Abstract
Neovascular glaucoma (NVG) is a secondary ocular pathological condition resulting from a myriad of ocular and systemic conditions with retinal ischemia as a mediator in over 95% of cases. NVG is caused by the growth of a fibrovascular membrane secondary to a local angiogenic stimulus over the trabecular meshwork obstructing aqueous outflow. This results in an initial secondary open-angle glaucoma stage that may be amenable to intraocular pressure (IOP)-lowering medications and modulation of the underlying ischemic process, often in combination with panretinal photocoagulation and adjunctive use of vascular endothelial growth factor inhibitors. In the more advanced stages of neovascularization, connective tissue myofibroblasts associated with new vessel growth contract causing progressive synechial closure of the anterior-chamber angle. Elevation of IOP, once significant secondary angle closure is established, tends to be refractory to topical and oral IOP-lowering medications and often requires glaucoma surgical interventions.
Coats [1] first demonstrated new blood vessel growth on the iris surface of eyes with prior central retinal vein occlusion (CRVO) in 1906. Salus [2] reported similar surface iris vessels in diabetic eyes in 1928. Weiss et al. [3] proposed the term ‘neovascular glaucoma’ as the elevated intraocular pressure (IOP) was shown to be related to the new vessels and associated connective tissue growth. Numerous systemic diseases and ocular conditions predispose to neovascularization (NV), but almost all share a common mediator of retinal ischemia and hypoxia. A proangiogenic milieu of cytokines and proteins promotes the development of a new network of fragile, leaky vessels with associated connective tissue scaffolding over the iris surface and anterior-chamber angle.
Early recognition and therapeutic intervention are imperative in providing the best possible long-term visual and IOP outcomes in eyes with NV. Depending on the stage at detection and presence of concurrent ocular findings, treatment may include topical and oral IOP-lowering medications, topical or intraocular steroids, panretinal photocoagulation (PRP), vascular endothelial growth factor (VEGF) inhibitors, cryotherapy, cyclophotocoagulation, trabeculectomy and glaucoma shunt placement. End-stage neovascular glaucoma (NVG) can lead to blind, hypertensive and painful eyes that may require retrobulbar injection of chlorpromazine or alcohol, or, more commonly, enucleation [4, 5].
Prevalence and Disease Burden
Data from the European Union estimated that 75,000–113,000 people are affected by NVG in Europe. NVG makes up approximately 3.9% of all glaucomas [6] . In the US, the overall prevalence of NVG is low but contributes to significant visual loss and morbidity nonetheless. It is more prevalent among elderly patients [7] . The most frequent cause for the development of NVG is retinal ischemia from vascular occlusion or diabetic alterations in the retinal vasculature. Up to 60% of patients with ischemic CRVO develop anterior-segment NV within a few weeks to 1–2 years after disease onset, with an estimated incidence of 3,800 new cases per year. The overall prevalence of NV of the iris (NVI) in diabetic patients in the US is approximately 17,500 cases, with NVI occurring most often in the setting of proliferative diabetic retinopathy (PDR) [8]. The incidence of NVI among diabetic patients ranges from 1 to 17% [9, 10]. An incidence of NVI up to 65% has been reported in patients with PDR [11]. NVG can occur in over 20% of patients with PDR [12]. Diabetics with NVG in one eye have a 33% risk of developing NVG in their other eye [11].
Etiologies and Pathogenesis
The three most common predisposing conditions for NVG are diabetic retinopathy (DR) (33%), ischemic CRVO (33%) and the ocular ischemic syndrome (13%). Other forms of retinal vascular diseases resulting in significant ischemia [central retinal artery occlusion (CRAO), branch RVO, Eales’ disease and sickle cell retinopathy], intraocular neoplasms, chronic retinal detachment and severe intraocular inflammation are among the numerous disorders that cause anterior-segment NV [13].
In 1948, Michelson [14] proposed ‘there exists in the retina a factor or factors affecting the budding of new vessels’. Leung et al. [15] identified and purified a heparin-binding VEGF from media conditioned by bovine pituitary cells. This suggested that VEGF was a secreted molecule and that it was a soluble mediator of angiogenesis. VEGF is a strong mitogen for vascular endothelial cells in small and large vessels. There are significantly higher levels of VEGF in ocular fluids of patients with PDR compared to patients without proliferative disease. Extremely high levels of VEGF are present in patients with NVG [16]. In addition to VEGF, several other molecules have been associated with the development of NVG, including basic fibroblast growth factor, platelet-derived growth factor, insulin-like growth factor-1 and interferon-α [17].
Vascular proliferation first occurs with endothelial budding at the capillary level not only of the vasculature of the minor arterial circle of the iris but also the major arterial circle at the iris base. These endothelial buds progress to glomerulus-like vascular tufts, resembling renal micro-vasculature. The new vascular tissue is composed of endothelial cells without a muscular layer and with little adventitial or supportive tissue. The vessels are thin walled and tend to be located near or on the iris surface but can be seen histologically at any level within the iris [18]. The fibrovascular membrane in NVI also contains proliferating myofibroblasts with smooth muscle differentiation. This clinically transparent and contractile membrane causes a flattening and effacement of iris surface architecture, ectropion uveae, development of peripheral anterior synechiae (PAS) and subsequent secondary angle closure [19].
Clinical Features
Despite having many potential causes, the clinical course of NVG follows a relatively common pathway with variable intensities and time courses. When a patient has a known disorder with the potential to cause NVG, the first sign that NV is occurring is leakage of intravenously injected fluorescein from vessels at the pupillary margin. The leakage can be seen even when the iris appears completely normal with a detailed slit lamp examination. Once vascular proliferation occurs, it is commonly first seen at the pupillary margin but may first appear in the anterior-chamber angle. As vessels progress from the base of the iris and cross the ciliary body band, scleral spur and finally the trabecular meshwork, IOP elevation is not uncommon. Once on the meshwork, the vessels create a complex network of fine vessels that eventually involve 360° of the filtration angle. This transparent fibrous tissue results in a progressive decrease in outflow facility and corresponding increase in IOP while the angle appears gonioscopically open [20] . A contractile process then occurs, initially along a major vessel emanating from the iris base to the trabecular meshwork. This creates localized PAS that spread and eventually become continuous, causing a ‘zippered-up’ secondary angle closure. The rate of PAS formation and progression is highly variable. In some cases, angle closure can progress to complete closure in 13 week, while other cases progress very slowly over months and years. Nonetheless, the onset of elevated IOP is often acute and painful, and exacerbated by associated anterior-chamber hemorrhage and inflammation. Clinically, there can be diffuse conjunctival injection and limbal flush, diffuse microcystic corneal edema, iris rubeosis and ectropion uveae, with variable pupil response to light and possibly an afferent papillary defect depending on the symmetry and degree of optic nerve and retinal pathology. Vision is commonly at the level of counting fingers to hand motions, and IOP ranges from 40 to 60 mm Hg or higher. Gonioscopy demonstrates NV of the angle (NVA) with angle anatomy ranging from completely open to focal or complete synechial closure. The view to the angle is often obscured by corneal edema during the acute IOP elevation phase.
Early diagnosis is critical in the prognostication and initiation of prompt appropriate treatment. Every patient with NVG should undergo a comprehensive medical and ocular evaluation. Attention should be paid to pupillary responses (looking for an afferent pupillary defect), and detailed slit lamp, gonioscopic and dilated fundus examinations are required. Early undilated gonioscopy is essential in identifying early NVA and PAS. In over 10% of cases presenting with CRVO, NVA can develop before NVI is clinically apparent. Anterior-chamber cells may be seen, pointing to vascular endothelial dysfunction, and may lead to a misdiagnosis of uveitis. Early in the process, iris fluorescein angiography can be used to identify vascular endothelial dysfunction and NVI. New wide-angle imaging systems allow visualization and transit video of the peripheral retina to the level of the ora serrata, providing more information in defining the entire area of retinal ischemia and capillary nonperfusion.
Central Retinal Vein Occlusion
Several studies have reported the proportion of ischemic CRVO (over 10 disk areas of capillary nonperfusion on fluorescein angiogram) to be approximately 25%. Eighteen to 60% of ischemic CRVO cases develop NVG. It is rare for a non-ischemic CRVO to develop NVG without ischemic transition [21] . NVG has been called ‘90-’ or ‘100-day glaucoma’ in the past due to its typical development 3 months after the onset of CRVO. In reality, NVG can occur anywhere between 2 weeks and 2 years after initial RVO. Over 80% of NVI and NVG occur within 6 months of RVO however. Eyes with indeterminate fluorescein angiograms should be followed as ischemic CRVO as there is a higher incidence of NV in this group. There is also a 16% conversion rate from nonischemic to ischemic CRVO within the first 4 months of the initial occlusion [22].
Glaucoma is a recognized risk factor for CRVO. A prior diagnosis of glaucoma increases the risk of having an ischemic CRVO and has demonstrated significantly worse visual acuity outcomes when present compared to patients that experience CRVO without a prior diagnosis of glaucoma [23]. An increased cup-to-disk ratio was also shown to carry an increased risk of RVO in the Beaver Dam Eye Study [24] . Ocular hypertension and glaucomatous optic nerve cupping has been shown to be associated with branch RVO occurring at the optic nerve head or optic cup without optic nerve head edema. In contrast, ocular hypertension and glaucomatous cupping were not associated with branch RVO at the arteriovenous crossing site or with RVO associated with optic nerve swelling [25].
Diabetic Retinopathy
The majority of blindness in diabetics is a result of PDR, while NVG accounts for approximately 5% of blindness in diabetics. If a diabetic patient develops NVG in one eye, there is a relatively high risk of NVG development in the fellow eye without prophylactic treatment. Bilateral NVI and NVG are almost exclusively due to DR (fig. 1). The time interval from the development of NVI to NVG in untreated eyes is unpredictable, varying from 1 month to over 3 years. NVI has been reported to regress spontaneously in up to 26% of cases associated with DR [26]. Surgical procedures in diabetics that disrupt the anterior hyaloid or posterior capsule increase the risk for the development of anterior-segment NV (including cataract surgery and posterior capsulotomy) [27]. These structures are considered barriers to the diffusion of angiogenic factors from the posterior segment. Thus, if the view allows, a thorough evaluation of the retina is necessary to allow appropriate treatment with PRP if there is evidence of active PDR or NV prior to cataract extraction.
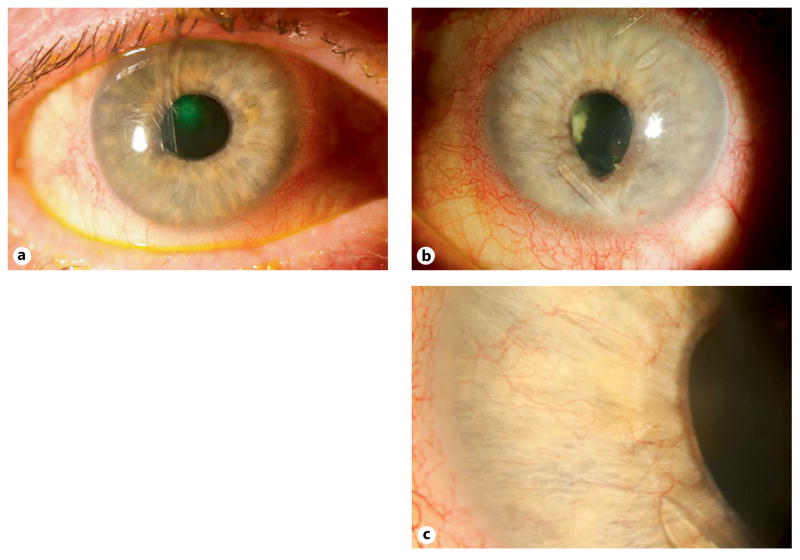
Fig. 1.
a–c : Right (a ) and left eye (b ) of a patient with severe PDR and bilateral NVG refractory to topical and oral IOP-lowering therapy requiring bilateral glaucoma drainage device implantation. Two tube shunts are present in the left eye with persistent NVI with inferior dyscoria and ectropion uvea after dense PRP and repeated VEGF inhibitor injection. c High magnification of the left eye shows NVI and the tip of tube shunt.
Carotid Artery Occlusive Disease
Carotid artery occlusive disease (CAOD) is currently the third most common cause of NVG, accounting for 13% of cases. Chronic ocular ischemia can be found in 4–18% of patients with CAOD. Chronic ciliary body hypoperfusion results in decreased aqueous production. Thus, despite extensive synechial closure, IOP may remain low or normal. CAOD should be considered in cases of NVI without a defined ocular cause, in cases of marked asymmetry of retinopathy between the eyes or if NVI does not regress after PRP.
Central Retinal Artery Occlusion
Ocular NV has been reported to occur in 2.5–31.6% cases of CRAO. NVI occurs in 3–18% and typically presents within the first 3 months after onset, but can develop much later [28]. With CRAO, there is ischemia to the inner two thirds of the retina and damage to capillary endothelial cells while outer retinal and retinal pigment epithelial perfusion from the choroid is typically intact. This helps account for why NV of the disk and elsewhere is less frequent in cases of isolated CRAO without CAOD. It has been proposed that preexisting CAOD may cause additional ocular ischemia, promoting the development of NVI and secondary NVG in cases of CRAO.
Intraocular Neoplasia
NVI has also been described in eyes with meta-static disease and primary intraocular malignancies. In these cases, the direct effect of tumor-secreted angiogenesis factors may be the cause of the NV. The incidence of NVG in the setting of melanoma is low. It is important to visualize or image the posterior pole, peripheral retina and anterior uveal tract in NVG, especially in the absence of DR in the fellow eye or a history suggestive of CRVO in the same eye. Malignant melanoma must be considered and evaluated for in these cases as the treatment will vary according to the location and size of the melanoma. The most common cause of glaucoma in the setting of retinoblastoma is NVG. Walton and Grant [29] found 38 of 56 children with retinoblastoma to have NVI, and correlated its presence with advanced tumor stage. They also noted a correlation between the duration of tumor presence and the presence of NVI. In 88 eyes enucleated for retinoblastoma, 39 (44%) had histologically confirmed NVI and choroidal involvement by the tumor.
Miscellaneous Causes
The many potential causes for NVI and NVG share the common pathway of extensive capillary nonperfusion and resulting retinal hypoxia. A listing of over 40 causes has been published previously [5]. More localized anterior-segment hypo-perfusion has been hypothesized to be the cause of the fine NVI associated with Fuch’s hetero-chromic iridocyclitis and pseudoexfoliation syndrome, but progression to NVG is extremely rare in these clinical entities.
Treatment
There are two key aspects to the management of NVG: treatment of the underlying disease responsible for the development of NV at its earliest possible stage and treatment of elevated IOP once established.
Preventive Measures
Treatment of NV is directed at the ablation of ischemic retina in most cases. PRP is considered the treatment of choice currently. Several authors have demonstrated regression of NVI due to different primary mechanisms after PRP [21, 30–33] . With the characterization of VEGF and other angiogenic factors and the agents that block their effect, treatment paradigms in the clinical management of NV are changing. In current clinical practice, the off-label use of adjunctive injection of VEGF inhibitors is becoming more common. They induce a rapid involution of NV compared with PRP, allowing for earlier resolution of anterior-segment NV while providing more time to allow for the effect of PRP or other treatment aimed at the underlying proangiogenic pathology. Initial small trials of intravitreal injection of VEGF inhibitors have shown a dramatic reduction in anterior-chamber NV, and subsequent larger series have confirmed these initial findings [34]. Iris fluorescein angiography leakage from NVI has been shown to decrease 1 day after intracameral bevacizumab [35]. Data from these series and trials suggest that VEGF inhibitors are an effective although temporary means of reducing iris and angle NV and lowering IOP. There has yet to be a large, prospective, randomized trial evaluating the use, dosing, route and timing of VEGF inhibitor treatment, and combination treatment of VEGF inhibitors with retinal ablative procedures in the prevention and treatment of NVG [36]. Despite the success with VEGF inhibitors, the large-scale destruction of ischemic retinal tissue with PRP remains the preferred standard in the large majority of eyes with NV.
When an indication for PRP exists, timely treatment may decrease the angiogenic stimulus, and prevent or induce NV regression. When NVI and NVA are already present, prompt treatment with PRP may lead to vascular regression and preservation of angle anatomy. Reports show that without PRP, 40% of ischemic CRVO will progress to NVG [7, 37] . According to Central Vein Occlusion Study recommendations, all eyes with CRVO should undergo fluorescein angiography to identify whether an ischemic or nonischemic CRVO is present. This study demonstrated that PRP was an effective means of reducing the risk of NVG after ischemic CRVO and NV. Likewise, in diabetic patients, once PDR is present, there is strong evidence that PRP is the treatment of choice for the prevention of NVG, in addition to vitreous hemorrhage and tractional retinal pathology and related vision loss [38, 39]. PRP not only reduces the risk of NVI and NVG development in PDR, it can also induce regression of established NVI and NVA when promptly administered. Large areas of retinal ischemia can still exist in eyes with PDR after otherwise seemingly adequate PRP treatment; therefore, the risk for anterior-segment NV persists. Continued monitoring for NVI and repeat gonioscopy are needed to allow timely further retinal ablation. In some cases with delayed presentation or aggressive disease, the progressive angle closure occurs at a rate too fast to wait for the eye to respond to PRP alone, and adjunctive measures are required, e.g. VEGF inhibitors.
In patients with CAOD and neurologic symptoms, including amaurosis fugax, endarterectomy is recommended in patients with significant carotid stenosis. Resolution of NVI and NVG has been reported with this procedure alone [40]. Endarterectomy is not recommended for asymptomatic patients, but risk factor control and anti-platelet therapy should be considered by the patient’s primary medical doctor.
There are various treatment options to prevent progressive NV and the development of NVG. It is thus important to identify eyes at risk for, or in the early stages of, NV to allow prompt treatment and preserve the anterior-chamber angle anatomy before there is IOP elevation.
Medical Treatment for Elevated Intraocular Pressure
Medications that effectively reduce aqueous production such as topical β-blockers, topical and oral carbonic anhydrase inhibitors and topical α-agonists may be beneficial in IOP reduction. Prostaglandin analogues are often used in an effort to enhance uveoscleral outflow, but they may have less than the typical IOP-lowering effect in NVG. Pilocarpine and other anti-cholinergic agents are relatively contraindicated due to a potential increase in inflammation, induced miosis, further anterior movement of the iris-lens diaphragm exacerbating angle closure and decreased uveoscleral outflow. Topical corticosteroid and cycloplegic therapy is often used to treat the inflammatory changes in the anterior segment, and thus improve patient comfort in addition to the effect of steroids to reduce vascular permeability and angiogenesis. Intravenous and oral hyperosmotic agents (mannitol and glycerol) can also be used as temporizing measures.
Surgical Treatment
Surgical management of refractory NVG is complicated by higher failure rates and more difficult tissue anatomy than in primary glaucomas, and has historically been a major clinical challenge. When the anterior-chamber angle is completely and irreversibly closed, the eye can be prepped for surgery to optimize the outcome with the use of aqueous suppressants to reduce and temporize IOP, corticosteroids and cycloplegics to reduce inflammation, and PRP and VEGF inhibitors to reduce or eliminate the angiogenic stimulus [41].
The data supporting the use of VEGF inhibitors as adjuncts to glaucoma filtration surgery is mounting, but as of now long-term data are scarce [42–44]. Routes of administration have included intravitreal, intracameral, subconjunctival and even topical routes with varying success in case reports and series [42–47]. The authors advocate caution as the immediate IOP spike after injection can be higher, more prolonged and detrimental in patients with compromised outflow such as NVG compared to patients with a normal outflow. A paracentesis before or after injection is frequently required and can be complicated by a hyphema related to the thin friable vessels in the anterior segment.
The surgery of choice for NVG has changed with time and the availability of antimetabolites for filtering surgeries and an increasing selection of glaucoma drainage devices. The improvements in glaucoma filtering and tube shunt procedures have facilitated a reduction in the number of cyclodestructive procedures that are performed as initial surgical procedures. Trabeculectomy remains an option preferred by some [48]. Success rates have improved with the adoption of intra- and postoperative use of anti-metabolites and antifibrotics. Despite these improvements, failure rates of trabeculectomy in NVG remain high. Anatomic alterations that favor the use of glaucoma drainage implantation in NVG include scarred and inflamed conjunctiva, potential for occlusion of the sclerotomy with the neovascular membrane, vigorous new vessel growth postoperatively and overall increased failure rates of trabeculectomy in NVG. Nonetheless, there is no large randomized trial to serve as the basis for choosing trabeculectomy over a tube drainage device or vice versa. The selection of the surgery type and tube model is based primarily on the individual surgeon’s judgment and consideration of all patient variables [49].
Cyclodestructive procedures are an option for initial or secondary intervention in eyes with little visual potential, or recurrent and refractory IOP elevation. This is most often completed using a transscleral diode laser. Despite advances in medical, laser and surgical therapy, it is not uncommon that NVG progresses to total blindness with pain. In these cases, retrobulbar injection of alcohol remains an option, but often evisceration or enucleation is required to achieve pain control.
Tremendous progress has been made in the management of NVG over the past decades, improving the prognosis for preserving vision for these patients, but improvements and advances in both diagnostic and therapeutic modalities are needed to further reduce the ocular morbidity and vision loss associated with NVG.
Conclusion and Key Points.
In patients at risk for developing NVG, high clinical suspicion, repeated clinical examination (including gonioscopy) and recognition of NV at an early stage are necessary to provide prompt treatment to prevent progression to NVG.
Topical, oral and intravenous medications are useful but often not sufficient, and filtration or shunt surgery is often required when significant synechial angle closure has occurred.
While the role of VEGF inhibitors in NVG is not well defined yet, they show promise as part of a clinical paradigm of earlier recognition, prompt treatment with PRP and surgical interventions with adjunctive medical therapies.
Acknowledgments
Supported in part by NEI K23EY023266 (VG) and an unrestricted grant from Research to Prevent Blindness, New York, NY.
References
- 1.Coats G. Further cases of thrombosis of the central vein. Roy Lond Ophthal Hosp Rep. 1906;16:516. [Google Scholar]
- 2.Salus R. Rubeosis iridis diabetica, eine bisher unbekannte diabetische Iris-veränderung. Med Klin. 1928;24:256. [Google Scholar]
- 3.Weiss DI, Shaffer RN, Nehrenberg TR. Neovascular glaucoma complicating carotid-cavernous fistula. Arch Ophthalmol. 1963;69:304–307. doi: 10.1001/archopht.1963.00960040310007. [DOI] [PubMed] [Google Scholar]
- 4.Fiore C, Lupidi G, Santoni G. Retrobulbar injection of chlorpromazine in the absolute glaucoma (in French) J Fr Ophtalmol. 1980;3:397–399. [PubMed] [Google Scholar]
- 5.Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108:1767–1776. doi: 10.1016/s0161-6420(01)00775-8. quiz 1777, 1800. [DOI] [PubMed] [Google Scholar]
- 6.Mocanu C, Barascu D, Marinescu F, Lacrateanu M, Iliusi F, Simionescu C. Neovascular glaucoma – retrospective study (in Romanian) Oftalmologia. 2005;49:58–65. [PubMed] [Google Scholar]
- 7.Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion. III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 9.Armaly MF, Baloglou PJ. Diabetes mellitus and the eye. 1. Changes in the anterior segment. Arch Ophthalmol. 1967;77:485–492. doi: 10.1001/archopht.1967.00980020487011. [DOI] [PubMed] [Google Scholar]
- 10.Madsen PH. Haemorrhagic glaucoma. Comparative study in diabetic and non-diabetic patients. Br J Ophthalmol. 1971;55:444–450. doi: 10.1136/bjo.55.7.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohrt V. The frequency of rubeosis iridis in diabetic patients. Acta Ophthalmol (Copenh) 1971;49:301–307. doi: 10.1111/j.1755-3768.1971.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen NV. The prevalence of glaucoma and ocular hypertension in type 1 and 2 diabetes mellitus. An epidemiological study of diabetes mellitus on the Island of Falster, Denmark. Acta Ophthalmol (Copenh) 1983;61:662–672. doi: 10.1111/j.1755-3768.1983.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahook M, Shuman J. Thorofare, Slack. 2013. Chandler and Grant’s Glaucoma; pp. 309–317. [Google Scholar]
- 14.Michelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc UK. 1948;68:137–180. [Google Scholar]
- 15.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 16.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 17.Ayaki M. Development of neovascular glaucoma in the course of interferon alfa therapy for hepatitis type C. Br J Ophthalmol. 1994;78:238. doi: 10.1136/bjo.78.3.238-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gartner S, Henkind P. Neovascularization of the iris (rubeosis iridis) Surv Ophthalmol. 1978;22:291–312. doi: 10.1016/0039-6257(78)90175-3. [DOI] [PubMed] [Google Scholar]
- 19.John T, Sassani JW, Eagle RC., Jr The myofibroblastic component of rubeosis iridis. Ophthalmology. 1983;90:721–728. doi: 10.1016/s0161-6420(83)34520-6. [DOI] [PubMed] [Google Scholar]
- 20.Grant WM. Management of neovascular glaucoma. In: Leopold IH, editor. Symposium on Ocular Therapy. St. Louis: Mosby; 1974. [Google Scholar]
- 21.Laatikainen L, Kohner EM, Khoury D, Blach RK. Panretinal photocoagulation in central retinal vein occlusion: a randomised controlled clinical study. Br J Ophthalmol. 1977;61:741–753. doi: 10.1136/bjo.61.12.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087–1095. doi: 10.1001/archopht.1993.01090080083022. [DOI] [PubMed] [Google Scholar]
- 23.Moisseiev J, Desatnik H, Cohen Y, Lusky A, Melamed S. Glaucoma and visual outcome in central retinal vein occlusion. Acta Ophthalmol Scand. 1996;74:368–371. doi: 10.1111/j.1600-0420.1996.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein BE, Meuer SM, Knudtson MD, Klein R. The relationship of optic disk cupping to retinal vein occlusion: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;141:859–862. doi: 10.1016/j.ajo.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Beaumont PE, Kang HK. Cup-to-disc ratio, intraocular pressure, and primary open-angle glaucoma in retinal venous occlusion. Ophthalmology. 2002;109:282–286. doi: 10.1016/s0161-6420(01)00922-8. [DOI] [PubMed] [Google Scholar]
- 26.Madsen PH. Rubeosis of the iris and haemorrhagic glaucoma in patients with proliferative diabetic retinopathy. Br J Ophthalmol. 1971;55:368–371. doi: 10.1136/bjo.55.6.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinreb RN, Wasserstrom JP, Parker W. Neovascular glaucoma following neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1986;104:730–731. doi: 10.1001/archopht.1986.01050170120035. [DOI] [PubMed] [Google Scholar]
- 28.Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol. 2010;20:1042–1046. doi: 10.1177/112067211002000603. [DOI] [PubMed] [Google Scholar]
- 29.Walton DS, Grant WM. Retinoblastoma and iris neovascularization. Am J Ophthalmol. 1968;65:598–599. doi: 10.1016/0002-9394(68)93882-8. [DOI] [PubMed] [Google Scholar]
- 30.Callahan MA. Letter: photocoagulation and rubeosis iridis. Am J Ophthalmol. 1974;78:873–874. doi: 10.1016/0002-9394(74)90338-9. [DOI] [PubMed] [Google Scholar]
- 31.Laatikainen L. Preliminary report on effect of retinal panphotocoagulation on rubeosis iridis and neovascular glaucoma. Br J Ophthalmol. 1977;61:278–284. doi: 10.1136/bjo.61.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy RP, Egbert PR. Regression of iris neovascularization following pan-retinal photocoagulation. Arch Ophthalmol. 1979;97:700–702. doi: 10.1001/archopht.1979.01020010352013. [DOI] [PubMed] [Google Scholar]
- 33.Tasman W, Magargal LE, Augsburger JJ. Effects of argon laser photocoagulation on rubeosis iridis and angle neovascularization. Ophthalmology. 1980;87:400–402. doi: 10.1016/s0161-6420(80)35225-1. [DOI] [PubMed] [Google Scholar]
- 34.Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21:112–117. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- 35.Grisanti S, Biester S, Peters S, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158–160. doi: 10.1016/j.ajo.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 36.SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol. 2013;28:165–172. doi: 10.3109/08820538.2012.730103. [DOI] [PubMed] [Google Scholar]
- 37.Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion. A 10-year prospective study. Graefes Arch Clin Exp Ophthalmol. 1990;228:281–296. doi: 10.1007/BF00920049. [DOI] [PubMed] [Google Scholar]
- 38.Preliminary report on effects of photo-coagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 39.Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. The Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1979;97:654–655. doi: 10.1001/archopht.1979.01020010310003. [DOI] [PubMed] [Google Scholar]
- 40.Neupert JR, Brubaker RF, Kearns TP, Sundt TM. Rapid resolution of venous stasis retinopathy after carotid endarterectomy. Am J Ophthalmol. 1976;81:600–602. doi: 10.1016/0002-9394(76)90123-9. [DOI] [PubMed] [Google Scholar]
- 41.Allen RC, Bellows AR, Hutchinson BT, Murphy SD. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982;89:1181–1187. doi: 10.1016/s0161-6420(82)34672-2. [DOI] [PubMed] [Google Scholar]
- 42.Cornish KS, Ramamurthi S, Saidkasimova S, Ramaesh K. Intravitreal bevacizumab and augmented trabeculectomy for neovascular glaucoma in young diabetic patients. Eye (Lond) 2009;23:979–981. doi: 10.1038/eye.2008.113. [DOI] [PubMed] [Google Scholar]
- 43.Grewal DS, Jain R, Kumar H, Grewal SP. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy: a pilot study. Ophthalmology. 2008;115:2141e2–2145.e2. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Kapetansky FM, Pappa KS, Krasnow ND, Baker ND, Francis CD. Subconjunctival injection(s) of bevacizumab for failing filtering blebs. Invest Ophthalmol Vis Sci 2007, ARVO Meeting Abstracts; p. E-837. [Google Scholar]
- 45.Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144–146. [PubMed] [Google Scholar]
- 46.Kahook MY, Schuman JS, Noecker RJ. Needle bleb revision of encapsulated filtering bleb with bevacizumab. Ophthalmic Surg Lasers Imaging. 2006;37:148–150. [PubMed] [Google Scholar]
- 47.Choi JY, Choi J, Kim YD. Subconjunctival bevacizumab as an adjunct to trabeculectomy in eyes with refractory glaucoma: a case series. Korean J Ophthalmol. 2010;24:47–52. doi: 10.3341/kjo.2010.24.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi AB, Parrish RK, 2nd, Feuer WF. 2002 survey of the American Glaucoma Society: practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172–174. doi: 10.1097/01.ijg.0000151684.12033.4d. [DOI] [PubMed] [Google Scholar]
- 49.Netland PA. The Ahmed glaucoma valve in neovascular glaucoma (an AOS thesis) Trans Am Ophthalmol Soc. 2009;107:325–342. [PMC free article] [PubMed] [Google Scholar]