dialyzed parallel to HNE-Ro, served as the control (17).
Ro 60 rabbit immunization
New Zealand White rabbits were immunized with either unmodified Ro60 or HNE-modified Ro60. On day 1, 500 µg of either Ro60 or HNE-modified Ro60 was emulsified in 500 µl of complete Freund’s adjuvant and injected intraperitoneally and subcutaneously into the rabbit. Subsequent boosts, with 500 µg antigen in incomplete Freund’s adjuvant each time, were administered on day 26, 53, 99 with a final intravenous boost on day 152. The animals were bled weekly and sera obtained were stored at −20° C. Pre-immunization serum was collected from all animals.
Anti-spectrin antibody
Anti-spectrin antibody (Sigma Chemical Co.) was made by the manufacturer by immunizing rabbits with spectrin purified from freshly isolated red cell membranes. This antibody interacts specifically with the α and β chains of human erythrocyte spectrin.
Enzyme linked immunosorbant assay (ELISA)
Solid phase ELISA assays for anti-spectrin and anti-spectrin binding to Ro60 MAPs, Ro60 or La were performed as previously described (23,24). For inhibition studies, serum samples were incubated one hour at RT with inhibitor, Ro60, spectrin or La, at 10 µg/ml
Detection of antibodies to double stranded DNA by CLIF
Anti-dsDNA assays using Crithidia Lucillae Immunofluorescence (CLIF) dsDNA kit was carried out according to manufacturer’s instructions.
Anti-nuclear antibody (ANA) testing
ANA using HEp-2 cells was carried out following the instructions of the manufacturer.
Preparation of inside-out red cell membrane
Inside-out cell RBC membranes have been prepared using polylysine coated glass beads (25,26). We basically adapted this procedure to obtain inside-out RBC membranes on ELISA plates.
Peripheral blood collected in a heparinized tube was spun to remove plasma. Lymphocytes were removed using Lymphoprep according to instructions of the manufacturer.
200 µl of packed RBC’s were washed twice with PBST, containing 220 mM sucrose. From the washed packed cells, 12.5 µl was removed and diluted to 20 ml. A poly-D-lysine coated ELISA plate (96-well) was washed with PBST. 200 µl of diluted cells were added to the buffer-washed polylysine plate and incubated overnight at 4° C. The following day, the plate was washed once with PBS and blocked for 2 h with 0.03% milk. One half of the plate was sonicated (10 sec/well; setting # 4, Branson Sonicator, Danbury, CT) . The wells were washed twice with PBST and then blocked with 3% milk for 2 h at room temperature. Samples were added to the wells (1:100 dilution in 0.03% milk) and incubated at room temperature for two hours. The plate was then washed with PBST, followed by the addition of secondary antibody conjugated with alkaline phosphatase (1:5000) and incubated further for 1 hour at room temperature. The plate was washed with PBST 4–5 times and color developed with p-nitrophenyl phosphate substrate.
Results
Earlier data from our laboratory show that autoimmunity is induced in experimental animals upon immunization with either the whole Ro60 autoantigen or peptides derived from Ro60 antigen (17,18,22,24,27–29). Immunization with Ro60 or HNE-modified Ro60 led to epitope spreading to La, Sm B/B’ and 70K autoantigens. Here, we investigated epitope spreading to human spectrin in Ro60 and HNE-Ro60 immunized animals as well as epitope spreading to Ro60 and La in animals immunized with spectrin.
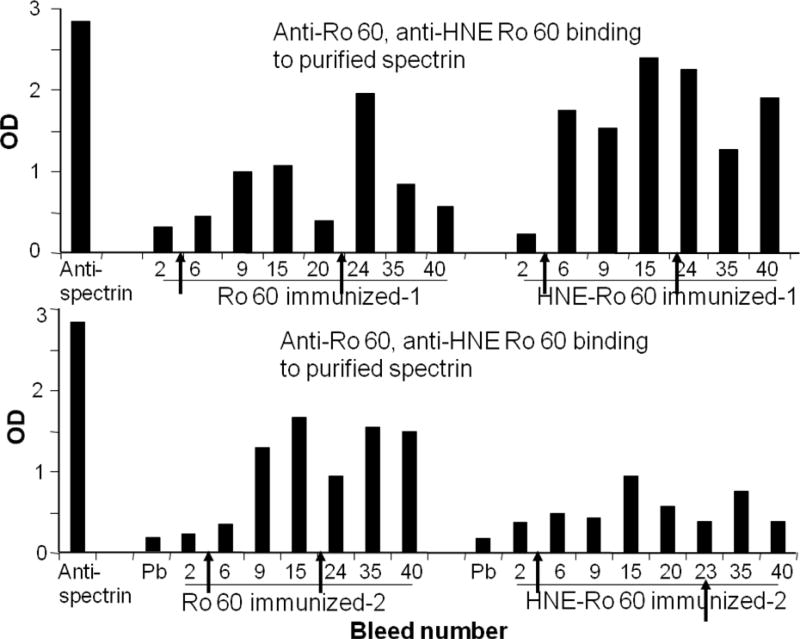
Figure 1 shows the binding of anti-Ro60 and anti-HNE Ro60 antibodies to purified spectrin by ELISA. Commercial anti-spectrin binds to solid phase spectrin with an OD of about 2.9. Anti-Ro60 as well as anti-HNE Ro60 antibodies bound to spectrin. Anti-spectrin antibodies were induced strongly by the 9th bleed in the Ro60 immunized rabbits, while it was induced by the 6th bleed in HNE Ro60 immunized rabbits.
Figure 1.
Serial bleeds of rabbits immunized with Ro60 or 4-hydroxy nonenal (HNE)-modified Ro 60 analyzed for binding to purified spectrin. Spectrin was coated on ELISA plates and binding of the rabbit anti-sera to spectrin was determined by ELISA. Anti-spectrin was used as the positive control. Arrow indicates time of boost given to the animals.
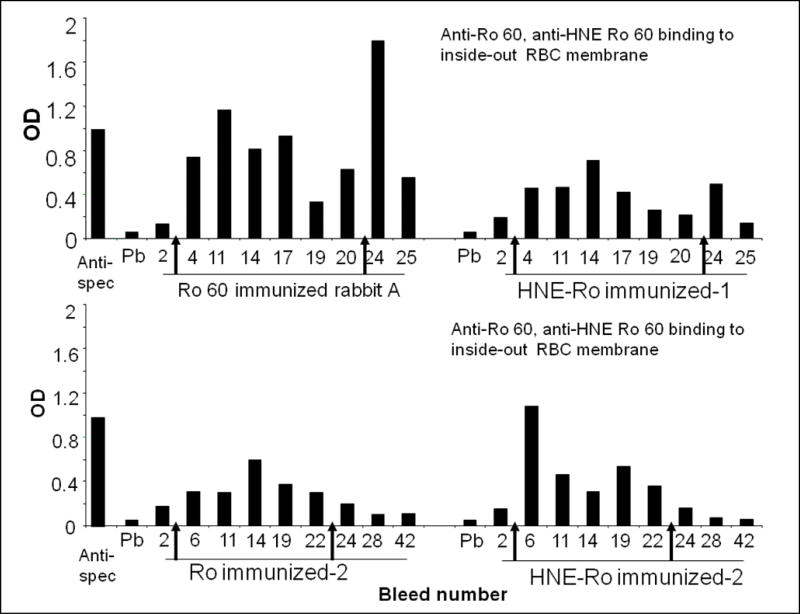
We prepared inside-out red cell membrane, to see whether anti-Ro60 and anti-HNE Ro60 rabbit sera would bind to spectrin lining the inner side of the cells. Commercial anti-spectrin antibody bound to the spectrin in the inside-out cell membrane preparation (Figure 2). However, the binding was not robust as the binding to purified spectrin observed in Figure 1. Anti-Ro60 and anti-HNE Ro60 antibodies also bound to the inside-out cell membrane preparation.
Figure 2.
Serial bleeds of rabbits immunized with Ro 60 or HNE-modified Ro 60 analyzed for binding to inside-out cell membrane immobilized to polylysine coated ELISA plates. Inside-out cell membrane was prepared as mentioned in Materials and methods. The plates were blocked and anti-Ro60 or anti-HNE Ro60 rabbit sera were added (1: 100 dilution). After addition of appropriate alkaline phosphatase conjugate, paranitrophenol phosphate substrate was added and the color developed was read.
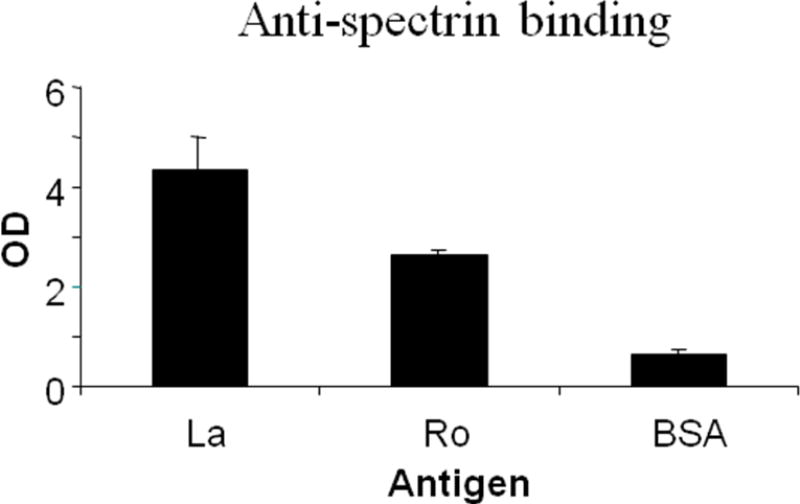
Figure 3 shows the binding of commercial anti-spectrin sera to La, Ro60 and BSA. As hypothesized, anti-spectrin bound very significantly to La and Ro60 (p< 0.0001), but not to BSA (Figure 3).
Figure 3.
Anti-spectrin binding to purified recombinant human La and to bovine serum albumin by ELISA. Ro 60, La or BSA were coated on ELISA plates and binding by anti-spectrin to these antigens was determined.
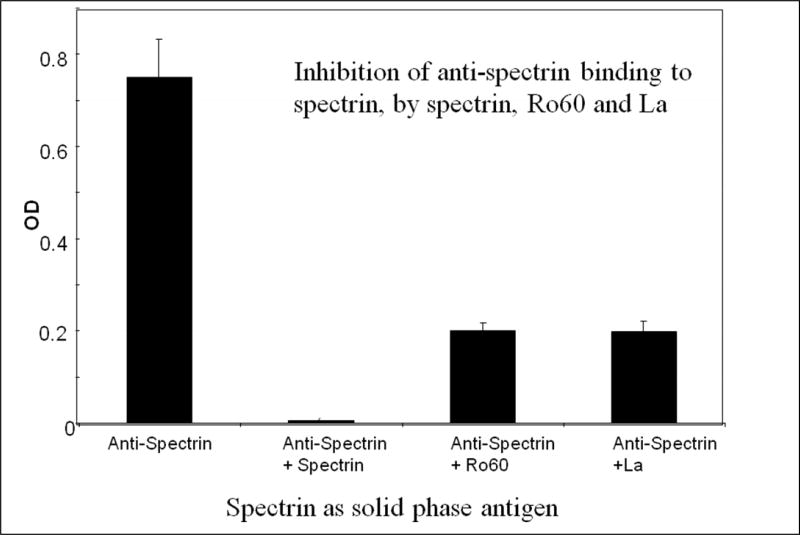
To test the specificity of binding, anti-spectrin was incubated with La, Ro60 or spectrin and tested for binding to spectrin used as solid phase antigen. As shown in Figure 4, spectrin completely inhibited the binding of anti-spectrin to spectrin. However, Ro60 or La inhibited the binding of anti-spectrin to spectrin by only 70%.
Figure 4.
Inhibition of anti-spectrin binding to spectrin in the absence and presence of spectrin, Ro or La. The antigens were incubated at room temperature for one hour with anti-spectrin (1:500 dilution) and binding of anti-spectrin or anti-spectrin + antigen to spectrin was investigated.
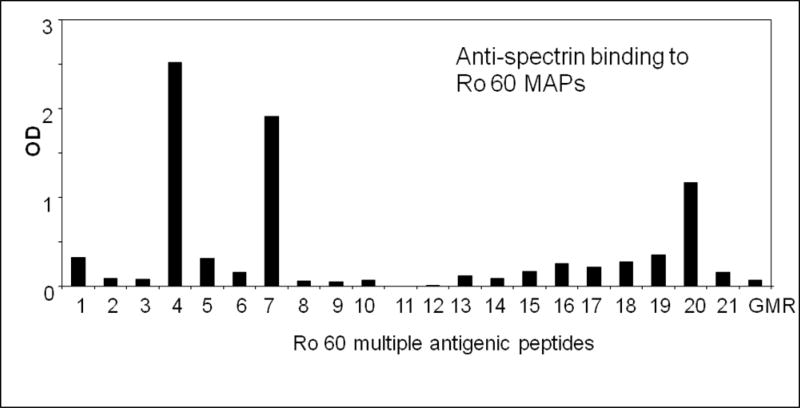
Since anti-spectrin bound Ro60 and La, we were interested to identify the epitopes recognized by anti-spectrin on Ro60 and La. We used 21 Ro60 multiple antigenic peptides (Table 1) as the solid phase antigen and investigated the binding of anti-spectrin to these peptides. We found three specific epitopes recognized by the anti-spectrin sera (Figure 5), namely TFIQFKKDLKES, LAVTKYKQRNGWSHK and LPMIWAQKTNTP (MAP#4, 7 and 20-Table 1). However, anti-spectrin did not bind to a peptide PPPGMRPP derived from Sm B/B’. Figure 6 shows anti-spectrin binding to human La recombinant fragments, coated on an ELISA plate. N4 fragment, spanning C-terminal 256 aa (aa 159 to 408) amino acids bound to anti-spectrin the strongest.
Table 1.
Ro 60 multiple antigenic peptides (MAPs), their sequence, amino acid position and molecular weight (measured by mass spectrometry)
| Map # | Amino acid sequence | Location on protein |
M. Wt. by mass spec. (in kD) |
|---|---|---|---|
| 1 | TYYIKEQKLGL | 45–55 | 11.69 |
| 2 | SQEGRTTKQ | 81–89 | 9.12 |
| 3 | STKQAAFKAV | 106–115 | 9.25 |
| 4 | TFIQFKKDLKES | 126–137 | 12.7 |
| 5 | MKCGMWGRA | 139–147 | 9.2 |
| 6 | MWGRALRKAIA | 143–153 | 11.02 |
| 7 | LAVTKYKQR-NGWSHK | 166–180 | 15.37 |
| 8 | LRLSHLKPS | 183–191 | 9.25 |
| 9 | VTKYITKGWKEVH | 198–210 | 13.55 |
| 10 | LYKEKALS | 212–219 | 8.45 |
| 11 | TEKLLKYL | 222–229 | 8.9 |
| 12 | EAVEKVKRTKD-ELE | 230–243 | 14.23 |
| 13 | HLLTNHLKSKE-VWKAL | 257–272 | 16.18 |
| 14 | ALLRNLGKMTA | 280–290 | 10.34 |
| 15 | NEKLLKKARIHPFH | 310–323 | 14.69 |
| 16 | YKTGHGLRGK-LKWRP | 331–345 | 15.22 |
| 17 | AAFYKTFKTV | 355–364 | 10.25 |
| 18 | VEPTGKRFL | 364–372 | 9.21 |
| 19 | MVVTRTEKDSY | 401–411 | 11.4 |
| 20 | LPMIWAQKTNTP | 449–460 | 12.04 |
| 21 | ALREYRKKMD-IPAK | 482–495 | 14.59 |
| 22 | PPPGRRPP | Sm | 7.63 |
Figure 5.
Intermolecular epitope spreading of anti-spectrin to Ro MAPs. MAPs constructed from the 60 kD Ro sequence were coated on ELISA plates, blocked and incubated with anti-spectrin rabbit antisera. A MAP from the Sm autoantigen, PPPGMRPP was used as control.
Figure 6.
Anti-spectrin binding to fragments of La showing intermolecular epitope spreading. BSA, La and fragments of La were coated on ELISA plates and the binding of anti-spectrin to these antigens was determined by ELISA. N4 corresponds to the C-terminal 256 amino acids (aa 159 to 408). N9 corresponds to the C-terminal 46 amino acids (aa 373 to 408).
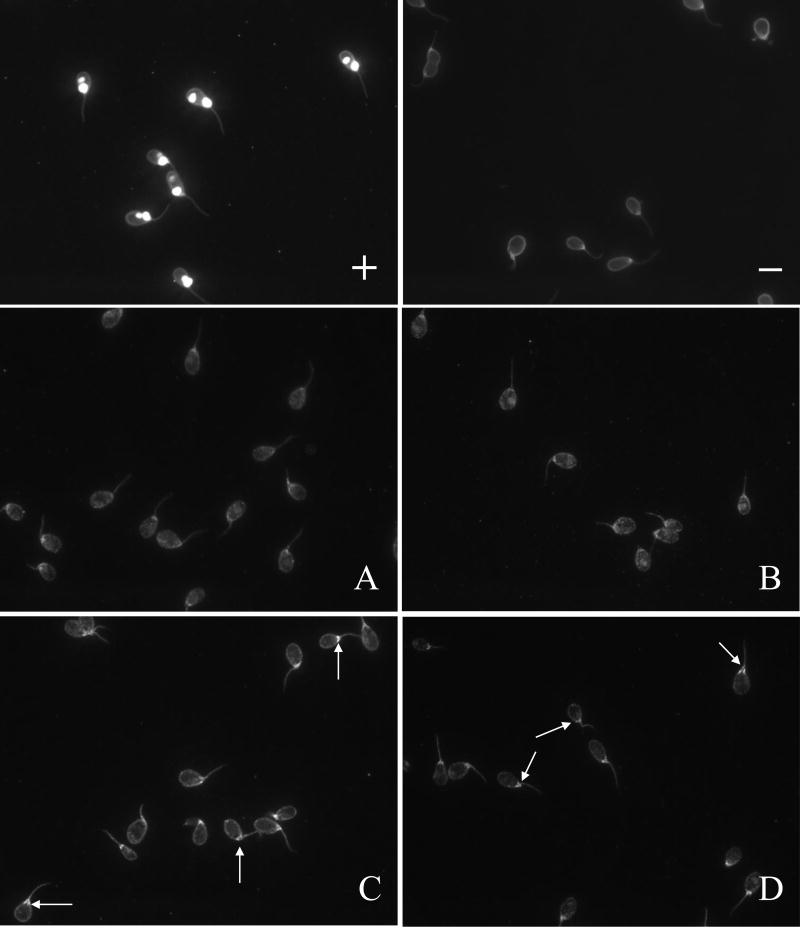
Finally we determined whether immunization with spectrin would bring about a lupus-like phenotype, in the form of induction of antibodies to double stranded DNA. When we tested anti-dsDNA levels in the anti-spectrin anti-sera we found that anti-dsDNA antibodies were induced upon immunization with spectrin (Figure 7).
Figure 7.
Antibodies to dsDNA detected by CLIF assay in rabbits immunized with spectrin, hemoglobin, or Freund’s control. A: Hemoglobin immunized; B: Freund’s immunized. C: Spectrin immunized; D: Spectrin immunized.
Discussion
Spectrin plays a vital role in the structural organization of plasma membranes of erythrocytes, and also in other cells in the form of its structural homologue fodrin. Spectrin has been shown to be associated with the membrane of organelles like exocytotic vesicles (30,31) or with electron-dense cytoplasmic islets (32). In addition, a β-spectrin homologue (bIS*) is associated with the Golgi apparatus (33).
α-fodrin genes are strictly conserved across species, while the mammalian spectrin genes have diverged rapidly. The alpha-chains of spectrin and fodrin are mainly composed of homologous 106-amino-acid repeat units. Spectrin α chain lacks a 37 amino-acid sequence bearing the calmodulin-binding site of α-fodrin. The prominent degree of homology between the alpha-chains of spectrin and fodrin resides in a central atypical segment that is not related to the canonical repeat sequence. The important central portion of β-spectrin is comprised of repeat units of 106 amino-acids, just like α-spectrin (34).
In addition to its structural role, fodrin has been reported to play an important regulatory role in secretion and exocytosis (35). Perrin et al, using permeabilized chromaffin cells, found that anti-fodrin inhibited secretion (36), showing that fodrin and the cytoskeleton take part in the exocytosis mechanism.
Owing to the position of spectrin or fodrin on the internal side of the membrane should, in theory, exclude any interaction with antibodies. However, data in literature and our experience both indicate the interaction of anti-spectrin antibodies with the RBC under certain conditions. Membrane complexes containing IgG, globin, band 3 and other polypeptides including spectrin, have been found in the denser RBC population (37), in sickle RBCs (38) and also in RBCs from β-thalassemic subjects (39). The ascertained anti-spectrin specificity of some RBC-bound IgG (40–42), as well as the appearance of antibodies against spectrin in some experimental hematological conditions (43) show that antibodies can interact with spectrin.
Haneji et al first identified the 120 kDa cleavage product of alpha-fodrin as a candidate autoantigen in experimental Sjögren’s syndrome (44) and cleaved alpha-fodrin has since been found as apopoptic marker in salivary glands of SS patients (45) (salivary glands specifically expresses alpha-fodrin). Antibodies targeting α-fodrin have been found in SS and SLE patients (44,46–48). SS has been induced by immunization with α-fodrin in an animal model (10). Mucosal administration of α-fodrin inhibits experimentally induced SS in mice (11). Savuz et al observed IgA and IgG anti-spectrin with a frequency similar to anti-Ro/SSA and anti-La/SSB in the serum of patients with SS (47). Haneji et al first showed (44) showed that anti-alpha-fodrin have a higher specificity and sensitivity than anti-Ro60 and anti-La antibodies in adult SS patients.
Ro60 is a common target in SLE and SS. Immunization of experimental animals with Ro60 or HNE-Ro60 induces intermolecular epitope spreading to La and other autoantigens (17,18,24,28,29). Similarly immunization with La brings about intermolecular epitope spreading to the Ro60 autoantigen (49).
In this work we demonstrate that immunization with Ro60 or HNE Ro60 induces intermolecular epitope spreading to the spectrin autoantigen. We also show that the reverse is possible. That is, immunization with spectrin leads to cross-reactive intermolecular epitope spreading to the Ro60 and La autoantigens. The interaction of anti-spectrin with Ro60 is mediated via binding through three specific sequences on Ro60, namely amino acids 126–137, 166–180 and 449–460. Interestingly, anti-spectrin binds to two epitopes of Ro60 (Ro 166–180 and Ro 126–137) that are among the targets bound by some human SLE patients that are positive for anti-Ro60 (50). Ro 169–180 sequence is the epitope targeted first by these SLE patients, as they develop the disease. Following this, the antibody binding spreads to other regions of the Ro60 molecule in these patients, including Ro 126–137 and Ro 449–460 (50). The interaction with La is mediated by binding to amino acids 159 to 408 on the La autoantigen. It is interesting that Ro 60 or La do not share any significant sequence similarity with spectrin or fodrin, and yet these antigens are able to inhibit anti-spectrin binding to spectrin by 70%. The observed reactivity is likely a result of cross-reactive conformational epitopes brought about by epitope spreading. The Ro60, especially the HNE Ro60, immunized animals developed anti-Ro60, anti-La, anti-Sm, anti-RNP A and anti-double stranded DNA, thus developing a lupus-like condition. The fact that spectrin immunization induced anti-Ro60, anti-La and antibodies to double stranded DNA also suggests the development of a lupus phenotype.
We have not monitored salivary flow or lymphocytic infiltration in the salivary glands of the Ro60, HNE-Ro60 or the spectrin immunized animals. However, it is our hypothesis that these animals will have a normal salivary flow and would not have lymphocytic infiltration of the salivary glands owing to the observed development of anti-dsDNA antibodies in these animals. Anti-dsDNA is normally not seen in primary SS patients. Therefore, anti-spectrin development in the Ro60/HNE-Ro60 immunized animals and anti-Ro60/anti-La development in the spectrin immunized mice points more to these animals as being a model of SLE rather than primary SS.
Even though studies have shown elevated anti-fodrin activity in Sjögrens syndrome, other studies report the non-specificity of these antibodies in SS (51,52). Anti-spectrin also has been shown to be part of the normal repertoire in human and animals (51,53–57). A more recent study (58) has shown that anti-alpha fodrin is present in almost twice as many non-SS (with sicca symptoms only) compared to SS patients with anti-Ro60, anti-La and sicca. This study also showed using sialoscintigraphy that almost thrice as many of the non-SS subjects had grade III impairment of the salivary gland compared to SS subjects. The authors suggest that sialoscintigraphy could be used along with serum anti-alpha-fodrin, anti-Ro and anti-La to distinguish between SS and non-SS sicca (58).
Nordmark et al found anti-fodrin antibodies in 16/56 (29%) of subjects with primary SS and in 25/53 (47%) of subjects with SLE (without secondary SS). However, these authors suggest that alpha-fodrin autoantibodies are mainly related to non-organ-specific autoimmunity in primary SS and SLE and only have limited discriminating value (48). SLE serum, mediated by complement fragment deposition on RBC membrane, has been reported to induce dephosphorylation of β-spectrin bringing about reduced RBC membrane deformability and increase in RBC production of nitric oxide (59). Thus, complement activation in SLE patients is thought to lead to calcium-dependent changes in the RBC cytosketeleton making RBCs less deformable. This can impair the flow through capillaries and negatively affect oxygen delivery to the tissues (59).
Anti-spectrin has not been described in animal models of autoimmunity, as far as we know. The fact that we have observed anti-spectrin antibodies in our animal models of autoimmunity suggests that these antibodies might have a pathogenic role.
References
- 1.Yamagata H, Harley JB, Reichlin M. Molecular properties of the Ro/SSA antigen and ELISA for quantitation of antibody. Journal of Clinical Investigation. 1984;74:625–33. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harley JB, Yamagata H, Reichlin M. Anti-La/SSB antibody is present in some normals and is coincident with anti-Ro/SSA precipitins in systemic lupus erythematosus. Journal of Rheumatology. 1984;11:309–14. [PubMed] [Google Scholar]
- 3.Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren’s syndrome and systemic lupus erythematosus. Rheumatic Disease Clinics of North America. 1992;18:337–58. [PubMed] [Google Scholar]
- 4.Harley JB, Sestak AS, Willis LG, Fu SM, Hansen JA, Reichlin M. A model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies and lymphopenia or renal disease. Arthritis and Rheumatism. 1989;32:826–36. [PubMed] [Google Scholar]
- 5.Kurien BT, Newland JG, Paczkowski C, Moore KL, Scofield RH. Anti-Ro mediates neutropenia in systemic lupus erythematosus by binding a 60,000 MW antigen on the surface of neutrophils. Clinican and Experimental Immunology. 2000;120:209–17. doi: 10.1046/j.1365-2249.2000.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scofield RH, Asfa S, Obeso D, Jonsson R, Kurien BT. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren’s syndrome. Journal of Immunology. 2005;175:8409–14. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- 7.Bowman SJ. Patient-reported outcomes including fatigue in primary Sjögren’s syndrome. Rheumatic Diseases Clinics of North America. 2008;34:949–62. doi: 10.1016/j.rdc.2008.08.010. ix. Review. [DOI] [PubMed] [Google Scholar]
- 8.Priori R, Iannuccelli C, Alessandri C, Modesti M, Antonazzo B, Di Lollo AC, Valesini G, Di Franco M. Fatigue in Sjogren’s syndrome: relationship with fibromyalgia, clinical and biologic features. Clinical and Experimental Rheumatology. 2010;28(6 Suppl 63):S82–6. [PubMed] [Google Scholar]
- 9.Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjögren’s syndrome. Journal of Dental Research. 2008;87:308–18. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 10.He J, Wang H, Zhao JX, Li ZG. [Establishment of Sjögren’s syndrome models by immunization with alpha-Fodrin: experiment with mice] Zhonghua Yi Xue Za Zhi. 2008;88:2360–3. [PubMed] [Google Scholar]
- 11.He J, Zhao JX, Wang H, Li ZG. [Mucosal administration of alpha-fodrin inhibits experimental Sjögren’s syndrome autoimmunity] Zhonghua Yi Xue Za Zhi. 2008;88:625–9. [PubMed] [Google Scholar]
- 12.Bennett V, Gilligan DM. The Spectrin-Based Membrane Skeleton and Micron-Scale Organization of the Plasma Membrane. Annual Review of Cell Biology. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- 13.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radical Biology and Medicine. 1997;23:357–60. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 14.Turi S, Nemeth I, Torkos A, Saghy L, Vargha I, Matcovics B, Nagy J. Oxidative stress and antioxidant defense mechanism in glomerular diseases. Free Radical Biology and Medicine. 1997;22:161–8. doi: 10.1016/s0891-5849(96)00284-5. [DOI] [PubMed] [Google Scholar]
- 15.Serban MG, Tanaseanu S, Bara C. Oxidant stress and antioxidant protection in lupus nephropathy. Romanian Journal of Internal Medicine. 1996;34:105–9. [PubMed] [Google Scholar]
- 16.Uchida K, Kanamatsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation. Formation of free acrolein and its conjugation with lysine residues in oxidized low density lipoproteins. Journal of Biological Chemistry. 1998;273:16058–66. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 17.Scofield RH, Kurien BT, Ganick S, McClain MT, Pye Q, James JA, Schneider RI, Broyles RH, Bachmann M, Hensley K. Modification of lupus-associated 60 kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radical Biology and Medicine. 2005;38:719–28. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Kurien BT, Porter A, Dorri Y, Iqbal S, D’Souza A, Singh A, Asfa S, Cartellieri M, Matsumoto H, Bachmann M, Hensley K, Scofield RH. Degree of modification of Ro60 by the lipid peroxidation by-product 4-hydroxy-2-nonenal may differentially induce Sjögren syndrome or systemic lupus erythematosus in BALB/c mice. Free Radical Biology and Medicine. 2011;50:1222–33. doi: 10.1016/j.freeradbiomed.2010.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60 kDa human Ro ribonucleoprotein. Proceedings of the National Academy of Sciences USA. 1988;85:9479–83. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scofield RH, Kurien BT, Zhang F, Mehta P, Kaufman K, Gross T, Bachmann M, Gordon T, Harley JB. Protein-protein interaction of the Ro-ribonucleoprotein particle using multiple antigenic peptides. Molecular Immunology. 1999;36:1093–106. doi: 10.1016/s0161-5890(99)00095-4. [DOI] [PubMed] [Google Scholar]
- 21.Kurien BT, Scofield RH. Immunoblotting of multiple antigenic peptides. Electrophoresis. 1998;19:1659–61. doi: 10.1002/elps.1150191023. [DOI] [PubMed] [Google Scholar]
- 22.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. Journal of Experimental Medicine. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang SC, Pan Z, Kurien BT, James JA, Harley JB, Scofield RH. Immunization with Vesicular stomatitis virus nucleocapsid protein induces autoantibodies to the 60 kD Ro ribonucleoprotein particle. Journal of Investigative Medicine. 1995;43:151–8. [PubMed] [Google Scholar]
- 24.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides derived from the systemic lupus erythematosus associated 60 kDa Ro autoantigen results in autoimmunity directed towards the Ro ribonucleoprotein. Journal of Immunology. 1996;156:4059–66. [PubMed] [Google Scholar]
- 25.Kalish DI, Cohen CM, Jacobson BS, Branton D. Membrane isolation on polylysine- coated glass beads. Asymmetry of bound membrane. Biochimica et Biophysica Acta. 1978;506:97–110. doi: 10.1016/0005-2736(78)90437-6. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson BS, Branton D. Plasma membrane: rapid isolation and exposure of the cytoplasmic surface by use of positively charged beads. Science. 1977;195:302–4. doi: 10.1126/science.831278. [DOI] [PubMed] [Google Scholar]
- 27.Kurien BT, Asfa S, Li C, Dorri Y, Jonsson R, Scofield RH. Induction of oral tolerance in experimental Sjogren’s syndrome autoimmunity. Scandinavian Journal of Immunology. 2005;61:418–25. doi: 10.1111/j.1365-3083.2005.01593.x. [DOI] [PubMed] [Google Scholar]
- 28.Scofield RH, Pierce PG, James JA, Kaufman KM, Kurien BT. Immunization with Peptides from 60 kDa Ro in Diverse Mouse Strains. Scandinavian Journal of Immunology. 2002;56:477–483. doi: 10.1046/j.1365-3083.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 29.Scofield RH, Kaufman KM, Baber U, James JA, Harley JB, Kurien BT. Immunization of mouse with 60 kD Ro peptides results in epitope spreading if peptides are highly homologous between man and mouse. Arthritis Rheum. 1999;42:1017–1024. doi: 10.1002/1529-0131(199905)42:5<1017::AID-ANR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Hirokawa NR, Cheney E, Villard M. Localization of a protein of the fodrin-spectrin-TW 260/240 family in the mouse intestinal brush border. Cell. 1983;32:953–965. doi: 10.1016/0092-8674(83)90080-6. [DOI] [PubMed] [Google Scholar]
- 31.Fishkind DJ, Bonder EM, Begg DA. Subcellular localization of sea urchin spectrin: evidence for assembly of the membraneskeleton on unique classes of vesicles in eggs and embryos. Develepmental Biology. 1990;142:439–452. doi: 10.1016/0012-1606(90)90366-q. [DOI] [PubMed] [Google Scholar]
- 32.Black JD, Koury ST, Bankert RB, Repasky EA. Heterogeneity in lymphocyte spectrin distribuition: Ultrastructural identification of a new spectrin-rich cytoplasmic structure. Journal of Cell Biology. 1988;106:97–109. doi: 10.1083/jcb.106.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: identification of an erythroid beta-spectrin homolog associated with the Golgi complex. Journal of Cell Biology. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhermy D. The spectrin super-family. Biology of the Cell. 1991;71:249–54. [PubMed] [Google Scholar]
- 35.Aunis D, Bader MF. The cytoskeleton as a barrier to exocytosis in secretory cells. Journal of Experimental Biology. 1988;139:253–66. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- 36.Perrin D, Langely K, Aunis D. Anti-alpha fodrin inhibits secretion from Permeabilized chromaffin cells. Nature. 1987;326:498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- 37.Kannan R, Yuan J, Low PS. Isolation and partial characterization of antibody-and globin-enriched complexes from membranes of dense human erythrocytes. Biochemical Journal. 1991;278:57–62. doi: 10.1042/bj2780057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kannan R, Labotka R, Low PS. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. Journal of Biological Chemistry. 1988;263:13766–73. [PubMed] [Google Scholar]
- 39.Yuan J, Kannan R, Shinar E, Rachmilewitz EA, Low PS. Isolation, characterization, and immunoprecipitation studies of immune complexes from membranes of beta-thalassemic erythrocytes. Blood. 1992;79:3007–13. [PubMed] [Google Scholar]
- 40.Wiener E, Hughes-Jones NC, Irish WT, Wickramasinghe SN. Elution of Antispectrin antibodies from red cells in homozygous beta-thalassaemia. Clinical and Experimental Immunology. 1986;63:680–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Sorette MP, Clark MR. Specificity of autologous antibody binding to phenylhydrazine-treated human erythrocytes: implications for models of red blood cell aging. Journal of Laboratory and Clinical Medicine. 1991;117:477–84. [PubMed] [Google Scholar]
- 42.Wiener E, Wanachiwanawin W, Kotipan K, Fucharoen S, Wasi P, Wickramasinghe SN. Erythroblast- and erythrocyte-bound antibodies in alpha and beta thalassaemia syndromes. Transfusion Medicine. 1991;1:229–38. doi: 10.1111/j.1365-3148.1991.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 43.Day MJ, Russell J, Kitwood AJ, Ponsford M, Elson CJ. Expression and regulation of erythrocyte auto-antibodies in mice following immunization with rat erythrocytes. European Journal of Immunology. 1989;19:795–801. doi: 10.1002/eji.1830190503. [DOI] [PubMed] [Google Scholar]
- 44.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Virji AS, Howard P, Sayani Y, Zhang J, Achu P, McArthur C. Detection of cleaved alpha-fodrin autoantigen in Sjögren’s syndrome: apoptosis and co-localisation of cleaved alpha-fodrin with activated caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP) in labial salivary glands. Archives of Oral Biology. 2006;51:558–66. doi: 10.1016/j.archoralbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Ulbricht KU, Schmidt RE, Witte T. Antibodies against alphafodrin in Sjogren’s syndrome. Autoimmunity Reviews. 2003;2:109–13. doi: 10.1016/s1568-9972(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 47.Yavuz S, Toker E, Bicakcigil M, Mumcu G, Cakir S. Comparative analysis of autoantibodies against a-fodrin in serum, tear fluid, and saliva from patients with Sjögren’s syndrome. Journal of Rheumatology. 2006;33:1289–92. [PubMed] [Google Scholar]
- 48.Nordmark G, Rorsman F, Rönnblom L, Cajander S, Taussig MJ, Kämpe O, Winqvist O. Autoantibodies to alpha-fodrin in primary Sjögren’s syndrome and SLE detected by an in vitro transcription and translation assay. Clinical and Experimental Rheumatology. 2003;21:49–56. [PubMed] [Google Scholar]
- 49.Farris AD, Brown L, Reynolds P, Harley JB, James JA, Scofield RH, McCluskey J, Gordon TP. Induction of autoimmunity by multivalent immunodominant and Subdominant T cell determinants of La (SS-B) Journal of Immunology. 1999;162:3079–87. [PubMed] [Google Scholar]
- 50.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in Lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature Medicine. 2005;11:85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 51.Moody M, Zipp M, Al-Hashimi I. Salivary anti-spectrin autoantibodies in Sjögren’s syndrome. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology. 2001;91:322–7. doi: 10.1067/moe.2001.112498. [DOI] [PubMed] [Google Scholar]
- 52.Turkçapar N, Olmez U, Tutkak H, Duman M. The importance of alpha-fodrin antibodies in the diagnosis of Sjögren’s syndrome. Rheumatology International. 2006;26:354–9. doi: 10.1007/s00296-005-0607-9. [DOI] [PubMed] [Google Scholar]
- 53.Giuliani AL, Graldi G, Veronesi M, Previato A, Simoni M, Bergamini C, Berti G. Binding of anti-spectrin antibodies to red blood cells and vesiculation in various in vivo and in vitro ageing conditions in the rat. Experimental Gerontology. 2000;35:1045–59. doi: 10.1016/s0531-5565(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 54.Graldi G, Giuliani AL, Unis L, Pora R, Verenini M, Lorenzini F, Melandri P, Torboli M, Bergamini C, Berti G. Accelerated elimination from the circulation of homologous aged red blood cells in rats bearing anti-spectrin antibodies. Mechanisms of Ageing and Development. 1999;107:21–36. doi: 10.1016/s0047-6374(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 55.Berti G, Govoni M, Ventrelli I. Autoantibodies against spectrin in rats. Bollettino-Societa Italiana Biologia Sperimentale (Napoli) 1989;65:29–36. [PubMed] [Google Scholar]
- 56.Ballas SK. Spectrin autoantibodies in normal human serum and in polyclonal blood grouping sera. British Journal of Haematology. 1989;71:137–9. doi: 10.1111/j.1365-2141.1989.tb06287.x. [DOI] [PubMed] [Google Scholar]
- 57.Lutz HU, Flepp R, Stammler P, Baccalà R. Red cell associated, naturally occurring anti-spectrin antibodies. Clinical and Experimental Immunology. 1987;67:674–6. [PMC free article] [PubMed] [Google Scholar]
- 58.Chen KS, Jiang MC, Li CJ, Liu OK, Tsai CS. Discrimination between Sjögren’s and non-Sjögren’s sicca syndrome by sialoscintigraphy and antibodies against alpha-fodrin and Ro/La autoantigens. Journal of International Medical Research. 2009;37:1088–96. doi: 10.1177/147323000903700413. [DOI] [PubMed] [Google Scholar]
- 59.Ghiran IC, Zeidel ML, Shevkoplyas SS, Burns JM, Tsokos GC, Kyttaris VC. Systemic lupus erythematosus serum deposits C4d on red blood cells, decreases Red blood cell membrane deformability, and promotes nitric oxide production. Arthritis and Rheumatism. 2011;63:503–12. doi: 10.1002/art.30143. [DOI] [PMC free article] [PubMed] [Google Scholar]