Abstract
The unfolded protein response (UPR) is an evolutionarily conserved stress response to intra- and extracellular conditions that disrupt endoplasmic reticulum (ER) protein-folding capacity. The UPR is engaged by a variety of disease conditions, including most cancers as well as both metabolic and neurodegenerative disorders. Three transmembrane transducers—PERK, IRE1, and ATF6—are responsible for activating downstream signaling pathways that mediate the UPR and subsequent stress response pathways. PERK, an ER resident transmembrane protein kinase, initiates both pro-apoptotic and pro-survival signaling pathways. In the context of neoplasia, PERK and its downstream targets alter gene expression that can be both pro- and anti-tumorigenic. In this review, we discuss recent advances in understanding how canonical and non-canonical PERK-mediated signaling pathways influence cell fate, tumor progression, and tumor suppression and avenues for therapeutic intervention.
Keywords: PERK, Unfolded Protein Response, tumorigenesis, protein translation
Introduction
The endoplasmic reticulum (ER) is the site of post-translational modification, folding, maturation, and secretion for transmembrane and secreted proteins. The rate of protein transport into the ER and its folding capacity fluctuates on the basis of intra- and extracellular conditions and varies among cell type. Cells can adapt to increased nascent protein import into the ER lumen and folding demands by preferentially increasing the overall size of the ER and upregulating translation of chaperone proteins 1. However, under stressful conditions, such as tumorigenesis, protein translation exceeds ER folding capacity, resulting in the accumulation of misfolded proteins within the ER. As a result of the accumulation of misfolded proteins, an evolutionarily conserved stress response known as the unfolded protein response (UPR) is activated. The function of the UPR is to either re-establish homeostasis or trigger cell death in order to prevent accumulation of damaged, non-functional cells.
Mammalian cells have three ER resident transmembrane proteins—protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 alpha/beta (IRE1α/β), and activating transcription factor 6 (ATF6)—that function as signal transducers of the UPR. All three transmembrane proteins contain a single transmembrane domain, luminal domain, and are present at a basal level in their inactive state. PERK and IRE1α also consist of a cytosolic tail that has kinase activity and kinase and ribonuclease activity, respectively. The inactive states of PERK, IRE1α, and ATF6 are characterized by the binding of binding immunoglobulin protein (BiP)/glucose-regulated protein 78 kDa (GRP78) ATPase domain to their luminal domain 2. Decreased expression of BiP/GRP78 via repression of its coding gene, HSPA5, activates all three UPR sensors 3. Furthermore, accumulation of misfolded proteins sequesters BiP/GRP78 away from PERK, IREα, and ATF6. BiP sequestration may occur through the interaction of unfolded proteins, such as CH1. CH1 interacts with the substrate-binding domain of BiP/GRP78 where it potentially triggers the dissociation of BiP/GRP78 from the luminal domains of PERK, IRE1α, and ATF6 2, 4. Ultimately, reduced BiP/GRP78 interaction is essential for activation of PERK, IRE1α, and ATF6.
Sequestration of BiP/GRP78 from the luminal domain of PERK triggers oligomerization and autophosphorylation 5. PERK is found in both a dimer and a transient tetramer state, and the tetramer state is a higher state of activation than the dimer. PERK luminal domains oligomerize to form stable dimers and then a helix swap, or the intertwining of two dimers via helical subunit, produces the tetramer configuration 6. The tetramer interface is primarily composed of hydrophobic residues that are thought to help stabilize the tetramer structure and increase phosphorylation efficiency 6. Transient configuration changes between the dimer and the tetramer may represent an intrinsic form of regulation based on the level of misfolded proteins in the ER lumen. Activated PERK phosphorylates eukaryotic initiation factor 2α (eIF2α), thereby reducing translation initiation for a majority of cellular proteins. However, a select set of mRNAs that typically encode short open reading frames within the 5′ untranslated region (UTR) (upstream open reading frames, or uORFs), such as basic leucine zipper protein family member activating transcription factor 4 (ATF4), are translationally induced 7.
Similar to PERK, titration of BiP/GRP78 leads to IRE1α oligomerization and activation via trans-autophosphorylation. In addition to functioning as a protein kinase, the IRE1α cytosolic domain harbors ribonuclease activity which mediates the degradation of ER-localized mRNAs through a process known as regulated IRE1-dependent decay (RIDD). While RIDD triggers decay of both non-coding and coding RNA, IRE1 ribonuclease activity also specifically splices an intron from XBPI mRNA, thereby increasing XBP1 translation. Genes downstream of XBP1s influence protein secretion, cell survival and apoptosis, and DNA damage and repair 8. IREα associates with the Sec61 translocon to locate XBP1 unspliced mRNA 9. Interestingly, repression of translocon subunits specifically activated IRE1α and leads to XBP1 mRNA splicing equal to that of decreased BiP/GRP78 interaction 10. Activation of IRE1α through loss of translocon interaction suggests UPR signaling pathway activation specificity. PDIA6 influence on IRE1 and ATF6 activation independent of BiP, but not PERK activation, further suggests UPR signaling pathway activation specificity 11, 12.
ATF6 is a transmembrane transcription factor. ER stress causes export of ATF6 to the Golgi followed by two sequential cleavage events by protease site-1 protease (S1P) and protease site-2 protease (S2P). The two cleavage events expose ATF6’s transcriptionally active cytosolic domain, which translocates to the nucleus. ATF6 also induces XBP1 mRNA, highlighting the cross-talk between UPR pathways 13.
The pathways described above represent the canonical ER stress and UPR signaling pathway. In recent years, great strides have been made to better understand the canonical and non-canonical signaling pathways that influence other stress response mechanisms and cell fate and ultimately revealed potential roles of both canonical and non-canonical pathways in disease. In the following sections, we will highlight recent novel findings pertaining to the role of UPR in regulating cell fate following exposure of cells to tumor-associated stresses and the potential for therapeutic development.
UPR signaling and cell fate
Signaling through eIF2α
The integrative stress response (ISR) is a complex signaling pathway that is activated by both intracellular and extracellular stressors. Stress-specific protein kinases recognize stress induction and mediate ISR downstream signaling pathways. For example, amino acid deprivation, heme deprivation, viral infection, and misfolded proteins are recognized by GCN2, HRI, PKR, and PERK, respectively 14. While there is inherent stress recognition specificity, there is also considerable redundancy among these protein kinases that has been elucidated through gene-specific knockout mice 15. Interestingly, the ISR downstream signaling pathways of all four previously mentioned kinases converge on the phosphorylation of eIF2α. Of particular interest in the present review is the PERK-eIF2α UPR signaling pathway.
eIF2α coordinates the formation of eIF2α-GTP-Met-tRNA i Met, a component of the 43S pre-initiation complex necessary for formation of the 80 S ribosome complex. Formation of the 80S ribosome complex requires hydrolysis of eIF2-GTP to eIF2-GDP, which leads to dissociation of the ribosomal complex for translation termination. The eIF2α guanine exchange factor, eIF2β, is responsible for exchanging GDP for GTP to enable eIF2α-GTP-Met-tRNA i Met complex reformation and ultimately re-initiate protein translation 16. However, phosphorylation of eIF2α by PERK (HRI, PKR, or GCN2) inhibits eIF2β-dependent exchange of GDP for GTP, thereby preventing reformation of the 43 S pre-initiation complex and subsequent ribosome complex. mRNAs containing uORFs will stall 43S pre-initiation complexes to block translation, but at low concentrations the 43 S pre-initiation complex will skip the uORFs, enabling mRNA translation 17, 18.
PERK mediates pro-death and pro-survival signaling
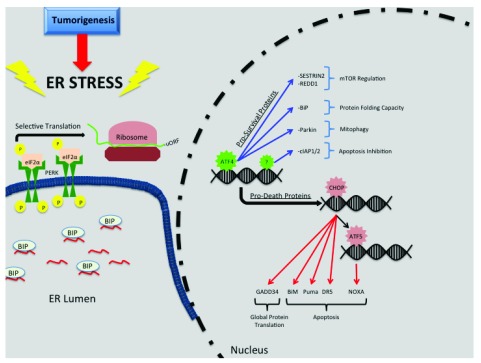
PERK-mediated eIF2α phosphorylation directly regulates expression of cell fate–determining genes through several mechanisms. ATF4, which is translationally induced by PERK is the most heavily studied. The ATF4 mRNA contains overlapping uORFs in its 5′ UTR which are necessary or and preferentially ATF4 translation upon phosphorylation of eIF2α ( Figure 1). ATF4 directs transcription of a complex network of genes that ultimately determine cell fate. ATF4 induces expression of multiple ER resident chaperone proteins (such as BiP/GRP78) to increase folding capacity, mediate amino acid metabolism and glutathione synthesis, and increase resistance to oxidative stress 19. ATF4 also induces autophagy genes that are important in autophagosome formation and function 20. Inhibitors of apoptosis, cIAP1 and 2, are also induced during ER stress in a PERK-dependent but ATF4-independent manner 21 ( Figure 1).
Figure 1. Activation of unfolded protein response and downstream pro-survival and pro-death proteins.
Tumorigenesis induces protein synthesis that exceeds the endoplasmic reticulum (ER) folding capacity and increases the number of nascent proteins within the ER lumen leading to ER stress. Sequestration of binding immunoglobulin protein (BiP) from the luminal domain of protein kinase RNA-like ER kinase (PERK) to nascent proteins enables PERK oligomerization, autophosphorylation, and activation. Activated PERK phosphorylates eukaryotic initiation factor 2α (eIF2α) at Ser51 and causes selective translation of proteins containing upstream open reading frames (uORFs). Selectively translated proteins, like transcription factor activating transcription factor 4 (ATF4), directly or indirectly regulate expression of pro-survival and pro-death proteins. Red arrows represent pro-death pathways, and blue arrows represent pro-survival pathways.
A well-known ATF4 pro-death target gene is the transcription factor C/EBP homologous protein (CHOP), which further promotes transcription of pro-death genes 22 ( Figure 1). CHOP is directly responsible for inducing expression of two BH3-only pro-apoptotic Bcl-2 family members: Bim and Puma. Bim and Puma mediate cell death by negatively regulating the activity of pro-survival Bcl-2 family members 23. Negative regulation of pro-survival Bcl-2 family member activity via Bim and Puma enables Bax/Bak oligomerization mediating the apoptotic release of cytochrome C 24, 25. CHOP indirectly induces expression of another BH3-only pro-apoptotic Bcl-2 family member, NOXA, through induction of the transcription factor ATF5 26. Similar to Bim and Puma, NOXA negatively regulates pro-survival Bcl-2 family proteins. Furthermore, CHOP induces expression of death receptor 5 (DR5) which will bind Fas-associated death domain (FADD) independent of the DR5 ligand, Apo2/TRAIL 27. FADD transduces pro-death signals by activating caspase 8 28. Interestingly, DR5 mRNA can be degraded via RIDD, highlighting regulatory cross-over between UPR signaling pathways 27.
ATF4 also impacts cell fate through modulation of apoptosis inhibitors such as XIAP. Here, transcriptional induction of ubiquitin ligases increases XIAP proteasomal degradation, thereby increasing apoptosis. In this instance, however, induction of ubiquitin ligase and subsequent XIAP proteasomal degradation is CHOP-independent 29. ATF4/CHOP induces GADD34, which encodes a protein that directs protein phosphatase 1 (PP1) to eIF2α 30. The induction of Gadd34 creates a negative feedback loop to re-establish global protein translation and can increase protein load during ER stress, exacerbating stress and causing cell death 31.
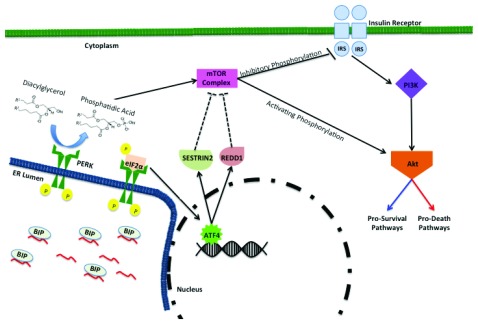
PERK also mediates pro-survival and pro-death signaling through the mechanistic target of rapamycin (mTOR) pathway ( Figure 2). mTOR is a kinase that regulates cell growth and proliferation via nutrient availability and protein translation and is dysregulated in many cancers 32, 33. PERK has intrinsic lipid kinase activity that mediates production of the pro-mitogenic phospholipid, phosphatidic acid (PA), via phosophorylation of diacylglycerol 34. PA has been implicated in mTOR activation via competition with rapamycin and is essential for mTOR complex (mTORC) formation 35– 37. Therefore, PERK’s lipid kinase activity produces PA-mediating mTORC formation and subsequent Akt activating phosphorylation ( Figure 2). Akt downstream signaling pathways are highly complex as they both mediate survival and apoptotic responses 35, 36. Another example of PERK-mediated mTOR regulation is the ATF4-dependent expression of DNA damage and development 1 (REDD1) and SESTRIN2, both of which can suppress mTOR 38– 41 ( Figure 2). One of mTOR’s functions is to mediate inhibitory phosphorylation of insulin receptor substrate (IRS) docking proteins that activate the PI3K pathway 42, 43. Suppression of mTOR prevents inhibitory phosphorylation of IRS docking proteins thereby activating PI3K and subsequent Akt signaling pathways ( Figure 2).
Figure 2. PERK-mediated regulation of the mTOR-PI3K-Akt pathway.
Endoplasmic reticulum (ER) stress sequesters ER resident chaperone binding immunoglobulin protein (BiP) away from luminal domain of PERK, leading to PERK activation. Activated PERK contains lipid kinase activity and converts diacylglycerol to phosphatidic acid (PA). PA is instrumental in mammalian target of rapamycin (mTOR) complex formation. mTOR activation can inhibit insulin receptor substrates via phosphorylation and block PI3K-Akt activation. Alternatively, mTOR complex can activate Akt and downstream pathways via phosphorylation. Activating transcription factor 4 (ATF4)-dependent expression of SESTRIN2 and regulated IRE1-dependent decay 1 (RIDD1) can indirectly suppresses mTOR activity (indirect regulation illustrated by dashed lines). Akt-mediated downstream signaling pathways can have both pro-survival and pro-death impacts on cell fate. mTOR, mechanistic target of rapamycin; PERK, protein kinase RNA-like endoplasmic reticulum kinase.
PERK signaling can also play a role in mitochondrial pro-survival signaling 44. The ER and mitochondrial membranes are connected via mitochondria-associated ER membranes (MAMs) 44. Interestingly, activated PERK has been localized to MAMs, suggesting that PERK’s activation and downstream signaling pathways can influence mitochondrial mediated cell survival 45. Furthermore, PERK-ATF4 transcriptionally induces expression of Parkin, a protein that mediates autophagy of mitochondria (mitophagy) 46, 47. Mitophagy promotes cell survival by maintaining mitochondrial homeostasis.
Non-coding RNA mediated pro-death and pro-survival signaling
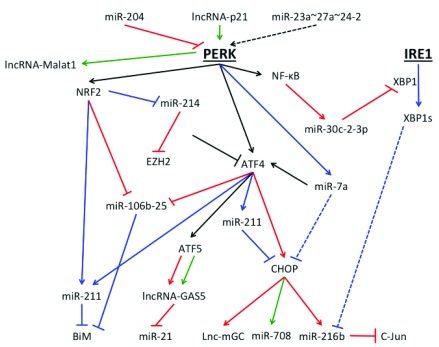
The role of non-coding RNAs in cell fate has become an area of intense investigation. Two non-coding RNAs—microRNA (miRNA) and long non-coding RNA (lncRNA)—are regulated by the UPR and play significant roles in cell survival and cell death signaling. miRNAs are small regulatory non-coding RNA molecules approximately 22 nucleotides in length with a seven-nucleotide seed sequence that recognizes complementary sequences in target mRNA. Upon recognition, the most miRNAs mediate target mRNA degradation or inhibit their translation. However, some miRNAs can lead to mRNA-specific upregulation 48. Advances in high-throughput sequencing technology facilitated the identification of up to 86 differentially expressed miRNAs following ER stress induction 49. Much of the miRNA regulation that influences cell fate occurs through the PERK arm of the UPR. Interestingly, one miRNA, miR-204, directly targets and inhibits PERK signaling 50. Repression of PERK signaling blocks expression of most genes necessary to overcome ER stress and leads to cell death.
Downstream of PERK, ATF4-dependent expression of miR-211 and NRF2-dependent repression of miR-214 promote cell survival 51, 52 ( Figure 3). MiR-211 contains a seed sequence targeting the promoter region of chop 51. Repression of chop inhibits pro-apoptotic signaling and promotes cell survival. MiR-214 targets both ATF4 and EZH2. Decreased expression of miR-214 enables ATF4 and EZH2 expression, which increases transcription of pro-survival genes and represses pro-apoptotic protein BiM, respectively 52. Cell survival is also promoted by ER stress-induced expression of miR-7a and subsequent indirect repression of pro-apoptotic transcription factor CHOP 49. CHOP induces expression of miR-216b, which mediates translational repression of c-Jun and sensitizes cells to ER stress-induced apoptosis 53. Interestingly, miR-216b is indirectly regulated by the IRE1 branch of the UPR, suggesting cross-over in miRNA regulation 53 ( Figure 3).
Figure 3. Activation of unfolded protein response and downstream pro-survival, pro-death, and tumorigenic related non-coding RNA.
Endoplasmic reticulum (ER) stress induces complex non-coding RNA, both microRNA (miRNA) and long non-coding RNA (lncRNA), regulatory pathways that influence cell fate and tumorigenesis. Two miRNAs—miR-204 and miR023a~27a~24-2—repress and activate protein kinase RNA-like ER kinase (PERK) activity, respectively. Furthermore, multiple miRNAs and lncRNAs are induced or repressed following PERK activation. Black arrows represent pathways that can lead to either pro-survival or pro-death signaling, blue arrows represent pathways that lead to pro-survival signaling, red arrows represent pathways that lead to pro-death signaling, and green arrows represent pathways that influence tumorigenesis. Dotted lines represent indirect regulatory pathways.
miRNAs that are within adjacent regions and transcribed in the same orientation can form miRNA clusters. Most clusters contain two to three miRNAs, and different miRNAs within a cluster can have different targets 54. PERK-mediated induction of ATF4 and NRF2 downregulates expression of miR-106b-25 cluster and increases cell death 55. Repression of this cluster enhances ER stress-induced apoptosis because miR-106b-25 cluster antagonizes pro-apoptotic protein BiM translation. Furthermore, PERK/NF-κB induces miR-30c-2-3p expression which targets the IRE1-dependent transcription factor XBP1 56. Spliced XBP1 (XBP1s) induces transcription of important pro-survival genes; therefore, decreased XPB1 can induce cell death. Regulation of miR-30c-2-3p also demonstrates cross-talk between arms of the UPR. Interestingly, in contrast to ER stress inducing miRNA expression, miR-23a~27a~24-2 cluster induces ER stress and increases expression of pro-apoptotic proteins CHOP and Bim 57 ( Figure 3).
lncRNAs, RNA molecules of more than 200 base pairs, regulate cellular processes at both the transcription and translational level, and some evidence suggests regulation at the post-translational level 58. Multiple lncRNAs are regulated under ER stress states and play a role in UPR-mediated cell fate. The lncRNA TUG1 protects hepatocytes from apoptosis by repressing expression of essential UPR proteins BiP/GRP78, PERK, phosphorylated eIF2α, and CHOP 59. CHOP induces expression of lnc-mGC, a lncRNA that binds a megacluster of 40 miRNAs and promotes cell death through changes in multiple miRNA-mediated translation regulation pathways 60. Furthermore, PERK-dependent ATF4 expression induces expression of ATF5, which subsequently induces expression of GAS5 26 ( Figure 3). GAS5 expression can promote apoptosis by multiple hypothesized mechanisms, including repression of steroid receptor–induced transcriptional activation, inhibition of miR-21, and sensitizing cells to external stressors 61. More research is needed to identify UPR-mediated lncRNA and elucidate their highly complex role in determining cell fate.
ER stress-induced tumor suppression versus tumor progression
Malignant transformation and tumor progression must bypass ER stress, which is induced by factors including aberrant expression of oncogenes and a microenvironment with disordered vasculature that contributes to nutrient restriction, hypoxia, and increased ROS 62, 63. Given the propensity of the UPR and PERK to induce pro-survival signaling, significant efforts have been devoted to elucidate its contribution to tumor progression with the underlying assumption that PERK will be pro-tumorigenic; if so, the expectation was tumor addiction to PERK signaling. However, many genetic experiments support both a tumor-suppressive and tumor-promoting function for PERK.
Activation of the potent oncogene, HRAS, in non-cancerous melanocytes increased cellular senescence in a PERK-dependent manner, suggesting that PERK mediates senescence in pre-malignant cells 64. Furthermore, some cancer cell lines display decreased PERK-mediated eIF2α signaling, suggesting that PERK activation and CHOP expression may attenuate malignant transformation and progression under certain conditions or in a cell type–dependent manner 62, 65. Consistent with this notion, PERK displays characteristics of a haploinsufficient tumor suppressive in melanocytes 66. ER stress-induced PERK activation can also influence immunological recognition of malignant cells. For example, PERK activation upregulates the ER chaperone calreticulin, which can be translocated to the cell surface of malignant cells and act as a phagocytic signal for immune cells 67.
PERK-dependent non-coding RNAs can also act as tumor suppressors. For example, CHOP-dependent miR-708 targets neuronatin, which decreases intracellular calcium levels, resulting in reduced metastasis 68, 69. Likewise, lncRNA-p21 induces ER stress through PERK activation and mediates hepatocellular carcinoma apoptosis 70. In addition, CHOP mediates expression of ATF5, which induces expression of lncRNA GAS5, which is pro-apoptotic in different cancers 61, 71. Ultimately, PERK activation- or deactivation-mediated tumor suppression signaling may be cancer-specific and further investigation is needed.
PERK-mediated tumor progression can occur at multiple stages in cancer development. PERK activation can help pre-malignant cells cope and survive ER stress conditions enabling neoplastic transformation 72. Recent studies have demonstrated the importance of PERK activation in tumor metastasis by mediating pathways that promote tumor cell epithelial-to-mesenchymal transition (EMT) and detachment and invasion 73– 76. The PERK-dependent lncRNA Malat1 is a marker in numerous cancers and plays an important role in lung cancer progression and metastasis 77– 79. Tumor angiogenesis can be indirectly induced by ATF4-dependent induction of the aryl hydrocarbon receptor and subsequent expression of vascular endothelial growth factor in hepatoblastoma cells 80. Interestingly, PERK activation can further promote therapy resistance and resistance to hypoxia 81. The dynamic role of PERK-mediated signaling in tumor progression and resistance makes PERK and its downstream pathways attractive for therapeutic intervention.
Therapeutic potential
The contribution of PERK-dependent gene expression to cell survival stimulated the development of strategies for therapeutic intervention. Recently, a potent PERK inhibitor, GSK2606414, was synthesized and specifically inhibits PERK activation as well as decreases tumor growth in human tumor xenograft mice 82. GSK2606414 was later modified to a more pharmacological stable form, GSK2656157, which demonstrated dose-dependent inhibition of human tumor xenograft growth in mice and the potential for further clinical implementation 83, 84.
Targeting PERK may not be without consequence. PERK signaling is critical for enabling normal cells to overcome ER stress. PERK also elicits anti-proliferative signals through silencing of G 1 cyclins and induction of pro-apoptotic pathways 85, 86. In addition, while PERK is non-essential in most adult tissues, PERK deletion or inhibition can lead to pancreatic failure and hyperglycemia 87. Further investigation of PERK inhibition–associated risks in in vivo models is needed to determine therapeutic potential.
Other approaches to therapeutic intervention have demonstrated promising anti-tumor potential. For example, the eIF2α phosphatase complex inhibitor, salubrinal, induced apoptosis, increased chemotherapy efficiency, and restored treatment sensitivity in chemo-resistant cancer cells 88– 91. Also, salubrinal treatment in conjunction with a proteasome inhibitor increased apoptosis in leukemic cells 88. Inhibition of GRP78 demonstrated anti-angiogenic potential, and indirect inhibition of the PERK/eIF2α/ATF4 pathway inhibited EMT 92, 93.
Additionally, two small molecules—guanabenz and Sephin1—have been reported to selectively inhibit the eIF2α phosphatase complex 94, 95. Decreased dephosphorylation of eIF2α prolongs global protein translation repression in stressed cells and increases chaperone-folding ability. Guanabenz and Sephin1 blocked eIF2α dephosphorylation by inducing a conformational change that disrupts recruitment of eIF2α to its phosphatase complex 96. Efficacy of the aforementioned therapeutics, though promising, may hinder their clinical impact as some have suggested that guanabenz and Sephin1 do not interfere with eIF2α dephosphorylation 97. Combinatorial studies of PERK and downstream target inhibitors with other drugs such as proteasome inhibitors may be one approach to increasing efficacy and reducing toxicities.
Concluding remarks
PERK-mediated signaling pathways are complex and vary between tissue and cell type but play a clear role in cell fate and tumorigenesis. PERK activation and downstream signaling pathways have also been implicated to play a role in other pathologies such as neurodegeneration and diabetes 87, 98, 99. The findings presented in this review demonstrate the tremendous progress toward understanding PERK biology. However, while we are beginning to elucidate signals that drive these PERK-mediated responses, there remain significant gaps in our knowledge that will compromise our ability to translate inhibitors successfully into the clinic. Furthermore, the role and mechanisms of ER stress-induced non-coding RNAs remain incompletely understood. Ultimately, a deeper understanding of these remaining questions will help elucidate the role of PERK in different pathologies for more effective therapeutic intervention.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David Wiest, Fox Chase Cancer Center, Philadelphia, PA, 19111-2497, USA
Linda Hendershot, Department of Tumor Cell Biology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA
Eric Chevet, INSERM, Université Rennes 1, Rennes, France
Funding Statement
This work was supported by National Institutes of Health grants P01CA104838 (JAD) and T32DE017551 (ASM).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Schuck S, Prinz WA, Thorn KS, et al. : Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187(4):525–36. 10.1083/jcb.200907074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrara M, Prischi F, Nowak PR, et al. : Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife. 2015;4:e03522. 10.7554/eLife.03522 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Wang M, Wey S, Zhang Y, et al. : Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11(9):2307–16. 10.1089/ars.2009.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen J, Snapp EL, Lippincott-Schwartz J, et al. : Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol. 2005;25(3):921–32. 10.1128/MCB.25.3.921-932.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma K, Vattem KM, Wek RC: Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277(21):18728–35. 10.1074/jbc.M200903200 [DOI] [PubMed] [Google Scholar]
- 6. Carrara M, Prischi F, Nowak PR, et al. : Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. EMBO J. 2015;34(11):1589–600. 10.15252/embj.201489183 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Zhang K, Kaufman RJ: Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279(25):25935–8. 10.1074/jbc.R400008200 [DOI] [PubMed] [Google Scholar]
- 8. He Y, Sun S, Sha H, et al. : Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr. 2010;15(1):13–25. 10.3727/105221610X12819686555051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plumb R, Zhang ZR, Appathurai S, et al. : A functional link between the co-translational protein translocation pathway and the UPR. eLife. 2015;4:e07426. 10.7554/eLife.07426 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Adamson B, Norman TM, Jost M, et al. : A Multiplexed Single-Cell CRISPR Screening Platform Enables Systematic Dissection of the Unfolded Protein Response. Cell. 2016;167(7):1867–1882.e21. 10.1016/j.cell.2016.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Groenendyk J, Peng Z, Dudek E, et al. : Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal. 2014;7(329):ra54. 10.1126/scisignal.2004983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Hossain MM, Read DE, et al. : PERK regulated miR-424(322)-503 cluster fine-tunes activation of IRE1 and ATF6 during Unfolded Protein Response. Sci Rep. 2015;5: 18304. 10.1038/srep18304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida H, Matsui T, Yamamoto A, et al. : XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–91. 10.1016/S0092-8674(01)00611-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Ron D, Harding H: eIF2 Phosphorylation in Cellular Stress Responses and Disease. Transl Control Biol Med. 2007;349–372. Reference Source [Google Scholar]
- 15. Rothenburg S, Georgiadis MM, Wek RC: Evolution of eIF2α Kinases: Adapting Translational Control to Diverse Stresses. In: Evolution of the Protein Synthesis Machinery and Its Regulation (eds. Hernandez G, Jagus R), Springer International Publishing.2016;235–260. 10.1007/978-3-319-39468-8_11 [DOI] [Google Scholar]
- 16. Hinnebusch AG: The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem. 2014;83:779–812. 10.1146/annurev-biochem-060713-035802 [DOI] [PubMed] [Google Scholar]
- 17. Young SK, Baird TD, Wek RC: Translation Regulation of the Glutamyl-prolyl-tRNA Synthetase Gene EPRS through Bypass of Upstream Open Reading Frames with Noncanonical Initiation Codons. J Biol Chem. 2016;291(20):10824–35. 10.1074/jbc.M116.722256 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Young SK, Palam LR, Wu C, et al. : Ribosome Elongation Stall Directs Gene-specific Translation in the Integrated Stress Response. J Biol Chem. 2016;291(12):6546–58. 10.1074/jbc.M115.705640 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Tsai YC, Weissman AM: The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1(7):764–78. 10.1177/1947601910383011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. B'chir W, Maurin AC, Carraro V, et al. : The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–99. 10.1093/nar/gkt563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamanaka RB, Bobrovnikova-Marjon E, Ji X, et al. : PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28(6):910–20. 10.1038/onc.2008.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harding HP, Novoa I, Zhang Y, et al. : Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. 10.1016/S1097-2765(00)00108-8 [DOI] [PubMed] [Google Scholar]
- 23. Dai H, Meng W, Kaufmann S: BCL2 Family, Mitochondrial Apoptosis, and Beyond. Cancer Transl Med. 2016;2(1):7–20. 10.4103/2395-3977.177558 [DOI] [Google Scholar]
- 24. Puthalakath H, O'Reilly LA, Gunn P, et al. : ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–49. 10.1016/j.cell.2007.04.027 [DOI] [PubMed] [Google Scholar]
- 25. Willis SN: Apoptosis Initiated When BH3 Ligands. Science (80-.). 2011;856. [DOI] [PubMed] [Google Scholar]
- 26. Teske BF, Fusakio ME, Zhou D, et al. : CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. Mol Biol Cell. 2013;24(15):2477–90. 10.1091/mbc.E13-01-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu M, Lawrence DA, Marsters S, et al. : Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. 10.1126/science.1254312 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Wilson NS, Dixit V, Ashkenazi A: Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10(4):348–55. 10.1038/ni.1714 [DOI] [PubMed] [Google Scholar]
- 29. Hiramatsu N, Messah C, Han J, et al. : Translational and posttranslational regulation of XIAP by eIF2α and ATF4 promotes ER stress-induced cell death during the unfolded protein response. Mol Biol Cell. 2014;25(9):1411–20. 10.1091/mbc.E13-11-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brush MH, Weiser DC, Shenolikar S: Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23(4):1292–303. 10.1128/MCB.23.4.1292-1303.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han J, Back SH, Hur J, et al. : ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–90. 10.1038/ncb2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Showkat M, Beigh MA, Andrabi KI: mTOR Signaling in Protein Translation Regulation: Implications in Cancer Genesis and Therapeutic Interventions. Mol Biol Int. 2014;2014: 686984. 10.1155/2014/686984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guertin DA, Sabatini DM: Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 34. Bobrovnikova-Marjon E, Pytel D, Riese MJ, et al. : PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol Cell Biol. 2012;32(12):2268–78. 10.1128/MCB.00063-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anjum R, Blenis J: The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–58. 10.1038/nrm2509 [DOI] [PubMed] [Google Scholar]
- 36. Song G, Ouyang G, Bao S: The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. 10.1111/j.1582-4934.2005.tb00337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toschi A, Lee E, Xu L, et al. : Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29(6):1411–20. 10.1128/MCB.00782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dennis MD, McGhee NK, Jefferson LS, et al. : Regulated in DNA damage and development 1 (REDD1) promotes cell survival during serum deprivation by sustaining repression of signaling through the mechanistic target of rapamycin in complex 1 (mTORC1). Cell Signal. 2013;25(12):2709–16. 10.1016/j.cellsig.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brüning A, Rahmeh M, Friese K: Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol. 2013;7(6):1012–8. 10.1016/j.molonc.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park HW, Park H, Ro SH, et al. : Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun. 2014;5: 4233. 10.1038/ncomms5233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saveljeva S, Cleary P, Mnich K, et al. : Endoplasmic reticulum stress-mediated induction of SESTRIN 2 potentiates cell survival. Oncotarget. 2016;7(11):12254–66. 10.18632/oncotarget.7601 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Rozengurt E, Soares HP, Sinnet-Smith J: Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13(11):2477–88. 10.1158/1535-7163.MCT-14-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanti JF, Jager J: Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9(6):753–62. 10.1016/j.coph.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 44. Malhotra JD, Kaufman RJ: ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3(9):a004424. 10.1101/cshperspect.a004424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verfaillie T, Rubio N, Garg AD, et al. : PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19(11):1880–91. 10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin SM, Youle RJ: PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125(Pt 4):795–9. 10.1242/jcs.093849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamacher-Brady A, Brady NR: Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016;73(4):775–95. 10.1007/s00018-015-2087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M: Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int J Genomics. 2014;2014: 970607. 10.1155/2014/970607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Read DE, Gupta A, Ladilov Y, et al. : miRNA signature of unfolded protein response in H9c2 rat cardiomyoblasts. Cell Biosci. 2014;4(1):56. 10.1186/2045-3701-4-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu G, Chen J, Jing G, et al. : miR-204 Targets PERK and Regulates UPR Signaling and β-Cell Apoptosis. Mol Endocrinol. 2016;30(8):917–24. 10.1210/me.2016-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Chitnis NS, Pytel D, Bobrovnikova-Marjon E, et al. : miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol Cell. 2012;48(3):353–64. 10.1016/j.molcel.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao M, Liu Y, Chen Y, et al. : miR-214 protects erythroid cells against oxidative stress by targeting ATF4 and EZH2. Free Radic Biol Med. 2016;92:39–49. 10.1016/j.freeradbiomed.2016.01.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Xu Z, Bu Y, Chitnis N, et al. : miR-216b regulation of c-Jun mediates GADD153/CHOP-dependent apoptosis. Nat Commun. 2016;7: 11422. 10.1038/ncomms11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lai X, Vera J: MicroRNA Clusters. In: Encyclopedia of Systems Biology (eds. Dubitzky W, Wolkenhauer O, Yokota H, Cho KH). (Springer New York).2013;1310–1314. 10.1007/978-1-4419-9863-7_1121 [DOI] [Google Scholar]
- 55. Gupta S, Read DE, Deepti A, et al. : Perk-dependent repression of miR-106b-25 cluster is required for ER stress-induced apoptosis. Cell Death Dis. 2012;3(6):e333. 10.1038/cddis.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Byrd AE, Aragon IV, Brewer JW: MicroRNA-30c-2* limits expression of proadaptive factor XBP1 in the unfolded protein response. J Cell Biol. 2012;196(6):689–98. 10.1083/jcb.201201077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chhabra R, Dubey R, Saini N: Gene expression profiling indicate role of ER stress in miR-23a~27a~24-2 cluster induced apoptosis in HEK293T cells. RNA Biol. 2011;8(4):648–64. 10.4161/rna.8.4.15583 [DOI] [PubMed] [Google Scholar]
- 58. Geisler S, Coller J: RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14(11):699–712. 10.1038/nrm3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Su S, Liu J, He K, et al. : Overexpression of the long noncoding RNA TUG1 protects against cold-induced injury of mouse livers by inhibiting apoptosis and inflammation. FEBS J. 2016;283(7):1261–74. 10.1111/febs.13660 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Kato M, Wang M, Chen Z, et al. : An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat Commun. 2016;7: 12864. 10.1038/ncomms12864 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Pickard MR, Mourtada-Maarabouni M, Williams GT: Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832(10):1613–23. 10.1016/j.bbadis.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 62. Ranganathan AC, Ojha S, Kourtidis A, et al. : Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68(9):3260–8. 10.1158/0008-5472.CAN-07-6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bu Y, Diehl JA: PERK Integrates Oncogenic Signaling and Cell Survival During Cancer Development. J Cell Physiol. 2016;231(10):2088–96. 10.1002/jcp.25336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Denoyelle C, Abou-Rjaily G, Bezrookove V, et al. : Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8(10):1053–63. 10.1038/ncb1471 [DOI] [PubMed] [Google Scholar]
- 65. Huber AL, Lebeau J, Guillaumot P, et al. : p58 IPK-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Mol Cell. 2013;49(6):1049–59. 10.1016/j.molcel.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 66. Pytel D, Gao Y, Mackiewicz K, et al. : PERK Is a Haploinsufficient Tumor Suppressor: Gene Dose Determines Tumor-Suppressive Versus Tumor Promoting Properties of PERK in Melanoma. PLoS Genet. 2016;12(12):e1006518. 10.1371/journal.pgen.1006518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wiersma VR, Michalak M, Abdullah TM, et al. : Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front Oncol. 2015;5:7. 10.3389/fonc.2015.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Behrman S, Acosta-Alvear D, Walter P: A CHOP-regulated microRNA controls rhodopsin expression. J Cell Biol. 2011;192(6):919–27. 10.1083/jcb.201010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ryu S, McDonnell K, Choi H, et al. : Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell. 2013;23(1):63–76. 10.1016/j.ccr.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 70. Yang N, Fu Y, Zhang H, et al. : LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget. 2015;6(29):28151–63. 10.18632/oncotarget.4661 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Mourtada-Maarabouni M, Pickard MR, Hedge VL, et al. : GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28(2):195–208. 10.1038/onc.2008.373 [DOI] [PubMed] [Google Scholar]
- 72. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH: Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell. 2017;168(4):692–706. 10.1016/j.cell.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, et al. : PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31(17):3616–29. 10.1128/MCB.05164-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mujcic H, Nagelkerke A, Rouschop KM, et al. : Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19(22):6126–37. 10.1158/1078-0432.CCR-13-0526 [DOI] [PubMed] [Google Scholar]
- 75. Feng YX, Sokol ES, Del Vecchio CA, et al. : Epithelial-to-mesenchymal transition activates PERK-eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014;4(6):702–15. 10.1158/2159-8290.CD-13-0945 [DOI] [PubMed] [Google Scholar]
- 76. Dey S, Sayers CM, Verginadis II, et al. : ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest. 2015;125(7):2592–608. 10.1172/JCI78031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gutschner T, Hämmerle M, Eissmann M, et al. : The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–9. 10.1158/0008-5472.CAN-12-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Bhattacharyya S, Vrati S: The Malat1 long non-coding RNA is upregulated by signalling through the PERK axis of unfolded protein response during flavivirus infection. Sci Rep. 2015;5: 17794. 10.1038/srep17794 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Yoshimoto R, Mayeda A, Yoshida M, et al. : MALAT1 long non-coding RNA in cancer. Biochim Biophys Acta. 2016;1859(1):192–9. 10.1016/j.bbagrm.2015.09.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Terashima J, Tachikawa C, Kudo K, et al. : An aryl hydrocarbon receptor induces VEGF expression through ATF4 under glucose deprivation in HepG2. BMC Mol Biol. 2013;14:27. 10.1186/1471-2199-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rouschop KM, Dubois LJ, Keulers TG, et al. : PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci U S A. 2013;110(12):4622–7. 10.1073/pnas.1210633110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Axten JM, Medina JR, Feng Y, et al. : Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1 H-indol-5-yl)-7 H-pyrrolo[2,3- d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55(16):7193–207. 10.1021/jm300713s [DOI] [PubMed] [Google Scholar]
- 83. Atkins C, Liu Q, Minthorn E, et al. : Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. 10.1158/0008-5472.CAN-12-3109 [DOI] [PubMed] [Google Scholar]
- 84. Axten JM, Romeril SP, Shu A, et al. : Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Med Chem Lett. 2013;4(10):964–8. 10.1021/ml400228e [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Brewer JW, Diehl JA: PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97(23):12625–30. 10.1073/pnas.220247197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brewer JW, Hendershot LM, Sherr CJ, et al. : Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci U S A. 1999;96(15):8505–10. 10.1073/pnas.96.15.8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gao Y, Sartori DJ, Li C, et al. : PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol Cell Biol. 2012;32(24):5129–39. 10.1128/MCB.01009-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Drexler HC: Synergistic apoptosis induction in leukemic cells by the phosphatase inhibitor salubrinal and proteasome inhibitors. PLoS One. 2009;4(1):e4161. 10.1371/journal.pone.0004161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Teng Y, Gao M, Wang J, et al. : Inhibition of eIF2 α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis. 2014;5:e1060. 10.1038/cddis.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schewe DM, Aguirre-Ghiso JA: Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res. 2009;69(4):1545–52. 10.1158/0008-5472.CAN-08-3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jeon YJ, Kim JH, Shin JI, et al. : Salubrinal-Mediated Upregulation of eIF2α Phosphorylation Increases Doxorubicin Sensitivity in MCF-7/ADR Cells. Mol Cells. 2016;39(2):129–35. 10.14348/molcells.2016.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Han KS, Li N, Raven PA, et al. : Inhibition of endoplasmic reticulum chaperone protein glucose-regulated protein 78 potentiates anti-angiogenic therapy in renal cell carcinoma through inactivation of the PERK/eIF2α pathway. Oncotarget. 2015;6(33):34818–30. 10.18632/oncotarget.5397 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Dekervel J, Bulle A, Windmolders P, et al. : Acriflavine Inhibits Acquired Drug Resistance by Blocking the Epithelial-to-Mesenchymal Transition and the Unfolded Protein Response. Transl Oncol. 2017;10(1):59–69. 10.1016/j.tranon.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Tsaytler P, Harding HP, Ron D, et al. : Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332(6025):91–4. 10.1126/science.1201396 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Das I, Krzyzosiak A, Schneider K, et al. : Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348(6231):239–42. 10.1126/science.aaa4484 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Carrara M, Sigurdardottir A, Bertolotti A: Decoding the selectivity of eIF2α holophosphatases and PPP1R15A inhibitors. Nat Struct Mol Biol. 2017;24(9):708–16. 10.1038/nsmb.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Crespillo-Casado A, Chambers JE, Fischer PM, et al. : PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. eLife. 2017;6: pii: e26109. 10.7554/eLife.26109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Ma T, Klann E: PERK: a novel therapeutic target for neurodegenerative diseases? Alzheimers Res Ther. 2014;6(3):30. 10.1186/alzrt260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Harding HP, Zeng H, Zhang Y, et al. : Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–63. 10.1016/S1097-2765(01)00264-7 [DOI] [PubMed] [Google Scholar]