Abstract
Biased attention to emotional stimuli plays a key role in the RDoC constructs of Sustained Threat and Loss. In this article, we review approaches to assessing these biases, their links with psychopathology, and the underlying neural influences. We then review evidence from twin and candidate gene studies regarding genetic influences on attentional biases. We also discuss the impact of developmental and environmental influences and end with a number of suggestions for future research in this area.
A key goal of the Research Domain Criteria (RDoC) project, which grew out of Strategy 1.4 of the 2008 NIMH Strategic Plan, is to “develop new ways of classifying disorders based on dimensions of observable behaviors and brain functions”. A promising line of research toward this goal focuses on individual differences in the processing of emotional information. The ability to flexibly allocate one’s attention to relevant social cues in the environment is essential for adaptive functioning and deficits in this ability play a central role in various domains of psychopathology, including depression and anxiety. Indeed, as highlighted by the workshop on the RDoC Negative Valence Systems Domain, the constructs of Loss and Sustained Threat are both characterized by biases in attention to specific types of affectively-salient stimuli. 1
The RDoC matrix defines constructs across multiple units of analysis including genes, molecules, cells, neural circuits, physiology, behavior, and self report. Attentional biases can be assessed at the behavioral (via reaction times or eye tracking) or physiological (via event-related potentials [ERPs]) unit of analysis. At the neural circuit unit of analysis, attentional biases relevant to both constructs are thought to be driven by a combination of bottom-up and top-down processes within the brain’s emotional circuitry (particularly amygdala and regions of prefrontal cortex; for reviews, see Bishop, 2008; De Raedt & Koster, 2010; Disner, Beevers, Haigh, & Beck, 2011; Frewen, Dozois, Joanisse, & Neufeld, 2008).
Despite common neural influences, however, the focus and time course of these attentional biases is hypothesized to differ across the two RDoC constructs with the Loss construct associated specifically with difficulty disengaging attention from stimuli reflecting themes of sadness or loss (e.g., sad faces) and the Sustained Threat construct associated specifically with quick initial orienting of attention to threat-relevant stimuli (e.g., angry or fearful faces). At the physiological unit of analysis for both the Loss and Sustained Threat constructs, the RDoC matrix highlights disruption in HPA axis activity, which is reciprocally associated with attentional biases (Browning, Holmes, Charles, Cowen, & Harmer, 2012; Hakamata et al., 2013; Pilgrim, Marin, & Lupien, 2010; Ursache & Blair, 2015; van Honk et al., 1998) and likely plays a role in the impact of environmental influences on these biases, further highlighting links across units of analysis for these constructs.
The primary goal of this review is to discuss what is currently known about genetic influences on attentional biases. Before doing this, however, we first review methods used to assess attentional biases, the relations of these measures with depression and anxiety, and neural influences thought to underlie these biases. We then review research examining genetic influences on attentional biases. In doing so, we highlight both twin and candidate gene studies. We also discuss the impact of developmental and environmental influences, highlighting how they may interact with genetic influences. Finally, we end with a number of suggestions for future research in this area. At the outset, we must acknowledge that although RDoC focuses on constructs that may cut across current diagnostic boundaries, most of the extant research on attentional biases has focused on differences between diagnostic groups rather than associations with more focused, continuous symptom domains (e.g., low positive affect or anxious arousal) that may be more specifically associated with disruptions across units of analysis in each construct. This said, a review of current research provides an important starting point for discussing the current state of the field and important areas of research for studies seeking to understand the role of attentional biases in the RDoC Loss and Sustained Threat constructs.
Measurement of Attentional Biases
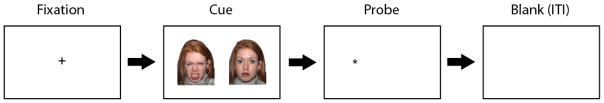
The method most commonly used by researchers to assess attentional biases in depression and anxiety is the dot probe task (MacLeod, Mathews, & Tata, 1986), which is depicted in Figure 1. As can be seen in the figure, each trial starts with a fixation cross, typically presented for 500ms. Next, two stimuli, one emotional and one neutral, are presented simultaneously on a computer screen. After a given amount of time (e.g., 500ms or 1000ms) the stimuli disappear and a probe appears in the same location as one of the stimuli. Participants are asked to respond as quickly as possible to the location of the probe (e.g., left or right side of screen) or the type of probe presented (e.g., letter “e” or “f”). Following this is a blank screen for a given inter-trial interval (ITI) before the start of the next trial.
Figure 1.
Sample trial in the dot probe task. Each trial starts with a fixation cross, typically presented for 500ms. Next, two stimuli, one emotional and one neutral, are presented simultaneously on a computer screen. After a given amount of time (e.g., 500ms or 1000ms) the stimuli disappear and a probe appears in the same location as one of the stimuli. Participants are asked to respond as quickly as possible to the location of the probe (e.g., left or right side of screen) or the type of probe presented (e.g., letter “e” or “f”). Following this is a blank screen for a given inter-trial interval (ITI) before the start of the next trial.
Traditionally, researchers have focused on reaction time (RT) indices of attentional bias derived from the dot-probe task based on the assumption that reaction times to the probe will be faster if one’s attention is already allocated to that side of the visual field. Therefore, preferential attention to emotional information is inferred when reaction times are quicker to probes occurring in the same location as the emotional stimuli than to probes occurring in the same location as the neutral stimuli. In contrast, attentional avoidance of emotional information is inferred when reaction times are quicker to probes occurring in the same location as the neutral stimuli than to probes occurring in the same location as the emotional stimuli. In addition to RT indices of attentional bias, more recent research has utilized eye tracking (for a review, see Armstrong & Olatunji, 2012) or event-related potentials (e.g., Holmes, Bradley, Kragh Nielsen, & Mogg, 2009; Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b)) to gain a more direct measure of attentional allocation and to differentiate initial orienting versus sustained attention in this task.
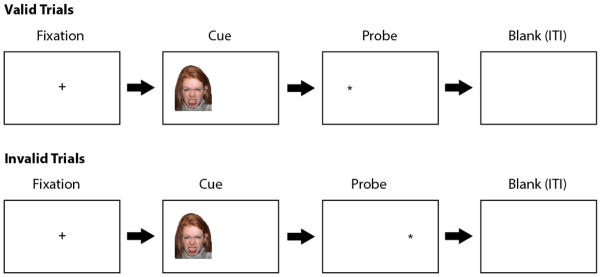
Another commonly used assessment of attentional bias is an emotional variant of the Posner spatial cueing task (Posner, 2007). As can be seen in Figure 2, this task is similar to the dot probe but only one stimulus appears on the screen at a time, which serves as either a valid or invalid cue for the location of the probe when it appears. On “valid” trials, which typically occur on approximately 80% of the trials, the probe appears on the same side of the screen as the stimulus. In contrast, on “invalid” trials (~20% of trials), the probe appears on the opposite side of the screen as the stimulus. As with the dot probe task, participants are asked to respond as quickly as possible to the location or type of probe presented. One potential benefit of this task compared to the dot probe is that it may provide a more specific index of attentional engagement versus disengagement since only one stimulus is presented on the screen at a time. Although researchers have typically focused on RT indices of attentional bias in this task, some studies have also used this task to collect ERP indices of attentional bias (e.g., Pollak & Tolley-Schell, 2003; Pollak, Klorman, Thatcher, & Cicchetti, 2001).
Figure 2.
Sample trials in the Posner spatial cueing task. Each trial starts with a fixation cross, typically presented for 500ms. Next, one stimulus, either emotional or neutral, is presented on one side of the computer screen. After a given amount of time (e.g., 500ms or 1000ms) the stimulus disappears and a probe appears on either the same side of the screen (“valid” trials; ~80% of the trials) or on the opposite side of the screen (“invalid” trials; ~20% of trials). Participants are asked to respond as quickly as possible to the location of the probe (e.g., left or right side of screen) or the type of probe presented (e.g., letter “e” or “f”). Following this is a blank screen for a given inter-trial interval (ITI) before the start of the next trial.
More recently, researchers have begun to use passive viewing tasks in which participants are shown multiple stimuli on a computer screen at the same time while their eye gaze patterns are recorded with an eye tracker. An example of this type of task is presented in Figure 3. As can be seen in the figure, each trial starts with a fixation cross and then four emotional stimuli are presented, one in each quadrant of the screen. These stimulus arrays can include emotional faces or scenes and are typically presented for 20–30 seconds during which participants are simply asked to look at the screen as they would a picture book or TV. An eye tracker is used to track patterns of gaze throughout each trial. Another variant of a passive viewing task includes only a single stimulus on the screen at a time. For these types of tasks, either an eye tracker is used to track fixations to various areas of interest within each stimulus or ERPs are used to index neural reactivity to the image.
Figure 3.
Sample trial in a passive viewing task. Each trial starts with a fixation cross and then four emotional stimuli are presented, one in each quadrant of the screen. These stimulus arrays can include emotional faces or scenes and are typically presented for 20–30 seconds during which participants are simply asked to look at the screen as they would a picture book or TV. An eye tracker is used to track patterns of gaze throughout each trial.
Finally, we should note that some researchers have used an emotional variation of the classic Stroop task (Stroop, 1935) as a measure of attention (or the resolution of attentional interference). In this task, participants are asked to name the colors of variously presented emotional and nonemotional words. The primary assumption for this task is that responses will be slower if attention is captured by the content of the word rather than the color. Words with greater self-relevance (i.e., threat words to someone who is anxious) should theoretically create greater attentional interference with naming the word color. Although this task has been used frequently, there is concern that the emotional Stroop test does not provide a clear or precise index of attentional bias (Algom, Chajut, & Lev, 2004). Other assessments that more directly measure the resolution of attentional interference should be considered, such as flanker tasks with emotion stimuli, for future research in this area (cf. Pe, Vandekerckhove, & Kuppens, 2013).
Psychometric Properties of Various Methods Used to Assess Attentional Biases
Before moving on, is it important to discuss the psychometric properties of the various indices of attentional bias used in research, particularly their reliability. This is important because reliability places an upper limit on validity (i.e., the observed correlation between two variables is equal to the true correlation of those two variables multiplied by the square root of the product of the reliability of each variable). If we hope to use attentional biases as (endo)phenotypes for genetic association studies, therefore, the reliability of our attention bias index can dramatically affect our risk of Type II (and Type I) errors.
As noted above, the vast majority of studies that have examined attentional biases relevant to depression and anxiety have utilized attentional bias scores derived from RTs in the dot probe task. However, recent research examining the psychometric properties of RT indices of attentional bias have shown that they have poor split-half and retest reliability (Brown et al., 2014; Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Price et al., 2015; Schmukle, 2005; Staugaard, 2009). For example, studies have yielded split-half reliability estimates of -.18 to .35 for RT indices of bias (Brown et al., 2014; Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Price et al., 2015; Staugaard, 2009). Although rarely examined, there is evidence that eye tracking (Price et al., 2015) and ERP (Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Kujawa, Klein, & Proudfit, 2013; Moran, Jendrusina, & Moser, 2013) indices of attentional bias have stronger reliability than RT indices. For example, studies have yielded split-half reliability values of .32 to .33 for eye-tracking indices of bias (Price et al., 2015) and .52 to .79 for ERP indices of bias (Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Moran et al., 2013). Further, RT indices of attention bias are not significantly correlated with indices of bias obtained via eye tracking (Mogg, Millar, & Bradley, 2000; Stevens, Rist, & Gerlach, 2011) or ERPs (Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b). Based on this, it appears that ERP indices of bias have the strongest reliability followed by eye tracking indices. In contrast, the reliability of RT indices of attention bias are highly variable, sometimes yielding a negative correlation between values obtained for even and odd trials. This said, new approaches that rely on trial level RT data have been proposed and appear promising (e.g., split half reliability ranged from .58 – .67; Zvielli, Bernstein, & Koster, 2014), although additional validation is needed.
Links with Depression and Anxiety
Studies Using Reaction Time Indices of Attentional Bias
Despite questions about the reliability of RT indices of attentional biases, research using these indices from the dot probe task has found strong support for the hypothesis that anxiety and depression are associated with attentional biases for disorder-specific stimuli. Specifically, symptoms and diagnoses of anxiety are associated with attentional biases for threat-relevant stimuli (e.g., angry or fearful faces) whereas symptoms and diagnoses of depression are associated with attentional bias for depression-relevant stimuli (e.g., sad faces; for reviews, see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Gotlib & Joormann, 2010; Joormann & Arditte, 2014; Peckham, McHugh, & Otto, 2010).
Studies have also supported cognitive theories regarding the time course of these biases. For example, consistent with the hypothesis that anxiety is associated with biases in the initial allocation of attention, but not sustained attention, findings are stronger with shorter stimulus presentation durations (≤500ms) than longer stimulus presentation durations (≥1000ms) (Bar-Haim et al., 2007). In contrast, studies examining attentional biases in depression have found stronger support when using relatively longer stimulus presentation durations (≥ 1000ms), which is consistent with the hypothesis that depression is associated with biases in sustained attention or difficulty disengaging attention from depression-relevant stimuli (Gotlib & Joormann, 2010; Joormann & Arditte, 2014). Importantly, these biases are not merely correlates of current symptoms, but also predict prospective change in depressive and anxious symptoms over time (Beevers & Carver, 2003; Gibb, Benas, Grassia, & McGeary, 2009; Van Bockstaele et al., 2014). In addition, there is growing evidence that variations of the dot probe designed to reduce attentional biases can be used to effectively treat anxiety and depression (Hakamata et al., 2010; Hallion & Ruscio, 2011; Kuckertz & Amir, 2015).
Studies Using Indices of Attentional Bias Derived from Eye Tracking or ERPs
Even if one were to ignore questions about the reliability of RT indices of attention bias, another limitation of this approach is that, at best, it provides only a snap shot of attentional processes – where attention was allocated at the precise moment the probe appeared (e.g., 500ms or 1000ms after stimulus onset). However, attention is a multi-faceted construct that can be decomposed into a number of distinct processes including involuntary capture of attention by a salient stimulus, voluntary shift of attention toward a stimulus, disengagement of attention, and inhibition of return to a recently-attended location.
To address this more nuanced view of attentional biases, researchers have begun to assess patterns of attentional processing across the entire time course of a trial using eye tracking. These studies have supported the hypothesis that, during free or passive viewing paradigms, anxious participants exhibit increased initial orienting to threat-relevant stimuli compared to nonanxious controls, but no differences in sustained attention (Armstrong & Olatunji, 2012). In contrast, whereas depressed individuals do not differ from controls in initial orienting of attention, they do exhibit biases in sustained attention, specifically increased sustained attention to depression-relevant stimuli and decreased sustained attention to positive stimuli (Armstrong & Olatunji, 2012). In addition, one study found that biases in sustained attention to sad faces during a passive viewing task assessed in soldiers prior to deployment to Iraq moderated the impact of war zone stress on prospective increases in depressive symptoms (Beevers, Lee, Wells, Ellis, & Telch, 2011a). Specifically, greater attention to sad faces during the passive viewing task (total fixation time, longer mean fixation time, number of fixations) predicted greater increases in depressive symptoms among those soldiers exposed to higher levels of war zone stress. Also in this study, shorter mean fixation time to fear faces (but not total fixation time or number of fixations) predicted increases in symptoms of posttraumatic stress disorder (PTSD) among those exposed to higher levels of war zone stress.
These latter findings are consistent with the larger body of research reviewed earlier suggesting that attentional biases are specific to disorder-relevant stimuli, but contradict other research in suggesting that threat avoidance (rather than preferential attention toward threat) may increase risk for PTSD. Providing a potential explanation for these contradictory results, there is evidence from one study that the direction of attention bias to threat-relevant stimuli exhibited may differ based on one’s proximity to real-world threat, with greater avoidance of threat-relevant stimuli and higher levels of internalizing symptoms observed among individuals living in closer proximity to war-related stress (Bar-Haim et al., 2010). Additional research in this area is clearly needed and may have important implications regarding differentiations among the different RDoC threat constructs within the Negative Valence Systems domain (i.e., Acute Threat versus Potential Threat versus Sustained Threat).
Finally, we should note that despite the strengths of eye tracking, it still relies on a behavioral index of attentional allocation (eye movements). To complement this research, the use of ERP measures may allow an examination of more covert indices of initial and sustained attention. Studies in this area have focused on a number of ERP components reflecting initial orienting of attention (e.g., P1, P2, N1, N170, N2pc) as well as sustained attention (e.g., late positive potential [LPP], sustained posterior contralateral negativity [SPCN], P3b) (for reviews, see Hajcak, Weinberg, MacNamara, & Foti, 2012; Luck, 2012; Luck & Kappenman, 2012).
The results from studies focused on ERP indices of attentional bias have differed somewhat from those obtained from studies using behavioral measures of attentional bias (i.e., reaction time or eye-tracking indices). The majority of this research has focused on ERP indices of biased attention in anxiety and has generally found that anxiety is associated with both early (e.g., P1, N170) and sustained (LPP) attention to threat-relevant stimuli (Bar-Haim, Lamy, & Glickman, 2005; Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010; Frenkel & Bar-Haim, 2011; A. Holmes, Nielsen, & Green, 2008; MacNamara & Hajcak, 2010; O’Toole, DeCicco, Berthod, & Dennis, 2013; Rossignol, Campanella, Bissot, & Philippot, 2013; Solomon, DeCicco, & Dennis, 2012; Staugaard, 2010). Although there is considerably less ERP research examining attentional biases in depression than anxiety, there is evidence that depression is associated with reduced sustained attention (smaller LPP and P3b magnitudes) to emotional stimuli. However, in contrast to findings from behavioral measures of attentional bias (reaction time or eye tracking), this bias is observed for emotional stimuli generally and does not appear to be specific to depression-relevant stimuli (Bruder, Kayser, & Tenke, 2012; Proudfit, Bress, Foti, Kujawa, & Klein, 2014).
This inconsistency brings up an important and as yet unresolved point. A key difference across tasks is the number of stimuli presented, which varies for the studies reviewed here from one stimulus at a time (Posner spatial cueing tasks and single stimulus passive viewing tasks with ERPs) to two stimuli (dot probe) to four stimuli (multi-stimulus passive viewing task with eye tracking). It is likely that stimulus competition plays an important role in what specific pattern of attentional allocation will be observed in terms of initial orienting, sustained attention, and difficulty disengaging attention. Future research that includes multiple tasks administered to the same participants is needed to disentangle these more nuanced effects. For example, it would be useful to know whether, for example, blunted late stage neural activity (e.g., LPP) in response to emotion stimuli is associated with visual gaze biases.
Attentional Biases in Comorbid Depression and Anxiety
Up to this point, we have focused on studies examining attention biases in depression or anxiety individually. In contrast, surprisingly few have examined attentional biases among individuals with comorbid depression and anxiety. This is somewhat surprising given that comorbidity is often the rule rather than the exception in epidemiological studies (Kessler, Chiu, Demler, Merikangas, & Walters, 2005; Merikangas et al., 2010). Among the studies that have been conducted, evidence for the impact of comorbidity is mixed. For example, one study found that individuals with pure social phobia, but not those with comorbid social phobia and depression, exhibited an attentional biases toward threat-relevant stimuli (social threat words) presented for 500ms (Musa, Lépine, Clark, Mansell, & Ehlers, 2003). In contrast, another study found that an attentional bias toward threat-relevant stimuli (angry faces presented for 1000ms) was only observed among individuals with comorbid social phobia and depression, but not those with pure social phobia (LeMoult & Joormann, 2012). Finally, one study found that whereas adolescents with a pure anxiety disorder (without comorbid depression) exhibited an attentional bias toward angry faces and adolescents with pure depression (without comorbid anxiety) exhibited an attentional bias toward sad faces, adolescents with comorbid depression and anxiety exhibited attentional biases toward both face types (Hankin, Gibb, Abela, & Flory, 2010b). Given the high level of comorbidity typically seen between depression and anxiety, this is clearly an area in need of additional research.
In this light, we should also acknowledge the obvious: anxiety disorders are a heterogeneous group. Indeed, there is some evidence that, although many of the anxiety disorders are characterized by biased attention for threat-relevant stimuli (e.g., angry or fearful faces), the direction of this bias may differ based on the specific disorder considered. For example, there is evidence from a sample of children that whereas distress-related anxiety disorders (e.g., GAD) are associated with preferential attention toward threat, fear-related anxiety disorders (e.g., social phobia, separation anxiety disorder, specific phobia) are associated with attentional avoidance of threat-relevant stimuli (Waters, Bradley, & Mogg, 2014). Therefore, consistent with the RDoC initiative, future research may benefit from a focus on specific symptom clusters (e.g., anxious arousal) rather than broader and more heterogeneous diagnostic categories.
Summary
In summary, research using a variety of tasks and methods has largely supported cognitive theories regarding the nature of attentional biases in depression and anxiety. Specifically, whereas both depression and anxiety are related to attentional biases for affectively salient stimuli, as highlighted in the RDoC matrix for the Loss and Sustained Threat constructs, the focus and time course of these biases appear to differ. Specifically, whereas anxiety appears to be characterized by biases in initial orienting of attention toward threat-relevant stimuli, depression appears to be characterized by biases in sustained attention rather than initial orienting such that depressed individuals exhibit difficulty disengaging their attention from depression-relevant stimuli (e.g., sad faces). This said, there is some evidence that the direction of the attentional bias for threat-relevant information in anxiety may vary based on contextual factors (Wald et al., 2013) or may follow a vigilance-avoidance pattern, at least for some forms of anxiety (Calvo & Avero, 2005; In-Albon, Kossowsky, & Schneider, 2010; Koster, Verschuere, Crombez, & Van Damme, 2005; but see Schofield, Inhoff, & Coles, 2013). Similarly, there is evidence that the direction and stimulus specificity of attentional biases in depression may be affected by the type of paradigm chosen, for example the presence versus absence of competing stimuli presented simultaneously. Finally, with regard to comorbid depression and anxiety, there is too little research to form any firm conclusions. Also, as noted above, and consistent with the general thrust of RDoC, research is needed that focuses on more specific symptom domains associated with the RDoC Loss and Sustained Threat constructs (e.g., anhedonia/low positive affect and anxious arousal, respectively) rather than broader symptoms or diagnoses of depression or anxiety. This will help to clarify which specific component (e.g., initial allocation versus sustained attention) and focus (threat versus loss) of attention are biased, and the direction of that bias (toward or away from) in relation to each RDoC construct.
Neural Underpinnings
Attentional biases are thought to be driven by disruptions in cortico-limbic circuitry. Specifically, attentional biases, broadly defined, are thought to result from hyperactivation in limbic areas (e.g., amygdala) in response to salient emotional stimuli that are not effectively downregulated by areas of the prefrontal cortex (Bishop, 2008; De Raedt & Koster, 2010; Disner et al., 2011; Frewen et al., 2008). Initial capture of attention is influenced by activation of the amygdala by salient emotional stimuli (Bishop, 2008; De Raedt & Koster, 2010; Disner et al., 2011; Frewen et al., 2008). Although it was initially thought that the amygdala only responds to threat-relevant stimuli, it is now clear that the amygdala is sensitive to various forms of emotional stimuli including fearful, happy, and sad facial stimuli (Fusar-Poli et al., 2009). Evidence from intracranial ERP suggests an early amygdala response to emotional stimuli (fearful faces) that is later modulated by attention (Pourtois, Spinelli, Seeck, & Vuilleumier, 2010), demonstrating that amygdala activation to emotional stimuli precedes attentional allocation to the stimuli.
Significant work has also examined the neural systems that support sustained attention to negative affective information. The lateral prefrontal cortex (lPFC), including the right inferior frontal gyrus, appears to have a central role in the shifting of attention away from emotional information. The lPFC is more generally implicated in cognitive control, especially when competing responses have to be inhibited or new information is selected (Aron & Poldrack, 2005; Helfinstein et al., 2014; Nee, Wager, & Jonides, 2007). The lPFC also contributes to action selection and execution using external (e.g., cues in the environment) rather than internal cues as a guide (Matsumoto & Tanaka, 2004). In line with these findings, prior research indicates that the lPFC is strongly engaged during successful cognitive regulation of emotional information (Ochsner & Gross, 2005; Ochsner, Bunge, Gross, & Gabrieli, 2002).
The lPFC is part of a larger neural system that is involved in attention control (Beevers, Clasen, Stice, & Schnyer, 2010a). Prior work has identified seven nodes involved in the shifting of attention from emotion stimuli and form an attention control network: right inferior frontal gyrus, right middle frontal gyrus, dorsal anterior cingulate gyrus, left middle frontal gyrus, right supramarginal gyrus, left supramarginal gyrus, and a node located in the precuneus region of the occipital lobe. The latter is representative of the occipital/temporal activation associated with visual object processing. We focus primarily on the lPFC in this section, as it has a central role in attention control and a number of imaging and genetic studies have investigated it in this context.
There is evidence that altered lPFC function contributes to negatively biased attention among adults with elevated symptoms of depression. Compared to women with few symptoms of depression, women with elevated depression symptoms showed weaker activation in the inferior frontal gyrus, middle frontal gyrus and the supramarginal gyrus, primarily in the right hemisphere, when required to shift attention away from negative stimuli. In contrast, no depression group differences were observed in the lateral prefrontal cortex for shifting attention away from non-emotional cues (Beevers et al., 2010a). Alterations within the lPFC and attention control network have also been observed among adolescents who are at risk for depression by virtue of having a parental history of MDD. Prior work has shown that this group displays a negative attention bias using a behavioral reaction time task (Joormann, Talbot, & Gotlib, 2007). Further, adolescents with a parental history of depression also had lower levels of functional connectivity within a circumscribed network of brain regions underlying attentional control, including the right lPFC (Clasen, Beevers, Mumford, & Schnyer, 2014). More specifically, whole-brain omnibus functional connectivity maps indicated lower levels of connectivity between the right inferior frontal gyrus seed and regions of right dorsal lateral prefrontal cortex and left and right mesial prefrontal cortex in those with a parental history of MDD relative to adolescents with no parental history of MDD. Similarly, using a priori unbiased ROIs from prior work (Beevers et al., 2010a), adolescents with a parental history of depression had lower levels of resting state connectivity between the right middle frontal gyrus and the right inferior gyrus region and right supramarginal gyrus (Clasen et al., 2014), compared to adolescents with no parental history of MDD.
Neural Activity During Tasks Designed to Manipulate Attentional Biases
Research has also sought to experimentally manipulate negative attention bias in an effort to identify causal associations between the attention control network and negative attention biases. This work is important because it can simultaneously demonstrate that activity within the lPFC and associated regions is important for attention control over emotion stimuli and document whether these cognitive systems are amenable to change (i.e., determine its plasticity). In a study with healthy controls (Browning, Holmes, Murphy, Goodwin, & Harmer, 2010), two attention training conditions, provided in a single training session, were used: attend towards threat stimuli or attend away from threat stimuli. Attention training induced attention biases in the expected direction and led to increased lPFC activation when participants directed their attention in the opposite direction from what they were trained (attending toward threat in the attend away condition). Analyses indicated that the rostral anterior cingulate cortex (ACC) displayed a similar pattern of activity as the vlPFC, consistent with the idea that that the lPFC and ACC are part of a larger network involved in attention control over emotional stimuli.
A similar attention training study was completed among fourteen adults high in social anxiety (Taylor et al., 2014). This study found that a single session of attention training away from threat-relevant stimuli reduced bilateral amygdala, insula, and subgenual anterior cingulate cortex activity in response to emotional faces relative to shapes. Further, ventral medial PFC (vmPFC) and orbitofrontal cortex (OFC) activity significantly increased for this same contrast from before to after attention training. Attention training also reduced stress reactivity and threat bias among those with greater increases in ventromedial PFC activation. This study suggests that the effects of attention training on symptom reduction may be mediated by its impact on reducing bottom-up reactivity and enhancing top-down regulation over threatening stimuli.
Additional evidence for the role of the lPFC comes from a study that examined the impact of transcranial direct current stimulation (tDCS) during attention bias modification. In this study (Clarke, Browning, Hammond, Notebaert, & MacLeod, 2014), the application of tDCS to the left dorsolateral prefrontal cortex (dlPFC) moderated the impact of attention retraining such that participants receiving active stimulation of the dlPFC exhibited greater change in attentional bias than those in the sham stimulation condition (Clarke et al., 2014).
Recent work has also examined whether attention training changes resting state connectivity within the attention control network among clinically depressed adults (Beevers, Clasen, Enock, & Schnyer, 2015). This study involved two training conditions: training attention away from negative stimuli and towards neutral stimuli or placebo attention training. Prior to attention training, weaker connectivity between the right middle frontal gyrus (rMFG) and dorsal anterior cingulate cortex (dACC) was associated with greater negative attention bias. Further, active attention training was associated with reductions in negative attention bias and greater increases in connectivity between the rMFG and dACC compared to the placebo attention training condition. These findings highlight the importance of the attention control network and point to interventions that may improve attention control.
Genetic Influences
Twin Studies
Only three twin studies of which we are aware have examined the heritability of attentional biases for emotional information. The first study focused on reaction time indices of attentional bias in the dot probe task in a sample of 300 eight-year-old twins (Brown et al., 2013). This study found no evidence of genetic or shared environmental influences on attentional biases or negative stimuli. However, as noted earlier, reaction time indices of attentional bias suffer from low reliability, which may have contributed to the null findings. Indeed, the split-half reliability of bias scores in negative-neutral trials from this study was almost zero (r = .02). Although the reliability of bias scores for positive-neutral trails was stronger (r = .55), it was still rather modest and the authors did not report heritability estimates for attentional bias for positive stimuli.
Focusing on ERP indices, two studies have examined the heritability of the P300 and LPP ERP components in response to emotional stimuli. These studies found heritability estimates of .40–.55 for the P300 (Anokhin, Golosheykin, & Heath, 2010; Weinberg, Venables, Proudfit, & Patrick, 2015b), with significant but weaker heritability estimates for the LPP (h2 = .20–.47) (Weinberg, Venables, Proudfit, & Patrick, 2015b). Importantly, there was also substantial genetic influence on the modulation of the P300 in response to emotional versus neutral stimuli (h2 = .22–.30), demonstrating genetic influences specifically on the increase in P300 observed in response to emotional relative to neutral stimuli (Weinberg et al., 2015). These heritability estimates are consistent with those reported for rumination (h2 = .37–.41; (Johnson, Whisman, Corley, Hewitt, & Friedman, 2014), which is a construct thought to underlie multiple forms of information processing biases including attentional biases, particularly with regard to biases in sustained attention and difficulty disengaging attention from negative stimuli (Donaldson, Lam, & Mathews, 2007; Duque, Sanchez, & Vazquez, 2014; Koster, De Lissnyder, & De Raedt, 2013; LeMoult, Arditte, D’Avanzato, & Joormann, 2013).
These results are complemented by a larger body of twin research that has demonstrated clear genetic influences on neural regions thought to underlie attentional biases. For example, in the only twin study of which we are aware to examine genetic influences on amygdala activation during emotional tasks, 57% of the variability in amygdala activation to emotional faces in adult male twins was attributable to genetic factors (Jacobson & Cremers, 2014). Studies have also suggested substantial genetic influences on the structure (e.g., volume) of brain regions thought to underlie attentional biases. Although the relation between structural and functional differences in brain is complex and remains an active area of research (Messé, Rudrauf, Benali, Marrelec, 2014), heritability studies of structural differences in the brain regions/circuits implicated in attentional bias suggest an important role for genetics in explaining this variability. For example, studies have suggested heritability estimates (h2) of .42–.66 for amygdala volume (Kremen et al., 2010; Lewis et al., 2014; Rentería et al., 2014), .69 for frontal lobe volume (Blokland, de Zubicaray, McMahon, & Wright, 2012), and .25–.61 for cortical thickness in prefrontal areas (Blokland et al., 2012), with significant variability across regions and evidence of region-specific genetic influences (Blokland et al., 2012; Eyler et al., 2011; Rentería et al., 2014). Twin studies have also documented significant genetic influences on uncinate fasciculus microstructure (h2 = .21) (Blokland et al., 2012), which is a white matter tract connecting limbic and prefrontal regions. Finally,
Candidate Gene Studies
Building from these studies, researchers have begun to examine specific genetic influences on attentional biases. These studies have focused on polymorphisms in candidate genes linked to activity in the cortico-limbic areas underlying the biases and/or to activity of the hypothalamic pituitary adrenal (HPA) axis. The most frequently studied of these is a polymorphism (5-HTTLPR) in the serotonin transporter gene (SLC6A4). Although the impact of 5-HTLPR genotype on depression risk remains a matter of debate (Karg, Burmeister, Shedden, & Sen, 2011; Risch et al., 2009), there is more consistent evidence of its impact on processes relevant to attentional biases. For example, studies have shown that individuals homozygous for the lower expressing (short [S] or LG) allele, compared to those homozygous for the higher expressing (LA) allele, exhibit greater amygdala activation negative stimuli (for a meta-analytic review, see Munafò, Brown, & Hariri, 2008), reduced structural (Pacheco et al., 2009) and functional (Pezawas et al., 2005) connectivity between amygdala and prefrontal regions, and increased HPA axis (cortisol) reactivity to laboratory-based stressors (for a meta-analytic review, see (Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013). Importantly, the results of a meta-analysis also showed that individuals homozygous for the lower expressing 5-HTTLPR alleles (S or LG) exhibit greater attention toward negative stimuli than individuals carrying one or two copies of the higher expressing alleles (LA) (Pergamin-Hight, Bakermans-Kranenburg, van IJzendoorn, & Bar-Haim, 2012). This relation was specific to attentional biases for negative stimuli and was not observed for positive stimuli.
In addition to studies focused on reaction time indices of attentional bias, more recent research has focused on eye tracking indices of attentional bias. Two studies have now shown that, within the context of a passive viewing task in which four images (e.g., threatening, dysphoric, positive, neutral) appear on computer screen for a relatively long stimulus presentation duration, individuals recruited from the community who with one or two copies of the 5-HTTLPR S or LG allele exhibit increasing attention specifically toward positive stimuli over the course of each 30 second trial (Beevers, Ellis, Wells, & McGeary, 2010b; Beevers et al., 2011b). These findings have been interpreted as an effort among to S/LG carriers to downregulate heightened reactivity, which is consistent with emotion regulation models highlighting the role of attentional allocation as a mood regulation strategy (Gross, 2014).
Although, as noted above, the overwhelming majority of candidate gene studies examining genetic influences on attentional biases has focused on 5-HTTLPR, there have been some studies that have examined polymorphisms in other genes. For example, building from research suggesting that norepinephrine reuptake inhibitors (NRIs), specifically reboxetine, may have similar effects on the neural mechanisms underlying attentional biases as those observed for SSRIs (Harmer et al., 2009b; Norbury, Mackay, Cowen, Goodwin, & Harmer, 2007), researchers have examined genes that affect norepinephrine activity. For example, there is evidence that a 9 base pair insertion/deletion polymorphism in the alpha-2B adrenergic receptor (ADRA2B) is related to emotional memory and attentional biases, with carriers of the deletion variant exhibiting more biased processing of emotional stimuli (Li, Weerda, Milde, Wolf, & Thiel, 2015; Markovic, Anderson, & Todd, 2014; Shoumin et al., 2014; Todd et al., 2013).
Finally, studies have examined the influence of genes associated with dopaminergic activity given its role in prefrontal function. For example, the dopamine receptor D4 (DRD4) is highly distributed in the prefrontal cortex (Oak, Oldenhof, & Van Tol, 2000) and DRD4 genotype has been associated with enhanced anterior cingulate cortex function (a dopamine rich brain region) and better executive attention (Fan, Fossella, Sommer, Wu, & Posner, 2003), which is involved in the control of cognition and emotion (Bush, Luu, & Posner, 2000). Carriers of the DRD4 long allele (7 or more repeats) show disruptions in various forms of attention, particularly sustained attention, and there is evidence from two studies showing that carriers of the DRD4 long allele exhibit greater increases in attentional bias to faces than short allele homozygotes following a sad mood induction (Wells, Beevers, Knopik, & McGeary, 2013), suggesting that it may play a role in the expression of mood-congruent attentional biases. Studies have also found links between other genes associated with dopaminergic function and attentional biases including variation in the gene coding for the catechol-O-methyltransferase (COMT) enzyme, which breaks down dopamine and is primarily responsible for dopamine clearance in the prefrontal cortex (Frank, Doll, Oas-Terpstra, & Moreno, 2009). The Val variant of the COMT (rs4680) genotype catabolizes dopamine up to four times the rate of the COMT Met variant (Egan et al., 2001). There is evidence that carriers of the COMT Met allele, compared to Val allele homozygotes, exhibit increased activation in limbic areas and areas of the PFC (vlPFC, dlPFC) when viewing negative stimuli (Smolka et al., 2005) and greater attentional biases for negative facial expressions (sad, fearful, and angry) (Gong et al., 2013) but see also (Lonsdorf, Juth, Rohde, Schalling, & Öhman, 2013).
Polygenic Influences
Although much of the research in psychiatric genetics generally, and in attentional bias research more specifically, has focused on the influence of single candidate genes, there is growing recognition that individual genes contribute a small amount of variance to a given (endo)phenotype (Vrieze, Iacono, & McGue, 2012). More recently, therefore, researchers have focused on polygenic influences, the combined influence of multiple variants across a number of genes (e.g., The International Schizophrenia Consortium, 2009; Wang, Li, & Hakonarson, 2010)). Although there are a number of methods that are possible for choosing a gene set for these analyses, one approach is to focus on the aggregate influence of genes within a given biological pathway.
For example, a number of studies have suggested that selective serotonin reuptake inhibitors (SSRIs) may exert their therapeutic effects by helping to normalize neural circuits (e.g., amygdala and medial PFC) underlying information-processing biases (Harmer, Goodwin, & Cowen, 2009a; Pringle, Browning, & Harmer, 2011; Roiser, Elliott, & Sahakian, 2012) including attentional biases (Wells, Clerkin, Ellis, & Beevers, 2014). These effects are rapid and can be seen after a week of treatment (Di Simplicio, Norbury, & Harmer, 2012; Godlewska, Norbury, Selvaraj, & Harmer, 2012; Harmer, Mackay, Reid, Cowen, & Goodwin, 2006; Harmer, Shelley, Cowen, & Goodwin, 2004) or even after a single dose (Schaefer et al., 2014).
Seeking to index disrupted serotonergic functioning more generally, therefore, researchers have begun to examine aggregate levels of influence across multiple genes known to influence serotonergic activity. In one study (Disner, McGeary, Wells, Ellis, & Beevers, 2014), a polygenic score (PGS) was calculated based on variation in the serotonin transporter gene (SLC6A4 5-HTTLPR) as well as variation in two serotonin receptor genes (HTR1A rs6295 and HTR2A rs6311) by summing the number of “risk” alleles across all three polymorphisms (i.e., the number of SLC6A4 5-HTTLPR S/LG alleles, HTR1A rs6295 C alleles, and HTR2A rs6311 C alleles). In this study, participants completed a passive-viewing eye-tracking task in which they were presented with four stimuli on the screen at a time for 30s (positive, dysphoric, threatening, and neutral) during which patterns of eye gaze to each stimulus were tracked. This assessment was administered before and after a sad mood induction. Participants with higher PGS scores who were more reactive to the sad mood induction exhibited greater increases in gaze to dysphoric images and decreases in gaze to positive images. Importantly, these effects were only significant for the PGS; results for the genes examined individually were not significant suggesting that a PGS approach may help to account for some differences in genetic background that might otherwise be obscured in a single locus analysis. This demonstrates the benefit of examining the cumulative influence of multiple genes within a given biological system, an approach that is seeing increasing traction in psychopathology research more generally (Derringer et al., 2010; McGeary et al., 2012; Nikolova, Ferrell, Manuck, & Hariri, 2011; Wang et al., 2010)
Summary
In summary, findings from candidate gene studies have suggested specific genetic influences on attentional biases. These studies largely build from imaging and pharmacological studies, which is consistent with the RDoC goal of building a coherent understanding of a given construct across multiple units of analysis from genes through molecules and neural circuits to physiology and behavior. This said, however, variation individual genes accounts for only a small percentage of the variance in attentional biases (e.g., 5-HTTLPR accounts for only 4–5% of the variance in attentional biases to negative stimuli, which is likely an overestimate of the true effect size given the relatively small sample size of the studies to date; (Pergamin-Hight et al., 2012) and twin studies have suggested that the genetic influences may account for as much as 55% of the variance in attentional biases (Weinberg, Venables, Proudfit, & Patrick, 2015a). Therefore, additional efforts focused on examining the combined influence across multiple genes within a give biological system is necessary for there to be significant progress in this field (e.g., (Disner et al., 2014).
Role of the Environment and Development
The domains/constructs and units of analysis of the RDoC matrix are part of a larger four-dimensional matrix that also includes environmental and developmental influences (Casey, Oliveri, & Insel, 2014; Cuthbert, 2014; Woody & Gibb, 2015). Forms of psychopathology are viewed as neurodevelopmental diseases that develop in, and are maintained by, specific environmental factors. Attentional biases are hypothesized to develop during childhood, stabilize in adolescence, and contribute to the development and maintenance of psychopathology across the lifespan. More specifically, theorists have suggested that biased processing of affective stimuli develops in response to specific, affectively-salient environmental influences (Gibb et al., 2011; Pollak, 2003) and that the impact of these environmental influences on the development of attentional biases may be stronger among those with more reactive genotypes (Gibb, Beevers, & McGeary, 2013). There is growing evidence for these types of gene × environment (G×E) models of risk for attentional biases. Again, though, most of the research to date has focused on the influence of 5-HTTLPR. These studies have shown that 5-HTTLPR genotype moderates the link between (i) expressed-emotion criticism and children’s attentional biases for angry faces (Gibb et al., 2011), (ii) histories of childhood physical abuse and women’s attentional biases for angry faces (Johnson, Gibb, & McGeary, 2010), (iii) maternal history of major depression and children’s attentional biases for sad faces (Gibb et al., 2009), and (iv) levels of war zone stress and increases in sustained attention to negative stimuli among soldiers (Disner et al., 2013). In each of these cases, the link between environmental stress and experience-specific attentional biases was stronger among those carrying the lower expressing 5-HTTLPR alleles (S or LG).
More recently, researchers examined polygenic influences across genes known to affect HPA axis reactivity (Owens et al., in press). This study focused on FKBP5, which regulates the sensitivity of the glucocorticoid receptor (Zannas, 2014), moderates the impact of early life stress on risk for MDD (Zannas, 2014), and is associated with attentional bias to threat (Fani et al., 2013), and CRHR1, which codes for the corticotropin releasing hormone receptor and affects the level of cortisol released in response to a laboratory-based stressor (Sheikh, Kryski, Smith, & Singh, 2013). There is evidence that three CRHR1 SNPs – rs7209436, rs110402, and rs242924 – form a protective TAT haplotype and carriers of this haplotype are at reduced risk for depression in the context of early life stress compared to individuals with no copies of the haplotype (Bradley et al., 2008; Laucht et al., 2013; Polanczyk et al., 2009). In this study, a PGS reflecting alleles associated with greater HPA axis reactivity across both genes (i.e., greater number of FKBP5 rs1360780 T alleles and a fewer number of copies of the CRHR1 TAT haplotype) moderated the link between maternal depression and eye-tracking indices of attentional bias in children during a passive viewing task (Owens et al., in press). Specifically, among children of mothers with a history of MDD, higher PGS scores were associated with greater gaze duration to happy faces and shorter gaze duration to sad faces. In contrast, among children of mothers with no history of MDD, PGS scores were not significantly associated with attentional bias for any of the facial displays of emotion (sad, happy, angry, neutral).
This study highlights an important point about potential developmental differences in the presentation or function of attentional biases, at least in terms of depression-relevant attentional biases. Specifically, whereas the relation between anxiety and attentional biases toward threat-relevant stimuli appears to be consistent across children, adolescents, and adults (Bar-Haim et al., 2007), there is evidence that depressed and at-risk children and infants may display attentional avoidance of, rather than preferential attention toward, sad stimuli (Boyd, Zayas, & McKee, 2006; Connell, Patton, Klostermann, & Hughes-Scalise, 2013; Field, 1995; Gibb et al., 2009; Harrison & Gibb, 2014; Hernandez-Reif, Field, Diego, Vera, & Pickens, 2006; but see also Joormann et al., 2007; Kujawa et al., 2011). However, at this point the evidence is still somewhat mixed and differences in findings across studies, at least among at-risk samples, may have been due to the inclusion of a negative mood induction prior to the assessment of attentional biases in some studies (Joormann et al., 2007; Kujawa et al., 2011) but not others (Gibb et al., 2009). There is also evidence that genetic variation may contribute to the mixed findings. For example, ignoring the potential influence of child genotype, one study found that children of depressed mothers, compared to children of never depressed mothers, exhibited more sustained attention toward sad faces (Owens et al., in press). However, child genotype moderated this relation such that children carrying more copies of polymorphisms in genes associated with greater HPA axis reactivity exhibited less sustained attention to sad faces and more attention to happy faces, which may have reflected a mood regulation strategy (see also (Harrison & Gibb, 2014). Finally, although less theoretically interesting, it is possible that the mixed findings were due to studies’ reliance on traditional reaction time indices of attentional bias (Gibb et al., 2009; Joormann et al., 2007; Kujawa et al., 2011), which, as reviewed earlier, tend to exhibit poor reliability (Brown et al., 2014; Kappenman, Farrens, Luck, & Proudfit, 2014a; Price et al., 2014; Waechter, Nelson, Wright, & Hyatt, 2014).
To the extent that the form or function of attentional biases is different in children than in adults, it would be consistent with a larger body of research showing development changes (and reversals) in a number of related processes including developmental shifts in the direction of amygdala-prefrontal connectivity (Gee et al., 2013), cortisol reactivity to stress (Hankin, Badanes, Abela, & Watamura, 2010a), and pupillary reactivity to affectively-salient stimuli (Silk, Siegle, Ostapenko, & Ladouceur, 2009). Finally, in terms of developmental influences, we should note that there are also differences in heritability estimates of different neural regions across development (Lenroot et al., 2009), though to date this research has focused on cortical but not subcortical areas and has focused on brain structure, not function. This said, it suggests age-related changes in the expression of different genes in different areas of the brain (i.e., gene × development interactions), which have not yet been examined in relation to attentional biases.
Summary
In summary, there is growing support for the role of specific environmental influences on attentional biases. There is also evidence that the impact of these environmental influences on attentional biases may be stronger among individuals carrying genetic variants associated with greater HPA axis reactivity. This line of research is still in its infancy, however, and there are a number of important questions remaining. For example, no study of which we are aware has examined prospective changes in attentional biases, which is essential if we want to know what factors contribute to the actual development of these biases. Additional research is also needed to understand how the nature or function of these biases may change across development as well as potential gene × development interactions.
Areas of Future Research
There are at least three key areas of future research in this area. First, as noted previously, there must be increased precision in our assessment of attentional biases. The reliability of reaction time indices of attentional bias is simply too low to provide a reliable marker (Brown et al., 2014; Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Price et al., 2015; Schmukle, 2005; Staugaard, 2009). Future studies should focus on eye tracking and ERP indices of attentional bias, which have stronger psychometric properties (Kappenman, Farrens, Luck, & Proudfit, 2014a; Kappenman, MacNamara, & Proudfit, 2014b; Kujawa et al., 2013; Moran et al., 2013; Price et al., 2015). Studies should also report the reliability of the attention bias indices in their samples (e.g., split half reliability). These studies should also seek to provide a more fine-grained assessment of the key aspects of attention thought to be disrupted in the RDoC constructs of Loss and Sustained Threat, specifically biases in initial orienting versus sustained attention, overt versus covert attention, and specificity regarding the target stimulus (e.g., threat versus loss relevant stimuli). In addition, it should be noted that the dot probe task has so dominated research in this area that “attentional bias” has become almost synonymous with “that which is measured with the dot probe”. Despite the strengths of this paradigm and the gains in theory and research it has enabled over the last three decades, it is only one task and there are other approaches that may also be useful depending on the researchers’ specific questions including the Posner spatial cueing task, passive viewing tasks including face-in-the-crowd tasks, rapid serial visual presentation tasks, and tasks designed to elicit steady state visual evoked potentials. Each of these tasks has strengths and limitations that may make each one a better versus worse fit for answering a specific research question and the use of multiple approaches within a given study will allow for a more complete and nuanced understanding of attentional biases related to Sustained Threat and Loss.
A second important direction for future research is better precision in the measurement of psychopathology. Depression is a heterogeneous disorder and the anxiety disorders are clearly heterogeneous. Indeed, the primary goal of RDoC is to determine key mechanisms that may cut across current diagnostic boundaries. Therefore, when examining symptom correlates of the RDoC Loss and Sustained Threat constructs, researchers should seek to examine symptom clusters that may be unique to each construct, including low positive affect/anhedonia for Loss and physiological hyperarousal for Sustained Threat, distinctions that were originally made in the tripartite model of depression and anxiety over two decades ago (Clark & Watson, 1991; Watson, Clark, et al., 1995a; Watson, Weber, et al., 1995b).
Third, significant advances are needed in our approach to examining genetic influences. To date, only three twin studies have been conducted examining attentional biases, one focused on reaction times and the other two focused on ERP indices. The reaction time study (Brown et al., 2013) found no genetic influence on attentional bias whereas the ERP studies (Anokhin et al., 2010; Weinberg et al., 2015) found heritability estimates (h2) as high as .55. Additional twin studies are needed to gain a better understanding of the amount of variance that can be explained in various measures of attentional bias by genetic and environmental influences. However, although twin studies have had a profound impact on the nature versus nurture question in psychology more generally and continue to have great utility in providing a foundation for genetic research, these methods also have some limitations. For example, it is challenging to find large enough numbers of twins to be adequately powered for unique or intensive research protocols. A further limitation of twin studies is that these methods usually provide an estimate of total heritability across all genetic influences, which does not necessarily point to biological systems that may underlie these influences. Although extensions to twin studies do allow for single genetic variants to be co-modeled with overall heritability, fundamental issues remain.
Specifically, there is a large disconnect between the overall heritability identified in twin studies and the limited amount of variance explained by individual candidate genes, raising questions about so-called “missing heritability” (Maher, 2008). Indeed, even 5-HTTLPR, which so far has demonstrated the strongest and most consistent link with attentional biases to negative stimuli only explains 4–5% of the variance (Pergamin-Hight et al., 2012), at best. Genetic studies of complex phenotypes with GWAS also have challenges. They require tens to hundreds of thousands of phenotyped individuals and result in small amounts of variance explained by individual single nucleotide polymorphisms (SNPs) (Park et al., 2010). Therefore, although the sum influence of genetic variance for a phenotype is captured by twin-based heritability analyses, GWAS and candidate gene studies account for a tiny fraction of the heritable factors, leaving a significant gap or discrepancy in the variance identified. This gap leaves unanswered a number of important questions about the relevance of rare versus common variants, epistasis, and G×E interaction.
A relatively new quantitative genetic technique seeks to fill this gap by estimating genetic influences directly from measured genotypes rather than indirectly from comparisons between groups that differ genetically, such as identical (MZ) and fraternal (DZ) twins. This technique, called genomic-relatedness-matrix restricted maximum likelihood (GREML; Visscher et al., 2014)), compares genetic similarity across hundreds of thousands of SNPs for each pair of individuals in a matrix of unrelated individuals. This relatedness matrix is then used to predict phenotypic similarity for each pair of individuals. That is, GREML provides a characterization of the cumulative additive effects of genetic variation measured using GWAS arrays. This method can also address critical questions about the utility of GWAS arrays relative to other unmeasured genetic variation such as rare variants that might be contributing to the missing heritability problem. In a hypothetical example, if the twin-based analysis identifies heritability of an outcome at .40, GREML may provide information that common SNPs account for a discrete proportion of that total (perhaps 25%). Such information is absolutely vital to our ability to bridge heritability and candidate gene studies because it provides an upper limit to the relevance of common SNPs genotyped on GWAS arrays and may inform the extent to which whole-genome-sequencing may or may not be cost effective relative to GWAS arrays. Finally, by comparing the twin-based heritability to the SNP-based GREML heritability, we derive a measure of genetic variation not characterized by SNP arrays. This difference may highlight the relative importance of genetic variation not captured by GWAS arrays such as rare variants, fragment length polymorphisms, or other influences such as non-additive genetic effects. However, it should be noted that GREML does not typically identify which SNPs are responsible for the heritability of a trait. That said, it can help to identify sets of genes within a biological system that contribute significantly to the variance of a particular phenotype.
Although GREML provides a method for identifying the cumulative genetic influence accounted for by a gene set, another approach builds from identified candidate polymorphisms. The polygenic score (PGS) approach reviewed earlier seeks to account for more variance by aggregating the influence of individual candidate polymorphisms. Initial primitive PGS methods (e.g., Derringer et al., 2010; Disner et al., 2014; McGeary et al., 2012; Nikolova et al., 2011; Owens et al., in press) aggregated the influence of multiple polymorphisms in a simple additive manner that outperformed individual candidate polymorphisms in the amount of variance explained. In addition to accumulating small individual effects, the PGS approach also begins to account for relevant ‘genetic background’ that may alter the effect of a single polymorphism. For example a single polymorphism may have a significantly different effect in individuals who have substantially different genetic variation in other genes within the same biological system. To the extent that a PGS models variation within this biological system, it may begin to account for this problem that has likely contributed to reliability issues within the single candidate gene literature. Advances in PGS methods allow for: the constituent polymorphisms to be weighted, polymorphisms to be summed in additive and non additive models, inclusion of haplotypes, inclusion of epistasis, and the flexibility to aggregate influence of risk-conferring, protective, and neutral variants. As these techniques mature and improve over time, they are likely to provide critical new insights into the nature of the neurocognitive traits that RDoC describes.
Finally, with regard to future research, we should note that the replicability of candidate gene findings has come under increasing scrutiny (Munafò, 2006) and candidate gene by environment interaction (cG×E) studies have been a particular focus (Duncan & Keller, 2011). Nevertheless, the existence of G×E in psychiatry is undeniable (e.g., genetic risk factors for addiction or PTSD are only manifest in the presence of the environmental exposures of substances and trauma respectively). Therefore improvements in methods are required to further understanding of this phenomenon. Fortunately, a consensus is building within the field as to the best way to study and model cGxE effects (Dick et al., 2015). As this ‘recipe’ for cG×E research begins to be implemented more broadly and the study of cG×E continues to mature, greater understanding of the etiology, maintenance, and treatment of psychopathological conditions seems likely to emerge.
Conclusions
In summary, there is growing evidence for the role of attentional biases related to the RDoC Negative Valence Systems constructs of Sustained Threat and Loss. Although early research in this area focused exclusively on reaction time indices of attentional bias, more recent research has utilized eye-tracking and ERP indices of attentional allocation. In combination, these studies generally support the hypothesis that anxiety is associated with rapid initial allocation of attention to threat-relevant stimuli whereas depression is associated with difficulty disengaging attention from depression-relevant stimuli, though there is some evidence that there may be important developmental differences in the nature and perhaps function of attentional biases related to depression. Biases associated with both constructs are driven by disruption in cortico-limbic circuitry and research has begun to identify genetic influences on these biases. This research is clearly still in its infancy and additional research with larger samples and more precise measures of attentional biases and symptom domains is needed to take full advantage of recent advances in psychiatric genetics such as GREML and PGS approaches. These studies will help to build a more complete understanding of the specific ways in which attention biases may be disrupted, how they may impact and be impacted by other levels of analysis within each construct, and how they may be modified to provide more targeted treatments for psychopathology.
Acknowledgments
This article was supported by National Institute of Mental Health grant MH098060 awarded to B. E. Gibb and National Institute for Drug Abuse grant DA032457 to C. G. Beevers.
Footnotes
In this review, we focus only on biases in visual attention to emotional stimuli.
The views in this article are the views of the authors and do not necessarily represent the position of the NIH or Department of Veteran Affairs.
The authors have no conflicts of interest.
References
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop phenomenon: A generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133(3):323–338. doi: 10.1037/0096-3445.133.3.323. http://doi.org/10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of individual differences in cortical processing of facial affect. Behavior Genetics. 2010;40(2):178–185. doi: 10.1007/s10519-010-9337-1. http://doi.org/10.1007/s10519-010-9337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. http://doi.org/10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1285–1292. doi: 10.1016/j.biopsych.2004.10.026. http://doi.org/10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, et al. Life-threatening danger and suppression of attention bias to threat. American Journal of Psychiatry. 2010;167(6):694–698. doi: 10.1176/appi.ajp.2009.09070956. http://doi.org/10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59(1):11–22. doi: 10.1016/j.bandc.2005.03.005. http://doi.org/10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. http://doi.org/10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognitive Therapy and Research. 2003;27(6):619–637. http://doi.org/10.1023/A:1026347610928. [Google Scholar]
- Beevers CG, Clasen PC, Enock PM, Schnyer DM. Attention bias modification for major depressive disorder: Effects on attention bias, resting state connectivity, and symptom change. Journal of Abnormal Psychology. 2015 doi: 10.1037/abn0000049. http://doi.org/10.1037/abn0000049. [DOI] [PMC free article] [PubMed]
- Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: A functional magnetic resonance imaging study. Neuroscience. 2010a;167(1):97–103. doi: 10.1016/j.neuroscience.2010.01.047. http://doi.org/10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Ellis AJ, Wells TT, McGeary JE. Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biological Psychology. 2010b;83(3):260–265. doi: 10.1016/j.biopsycho.2009.08.007. http://doi.org/10.1016/j.biopsycho.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Lee HJ, Wells TT, Ellis AJ, Telch MJ. Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. American Journal of Psychiatry. 2011a;168(7):735–741. doi: 10.1176/appi.ajp.2011.10091309. http://doi.org/10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Marti CN, Lee HJ, Stote DL, Ferrell RE, Hariri AR, Telch MJ. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of Abnormal Psychology. 2011b;120(1):187–197. doi: 10.1037/a0022125. http://doi.org/10.1037/a0022125. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. http://doi.org/10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Blokland GAM, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: A meta-analytical perspective on twin imaging studies. 2012;15(3):351–371. doi: 10.1017/thg.2012.11. http://doi.org/10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd RC, Zayas LH, McKee MD. Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Maternal and Child Health Journal. 2006;10(2):139–148. doi: 10.1007/s10995-005-0042-2. http://doi.org/10.1007/s10995-005-0042-2. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. http://doi.org/10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM, Eley TC, Broeren S, MacLeod C, Rinck M, Hadwin JA, Lester KJ. Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. Journal of Anxiety Disorders. 2014;28(1):97–107. doi: 10.1016/j.janxdis.2013.11.004. http://doi.org/10.1016/j.janxdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Brown HM, McAdams TA, Lester KJ, Goodman R, Clark DM, Eley TC. Attentional threat avoidance and familial risk are independently associated with childhood anxiety disorders. Journal of Child Psychology and Psychiatry. 2013;54(6):678–685. doi: 10.1111/jcpp.12024. http://doi.org/10.1111/jcpp.12024. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using attentional bias modification as a cognitive vaccine against depression. Biological Psychiatry. 2012;72(7):572–579. doi: 10.1016/j.biopsych.2012.04.014. http://doi.org/10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. http://doi.org/10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. Event-related brain potentials in depression: Clinical, cognitive and neurophysiologic implications. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 563–592. [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cognition and Emotion. 2005;19(3):433–451. doi: 10.1080/02699930441000157. http://doi.org/10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological Psychiatry. 2014;76(5):350–353. doi: 10.1016/j.biopsych.2014.01.006. http://doi.org/10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biological Psychiatry. 2014;76(12):946–952. doi: 10.1016/j.biopsych.2014.03.003. http://doi.org/10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Developmental Cognitive Neuroscience. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. http://doi.org/10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Patton E, Klostermann S, Hughes-Scalise A. Attention bias in youth: Associations with youth and mother’s depressive symptoms moderated by emotion regulation and affective dynamics during family interactions. Cognition and Emotion. 2013;27(8):1522–1534. doi: 10.1080/02699931.2013.803459. http://doi.org/10.1080/02699931.2013.803459. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. http://doi.org/10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, and Behavioral Neuroscience. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. http://doi.org/10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Saccone S, Grucza RA, Agrawal A, et al. Predicting sensation seeking from dopamine genes. A candidate-system approach. Psychological Science. 2010;21(9):1282–1290. doi: 10.1177/0956797610380699. http://doi.org/10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Norbury R, Harmer CJ. Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Molecular Psychiatry. 2012;17(5):503–510. doi: 10.1038/mp.2011.16. http://doi.org/10.1038/mp.2011.16. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Candidate gene-environment interaction research: Reflections and recommendations. 2015;10(1):37–59. doi: 10.1177/1745691614556682. http://doi.org/10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12(8):467–477. doi: 10.1038/nrn3027. http://doi.org/10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Lee HJ, Ferrell RE, Hariri AR, Telch MJ. War zone stress interacts with the 5-HTTLPR polymorphism to predict the development of sustained attention for negative emotion stimuli in soldiers returning from Iraq. Clinical Psychological Science. 2013;1(4):413–425. http://doi.org/10.1177/2167702613485564. [Google Scholar]
- Disner SG, McGeary JE, Wells TT, Ellis AJ, Beevers CG. 5-HTTLPR, HTR1A, and HTR2A cumulative genetic score interacts with mood reactivity to predict mood-congruent gaze bias. Cognitive, Affective, and Behavioral Neuroscience. 2014;14(4):1259–1270. doi: 10.3758/s13415-014-0267-x. http://doi.org/10.3758/s13415-014-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behaviour Research and Therapy. 2007;45(11):2664–2678. doi: 10.1016/j.brat.2007.07.002. http://doi.org/10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. http://doi.org/10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, Sanchez A, Vazquez C. Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition and Emotion. 2014;28(8):1347–1366. doi: 10.1080/02699931.2014.881327. http://doi.org/10.1080/02699931.2014.881327. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. http://doi.org/10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2010;85(2):252–257. doi: 10.1016/j.biopsycho.2010.07.010. http://doi.org/10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Prom-Wormley E, Fennema-Notestine C, Panizzon MS, Neale MC, Jernigan TL, et al. Genetic patterns of correlation among subcortical volumes in humans: Results from a magnetic resonance imaging twin study. Human Brain Mapping. 2011;32(4):641–653. doi: 10.1002/hbm.21054. http://doi.org/10.1002/hbm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7406–7411. doi: 10.1073/pnas.0732088100. http://doi.org/10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Mercer KB, Davis J, Glover E, et al. FKBP5 and attention bias for threat: Associations with hippocampal function and shape. JAMA Psychiatry. 2013;70(4):392–400. doi: 10.1001/2013.jamapsychiatry.210. http://doi.org/10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Infants of depressed mothers. Infant Behavior and Development. 1995;18:1–3. doi: 10.1016/j.infbeh.2009.04.004. http://doi.org/10.1016/0163-6383(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience. 2009;12(8):1062–1068. doi: 10.1038/nn.2342. http://doi.org/10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel TI, Bar-Haim Y. Neural activation during the processing of ambiguous fearful facial expressions: An ERP study in anxious and nonanxious individuals. Biological Psychology. 2011;88(2–3):188–195. doi: 10.1016/j.biopsycho.2011.08.001. http://doi.org/10.1016/j.biopsycho.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Joanisse MF, Neufeld RWJ. Selective attention to threat versus reward: Meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. http://doi.org/10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. http://doi.org/10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2013;27(2):193–216. doi: 10.1080/02699931.2012.712950. http://doi.org/10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. http://doi.org/10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Johnson AL, Benas JS, Uhrlass DJ, Knopik VS, McGeary JE. Children’s 5-HTTLPR genotype moderates the link between maternal criticism and attentional biases specifically for facial displays of anger. Cognition and Emotion. 2011;25(6):1104–1120. doi: 10.1080/02699931.2010.508267. http://doi.org/10.1080/02699931.2010.508267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Norbury R, Selvaraj S, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychological Medicine. 2012;42(12):2609–2617. doi: 10.1017/S0033291712000591. http://doi.org/10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Shen G, Li S, Zhang G, Fang H, Lei L, et al. Genetic variations in COMT and DRD2 modulate attentional bias for affective facial expressions. PloS One. 2013;8(12):e81446. doi: 10.1371/journal.pone.0081446. http://doi.org/10.1371/journal.pone.0081446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. http://doi.org/10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Conceptual and empirical foundations. In: Gross JJ, editor. Handbook of emotion regulation. 2. New York, NY: Guilford Press; 2014. pp. 3–20. [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 441–472. [Google Scholar]
- Hakamata Y, Izawa S, Sato E, Komi S, Murayama N, Moriguchi Y, et al. Higher cortisol levels at diurnal trough predict greater attentional bias towards threat in healthy young adults. Journal of Affective Disorders. 2013;151(2):775–779. doi: 10.1016/j.jad.2013.06.031. http://doi.org/10.1016/j.jad.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Ernst M. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. http://doi.org/10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. http://doi.org/10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010a;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. http://doi.org/10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JRZ, Flory K. Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010b;119(3):491–501. doi: 10.1037/a0019609. http://doi.org/10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. British Journal of Psychiatry. 2009a;195(2):102–108. doi: 10.1192/bjp.bp.108.051193. http://doi.org/10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. http://doi.org/10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. American Journal of Psychiatry. 2009b;166(10):1178–1184. doi: 10.1176/appi.ajp.2009.09020149. http://doi.org/10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161(7):1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harrison AJ, Gibb BE. Attentional biases in currently depressed children: An eye-tracking study of biases in sustained attention to emotional stimuli. Journal of Clinical Child and Adolescent Psychology. 2014:1–7. doi: 10.1080/15374416.2014.930688. http://doi.org/10.1080/15374416.2014.930688. [DOI] [PMC free article] [PubMed]
- Helfinstein SM, Schonberg T, Congdon E, Karlsgodt KH, Mumford JA, Sabb FW, et al. Predicting risky choices from brain activity patterns. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(7):2470–2475. doi: 10.1073/pnas.1321728111. http://doi.org/10.1073/pnas.1321728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Vera Y, Pickens J. Happy faces are habituated more slowly by infants of depressed mothers. Infant Behavior and Development. 2006;29(1):131–135. doi: 10.1016/j.infbeh.2005.07.003. http://doi.org/10.1016/j.infbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Bradley BP, Kragh Nielsen M, Mogg K. Attentional selectivity for emotional faces: Evidence from human electrophysiology. Psychophysiology. 2009;46(1):62–68. doi: 10.1111/j.1469-8986.2008.00750.x. http://doi.org/10.1111/j.1469-8986.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Green S. Effects of anxiety on the processing of fearful and happy faces: An event-related potential study. Biological Psychology. 2008;77(2):159–173. doi: 10.1016/j.biopsycho.2007.10.003. http://doi.org/10.1016/j.biopsycho.2007.10.003. [DOI] [PubMed] [Google Scholar]
- In-Albon T, Kossowsky J, Schneider S. Vigilance and avoidance of threat in the eye movements of children with separation anxiety disorder. Journal of Abnormal Child Psychology. 2010;38(2):225–235. doi: 10.1007/s10802-009-9359-4. http://doi.org/10.1007/s10802-009-9359-4. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Cremers H. Genetic and environmental influences on amygdala activation to facial/emotion processing: A twin study using fMRI. Behavior Genetics. 2014;44:646–690. [Google Scholar]
- Johnson AL, Gibb BE, McGeary J. Reports of childhood physical abuse, 5-HTTLPR genotype, and women’s attentional biases for angry faces. Cognitive Therapy and Research. 2010;34(34):380–387. doi: 10.1007/s10608-009-9269-3. http://doi.org/10.1007/s10608-009-9269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, Whisman MA, Corley RP, Hewitt JK, Friedman NP. Genetic and environmental influences on rumination and its covariation with depression. Cognition and Emotion. 2014;28(7):1270–1286. doi: 10.1080/02699931.2014.881325. http://doi.org/10.1080/02699931.2014.881325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Arditte KA. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3. New York: Guilford; 2014. pp. 259–276. [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. http://doi.org/10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: Poor reliability and lack of correlation with anxiety. Frontiers in Psychology. 2014a;5:1368. doi: 10.3389/fpsyg.2014.01368. http://doi.org/10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, Proudfit GH. Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cognitive and Affective Neuroscience. 2014b:nsu098. doi: 10.1093/scan/nsu098. http://doi.org/10.1093/scan/nsu098. [DOI] [PMC free article] [PubMed]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. http://doi.org/10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, De Raedt R. Rumination is characterized by valence-specific impairments in switching of attention. Acta Psychologica. 2013;144(3):563–570. doi: 10.1016/j.actpsy.2013.09.008. http://doi.org/10.1016/j.actpsy.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Verschuere B, Crombez G, Van Damme S. Time-course of attention for threatening pictures in high and low trait anxiety. Behaviour Research and Therapy. 2005;43(8):1087–1098. doi: 10.1016/j.brat.2004.08.004. http://doi.org/10.1016/j.brat.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. NeuroImage. 2010;49(2):1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. http://doi.org/10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Amir N. Attention bias modification for anxiety and phobias: current status and future directions. Current Psychiatry Reports. 2015;17(2):9. doi: 10.1007/s11920-014-0545-x. http://doi.org/10.1007/s11920-014-0545-x. [DOI] [PubMed] [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39(1):125–135. doi: 10.1007/s10802-010-9438-6. http://doi.org/10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Proudfit GH. Two-year stability of the late positive potential across middle childhood and adolescence. Biological Psychology. 2013;94(2):290–296. doi: 10.1016/j.biopsycho.2013.07.002. http://doi.org/10.1016/j.biopsycho.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmidt MH, Esser G, et al. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: Findings from a longitudinal study. European Neuropsychopharmacology. 2013;23(5):358–367. doi: 10.1016/j.euroneuro.2012.06.002. http://doi.org/10.1016/j.euroneuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Joormann J. Attention and memory biases in social anxiety disorder: The role of comorbid depression. Cognitive Therapy and Research. 2012;36(1):47–57. doi: 10.1007/s10608-010-9322-2. http://doi.org/10.1007/s10608-010-9322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Arditte KA, D’Avanzato C, Joormann J. State Rumination: Associations with Emotional Stress Reactivity and Attention Biases. Journal of Experimental Psychopathology. 2013;4(5):471–484. doi: 10.5127/jep.029112. http://doi.org/10.5127/jep.029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2009;30(1):163–174. doi: 10.1002/hbm.20494. http://doi.org/10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Panizzon MS, Eyler L, Fennema-Notestine C, Chen CH, Neale MC, et al. Heritable influences on amygdala and orbitofrontal cortex contribute to genetic variation in core dimensions of personality. NeuroImage. 2014;103(C):309–315. doi: 10.1016/j.neuroimage.2014.09.043. http://doi.org/10.1016/j.neuroimage.2014.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Weerda R, Milde C, Wolf OT, Thiel CM. ADRA2B genotype differentially modulates stress-induced neural activity in the amygdala and hippocampus during emotional memory retrieval. Psychopharmacology. 2015;232(4):755–764. doi: 10.1007/s00213-014-3710-3. http://doi.org/10.1007/s00213-014-3710-3. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Juth P, Rohde C, Schalling M, Öhman A. Attention biases and habituation of attention biases are associated with 5-HTTLPR and COMTval158met. Cognitive, Affective, and Behavioral Neuroscience. 2013;14(1):354–363. doi: 10.3758/s13415-013-0200-8. http://doi.org/10.3758/s13415-013-0200-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 329–360. [Google Scholar]
- Luck SJ, Kappenman ES. ERP components and selective attention. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 295–327. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety. 2010;27(3):234–243. doi: 10.1002/da.20679. http://doi.org/10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- Maher B. Nature. Nature Publishing Group; 2008. Nov 6, Personal genomes: The case of the missing heritability; pp. 18–21. http://doi.org/10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, Todd RM. Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research. 2014;259:229–241. doi: 10.1016/j.bbr.2013.11.018. http://doi.org/10.1016/j.bbr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Current Opinion in Neurobiology. 2004;14(2):178–185. doi: 10.1016/j.conb.2004.03.005. http://doi.org/10.1016/j.conb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: Preliminary results using an aggregate genetic risk score. Substance Abuse: Research and Treatment. 2012;6:107–114. doi: 10.4137/SART.S8866. http://doi.org/10.4137/SART.S8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Cui L, Benjet C, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. http://doi.org/10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messé A, Rudrauf D, Benali H, Marrelec G. Relating structure and function in the human brain: Relative contributions of anatomy, stationary dynamics, and non-stationarities. PLoS Computational Biology. 2014;10(3):e1003530. doi: 10.1371/journal.pcbi.1003530. http://doi.org/10/137/journal.pcbi.003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: a meta-analysis. Molecular Psychiatry. 2013;18(9):1018–1024. doi: 10.1038/mp.2012.124. http://doi.org/10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. http://doi.org/10.1037//0021-843X.109.4.695. [DOI] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, Moser JS. The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research. 2013;1516:66–75. doi: 10.1016/j.brainres.2013.04.018. http://doi.org/10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Munafò MR. Candidate gene studies in the 21st century: Meta-analysis, mediation, moderation. Genes, Brain, and Behavior. 2006;5(Suppl 1)(S1):3–8. doi: 10.1111/j.1601-183X.2006.00188.x. http://doi.org/10.1111/j.1601-183X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. http://doi.org/10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa C, Lépine JP, Clark DM, Mansell W, Ehlers A. Selective attention in social phobia and the moderating effect of a concurrent depressive disorder. Behaviour Research and Therapy. 2003;41(9):1043–1054. doi: 10.1016/s0005-7967(02)00212-7. http://doi.org/10.1016/S0005-7967(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36(9):1940–1947. doi: 10.1038/npp.2011.82. http://doi.org/10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. British Journal of Psychiatry. 2007;190:531–532. doi: 10.1192/bjp.bp.106.031393. http://doi.org/10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- O’Toole LJ, DeCicco JM, Berthod S, Dennis TA. The N170 to angry faces predicts anxiety in typically developing children over a two-year period. Developmental Neuropsychology. 2013;38(5):352–363. doi: 10.1080/87565641.2013.802321. http://doi.org/10.1080/87565641.2013.802321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: One decade of research. European Journal of Pharmacology. 2000;405(1–3):303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. http://doi.org/10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. http://doi.org/10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Owens M, Harrison AJ, Burkhouse KL, McGeary JE, Knopik VS, Palmer RHC, Gibb BE. Eye-tracking indices of attentional bias in children of depressed mothers: Polygenic influences help to clarify previous mixed findings. Development and Psychopathology. doi: 10.1017/S0954579415000462. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. Journal of Neuroscience. 2009;29(19):6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. http://doi.org/10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, Chatterjee N. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nature Genetics. 2010;42(7):570–575. doi: 10.1038/ng.610. http://doi.org/10.1038/ng.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe ML, Vanderkerckhove J, Kuppens P. A diffusion model account of the relationship between the emotional flanker task and rumination and depression. Emotion. 2013;13(4):739–747. doi: 10.1037/a0031628. http://doi.org/10.1037/a0031628. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. http://doi.org/10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van IJzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: A meta-analysis. Biological Psychiatry. 2012;71(4):373–379. doi: 10.1016/j.biopsych.2011.10.030. http://doi.org/10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Muñoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. http://doi.org/10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pilgrim K, Marin MF, Lupien SJ. Attentional orienting toward social stress stimuli predicts increased cortisol responsivity to psychosocial stress irrespective of the early socioeconomic status. Psychoneuroendocrinology. 2010;35(4):588–595. doi: 10.1016/j.psyneuen.2009.09.015. http://doi.org/10.1016/j.psyneuen.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: Replication and extension. Archives of General Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. http://doi.org/10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD. Experience-dependent affective learning and risk for psychopathology in children. Annals of the New York Academy of Sciences. 2003;1008:102–111. doi: 10.1196/annals.1301.011. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112(3):323–338. doi: 10.1037/0021-843x.112.3.323. http://doi.org/10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38(2):267–274. [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 2007;32(1):3–25. doi: 10.1080/00335558008248231. http://doi.org/10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: Intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive, Affective, and Behavioral Neuroscience. 2010;10(1):83–93. doi: 10.3758/CABN.10.1.83. http://doi.org/10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, Amir N. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. 2014 doi: 10.1037/pas0000036. http://doi.org/10.1037/pas0000036. [DOI] [PMC free article] [PubMed]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. 2015;27(2):365–376. doi: 10.1037/pas0000036. http://doi.org/10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle A, Browning M, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(7):1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. http://doi.org/10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN. Depression and event-related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology. 2014;4:1–6. doi: 10.1016/j.copsyc.2014.12.018. http://doi.org/10.1016/j.copsyc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería ME, Hansell NK, Strike LT, McMahon KL, de Zubicaray GI, Hickie IB, et al. Genetic architecture of subcortical brain regions: Common and region-specific genetic contributions. Genes, Brain, and Behavior. 2014;13(8):821–830. doi: 10.1111/gbb.12177. http://doi.org/10.1111/gbb.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117–136. doi: 10.1038/npp.2011.183. http://doi.org/10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M, Campanella S, Bissot C, Philippot P. Fear of negative evaluation and attentional bias for facial expressions: An event-related study. Brain and Cognition. 2013;82(3):344–352. doi: 10.1016/j.bandc.2013.05.008. http://doi.org/10.1016/j.bandc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, et al. Serotonergic modulation of intrinsic functional connectivity. Current Biology. 2014;24(19):2314–2318. doi: 10.1016/j.cub.2014.08.024. http://doi.org/10.1016/j.cub.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19:595–605. http://doi.org/10.1002/per.554. [Google Scholar]
- Schofield CA, Inhoff AW, Coles ME. Time-course of attention biases in social phobia. Journal of Anxiety Disorders. 2013;27(7):661–669. doi: 10.1016/j.janxdis.2013.07.006. http://doi.org/10.1016/j.janxdis.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Kryski KR, Smith HJ, Singh SM. Corticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivity. Neuroscience. 2013;229:1–11. doi: 10.1016/j.neuroscience.2012.10.056. http://doi.org/10.1016/j.neuroscience.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Shoumin X, Pingyuan G, She L, Kunpeng D, Haijun H, Peizhe Z, Fuchang Z. A 9-nucleotide Ins/Del in ADRA2B modulates orientation of attention to facial expressions and emotional words. Behavioural Pharmacology. 2014;25(8):717–724. doi: 10.1097/FBP.0000000000000089. http://doi.org/10.1097/FBP.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Ostapenko LJ, Ladouceur CD. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21(1):7–26. doi: 10.1017/S0954579409000029. http://doi.org/10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. Journal of Neuroscience. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. http://doi.org/10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, Dennis TA. Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience. 2012;2(1):110–119. doi: 10.1016/j.dcn.2011.04.002. http://doi.org/10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychology Science Quarterly. 2009;51:339–350. [Google Scholar]
- Staugaard SR. Threatening faces and social anxiety: A literature review. Clinical Psychology Review. 2010;30(6):669–690. doi: 10.1016/j.cpr.2010.05.001. http://doi.org/10.1016/j.cpr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Stevens S, Rist F, Gerlach AL. Eye movement assessment in individuals with social phobia: Differential usefulness for varying presentation times? Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(2):219–224. doi: 10.1016/j.jbtep.2010.11.001. http://doi.org/10.1016/j.jbtep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interferenec in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–662. [Google Scholar]
- The International Schizophrenia Consortium, The International Schizophrenia. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. http://doi.org/10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Müller DJ, Lee DH, Robertson A, Eaton T, Freeman N, et al. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychological Science. 2013;24(11):2244–2253. doi: 10.1177/0956797613492423. http://doi.org/10.1177/0956797613492423. [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C. Children’s cortisol and salivary alpha-amylase interact to predict attention bias to threatening stimuli. Physiology & Behavior. 2015;138:266–272. doi: 10.1016/j.physbeh.2014.10.002. http://doi.org/10.1016/j.physbeh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EHW. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140(3):682–721. doi: 10.1037/a0034834. http://doi.org/10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R. Baseline salivary cortisol levels and preconscious selective attention for threat. A pilot study. Psychoneuroendocrinology. 1998;23(7):741–747. doi: 10.1016/s0306-4530(98)00047-x. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hemani G, Vinkhuyzen AAE, Chen GB, Lee SH, Wray NR, et al. Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genetics. 2014;10(4):e1004269. doi: 10.1371/journal.pgen.1004269. http://doi.org/10.1371/journal.pgen.1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Iacono WG, McGue M. Confluence of genes, environment, development, and behavior in a post Genome-Wide Association Study world. Development and Psychopathology. 2012;24(4):1195–1214. doi: 10.1017/S0954579412000648. http://doi.org/10.1017/S0954579412000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter S, Nelson AL, Wright C, Hyatt A. Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research. 2014 http://doi.org/10.1007/s10608-013-9588-2.
- Wald I, Degnan KA, Gorodetsky E, Charney DS, Fox NA, Fruchter E, et al. Attention to threats and combat-related posttraumatic stress symptoms: Prospective associations and moderation by the serotonin transporter gene. JAMA Psychiatry. 2013;70(4):401–408. doi: 10.1001/2013.jamapsychiatry.188. http://doi.org/10.1001/2013.jamapsychiatry.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nature Reviews. Genetics. 2010;11(12):843–854. doi: 10.1038/nrg2884. http://doi.org/10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- Waters AM, Bradley BP, Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus “fear” diagnostic categorization. Psychological Medicine. 2014;44(3):607–616. doi: 10.1017/S0033291713000779. http://doi.org/10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995b;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfit GH, Patrick CJ. Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience. 2015a;10(3):424–434. doi: 10.1093/scan/nsu059. http://doi.org/10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfit GH, Patrick CJ. Heritability of the neural response to emotional pictures: evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience. 2015b;10(3):424–434. doi: 10.1093/scan/nsu059. http://doi.org/10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TT, Beevers CG, Knopik VS, McGeary JE. Dopamine D4 receptor gene variation is associated with context-dependent attention for emotion stimuli. International Journal of Neuropsychopharmacology. 2013;16(3):525–534. doi: 10.1017/S1461145712000478. http://doi.org/10.1017/S1461145712000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TT, Clerkin EM, Ellis AJ, Beevers CG. Effect of antidepressant medication use on emotional information processing in major depression. American Journal of Psychiatry. 2014;171(2):195–200. doi: 10.1176/appi.ajp.2013.12091243. http://doi.org/10.1176/appi.ajp.2013.12091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into depression research. Current Opinion in Psychology. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. http://doi.org/10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS. Gene-environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism. Genes, Brain, and Behavior. 2014;13(1):25–37. doi: 10.1111/gbb.12104. http://doi.org/10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zvielli A, Bernstein A, Koster EHW. Dynamics of attentional bias to threat in anxious adults: Bias towards and/or away? PloS One. 2014;9(8):e104025. doi: 10.1371/journal.pone.0104025. http://doi.org/10.1371/journal.pone.0104025. [DOI] [PMC free article] [PubMed] [Google Scholar]