Abstract
BACKGROUND
Advanced heart failure (HF) is characterized by high morbidity and mortality. Conventional therapy may not sufficiently reduce patient suffering and maximize quality of life.
OBJECTIVES
. We investigated whether an interdisciplinary palliative care intervention in addition to evidence-based HF care improves certain outcomes.
METHODS
We randomized 150 patients with advanced HF between August 15, 2012, and June 25, 2015, to usual care (UC; n =75) or UC plus a palliative care intervention (UC+PAL; n =75) at a single center. Primary endpoints were 2 quality-of-life measurements, the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary and the Functional Assessment of Chronic Illness Therapy - Palliative Care scale (FACIT-Pal), assessed at 6 months. Secondary endpoints included assessments of depression and anxiety (measured via the Hospital Anxiety and Depression Scale [HADS]), spiritual well-being (measured via the FACIT - Spiritual Well-Being scale [FACIT-Sp]), hospitalizations, and mortality.
RESULTS
Patients randomized to UC+PAL versus UC alone had clinically significant incremental improvement in KCCQ and FACIT-Pal scores from randomization to 6 months (KCCQ difference =9.49 points, 95% CI 0.94 to 18.05, p =0.030; FACIT-Pal difference =11.77 points, 95% CI 0.84 to 22.71, p =0.035). Depression improved in UC+PAL patients (HADS-depression difference =−1.94 points; p =0.020) versus UC-alone patients, with similar findings for anxiety (HADS-anxiety difference =−1.83 points; p =0.048). Spiritual well-being was improved in UC+PAL versus UC-alone patients (FACIT-Sp difference =3.98 points; p =0.027). Randomization to UC+PAL did not affect rehospitalization or mortality.
CONCLUSIONS
An interdisciplinary palliative care intervention in advanced HF patients showed consistently greater benefits in quality of life, anxiety, depression, and spiritual well-being compared with UC alone.
Trial Registration
ClinicalTrials.gov Identifier: NCT01589601
Keywords: Heart failure, quality of life, palliative care
INTRODUCTION
Important progress has been made over the last 25 years in the identification and use of prognosis-modifying therapies for patients with heart failure (HF) (1). Unfortunately, over time these therapies often fail to prevent disease progression. Acute decompensated HF remains the most common cause of hospitalization in the Medicare population, highlighting the public health importance of the problem (2,3). Further, HF progression is a frightening and uncomfortable experience for patients, with both physical and psychological sequelae. Regarding the latter, patients with HF commonly experience depression, poor quality of life, and spiritual distress (4,5). The high mortality rates and poor quality of life in HF patients, despite use of contemporary therapies, suggest that new thinking is required in the management of these patients (6–8). One approach that has shown promise for patients with advanced cancer and other serious illnesses is palliative care (9), an interdisciplinary approach designed to improve symptoms, pain, and quality of life. Assessing the added value of palliative care in patients with HF requires a clear focus on patient-centered outcomes, particularly relief of suffering and improving quality of life as the disease advances (10).
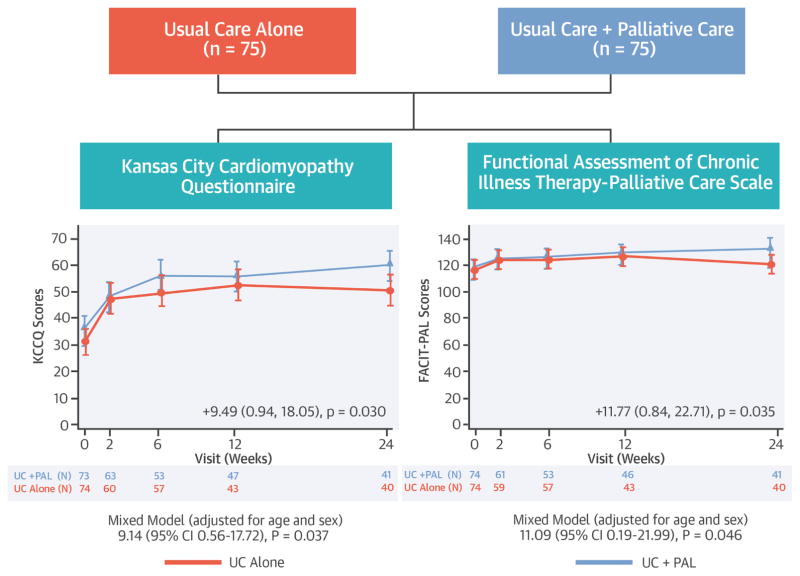
We conducted the Palliative Care in Heart Failure (PAL-HF) trial to assess the impact of an interdisciplinary palliative care intervention combined with usual HF management on HF-related and overall quality of life in patients with advanced HF (Central Illustration).
Central Illustration. The PAL-HF study randomized 150 patients with advanced heart failure to usual care or usual care + a multidimensional palliative care intervention.
The co-primary endpoints were quality of life measured by the Kansas City Cardiomyopathy Questionnaire and the Functional Assessment of Chronic Illness Therapy - Palliative Care scale. Patients who received the palliative care intervention had significantly better quality of life measured by both instruments.
METHODS
STUDY DESIGN
The design of the PAL-HF trial has been described previously (11). Briefly, PAL-HF was a prospective, 2-arm, single-center clinical trial, enrolling patients with advanced HF and a high 6-month mortality risk based on covariates measured at enrollment. Enrolled patients were randomized in a 1:1 allocation to usual care (UC) alone or UC plus palliative care intervention (UC+PAL) using a complete randomization scheme. The trial was unblinded, since blinding of the intervention was not feasible.
The duration of the intervention phase of the trial was 6 months, but patients in both groups were followed until death or end of the study.
PAL-HF INTERVENTION
Our interdisciplinary, guideline-driven, multicomponent palliative care intervention was administered in combination with contemporary HF management as previously detailed (11). In brief, the study team assessed and managed the multiple domains of quality of life for patients with advanced HF, including physical symptoms, psychosocial and spiritual concerns, and advance care planning. A certified palliative care nurse practitioner coordinated these aspects of the patient’s care in collaboration with a hospice and palliative medicine board-certified physician. The intervention was performed in collaboration with each patient’s clinical cardiology team and focused on shared goal-setting to combine HF symptom amelioration with palliative care goals. Following hospital discharge, the PAL-HF nurse practitioner actively participated in the ongoing management of the patients in the outpatient environment.
Patients were screened for depression and anxiety with the Hospital Anxiety and Depression Scale (HADS) (12). Patients who screened positive for these symptoms were considered for referral to a mental health provider as well as for possible use of symptomatic medical therapies, including anti-depressants, anxiolytics, stress management resources, and psychotherapy. Spiritual concerns were assessed by the study nurse practitioner using the FICA (Faith and Belief, Importance, Community, Address in Care) Spiritual History Tool, and these details were shared with the intervention team (13,14). Goals of care were iteratively assessed by the intervention nurse practitioner using communication techniques described in the VitalTalk Curriculum (vitaltalk.org). After the 6-month intervention period was completed, the nurse practitioner continued to contact the patients in the intervention arm every 3 months to provide ongoing support and clinical care.
USUAL CARE
Patients in the UC-alone arm were managed by a cardiologist-directed team with HF expertise. Inpatient care was focused on symptom relief and use of evidence-based therapies as detailed in current guidelines (1). Inpatient palliative care consultation was not denied to UC-alone patients. After discharge, these patients received outpatient follow-up with their general practitioners as well as an HF cardiologist or nurse practitioner with care focused on guidelines-based medication titration and serial monitoring of end-organ function.
STUDY PATIENT ELIGIBILITY
PAL-HF screened and enrolled both hospitalized patients (n =148) and recently discharged patients (n =2) who were at high risk of rehospitalization and mortality based on their ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk score (11,15). The most common reason for trial exclusion was failure to meet severity of illness criteria in the ESCAPE risk score. Hospitalized patients could be enrolled if they were expected to be discharged within 48 hours. Recently discharged patients were eligible to be enrolled if they were discharged from the hospital in the past 2 weeks and met the other study inclusion criteria. Exclusion criteria included anticipated heart transplant or ventricular assist device placement within 6 months and non-cardiac terminal illness. A complete list of inclusion and exclusion criteria has been previously reported (11). The Duke University Medical Center Institutional Review Board approved the study. All patients provided written, informed consent.
STUDY FOLLOW-UP AND QUALITY-OF-LIFE DATA COLLECTION
Following trial enrollment, subjects underwent reassessment of their clinical status and primary outcome questionnaires at weeks 2, 6, 12, and 24 (11).
OUTCOME MEASURES
The 2 primary endpoints were HF-specific quality of life (measured using the Kansas City Cardiomyopathy Questionnaire [KCCQ] overall summary score [16]) and general and palliative care-specific, health-related quality of life (measured using the Functional Assessment of Chronic Illness Therapy - Palliative Care scale [FACIT-Pal]). The KCCQ is a 23-item, disease-specific questionnaire scored from 0 to 100 with higher scores representing better health status. The overall summary score is derived from the physical function, symptom, social function, and quality-of-life domains. A 5-point change in the KCCQ overall summary score is considered a clinically meaningful difference (17). The FACIT-Pal is a 46-item measure of self-reported generic quality of life (27 general; 19 palliative care) that assesses quality of life in several domains, including physical well-being, social/family well-being, emotional well-being, functional well-being, and palliative care with a range of 0 to 184. Higher scores indicate better quality of life. A 10-point change in the overall summary score represents a clinically meaningful difference (18).
Secondary quality of life endpoints included scoring on the FACIT –Spiritual Well-Being scale (FACIT-Sp) and HADS. FACIT-Sp (19) is a 12-item scale that assesses the role of faith in illness and meaning, peace, and purpose in life. The range of the score is 0 to 48 with higher scores representing increased spirituality across the range of religious traditions. HADS is a 14-item scale divided into anxiety and depression subscales (20). Questions are scored from 0 to 3 with a cut-off point of 11 on each subscale, giving the optimal sensitivity and specificity for the presence of the corresponding psychiatric symptoms. The range of the HADS total score is 0 to 42 with depression and anxiety sub-scores each ranging from 0 to 21 and higher scores indicating worse symptoms. A score of 11 or higher on each subscale gives the optimal sensitivity and specificity for the presence of the corresponding psychiatric symptoms. These questionnaires were administered at weeks 2,12, and 24.
STATISTICAL ANALYSIS
The KCCQ overall summary and FACIT-Pal scores were selected as the co-primary endpoints of the PAL-HF study. Assuming a common standard deviation of 12 points for the KCCQ overall summary score, the planned sample size of 200 subjects (100 per arm) was projected to provide 80% power to detect a difference of 4.8 points. As noted earlier, a 5-point change in this score is the smallest change that is clinically significant at the individual patient level (17). For the FACIT-Pal co-primary endpoint, the sample size of 200 subjects was projected to provide 80% power to detect a difference of 10 points assuming a standard deviation of 25. As noted earlier, a 10-point change in this score is the smallest change that is clinically significant at the individual patient level. Interpretive benchmarks for quality-of-life scores based on clinically significant individual patient changes must be used with the understanding that, in group-level data, group mean values typically depend on the proportion of responders in the sample (10). Sample size calculations were based on a 2-sample t-test with a type I error rate of 0.05. The data and safety monitoring board, in consultation with the sponsoring agency, recommended a sample size reduction to 150 subjects on January 14, 2015, based upon enrollment rates, a mortality rate that was lower than predicted, and observed outcomes differences at that intermediate time point.
Baseline characteristics between treatment groups are presented using means (standard deviations) for continuous measures and counts (percentages) for categorical variables. The primary analyses of the longitudinal data from the KCCQ overall summary and FACIT-Pal scores are based on linear mixed models with an indicator variable for the treatment group. Mixed model analysis provides an approach that considers sources of variation and correlation among repeated measurements within a subject. In the analysis of repeated measurements, subjects can still be included even when some observations are missing; thus, it has advantage over the general linear model analyses in which no missing observations are allowed. The treatment effect at 6 months was calculated by using the appropriate contrast statements. Both co-primary endpoints were tested at the 2-sided type-I 0.05 level, because the KCCQ and FACIT-Pal assessments measure 2 different dimensions that are both important to patients. Mortality rates were estimated using the Kaplan-Meier method, and p-values were computed using the log-rank test. All reported analyses were performed using the intention-to-treat principle.
RESULTS
Overall, 150 patients were enrolled in PAL-HF with 75 patients in each study arm (Online Figure 1). The baseline characteristics of the study population are shown in Table 1. The mean age was 71 years, 47% were women, and 41% were African-American. The mean HF duration was 66.9 months, and patients had an average of 2.2 hospitalizations in the 12 months prior to enrollment. In addition, 45% of patients had an ejection fraction >40%, and the mean baseline NT-proBNP (N-terminal B-type natriuretic peptide) was 11,576 pmol/L. Most participants (82%) were sedentary >50% of the time, and 85% rated their overall health as poor/fair. There were no significant differences in baseline demographic characteristics by treatment assignment.
Table 1.
Baseline Characteristics
| Characteristic | UC+PAL N = 75 | UC Alone N = 75 |
|---|---|---|
| Age, yrs | 71.9 (12.4) | 69.8 (13.4) |
| Female | 33 (44.0%) | 38 (50.7%) |
| Race | ||
| Black | 36 (48.0%) | 26 (34.7%) |
| Asian | 1 (1.3%) | 1 (1.3) |
| White | 38 (50.7%) | 48 (64.0%) |
| Other | 0 (0.0%) | 0 (0.0%) |
| History of coronary artery disease | 38 (50.7%) | 47 (62.7%) |
| History of stroke | 18 (24.0%) | 10 (13.3%) |
| History of hypertension | 61 (81.3%) | 52 (69.3%) |
| History of diabetes mellitus | 42 (56.0%) | 38 (50.7%) |
| NYHA class III | 54 (72.0%) | 58 (77.3%) |
| NYHA class IV | 15 (20.0%) | 5 (6.7%) |
| Ischemic heart failure | 34 (45.3%) | 38 (50.7%) |
| Level of impairment of most recent ejection fraction | ||
| Normal (>55%) | 21 (28.0%) | 14 (18.7%) |
| Mildly impaired (40–55%) | 14 (18.7%) | 19 (25.3%) |
| Moderately impaired (25–40%) | 17 (22.7%) | 14 (18.7%) |
| Severely impaired (<25%) | 23 (30.7%) | 28 (37.3%) |
| Prior ICD / pacemaker implantation | 35 (46.7%) | 34 (45.3%) |
| ICD only | 8 (10.7%) | 12 (16.0%) |
| Pacemaker only | 9 (12.0%) | 7 (9.3%) |
| Biventricular pacer only | 1 (1.3%) | 2 (2.7%) |
| Biventricular pacer and ICD | 17 (22.7%) | 13 (17.3%) |
| NT-proBNP, pg/mL | 10040.2 (9434.2) | 13212.4 (14698.2) |
| Duration of HF, months | 64.7 (70.0) | 69.1 (76.5) |
| Importance of religion/spirituality | ||
| Fairly | 15 (20.0%) | 13 (17.3%) |
| Deeply | 54 (72.0%) | 49 (65.3%) |
| Time spent in bed/couch/chair in past month | ||
| More than half | 16 (21.3%) | 20 (26.7%) |
| Almost all | 25 (33.3%) | 29 (38.7%) |
| Depression treated with medications | 12 (16.0%) | 13 (17.3%) |
| Alcohol abuse | 6 (8.0%) | 8 (10.7%) |
| Drug abuse | 5 (6.7%) | 5 (6.7%) |
| Creatinine, mg/dL | 1.8 (0.83) | 1.9 (0.81) |
| ACE inhibitor | 17 (22.7%) | 16 (21.3%) |
| ARB | 4 (5.3%) | 7 (9.3%) |
| Aldosterone antagonist | 30 (40.0%) | 22 (29.3%) |
| Aspirin | 54 (72.0%) | 46 (61.3%) |
| Beta-blocker | 51 (68.0%) | 48 (64.0%) |
| Diuretics | ||
| Bumetanide | 1 (1.3%) | 1 (1.3%) |
| Furosemide | 39 (52.0%) | 49 (65.3%) |
| Torsemide | 27 (36.0%) | 14 (18.7%) |
| Statin | 43 (57.3%) | 41 (54.7%) |
Values presented are means (standard deviations) for continuous variables and counts (percentages) for categorical variables. All p-values comparing treatment groups >0.05.
Abbreviations: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; HF = heart failure; ICD = implantable cardioverter defibrillator; NT-proBNP = N-terminal B-type natriuretic peptide; NYHA = New York Heart Association Heart Failure Classification; PAL = palliative car intervention; UC = usual care; ICD = implantable cardioverter defibrillator.
The frequency of interactions that occurred between patients and providers is shown in Table 2. The specific components of the complex interactions designed to address advanced care planning, symptom amelioration, and psychosocial and spiritual support are difficult to capture from clinical records and study documents.
Table 2.
PAL-HF Patient-Provider Encounters*
| Summary Statistics: Mean (SE) of per/patient record-counts from each data source | |||
|---|---|---|---|
| Control (N = 75) | Intervention (N = 75) | Total (N = 150) | |
| Total number of hospital encounter records | 2.4 (0.35) | 2.5 (0.45) | 2.4 (0.28) |
| Total number of clinic encounter records | 20.8 (1.92) | 21.9 (1.99) | 21.3 (1.38) |
| Primary care | 5.2 (0.82) | 4.4 (0.93) | 4.8 (0.62) |
| Cardiology | 3.2 (1.00) | 2.3 (0.55) | 2.8 (0.57) |
| Telephone contact | 10.6 (0.88) | 12.6 (1.20) | 11.6 (0.74) |
| Rehabilitation clinic | 0.9 (0.48) | 1.4 (0.68) | 1.1 (0.41) |
| Emergency department / urgent care | 0.5 (0.11) | 0.4 (0.12) | 0.4 (0.08) |
The data displayed in this table represent the number of unique encounters documented on case report forms and hospital billing records over the 6-month study period. Home visits, telephonic encounters, and some types of interventions delivered (spiritual, advanced care planning, caregiver support) were not distinctly or differentially collected.
During the 6-month follow-up, 30% of patients were hospitalized for HF and 29% of patients died. No differences were seen between the 2 treatment groups in either of these clinical endpoints through the 6-month follow-up point.
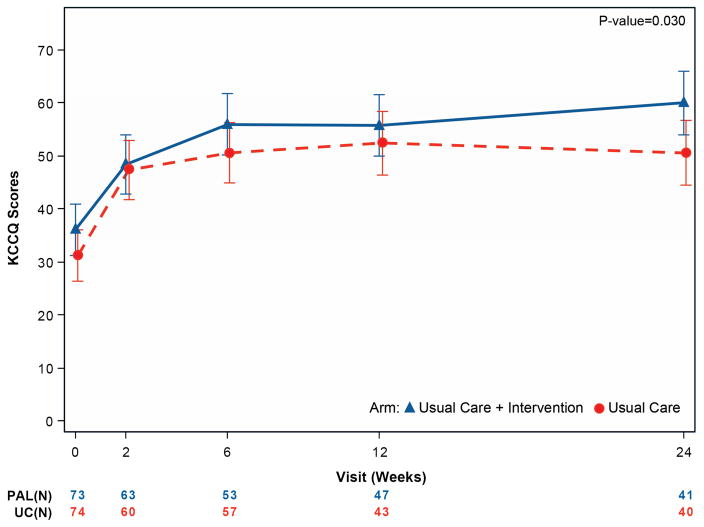
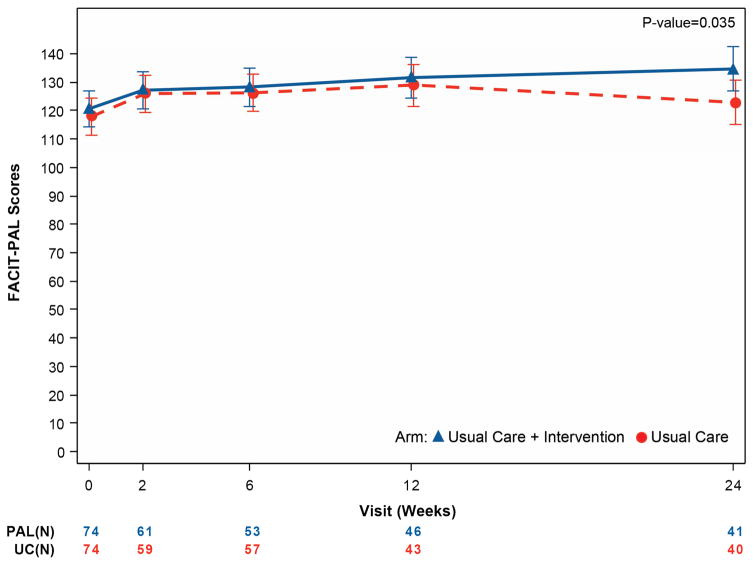
The primary endpoints are presented in Table 3 and Figure 1. At baseline, the mean KCCQ score was 33.7 and the mean FACIT-Pal score was 119.3. Patients randomized to UC+PAL had greater improvements in the KCCQ overall summary score from randomization to 6 months compared with patients randomized to UC alone (9.49 point difference, 95% confidence interval [CI] 0.94 to 18.05; p =0.03). Patients randomized to UC+PAL had greater improvement in FACIT-Pal scores over 6 months compared with those randomized to UC alone (11.77 point difference, 95% CI 0.84 to 22.71; p =0.035).
Table 3.
Primary Endpoint Results
| Endpoints | PAL | UC | ||
|---|---|---|---|---|
| N Assessed / N at Risk (%) | Mean (SD) | N Assessed / N at Risk (%) | Mean (SD) | |
| KCCQ – Overall summary score | ||||
| Baseline | 73/75 (97.3%) | 36.1 (19.8) | 74/75 (98.7%) | 31.4 (16.4) |
| Week 2 | 63/69 (91.3%) | 49.1 (22.0) | 60/71 (84.5%) | 48.4 (22.3) |
| Week 6 | 53/64 (82.8%) | 57.3 (24.4) | 57/63 (90.5%) | 51.2 (23.7) |
| Month 3 | 47/55 (85.5%) | 58.5 (25.9) | 43/51 (84.3%) | 53.8 (21.6) |
| Month 6 | 41/41 (100%) | 63.1 (20.4) | 40/44 (90.9%) | 52.1 (25.0) |
| Change from baseline to 6 months | 41/41 (100%) | 26.3 (19.42) | 39/43 (90.7%) | 22.2 (24.69) |
| Mixed model results – difference at 6 months (95% CIs), unadjusted | 9.49 (0.94, 18.05) p =0.030 |
|||
| Mixed model results – difference at 6 months (95% CIs), adjusted for age and sex | 9.14 (0.56, 17.72) p =0.037 |
|||
| FACIT – Pal | ||||
| Baseline | 74/75 (98.7%) | 120.6 (27.0) | 74/75 (98.7%) | 118.0 (25.1) |
| Week 2 | 61/69 (88.4%) | 128.1 (25.3) | 59/71 (83.1%) | 125.6 (26.6) |
| Week 6 | 53/64 (82.8%) | 128.7 (28.3) | 57/63 (90.5%) | 126.4 (29.0) |
| Month 3 | 46/55 (83.6%) | 132.9 (32.6) | 43/51 (84.3%) | 130.5 (27.7) |
| Month 6 | 41/41 (100%) | 136.5 (28.6) | 40/44 (90.9%) | 125.8 (30.7) |
| Change from baseline to 6 months | 41/41 (100%) | 16.7 (21.1) | 39/43 (90.7%) | 8.3 (29.1) |
| Mixed model results – difference at 6 months (95% CIs), unadjusted | 11.77 (0.84, 22.71) p =0.035 |
|||
| Mixed model results – difference at 6 months (95% CIs), adjusted for age and sex | 11.09 (0.19, 21.99) p =0.0462 |
|||
Values shown are number assessed/number alive and uncensored (%), mean (standard deviation).
Abbreviations: CI = confidence interval; FACIT-Pal = Functional Assessment of Chronic Illness Therapy with Palliative Care Subscale; KCCQ = Kansas City Cardiomyopathy Questionnaire; PAL = palliative care intervention; UC = usual care.
Adjusted analyses were not pre-specified in the PAL-HF statistical analysis plan.
Figure 1. Mean quality-of-life measures by treatment group over time for the primary outcome measures.
Panel A displays the KCCQ overall summary score. Panel B displays the FACIT-Pal score. For both scales, higher values represent better quality of life. A 5-point change for the KCCQ overall summary score is considered clinically significant. A 10-point change is considered clinically meaningful for the FACIT-Pal score.
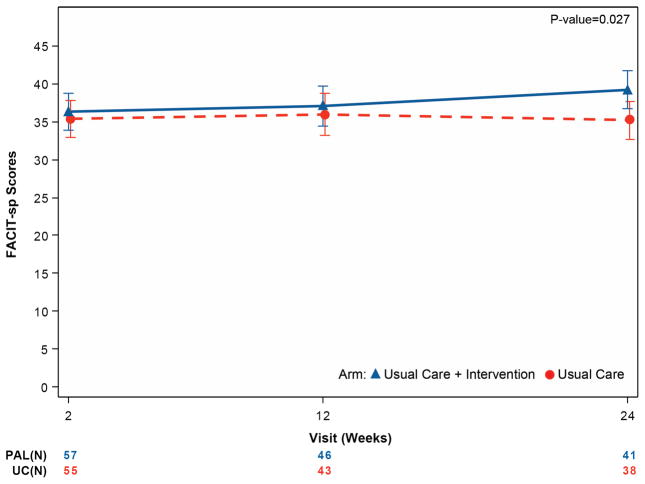
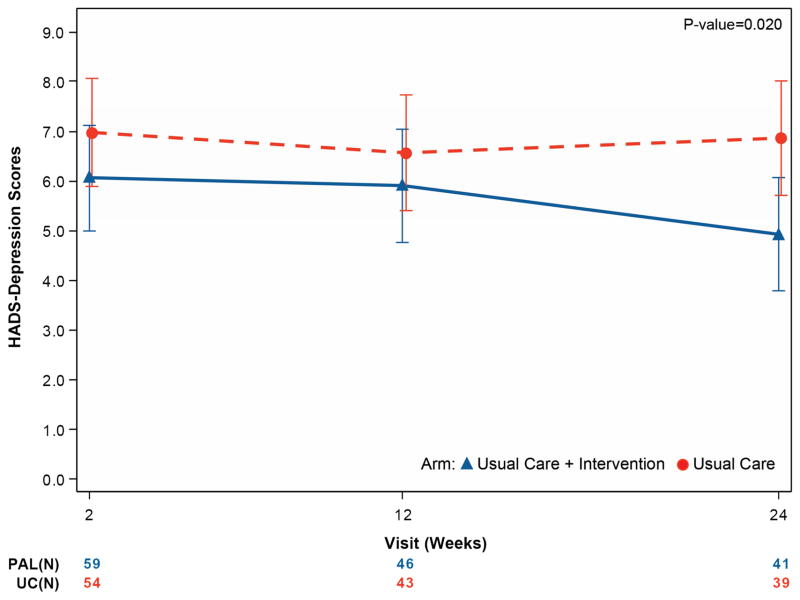
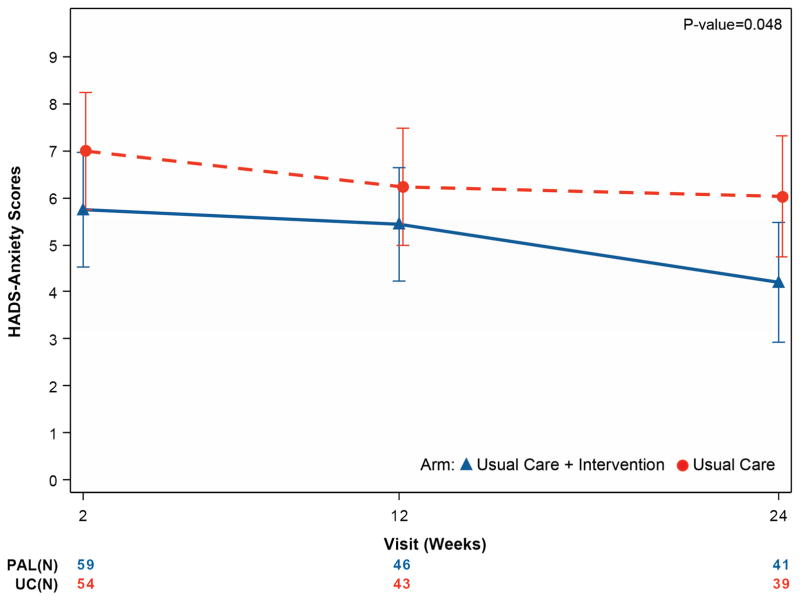
Table 4 and Figure 2 summarize secondary endpoints. From initial assessment through 6 months, depressive symptoms improved more in UC+PAL patients (HADS-depression difference of −1.94 points; 95% CI 3.57 to −0.31; p =0.02) compared with UC-alone patients, with similar findings for anxiety (HADS-anxiety difference of -1.83 points; 95% CI -3.64 to -0.02; p =0.048). Spiritual well-being was improved in UC+PAL patients compared with UC-alone patients (FACIT-Sp difference of 3.98 points, 95% CI 0.46 to 7.50; p =0.027).
Table 4.
Secondary Endpoint Results
| Endpoints | PAL | UC | ||
|---|---|---|---|---|
| N Assessed / N at Risk (%) | Mean (SD) | N Assessed / N at Risk (%) | Mean (SD) | |
| HADS – Depression | ||||
| Week 2 | 59/69 (85.5%) | 6.0 (3.9) | 54/71 (76.1%) | 7.3 (4.3) |
| Month 3 | 46/55 (83.6%) | 5.6 (4.1) | 43/51 (84.3%) | 6.3 (4.2) |
| Month 6 | 41/41 (100%) | 4.6 (3.6) | 39/44 (88.6%) | 6. 4 (4.3) |
| Mixed model results – difference at 6 months (95% CIs), unadjusted | −1.94 (−3.57, −0.31) p =0.020 |
|||
| Mixed model results – difference at 6 months (95% CIs), adjusted for age and sex | −1.94 (−3.58, −0.30) p =0.021 |
|||
| HADS – Anxiety | ||||
| Week 2 | 59/69 (85.5%) | 5.7 (4.9) | 54/71 (76.1%) | 7.2 (4.4) |
| Month 3 | 46/55 (83.6%) | 5.0 (4.6) | 43/51 (84.3%) | 6.0 (4.2) |
| Month 6 | 41/41 (100%) | 3.7 (4.0) | 39/44 (88.6%) | 6.2 (4.8) |
| Mixed model results – difference at 6 months (95% CIs), unadjusted | −1.83 (−3.64, −0.02) p =0.048 |
|||
| Mixed model results – difference at 6 months (95% CIs), adjusted for age and sex | −1.70 (−3.50, 0.09) p =0.063 |
|||
| FACIT – Spirituality | ||||
| Week 2 | 57/69 (82.6%) | 36.4 (9.6) | 55/71 (77.5%) | 35.3 (8.8) |
| Month 3 | 46/55 (83.6%) | 37.1 (10.0) | 43/51 (84.3%) | 35.9 (9.8) |
| Month 6 | 41/41 (100%) | 39.6 (8.1) | 38/44 (86.4%) | 35.5 (10.3) |
| Mixed model results – difference at 6 months (95% CIs), unadjusted | 3.98 (0.46, 7.50) p =0.027 |
|||
| Mixed model results – difference at 6 months (95% CIs), adjusted for age and sex | 3.93 (0.37, 7.48) p =0.031 |
|||
|
PAL
N=75 |
UC
N=75 |
|||
| 6-Month Mortality (%) | 23 (30.7%) | 20 (26.7%) | ||
| 6-Month Rehospitalizations (%) | ||||
| Heart failure | 23 (30.7%) | 22 (29.3%) | ||
| Non-heart failure cardiovascular | 12 (16.0%) | 10 (13.0%) | ||
| Non-cardiovascular | 8 (10.7%) | 18 (24.0%) | ||
Values shown are number assessed/number of patients alive and uncensored (%), mean (standard deviation). Note: baseline assessments were not collected for these endpoints. Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy with Palliative Care Subscale; HADS = Hospital Anxiety and Depression Scale; PAL = palliative car intervention; UC = usual care.
Adjusted analyses were not pre-specified in the PAL-HF statistical analysis plan.
Figure 2. Mean quality-of-life measures by treatment group over time for the key secondary outcome measures.
Panel A displays the FACIT-Sp score, panel B displays the HADS-depression score, and panel C displays the HADS-anxiety score. For the FACIT-Sp scale, higher scores represent increased spirituality across the range of religious traditions. The HADS scale is divided into anxiety and depression subscales, giving the optimal sensitivity and specificity for the presence of the corresponding psychiatric symptoms. Higher scores indicate worse symptoms.
DISCUSSION
PAL-HF is the first randomized, controlled trial of a longitudinal palliative care intervention to demonstrate the significant clinical benefit of embedding such an interdisciplinary intervention in the overall management of patients with advanced HF. The addition of palliative care principles in this vulnerable population improved physical, psychosocial (anxiety/depression), and spiritual quality-of-life measures, the key domains of patient experience in serious illness. While patient-reported outcomes are historically underused as primary outcome measures in cardiology clinical trials as compared with more traditional, objective measures (such as mortality and hospitalization rates) (20), these “hard” endpoints are often less important to patients with incurable, advanced diseases (21). Instead, many of these patients have a strong desire for relief of suffering and assistance with end-of-life planning (22).
One of the primary goals of PAL-HF was to select a diverse and high-risk HF cohort that mirrors the broader population commonly admitted to the hospital. The age, sex, and race/ethnic profile of our study cohort enhances the applicability of our findings. Trial inclusion criteria were based on symptom burden in chronic HF rather than ejection fraction, and as such, 45% of patients had preserved or mildly reduced left ventricular function. Prognostic biomarkers, including NT-proBNP and serum creatinine levels, were markedly elevated in both trial arms, suggestive of poor prognosis (23,24), and the KCCQ scores were demonstrative of a poor quality of life, heavy symptom burden, and high mortality risk (25). The 6-month rehospitalization (30%) and mortality (29%) rates and relatively low use of standard guideline-directed medical therapies related to hypotension and/or renal dysfunction confirmed the selection of a high-risk population.
Previous studies of palliative care interventions in HF are few. Most have focused on providing decision-making interventions in an inpatient setting and have not extended multidisciplinary care beyond the hospital (5,6). In the only other randomized trial of HF and palliative care, Sidebottom and colleagues demonstrated improvements in quality of life and symptom burden in patients managed with standard palliative care processes (26). PAL-HF advances the science by defining the value of a systematic approach for outpatient delivery of palliative care in the HF population. The findings support the patient-reported benefits of a care delivery model that transitions and changes, much like symptoms do, beyond the walls of the health system to the community and home settings. Though patients have reported the value of similar models in other chronic illnesses, such as HIV and oncology, the palliative care interventions have been empiric and difficult to scale (27). The value of PAL-HF is that it demonstrates the effectiveness of an intervention that is well described and primarily dependent on a trained nurse practitioner and, therefore, could likely be more broadly applicable.
The pattern of improvement in the primary endpoints is interesting and has previously been observed in palliative care clinical investigation (28). First, both patient cohorts improved relative to the baseline assessment, and these changes were anticipated as the acute exacerbation of HF is treated and patients leave the hospital. The real benefit of the palliative care intervention was seen after 3 months. We posit that this finding may be related to the sustained involvement of palliative care and the relationships that develop between patients, their families, and care teams. Changes in KCCQ and FACIT-Pal scores over the 6-month follow-up period were clinically relevant and as robust as any prior trial (22).
Concordance of the 2 epistemologically distinct quality-of-life measures (the HF domains assessed by the KCCQ, and the palliative care domains assessed by FACIT-Pal) highlights the intersection of the contributions made by each of these disciplines to the common patient journey in advanced HF. While previous studies have examined the symptom experience from an HF perspective (8,10) and reported interventions to address palliative care communication gaps (14,29,30), a persistent gap has been the lack of randomized, controlled trials demonstrating the effectiveness of interventions that target the intersection of advanced HF and palliative care.
LIMITATIONS
There are several limitations to this study that should be acknowledged. First, it is a single-center trial. The imperative for wider dissemination of palliative care interventions in heart failure will be enhanced when others replicate our findings in additional cohorts at other centers. Our data certainly provide strong support for a larger, multicenter study. Second, while our palliative care intervention was based on approaches adapted from those used in other disease states such as oncology, it was implemented by a single nurse practitioner and palliative care specialist. Third, the intervention was delivered in an HF program that has already adopted many palliative care principles, so it is possible that the control group received care that might vary from that provided in other programs. Finally, in addition to the anticipated high mortality rate, 12% of patients were either lost to follow-up or withdrew consent for participation, due in part to advanced disease; this is consistent with other studies and reflects the difficulty of retaining seriously ill patients in clinical trials. This reduced the proportion of the study population able to contribute data to the primary endpoints. While such types of missing data can create biases in the analysis of quality-of-life data, we believe this is unlikely in our study due to the equal mortality rate in the two arms and the equivalent loss to follow-up for survivors.
CONCLUSIONS
Advanced HF imposes significant physical, psychosocial, and spiritual burdens on patients and their families. PAL-HF provides empirical evidence that palliative care improves health-related quality of life in end-stage HF patients. Palliative care represents an important component of the holistic management of patients with advanced HF.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The PAL-HF trial demonstrates that a multidisciplinary palliative care intervention improves quality of life and patient-reported symptoms, and it reduces the psychological burden of heart failure compared with usual care alone.
TRANSLATIONAL OUTLOOK
The morbidity and mortality associated with heart failure requires reconsideration of the care provided. Based upon the results of the PAL-HF trial, practitioners should consider adding palliative care to guideline-directed medical therapy in patients with advanced heart failure and a high risk for short-term mortality.
Acknowledgments
Funding: The PAL-HF study was funded by the National Institute of Nursing Research (NINR). Dr. Steinhauser is a Health Scientist with the Center for Health Services Research and Development in Primary Care, Durham VA Medical Center, Durham, NC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
We thank the PAL-HF data and safety monitoring board members Drs. Kirkwood Adams (chair), Laura Hanson, and Todd Schwartz for their careful review of the study data. We thank the patients for their participation in the study. Joseph Rogers and Kevin Anstrom had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- CI
confidence interval
- ESCAPE
Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial
- FACIT-Pal
Functional Assessment of Chronic Illness Therapy - Palliative Care scale
- FACIT-SP
Functional Assessment of Chronic Illness Therapy - Spiritual Well-Being scale
- FICA
Faith and Belief, Importance, Community, Address in Care survey
- HADS
Hospital Anxiety and Depression Survey
- HF
heart failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NT-proBNP
N-terminal B-type natriuretic peptide
- UC
usual care
- UC+PAL
usual care + palliative care intervention
Footnotes
Disclosures: Robert J. Mentz receives research support from the National Institutes of Health (U10HL110312 and R01AG045551-01A1), Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Medtronic, Novartis, Otsuka, and ResMed; honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, and Thoratec/St Jude; and has served on an advisory board for Luitpold Pharmaceuticals, Inc. and Boehringer Ingelheim. Kimberly Johnson received research support from projects funded by the NIA (RO1AG042130; K08AG028975). Arun Krishnamoorthy reports working on projects funded by research grants to the Duke Clinical Research Institute from the NIH, Novartis, Daiichi Sankyo, and Eli Lilly, and support to attend educational conferences from HeartWare, Thoratec, and Medtronic. James Tulsky receives research funding from PCORI (SC 14-1403-13975). None of the remaining authors have any disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016 May 17; pii: S0735-1097(16)33024-8. [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With the Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002594. pii:e002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selman L, Beynon T, Higginson IJ, Harding R. Psychological, social and spiritual distress at the end of life in heart failure patients. Curr Opin Support Palliat Care. 2007;1:260–6. doi: 10.1097/SPC.0b013e3282f283a3. [DOI] [PubMed] [Google Scholar]
- 5.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13:643–8. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA. 2004;291:2476–82. doi: 10.1001/jama.291.20.2476. [DOI] [PubMed] [Google Scholar]
- 7.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–96. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 8.Goodlin SJ, Hauptman PJ, Arnold R, et al. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–9. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 10.Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol. 2016;13:286–308. doi: 10.1038/nrcardio.2016.10. [DOI] [PubMed] [Google Scholar]
- 11.Mentz RJ, Tulsky JA, Granger BB, et al. The palliative care in heart failure trial: rationale and design. Am Heart J. 2014;168:645–51. e1. doi: 10.1016/j.ahj.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 13.Borneman T, Ferrell B, Puchalski CM. Evaluation of the FICA tool for spiritual assessment. J Pain Symptom Manage. 2010;40:163–73. doi: 10.1016/j.jpainsymman.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Steinhauser KE, Alexander SC, Byock IR, George LK, Olsen MK, Tulsky JA. Do preparation and life completion discussions improve functioning and quality of life in seriously ill patients? Pilot randomized control trial J Palliat Med. 2008;11:1234–40. doi: 10.1089/jpm.2008.0078. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–8. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 17.Flynn KE, Lin L, Ellis SJ, et al. Outcomes, health policy, and managed care: relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J. 2009;158:S64–71. doi: 10.1016/j.ahj.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-Pal) Scale. J Pain Symptom Manage. 2009;37:23–32. doi: 10.1016/j.jpainsymman.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 20.Kelkar AA, Spertus J, Pang P, et al. Utility of patient-reported outcome instruments in heart failure. JACC Heart Fail. 2016;4:165–75. doi: 10.1016/j.jchf.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lum HD, Carey EP, Fairclough D, et al. Burdensome physical and depressive symptoms predict heart failure-specific health status over one year. J Pain Symptom Manage. 2016;51:963–70. doi: 10.1016/j.jpainsymman.2015.12.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4:628–36. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–6. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18:134–42. doi: 10.1089/jpm.2014.0192. [DOI] [PubMed] [Google Scholar]
- 27.Bekelman DB, Rabin BA, Nowels CT, et al. Barriers and facilitators to scaling up outpatient palliative care. J Palliat Med. 2016;19:456–9. doi: 10.1089/jpm.2015.0280. [DOI] [PubMed] [Google Scholar]
- 28.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1438–45. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JA, Anderson RA, Docherty SL, Tulsky JA, Steinhauser KE, Bailey DE., Jr Nursing strategies to support family members of ICU patients at high risk of dying. Heart Lung. 2014;43:406–15. doi: 10.1016/j.hrtlng.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein NE, Kalman J, Kutner JS, et al. A study to improve communication between clinicians and patients with advanced heart failure: methods and challenges behind the working to improve discussions about defibrillator management trial. J Pain Symptom Manage. 2014;48:1236–46. doi: 10.1016/j.jpainsymman.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.