Abstract
The human cluster of differentiation (CD)1 system for antigen display is comprised of four types of antigen-presenting molecules, each with a distinct functional niche: CD1a, CD1b, CD1c, and CD1d. Whereas CD1 proteins were thought solely to influence T-cell responses through display of amphipathic lipids, recent studies emphasize the role of direct contacts between the T-cell receptor and CD1 itself. Moving from molecules to diseases, new research approaches emphasize human CD1-transgenic mouse models and the study of human polyclonal T cells in vivo or ex vivo in disease states. Whereas the high genetic diversity of major histocompatibility complex (MHC)-encoded antigen-presenting molecules provides a major hurdle for designing antigens that activate T cells in all humans, the simple population genetics of the CD1 system offers the prospect of discovering or designing broadly acting immunomodulatory agents.
Keywords: CD1, antigen display, TCR, T cell receptor
Molecules to disease
Scientific inquiry into major histocompatibility complex (MHC) and cluster of differentiation (CD)1 antigen presentation to T cells developed in separate, nearly anti-parallel tracks. Key biological roles of thymic “T cells” were known fifty years ago 1. The molecular identity of antigens and an understanding of how they are displayed by MHC proteins unfolded over the following three decades. Starting with clear evidence for a role of T cells in tissue transplantation and donor-restricted recognition of intracellular viruses 2, 3, subsequent experiments mapped MHC genes, detected MHC protein heterodimers, and solved the tricky business of proving that antigens bind within (rather than beside) MHC proteins 4.
Whereas MHC I and II research followed a disease-to-molecules trajectory, CD1 research is unfolding as a molecules-to-disease story. At the outset, CD1 proteins were inferred to be antigen-presenting molecules on the basis of their homology to MHC I genes 5, association with beta-2-microglobulin 6, and restricted expression on professional antigen-presenting cells. Experimental biologists proved a role for CD1 in antigen presentation through landmark discoveries relating to identification of lipid antigens for T cells 7, 8, identification of CD1d as the target receptor for natural killer T (NKT) cells 9, and generating crystallographic evidence that T-cell receptors (TCRs) directly contact and discriminate the structures of CD1-lipid complexes 10.
This brief history is instructive because it explains why the molecular understanding of lipid antigen recognition greatly exceeds knowledge of the roles of CD1-reactive T cells in disease. Nevertheless, the CD1 system is broadly conserved in mammalian evolution, and many studies now show that CD1-reactive T cells are common in human blood 11. Through new animal models and the recent development of human CD1a, CD1b, and CD1c tetramers, insight into the roles of CD1 and lipid antigens in human immunology are now emerging. The roles of CD1d, alpha-galactosyl ceramides and NKT cells in vivo have been thoroughly reviewed elsewhere 12, so this summary focuses on the relatively overlooked and functionally distinct roles of the CD1a, CD1b, and CD1c antigen display systems. Here, we explain CD1-lipid-TCR interactions, highlight two recent examples of antigen recognition that diverge from accepted models, and summarize recent studies of disease-focused work in humans and human transgenic mice.
Head group recognition model
The earliest analysis of CD1 sequences with Kyte-Doolittle plots predicted that CD1 proteins would fold to form hydrophobic clefts 13. Crystal structures of CD1a 14, 15, CD1b 16, CD1c 17, and CD1d 18, 19 have subsequently demonstrated that each of these proteins has hydrophobic clefts and pockets of different sizes, which accommodate the aliphatic hydrocarbon chains present in lipid, glycolipid, phospholipid, or lipopeptide antigens. Structure-function studies of these antigens initially pointed to a model in which the T-cell recognition was precisely controlled by the carbohydrate, peptide, or other polar elements present in the head group moiety, but the lipidic moieties could be changed with only a limited effect on T-cell activation 20– 22. Confirming these early functional results, later crystallographic studies of CD1d-glycolipid-TCR 10 and CD1b-glycolipid-TCR 23 revealed that the hydrophilic head groups protrude above the cleft, allowing the TCR to contact the head group moiety directly. In some cases, the TCR contacts the head group as it lies on the outer surface of CD1, and in other cases, the TCR induces substantial conformational change to the head group to press or “bulldoze” it down on CD1 24– 26. Collectively, these studies suggested a “head group recognition” model for CD1b and CD1d that emphasizes direct and extensive contact of the TCR with antigen ( Figure 1). These data prove that the TCR can specifically bind to and discern the structure of carbohydrate and other non-peptidic head groups, expanding prior views that TCRs react solely to peptides.
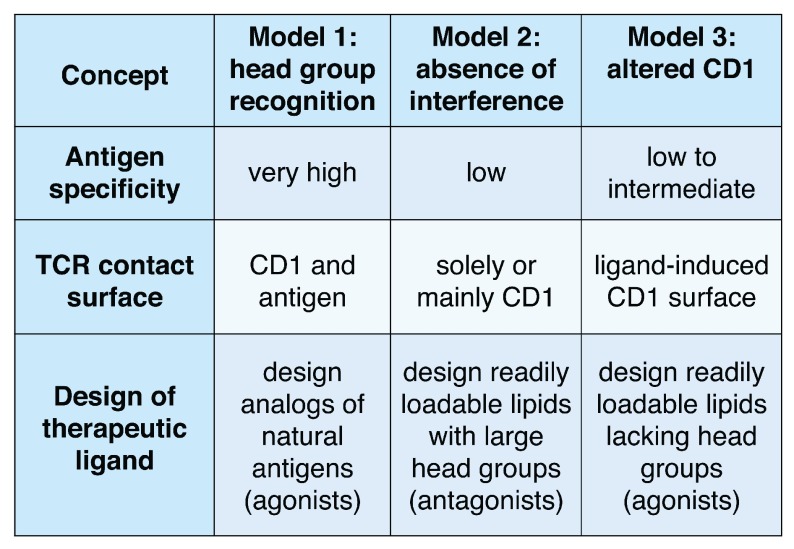
Figure 1. Three general models for CD1–lipid–T-cell receptor ternary interactions that highlight possible approaches to the design of therapeutic ligands.
TCR, T-cell receptor.
According to the head group recognition model, TCRs predominantly contact a hybrid epitope formed by the outer surface of CD1 and the exposed polar head groups of carried antigens. Because the lipid tails are largely hidden within CD1, natural or experimental alterations do not necessarily abrogate recognition. Altering the lipid anchor can produce no effect 22, relatively small effects based on ease of loading 27, or indirect effects based on changing the overall configuration of the CD1-lipid complex. For example, longer lipid anchors or lipid side chains can move the head group of CD1b-presented sulfolipids or cause local changes in the surface of CD1d 28– 32.
The head group recognition model is similar to basic MHC-peptide-TCR recognition because TCR contact with the MHC-peptide surface explains high specificity of T cells for both the antigen and antigen-presenting molecule ( Figure 1). Because the recognition of most known lipid antigens is very precisely controlled by the structure of the carbohydrate or peptide moieties present on glycolipids or lipopeptides, head group recognition was thought to be the universal mechanism of antigen recognition in the CD1 system. However, recent studies of CD1a and CD1c antigen complexes provide evidence for alternate models: “absence of interference” and “altered CD1”.
Absence of interference model
Isolation of autoantigens from skin and other tissues demonstrated that self-lipids like squalene could mediate CD1a autoreactivity 33. Squalene is a small alkane lipid, which lacks any hydrophilic head group moieties present in the previously described CD1 antigens. Furthermore, studies of a CD1a and squalene-responsive T-cell clone known as BC2 demonstrated cross-reactivity to other small hydrophobic molecules like wax esters and free fatty acids. Thus, the key premise (hydrophilic moieties in the antigen) and the key prediction (fine specificity for the hydrophilic moieties) of the head group recognition model were not fulfilled for CD1a-presented autoantigens. These functional results from T-cell assays invited consideration of a new structural model of CD1 recognition.
Because certain CD1a autoantigens were small relative to previously described antigens and the known molecular volume of CD1 clefts, an idea emerged that they might nest deeply within CD1a and allow recognition by “absence of interference” with the approaching TCR 33– 35. The first solved ternary structure of CD1a-lipid-TCR revealed that the TCR solely contacts epitopes on the CD1a protein and completely misses the carried lipids, lysophosphatidylcholine, and fatty acid. A binary structure of CD1a proteins bound to fatty acids and other molecules showed that the lipid density was seated wholly within CD1a, so fatty acids fulfill the original prediction of the absence of interference model. Lysophosphatidylcholine is not fully nested within CD1a, but instead protrudes from the cleft, but does so at an angle that pushed the head group laterally away from the TCR contact area of CD1a 36. These studies identified two mechanisms by which lipids could bind CD1a yet fail to interfere with the TCR-CD1a contact: lateral escape or seating below the plane of TCR contact.
Conversely, sulfatide and sphingomyelin, which have large polar head groups, could block recognition of CD1a by CD1a-autoreactive TCRs. In crystal structures of CD1a-sphingomyelin, the sphingomyelin head group substantially disrupted a triad of residues located with the A′ roof of the CD1a protein, which is located under the TCR footprint 36. This mechanism of interference involves alteration of CD1a itself. A somewhat different mechanism of interference likely occurs with other CD1 ligands with large head groups. In CD1d-ganglioside D3 complexes, the large head group presumably wedges between TCR and CD1d, creating stearic interference between CD1d and TCR 37. Thus, the large head groups interfere with the CD1a-TCR interaction by two distinct but related mechanisms.
These studies rule in the absence of interference model for three TCRs in the CD1a system and make some general predictions about TCR contact with lipid and CD1 that are different from the head group recognition model ( Figure 1). For these TCRs, small hydrophobic ligands could function to augment response, regardless of their fine structure, so long as they can efficiently load into CD1a and fail to interfere with TCR contact with CD1a. In contrast, many phospholipids and sphingolipids, which have large head groups, would be expected to block autoreactive TCRs 38. Thus, these structural studies raise the possibility that readily loadable lipids with small head groups could be developed as agonists for CD1 autoreactive T cells. Loadable lipids with large head groups might be antagonists. Currently, the absence of interference model is supported by fewer examples than those underlying head group recognition, but it has potential to explain immune surveillance by the high numbers of CD1a-autoreactive T cells circulating in human blood 38, 39.
Altered CD1 model
The first structure of CD1c was solved in complex with a foreign lipid known as mannosyl phosphomycoketide 17. This structure revealed that, rather than having a single point of access to the cleft, CD1c has a series of holes or portals, known as the F′ portal, D′ portal, and E′ portal. A recent article by Mansour and colleagues shows that self-cholesteryl esters trigger a major reorganization of CD1c upon binding. The cholesteryl ester triggers a motion in CD1c that is like a Venus flytrap closing on its prey 40. The looser structure of CD1c, dominated by three portals, might create a situation in which the dominant effect of lipid binding is the gross alteration of the three-dimensional surface of CD1c, an effect that is in addition to, or instead of, a lipid head group protruding to the surface 40.
This “altered CD1” model and the “absence of interference” model both predict that the TCR contacts solely, or mostly CD1 and not lipid. Both models predict lower TCR fine specificity for the structures of the antigens bound ( Figure 1). The latter emphasizes the role of blockers that prevent TCR contact of CD1a 36. The former predicts that the overall shape of CD1c changes depending on the presence or absence of lipids bound. Structurally, evidence for “altered CD1” is supported by binary structures of CD1c-lipid 17, 40 and functional studies of antigen recognition 41, 42. Formal structural proof of this model awaits the solution of ternary structures of CD1c-lipid-TCR.
Human CD1 tetramers
Tetramers represent a major technical advance in the study of T cells 43. Based on the antigen-specific nature of TCR interactions with tetramers of antigen-presenting molecules, antigen-loaded tetramers allow the direct tracking and sorting of individual antigen-specific T cells within complex mixtures of cells in ways that are otherwise not possible 44. Whereas mouse and human CD1d tetramers have been available for more than a decade 45, human CD1a, CD1b, and CD1c tetramers have only recently been validated 40, 42, 46– 49. A technical challenge has been the difficulty in loading hydrophobic lipids into CD1 proteins in aqueous solution. However, once loading has been accomplished for a particular CD1-lipid pair, the non-polymorphic nature of CD1 proteins creates a situation in which CD1 tetramers can be used on any donor without genetic (MHC locus) matching.
In this young field, most studies have focused on mycobacterial ligands, including dideoxymycobactin 47, phosphomycoketides 42, 48, mycolic acid 32, and glucose monomycolate 49, in staining T cells from the blood of patients with active or latent tuberculosis. These studies provide clear evidence for polyclonal lipid-reactive T cells in humans and certain aspects of their phenotypes, such as conserved TCRs, CD4 expression, and prominent secretion of tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ). A missing piece is the need to track CD1-reactive T cells over time during infection to determine whether such cells play a role in immunological memory.
Human CD1-transgenic mice
CD1a, CD1b, and CD1c are not expressed in mice and rats 50, so tractable small animal models for study of these three CD1 types in vivo were limited. However, guinea pigs express CD1a, CD1b, and CD1c 51, allowing analysis of mycobacterial infection and vaccination 52, 53. Also, transgenic mice expressing human CD1a, CD1b, and CD1c under control of endogenous human promoters show expression patterns similar to those of humans, allowing analysis of mycobacteria-reactive CD1-restricted T cells in vivo 54. Combining human transgenic CD1 proteins and the responding TCRs, mice co-expressing a CD1b-autoreactive TCR (HJ1) and CD1b displayed intrathymic-positive selection on CD1b-expressing hematopoietic cells and peripheral activation of HJ1 transgenic T cells in a CD1b-dependent manner 55. In response to CD1b expression and interleukin-12 (IL-12) exposure, HJ1 T cells secreted IFNγ and IL-17, contributing to local inflammation and tissue pathology. However, HJ1 T cells also conferred some anti-microbial control against listeria challenge 55, suggesting that they can be either pathogenic or protective, depending on the context.
CD1b is an attractive candidate for surveillance of pathogen-derived lipid antigens given its potent upregulation in vivo in response to bacteria 56, IL-1 57, or granulocyte-macrophage colony-stimulating factor (GM-CSF). Moreover, CD1b shows particularly efficient trafficking through acidic endosomal and lysosomal compartments, which are the usual sites for accumulation of exogenous bacteria or foreign lipid antigens 58, 59. Expression of a CD1b-restricted, mycolic acid–specific transgenic TCR (DN1) in human CD1-transgenic mice offered a practical model to study CD1b-mediated mycolic acid–specific T-cell responses 60. In these mice, DN1 T cells rapidly localized to the lungs and adjacent mediastinal lymph nodes following aerosol challenge with Mycobacterium tuberculosis and provided detectable, albeit modest, protection from infection 60.
Because CD1b proteins are abundantly expressed on foam cells in human atherosclerotic plaques 61, Wang and colleagues asked whether cells expressing HJ1 might influence atherosclerosis in a model of accelerated vascular disease in mice lacking apolipoprotein E (apoE) 62. Unexpectedly, apoE knockout mice expressing the human CD1b transgene and the HJ1 TCR spontaneously developed a severe psoriaform dermatitis 62. The authors traced the disease to ectopic phospholipid deposition in skin, occurring as a result of hyperlipidemia. Intradermal HJ1-autoreactive T cells secreted IL-17 and IL-22, driving recruitment of neutrophils to lesional skin 62. The extent to which these events mimic the pathophysiology of human psoriasis is unknown. However, human psoriatic lesions overexpress CD1b as compared with normal skin, and there is evidence for altered lipids in psoriatic skin 62, 63.
In vivo studies of CD1a in skin disease
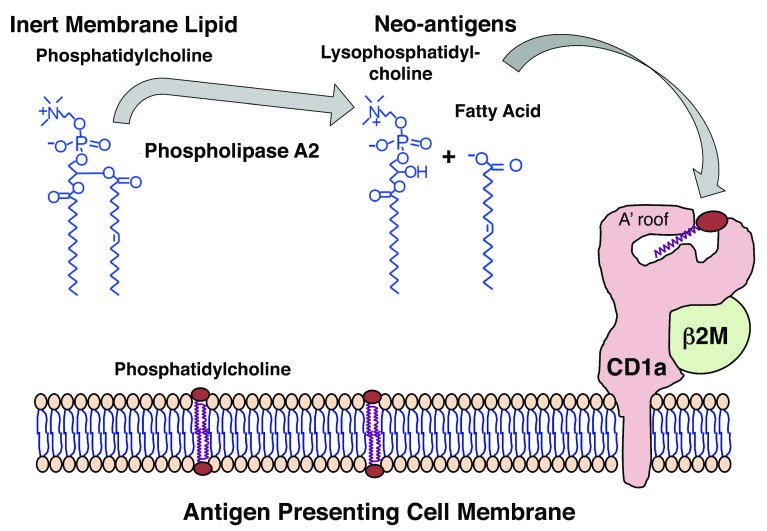
Recent studies have suggested a role for human CD1a in psoriasis. In humans, enumeration of CD1a-autoreactive T cells ex vivo provided evidence for increased cell number in patients with psoriasis. This work 64, and related mechanistic studies 65– 67, revealed that CD1a-autoreactivity was associated with higher expression of lesional mast cell–derived phospholipase A 2 (PLA 2), which can convert non-antigenic phosphatidic acid–containing molecules into lysolipid neo-antigens ( Figure 2). Separately, a second-generation knockin mouse expressing the human CD1a transgene showed constitutive and cytokine-inducible CD1a expression at high levels 68. This mouse showed that CD1a expression markedly exacerbated skin inflammation in response to imiquimod-induced dermatitis, which mimics certain downstream events in psoriasis 69.
Figure 2. Model for generation of lipid neo-antigens from intact membrane lipids.
The figure shows this through the action of phospholipases present in house dust mites 66 and bee and wasp venoms 65, 67 and viruses 71. Whereas cleavage of phosphatidylcholine is shown here, experimental evidence suggests that a similar mechanism could apply to other membrane phospholipids, including phosphatidylglycerol, phosphatidic acid, phosphatidylserine, and phosphatidylinositol. Newly generated lipid neo-antigens generated from self cellular lipids by phospholipases are thought to be loaded on CD1a through an unknown mechanism, where they can be presented to CD1a-reactive T cells.
CD1a, which is constitutively expressed on epidermal Langerhans cells and is by far the most abundant CD1 antigen-presenting molecule in human skin, has recently been implicated in promoting other skin diseases. Whereas all humans show immune response to bee and wasp venoms, certain patients have severe local and systemic inflammation or anaphylaxis 70. In a recent study, Ogg and colleagues showed that bee and wasp venom–derived PLA 2 released antigenic free fatty acids and lysophospholipids from non-antigenic phosphodiacylglycerol substrates 65. Interestingly, the PLA 2-dependent activation of CD1a-reactive T cells requires both CD1a and a cellular membrane to provide lipid substrates. Hence, Langerhans cells may serve a dual role of antigen presentation by both providing CD1a and acting as a reservoir of membrane-tethered self-lipid antigen substrates. In this emerging model, wasp stings penetrate the skin to allow PLA enzymes to reach cellular phospholipid membranes and release self-lipids for catalysis to generate CD1a lipid neo-antigens 65.
Furthermore, both venom-allergic individuals and house dust mite–allergic individuals have a higher frequency of CD1a-reactive T cells that also release IFNγ, GM-CSF and IL-13 in response to PLA 2 66, 67. Desensitization immunotherapy for venom-allergic patients, consisting of serial subcutaneous injections of increasing doses of wasp venom over a course of 8 weeks, initially increases the frequency of IFNγ-expressing CD1a-reactive T cells in the first 3 to 5 weeks, which decreases by the end of treatment 67. Filaggrin is a skin-barrier protein, which blocks PLA 2, and is thought to limit release of CD1a-reactive lipid neo-antigens 66. Finally, transgenic expression of human CD1a in the skin of mice strongly increases immune response to the allergenic compound, found in poison ivy, known as urushiol 69.
Combined, these recent clinically oriented studies offer candidate interventions to abrogate CD1a-mediated skin lesions. Antibodies that block PLA 2, lipid neo-antigens, or CD1a itself might limit pathogenic activation of T cells in lesional skin. Because lipid agonists and antagonists can be readily formulated in skin creams, the potential for topical treatment of skin diseases can now be investigated.
Designing lipid-based immunological therapies
The non-polymorphic nature of CD1 genes creates a situation in which nearly all humans express CD1 proteins with the same sequence and structure 72. As contrasted with highly polymorphic MHC-encoded genes, CD1-reactive T cells are not restricted to the genetic background of the donor. Thus, lipid ligands of CD1 proteins are attractive candidates as “one antigen for everyone” approaches for altering immune responses in humans.
Figure 1. highlights how the three modes of lipid recognition by T cells point toward distinct strategies to synthesize antigenic lipids such as immunomodulatory drugs that selectively alter human T-cell responses to CD1. The head group recognition model supports development of agonists or superagonists that mimic natural antigens but are chemically modified to increase, decrease, or alter the response of T cells 73, 74. This approach has been exploited to produce α-galactosyl ceramides with altered immunological properties (reviewed in 75). Absence of interference supports the development of lipids with large head groups that might be broadly acting antagonists of CD1-autoreactive T cells 34, 37. The altered CD1 model calls for agonists that are small lipids lacking head groups that readily load onto CD1 40.
Moving from theory to practice, several related approaches are testing the concept that mycobacterial lipids could alter systemic responses to tuberculosis disease. These studies are based on evidence that M. tuberculosis expresses many lipid antigens to which CD1-reactive T cells respond 76, 77 and that transgenic expression of human CD1b in mice provides some mycobacterial containment in vivo 60. As discussed above, guinea pigs express homologs for human CD1a, CD1b, and CD1c 50, so they can serve as models to define vaccine-induced responses. Proof-of-concept studies show that immunization with mycolic acid, diacylated sulfoglycolipids, or phosphatidylinositol dimannosides reduced lung inflammation and provided some reduction in bacterial burden compared with unvaccinated animals 52, 53. However, vaccination with lipids alone did not surpass protection induced by Bacille Calmette-Guérin, the current tuberculosis vaccine, suggesting that further development is needed. Although most studies of immune response to vaccination emphasize peptide antigens, whole cell vaccines, like Bacille Calmette-Guérin, also contain lipid antigens, raising the possibility that generating lipid-specific immune responses occur during current vaccination approaches in ways that are not currently appreciated or measured 78.
Summary
The discovery of CD1 antigen presentation pathways has provided a broader perspective about the natural targets of T cell response, which normally recognize both peptide and lipid antigens. The number of known CD1-presented antigens continues to increase, and three distinct molecular models for their recognition are emerging on the basis of strong structural and functional data ( Figure 1). Therefore, the key to harnessing this new basic insight that lipids are antigens for T cells will likely lie in generating better models for measuring the CD1-mediated T-cell response in vivo in animals and ex vivo in humans to understand and interrupt their disease-specific functions.
Acknowledgments
The authors thank Ildiko van Rhijn and Rachel Cotton for their advice.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Giulia Casorati, Experimental Immunology Unit, Division of Immunology, Transplantation and Infectious Diseases, San Raffaele Scientific Institute, Milan, Italy
Stephan Gadola, Faculty of Medicine, University of Southampton, Southampton, UK; F. Hoffman-La Roche Ltd, Basel, Switzerland
Dirk Zajonc, La Jolla Institute of Allergy & Immunology, La Jolla, California, USA
Funding Statement
This work is supported by grants from the Bill and Melinda Gates Foundation and the National Institutes of Health (AI043913, AI111224, and AR048632).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. McDevitt HO, Benacerraf B: Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. 10.1016/S0065-2776(08)60477-0 [DOI] [PubMed] [Google Scholar]
- 2. Zinkernagel RM, Doherty PC: Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248(5450):701–2. 10.1038/248701a0 [DOI] [PubMed] [Google Scholar]
- 3. Townsend AR, Gotch FM, Davey J: Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42(2):457–67. 10.1016/0092-8674(85)90103-5 [DOI] [PubMed] [Google Scholar]
- 4. Bjorkman PJ, Saper MA, Samraoui B, et al. : Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329(6139):506–12. 10.1038/329506a0 [DOI] [PubMed] [Google Scholar]
- 5. Calabi F, Milstein C: A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986;323(6088):540–3. 10.1038/323540a0 [DOI] [PubMed] [Google Scholar]
- 6. McMichael AJ, Pilch JR, Galfré G, et al. : A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979;9(3):205–10. 10.1002/eji.1830090307 [DOI] [PubMed] [Google Scholar]
- 7. Beckman EM, Porcelli SA, Morita CT, et al. : Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372(6507):691–4. 10.1038/372691a0 [DOI] [PubMed] [Google Scholar]
- 8. Porcelli S, Morita CT, Brenner MB: CD1b restricts the response of human CD4 –8 – T lymphocytes to a microbial antigen. Nature. 1992;360(6404):593–7. 10.1038/360593a0 [DOI] [PubMed] [Google Scholar]
- 9. Bendelac A, Lantz O, Quimby ME, et al. : CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–5. 10.1126/science.7538697 [DOI] [PubMed] [Google Scholar]
- 10. Borg NA, Wun KS, Kjer-Nielsen L, et al. : CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–9. 10.1038/nature05907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Godfrey DI, Uldrich AP, McCluskey J, et al. : The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–23. 10.1038/ni.3298 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Salio M, Silk JD, Jones EY, et al. : Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–66. 10.1146/annurev-immunol-032713-120243 [DOI] [PubMed] [Google Scholar]
- 13. Porcelli SA: The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. 10.1016/S0065-2776(08)60629-X [DOI] [PubMed] [Google Scholar]
- 14. Zajonc DM, Elsliger MA, Teyton L, et al. : Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat Immunol. 2003;4(8):808–15. 10.1038/ni948 [DOI] [PubMed] [Google Scholar]
- 15. Zajonc DM, Crispin MD, Bowden TA, et al. : Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22(2):209–19. 10.1016/j.immuni.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 16. Gadola SD, Zaccai NR, Harlos K, et al. : Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol. 2002;3(8):721–6. 10.1038/ni821 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Scharf L, Li NS, Hawk AJ, et al. : The 2.5 Å structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity. 2010;33(8):853–62. 10.1016/j.immuni.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Luoma AM, Castro CD, Mayassi T, et al. : Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. 2013;39(6):1032–42. 10.1016/j.immuni.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng Z, Castaño AR, Segelke BW, et al. : Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277(5324):339–45. 10.1126/science.277.5324.339 [DOI] [PubMed] [Google Scholar]
- 20. Kawano T, Cui J, Koezuka Y, et al. : CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–9. 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- 21. Moody DB, Young DC, Cheng TY, et al. : T cell activation by lipopeptide antigens. Science. 2004;303(5657):527–31. 10.1126/science.1089353 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Moody DB, Reinhold BB, Guy MR, et al. : Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278(5336):283–6. 10.1126/science.278.5336.283 [DOI] [PubMed] [Google Scholar]
- 23. Gras S, Van Rhijn I, Shahine A, et al. : T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat Commun. 2016;7: 13257. 10.1038/ncomms13257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pellicci DG, Clarke AJ, Patel O, et al. : Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12(9):827–33. 10.1038/ni.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Yu ED, Girardi E, Wang J, et al. : Cutting edge: structural basis for the recognition of β-linked glycolipid antigens by invariant NKT cells. J Immunol. 2011;187(5):2079–83. 10.4049/jimmunol.1101636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shahine A, Van Rhijn I, Cheng T, et al. : A molecular basis of human T cell receptor autoreactivity toward self-phospholipids. Sci Immunol. 2017;2(16): pii: eaao1384. 10.1126/sciimmunol.aao1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moody DB, Briken V, Cheng TY, et al. : Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol. 2002;3(5):435–42. 10.1038/ni780 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Garcia-Alles LF, Collmann A, Versluis C, et al. : Structural reorganization of the antigen-binding groove of human CD1b for presentation of mycobacterial sulfoglycolipids. Proc Natl Acad Sci U S A. 2011;108(43):17755–60. 10.1073/pnas.1110118108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Girardi E, Yu ED, Li Y, et al. : Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol. 2011;9(11):e1001189. 10.1371/journal.pbio.1001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kinjo Y, Tupin E, Wu D, et al. : Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–86. 10.1038/ni1380 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. McCarthy C, Shepherd D, Fleire S, et al. : The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204(5):1131–44. 10.1084/jem.20062342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Rhijn I, Iwany SK, Fodran P, et al. : CD1b-mycolic acid tetramers demonstrate T-cell fine specificity for mycobacterial lipid tails. Eur J Immunol. 2017;47(9):1525–34. 10.1002/eji.201747062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Jong A, Cheng TY, Huang S, et al. : CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol. 2014;15(2):177–85. 10.1038/ni.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kronenberg M, Havran WL: Immunology: oiling the wheels of autoimmunity. Nature. 2014;506(7486):42–3. 10.1038/506042a [DOI] [PubMed] [Google Scholar]
- 35. Van Rhijn I, Godfrey DI, Rossjohn J, et al. : Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15(10):643–54. 10.1038/nri3889 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Birkinshaw RW, Pellicci DG, Cheng TY, et al. : αβ T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat Immunol. 2015;16(3):258–66. 10.1038/ni.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Park JE, Wu DY, Prendes M, et al. : Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123(1):145–55. 10.1111/j.1365-2567.2007.02760.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Jong A, Peña-Cruz V, Cheng TY, et al. : CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11(12):1102–9. 10.1038/ni.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. de Lalla C, Lepore M, Piccolo FM, et al. : High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. 2011;41(3):602–10. 10.1002/eji.201041211 [DOI] [PubMed] [Google Scholar]
- 40. Mansour S, Tocheva AS, Cave-Ayland C, et al. : Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci U S A. 2016;113(9):E1266–75. 10.1073/pnas.1519246113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Roy S, Ly D, Li NS, et al. : Molecular basis of mycobacterial lipid antigen presentation by CD1c and its recognition by αβ T cells. Proc Natl Acad Sci U S A. 2014;111(43):E4648–57. 10.1073/pnas.1408549111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roy S, Ly D, Castro CD, et al. : Molecular Analysis of Lipid-Reactive Vδ1 γδ T Cells Identified by CD1c Tetramers. J Immunol. 2016;196(4):1933–42. 10.4049/jimmunol.1502202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Altman JD, Moss PA, Goulder PJ, et al. : Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–6. 10.1126/science.274.5284.94 [DOI] [PubMed] [Google Scholar]
- 44. Altman JD, Davis MM: MHC-Peptide Tetramers to Visualize Antigen-Specific T Cells. Curr Protoc Immunol. 2016;115:17.3.1–17.3.44. 10.1002/cpim.14 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Sidobre S, Kronenberg M: CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods. 2002;268(1):107–21. 10.1016/S0022-1759(02)00204-1 [DOI] [PubMed] [Google Scholar]
- 46. Kasmar AG, Van Rhijn I, Cheng TY, et al. : CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med. 2011;208(9):1741–7. 10.1084/jem.20110665 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Kasmar AG, Van Rhijn I, Magalhaes KG, et al. : Cutting Edge: CD1a tetramers and dextramers identify human lipopeptide-specific T cells ex vivo. J Immunol. 2013;191(9):4499–503. 10.4049/jimmunol.1301660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ly D, Kasmar AG, Cheng TY, et al. : CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. 2013;210(4):729–41. 10.1084/jem.20120624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Rhijn I, Kasmar A, de Jong A, et al. : A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14(7):706–13. 10.1038/ni.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reinink P, Van Rhijn I: Mammalian CD1 and MR1 genes. Immunogenetics. 2016;68(8):515–23. 10.1007/s00251-016-0926-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hiromatsu K, Dascher CC, Sugita M, et al. : Characterization of guinea-pig group 1 CD1 proteins. Immunology. 2002;106(2):159–72. 10.1046/j.1365-2567.2002.01422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dascher CC, Hiromatsu K, Xiong X, et al. : Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol. 2003;15(8):915–25. 10.1093/intimm/dxg091 [DOI] [PubMed] [Google Scholar]
- 53. Larrouy-Maumus G, Layre E, Clark S, et al. : Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine. 2017;35(10):1395–402. 10.1016/j.vaccine.2017.01.079 [DOI] [PubMed] [Google Scholar]
- 54. Felio K, Nguyen H, Dascher CC, et al. : CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206(11):2497–509. 10.1084/jem.20090898 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Li S, Choi HJ, Felio K, et al. : Autoreactive CD1b-restricted T cells: a new innate-like T-cell population that contributes to immunity against infection. Blood. 2011;118(14):3870–8. 10.1182/blood-2011-03-341941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roura-Mir C, Wang L, Cheng TY, et al. : Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J Immunol. 2005;175(3):1758–66. 10.4049/jimmunol.175.3.1758 [DOI] [PubMed] [Google Scholar]
- 57. Yakimchuk K, Roura-Mir C, Magalhaes KG, et al. : Borrelia burgdorferi infection regulates CD1 expression in human cells and tissues via IL1-β. Eur J Immunol. 2011;41(3):694–705. 10.1002/eji.201040808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jackman RM, Stenger S, Lee A, et al. : The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8(3):341–51. 10.1016/S1074-7613(00)80539-7 [DOI] [PubMed] [Google Scholar]
- 59. Sugita M, Jackman RM, van Donselaar E, et al. : Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science. 1996;273(5273):349–52. 10.1126/science.273.5273.349 [DOI] [PubMed] [Google Scholar]
- 60. Zhao J, Siddiqui S, Shang S, et al. : Mycolic acid-specific T cells protect against Mycobacterium tuberculosis infection in a humanized transgenic mouse model. eLife. 2015;4: pii: e08525. 10.7554/eLife.08525 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Melián A, Geng YJ, Sukhova GK, et al. : CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol. 1999;155(3):775–86. 10.1016/S0002-9440(10)65176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bagchi S, He Y, Zhang H, et al. : CD1b-autoreactive T cells contribute to hyperlipidemia-induced skin inflammation in mice. J Clin Invest. 2017;127(6):2339–52. 10.1172/JCI92217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li B, Tsoi LC, Swindell WR, et al. : Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol. 2014;134(7):1828–38. 10.1038/jid.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cheung KL, Jarrett R, Subramaniam S, et al. : Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213(11):2399–412. 10.1084/jem.20160258 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Bourgeois EA, Subramaniam S, Cheng TY, et al. : Bee venom processes human skin lipids for presentation by CD1a. J Exp Med. 2015;212(2):149–63. 10.1084/jem.20141505 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Jarrett R, Salio M, Lloyd-Lavery A, et al. : Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci Transl Med. 2016;8(325):325ra18. 10.1126/scitranslmed.aad6833 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Subramaniam S, Aslam A, Misbah SA, et al. : Elevated and cross-responsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur J Immunol. 2016;46(1):242–52. 10.1002/eji.201545869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kobayashi C, Shiina T, Tokioka A, et al. : GM-CSF-independent CD1a expression in epidermal Langerhans cells: evidence from human CD1A genome-transgenic mice. J Invest Dermatol. 2012;132(1):241–4. 10.1038/jid.2011.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim JH, Hu Y, Yongqing T, et al. : CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol. 2016;17(10):1159–66. 10.1038/ni.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Elieh Ali Komi D, Shafaghat F, Zwiener RD: Immunology of Bee Venom. Clin Rev Allergy Immunol. 2017. 10.1007/s12016-017-8597-4 [DOI] [PubMed] [Google Scholar]
- 71. Zeissig S, Murata K, Sweet L, et al. : Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18(7):1060–8. 10.1038/nm.2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Rhijn I, Moody DB: Donor Unrestricted T Cells: A Shared Human T Cell Response. J Immunol. 2015;195(5):1927–32. 10.4049/jimmunol.1500943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miyamoto K, Miyake S, Yamamura T: A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing T H2 bias of natural killer T cells. Nature. 2001;413(6855):531–4. 10.1038/35097097 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Silk JD, Salio M, Reddy BG, et al. : Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180(10):6452–6. 10.4049/jimmunol.180.10.6452 [DOI] [PubMed] [Google Scholar]
- 75. Kharkwal SS, Arora P, Porcelli SA: Glycolipid activators of invariant NKT cells as vaccine adjuvants. Immunogenetics. 2016;68(8):597–610. 10.1007/s00251-016-0925-y [DOI] [PubMed] [Google Scholar]
- 76. De Libero G, Mori L: The T-Cell Response to Lipid Antigens of Mycobacterium tuberculosis. Front Immunol. 2014;5:219. 10.3389/fimmu.2014.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Rhijn I, Moody DB: CD1 and mycobacterial lipids activate human T cells. Immunol Rev. 2015;264(1):138–53. 10.1111/imr.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scriba TJ, Kaufmann SH, Henri Lambert P, et al. : Vaccination Against Tuberculosis With Whole-Cell Mycobacterial Vaccines. J Infect Dis. 2016;214(5):659–64. 10.1093/infdis/jiw228 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation