Abstract
Traumatic spinal cord injury (SCI) is a devastating condition of motor, sensory, and autonomic dysfunction. The significant cost associated with the management and lifetime care of patients with SCI also presents a major economic burden. For these reasons, there is a need to develop and translate strategies that can improve outcomes following SCI. Given the challenges in achieving regeneration of the injured spinal cord, neuroprotection has been at the forefront of clinical translation. Yet, despite many preclinical advances, there has been limited translation into the clinic apart from methylprednisolone (which remains controversial), hypertensive therapy to maintain spinal cord perfusion, and early decompressive surgery. While there are several factors related to the limited translational success, including the clinical and mechanistic heterogeneity of human SCI, the misalignment between animal models of SCI and clinical reality continues to be an important factor. Whereas most clinical cases are at the cervical level, only a small fraction of preclinical research is conducted in cervical models of SCI. Therefore, this review highlights the most promising neuroprotective and neural reparative therapeutic strategies undergoing clinical assessment, including riluzole, hypothermia, granulocyte colony-stimulating factor, glibenclamide, minocycline, Cethrin (VX-210), and anti-Nogo-A antibody, and emphasizes their efficacy in relation to the anatomical level of injury. Our hope is that more basic research will be conducted in clinically relevant cervical SCI models in order to expedite the transition of important laboratory discoveries into meaningful treatment options for patients with SCI.
Keywords: Traumatic spinal cord injury, neuroprotective treatments, granulocyte colony stimulating fact
Introduction
Traumatic spinal cord injury (SCI), which is caused by external mechanical impact, results in impairment of motor, sensory, and autonomic function at and below the level of injury. Mechanical laceration, contusion, and compression result in cell death, which is further propagated by secondary injury mechanisms which include ischemia, sodium- and calcium-mediated cell injury, glutamatergic excitotoxicity, hemorrhage, and inflammation. The secondary injury amplifies the primary damage and promotes cystic degeneration and glial scar formation, thereby preventing functional recovery. Therefore, targeting secondary injury is a promising therapeutic intervention.
Despite several efficacious preclinical studies for SCI, there have been challenges in achieving successful translation into the clinic. While the disconnect between bench and bedside is not limited to SCI, it is important to recognize the underlying factors and identify solutions. Clinical heterogeneity, complexity of the disease, and the limited regenerative capacity of the spinal cord are among the key causes for poor translation and have been broadly discussed in the literature 1. Yet significantly less emphasis has been placed on the need to apply clinically relevant models of cervical SCI 2. Given that over 50% of human SCI cases occur at the cervical level 3 and the majority of preclinical work involves thoracic injuries ( Table 1), translation will require a greater understanding of injury-level subpopulation differences in pathophysiology and therapeutic benefits.
Table 1. Experimental evidence for the efficacy of promising neuroprotective therapies.
| Neuroprotective/
neural reparative therapy |
Injury model, species | Reference |
|---|---|---|
| Riluzole | Contusion, T7–T10, rat | 69 |
| Compression, T8, rat | 70 | |
| Compression, T6, rat | 71 | |
| Contusion, T10, rat | 72 | |
| Compression, T11, rat | 73 | |
| Contusion, T8, rat | 74 | |
| Unilateral contusion, C7, rat | 75 | |
| Hemisection, C2, rat | 35 | |
| Compression, C7, rat | 76 | |
| Unilateral contusion, C7, rat | 77 | |
| Compression, C7, rat | 78 | |
| Compression, C7, rat | 79 | |
| Transection, S2, rat | 34 | |
| Hypothermia | Contusion, T8, rat | 80 |
| Compression, T8, rat | 81 | |
| Compression, T8, rat | 82 | |
| Compression, T11, rat | 83 | |
| Contusion, T9, rat | 84 | |
| Contusion, T10, rat | 85 | |
| Unilateral contusion, C7, rat | 75 | |
| Contusion, C5, rat | 86 | |
| Glibenclamide | Contusion, T8, rat | 87 |
| Unilateral contusion, T9, mouse | 88 | |
| Unilateral contusion, C7, rat | 75 | |
| Unilateral contusion, C7, rat | 89 | |
| Contusion, C7, rat | 90 | |
| Unilateral contusion, C4, rat | 42 | |
| Unilateral contusion, C7, rat | 77 | |
| Unilateral contusion, C7, rat | 91 | |
| Granulocyte
colony-stimulating factor |
Contusion, T10, rat | 92 |
| Compression, T9, rat | 93 | |
| Contusion, T9, rat | 94 | |
| Hemisection, T10, mouse | 95 | |
| Contusion, T8, rat | 96 | |
| Contusion, T9, rat | 97 | |
| Contusion, T8, rat | 44 | |
| Compression, T8, mouse | 98 | |
| Compression, T7, mouse | 99 | |
| Transection, T8, mouse | 100 | |
| Contusion, T8, rat | 101 | |
| Compression, T8, rat | 102 | |
| Minocycline | Contusion, T7, rat | 103 |
| Contusion, T9, rat | 104 | |
| Contusion, T9, mouse | 105 | |
| Contusion, T9, rat | 106 | |
| Contusion, T9, rat | 107 | |
| Hemisection, T13, rat | 108 | |
| Contusion, T10, rat | 109 | |
| Contusion, T9, rat | 110 | |
| Contusion, T10, rat | 111 | |
| Contusion, T9, rat | 112 | |
| Dorsal transection, C7, rat | 113 | |
| Unilateral contusion, C5, rat | 114 | |
| Compression, T3, mouse | 115 | |
| Cethrin (VX-210) | Contusion, T8, mouse | 116 |
| Dorsal hemisection, T7, mouse | 117 | |
| Dorsal transection, T3, rat | 118 | |
| Contusion, T9, rat | 119 | |
| Anti-Nogo-A
antibody |
Hemisection, T10, rat | 120 |
| Dorsolateral hemisection, T8, rat | 121 | |
| T-shape transection, T9, rat | 122 | |
| Partial hemisection, T8, monkey | 123 | |
| T-shape transection, T8, rat | 124 | |
| T-shape transection, T8, rat | 125 | |
| T-shape transection, T8, rat | 126 | |
| Dorsal hemisection, T8, rat | 52 | |
| Partial dorsal transection, T6, rat | 53 | |
| Partial hemisection, C7, monkey | 54 | |
| Hemisection, C7, monkey | 55 |
The table summarizes the model, anatomical level of spinal cord injury, and the species used to evaluate the effectiveness and mechanisms of action of the neuroprotective therapies undergoing clinical trials. Although this list is not exhaustive, it highlights that thoracic models of spinal cord injury are most commonly applied at the preclinical level. All injury models are bilateral if not stated otherwise.
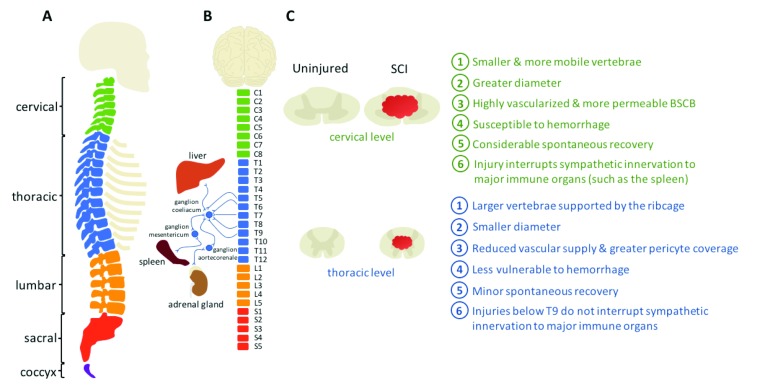
Differences between the cervical and thoracic cord anatomy, physiology, and immune response may affect the outcome of neuroprotective treatments ( Figure 1). Anatomically, the cervical spine has small vertebrae and increased mobility, which make it more susceptible to injury compared to the thoracic region. The cervical spinal cord also has a larger diameter, a greater blood supply, and larger gray and white matter areas 4. Relatedly, the cervical gray matter vasculature has less pericyte coverage than the thoracic cord, resulting in a blood spinal cord barrier predisposed to increased permeability 5, 6. Also, some of the most frequent conditions of the spine, such as central cord syndrome 7 and degenerative cervical myelopathy 8, affect primarily the cervical region. Of note, while the cervical spinal cord is especially vulnerable to injury and hemorrhage, it may also be more accessible to systemically administered therapeutics. Adding to this complexity, there is emerging evidence demonstrating level-dependent variations in the immune response 9, 10. For example, interestingly, higher-level injuries may be less prone to chronic autoimmunity 11, 12. Therefore, as SCI pathophysiology may differ between anatomical levels of injury, there is growing awareness that treatments should be tailored to the patient’s injury. Here, we review the most promising neuroprotective approaches, emphasizing their effect differences based on the level of injury ( Table 2).
Figure 1. There are several key differences between cervical and thoracic spinal cord injury.
( A) The cervical vertebrae are smaller and more mobile than their thoracic counterparts, which are further supported by the rib cage. ( B) The cervical spinal cord also has a larger diameter, and injuries at the cervical level interrupt the sympathetic innervation to major immune organs. ( C) Moreover, the greater vascularity of the cervical cord increases susceptibility to hemorrhage following trauma. Lastly, injuries at the cervical level allow for considerably more spontaneous recovery compared with injuries at the thoracic level 128. BSCB, blood spinal cord barrier; SCI, spinal cord injury.
Table 2. Neuroprotective strategies currently in clinical trials.
| Neuroprotective/
neural-reparative drug |
ClinicalTrials.gov
identifier |
Status | Enrollment (number of
patients, level of injury |
Results | Mechanism of action | Reference | |
|---|---|---|---|---|---|---|---|
| Thoracic | Cervical | ||||||
| Riluzole | NCT00876889 | Completed | 8 (T1–T11) | 28 (C4–C8) | Motor score improvement in patients
with cervical SCI, particularly with incomplete injuries. No significant effect in patients with thoracic SCI. |
Limit excitotoxicity | 36 |
| NCT01597518 | Recruiting | 0 | Est. enrollment
351 (C4–C8) |
37 | |||
| Therapeutic
hypothermia |
N/A | Completed | 0 | 14 (C4–C7) | Trend toward improvement of motor
scores compared with historical controls in the same institution. |
Reduce excitotoxicity,
inflammation, and vasogenic edema |
40 |
| NCT02991690 | Recruiting | 0 | Est. 120 (C1–C8) | ClinicalTrials.gov | |||
| Glibenclamide | NCT02524379 | Recruiting | 0 | Est. 10 (C2–C8) | Limit hemorrhage | ClinicalTrials.gov | |
| Granulocyte colony-
stimulating factor |
N/A | Completed | 0 | 28 (C2–C6) | Motor score improvement at 3 months
of treatment compared with MPSS historical controls. |
Promote neurogenesis
and angiogenesis, and reduce inflammation |
46 |
| N/A | Completed | 0 | 17 (C2–C6) | Motor score improvement from 1 week
to 1 year after SCI compared with placebo in an open non-randomized trial. |
47 | ||
| Minocycline | NCT00559494 | Completed | 17 (T1–T12) | 25 (C1–C8) | Motor score improvement in patients
with cervical SCI, particularly for motor incomplete. No benefit for patients with thoracic SCI. |
Reduce inflammation | 49 |
| NCT01828203 | Recruiting | 0 | Est. 248 (C0–C8) | ClinicalTrials.gov | |||
| Cethrin (VX-210) | NCT00500812 | Completed | 32 (T2–T12) | 16 (C4–T1) | Significant motor score improvement in
patients with cervical injury. No effect in patients with thoracic SCI. |
Inhibit axonal dieback
and reduce inflammation |
50 |
| NCT02669849 | Recruiting | 0 | Est. 150 (C5–C6) | ClinicalTrials.gov | |||
| Anti-Nogo-A antibody | NCT00406016 | Completed | 52 patients with injury between
C5 and T12; no data available about injury level classification |
No adverse effects. | Promote neurite sprouting | 127 | |
| ClinicalTrials.gov | |||||||
The table lists the discussed neuroprotective strategies for spinal cord injury (SCI) undergoing clinical evaluation. The status of trials and enrollment information, including level of injury and results, are summarized. This demonstrates that clinical trials are predominately focused on cervical SCI.
Est, estimated; N/A, not applicable; MPSS, methylprednisolone sodium succinate.
Neuroprotective strategies in current care
Early surgical decompression
The popularized phrase coined by the senior author, “Time is spine”, highlights the preclinical 13, 14 and clinical 15, 16 success of early surgical decompression, which aims to realign the spinal column and relieve bony or ligamentous spinal cord compression. Decompression of intradural pressure, by durotomy alone or durotomy combined with duraplasty, has also been evaluated in experimental SCI 17, 18. Yet mixed results warrant further research on the efficacy and standardization of these practices. In contrast, early extradural surgical decompression has been shown to reduce tissue damage and improve outcomes following SCI. Even with some concern regarding perioperative hemodynamic changes affecting cord perfusion, most spine surgeons have been, and continue to be, in favor of decompressing the acutely injured spinal cord 19, 20. As a result, early decompression remains recommended in clinical management guidelines by the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons 21. Similarly, the recent AOSpine guideline also recommends decompression within 24 hours of SCI 22. While there may be differential efficacy between injury-level subpopulations 23, current evidence is limited by substantial clinical heterogeneity, loss to follow-up, unclear adjustment for baseline factors, and a lack of statistical power. For these reasons, more work is needed to develop customizable treatment regimens and prioritized surgical access to the most benefitting patient subtypes.
Support of mean arterial pressure
Hypotension, hypoxemia, pulmonary dysfunction, and cardiovascular instability are common within the first 7 to 10 days of SCI 24. Hemodynamic instability not only limits the opportunity for early surgical intervention but also increases spinal cord ischemia and therefore secondary damage. For this reason, the current AANS and Congress of Neurological Surgeons guideline recommends continuous hemodynamic monitoring, interventions correcting hypotension (such as by vasopressor administration), and maintenance of mean arterial blood pressure (MAP) between 85 and 90 mmHg for the first 7 days following cervical injury 25. While these recommendations are largely based on a small group of uncontrolled and underpowered studies 26, a recent retrospective assessment largely confirmed the published guidelines as well as the neuroprotective potential of vasopressor administration 27. As these results need further prospective validation, it will be important to stratify patient populations and identify potential treatment effect differences based on the anatomical level of injury. It is also important to note that MAP support principally aims to maintain appropriate spinal cord perfusion pressure (SCPP), determined by the difference between MAP and intraspinal pressure (ISP). However, as ISP may increase independently of MAP, maintenance of a low ISP or a high SCPP (or both) is gaining increasing attention as an important practice in the acute clinical management of SCI 28, 29. While initial studies have shown encouraging results about the predictive value of low ISP or high SCPP in neurological recovery, larger multicenter studies are needed to validate these preliminary data 29.
Methylprednisolone sodium succinate
Methylprednisolone sodium succinate (MPSS) is a synthetic corticosteroid with potent anti-inflammatory effects and neuroprotective potential in acute traumatic SCI. Concerns about increased risk for infections following MPSS treatment have kept the drug at the forefront of continuous controversy. While it remains the only treatment option for acute SCI, debate regarding optimal dose, time of administration, efficacy, and adverse effects has dominated the field for decades and has dichotomized clinicians around the world. For this reason, there have been three National Acute Spinal Cord Injury Studies (NASCIS) to evaluate the clinical safety and efficacy of varying MPSS dose and timing. Moreover, NASCIS results have been retrospectively analyzed on numerous occasions to derive meaningful conclusions. One of the most recent publications on the topic concluded that MPSS does not increase the risk of infections and confers significant short-term effects when given within the first 8 hours of injury 30. Importantly, patients with cervical SCI and reduced baseline injury severity seem to benefit most from this treatment 31. Given the particularly debilitating nature of cervical injuries, these improvements have tremendous impact on patients’ quality of life. Thus, the most recent AOSpine guideline currently recommends a 24-hour treatment of intravenous MPSS when initiated within the first 8 hours of SCI, independently of injury level 22.
Promising neuroprotective and neural reparative therapies in clinical trials
Riluzole
Secondary injury involves ionic dysregulation and excitotoxicity. As cell membranes become highly permeable to sodium ions, there is increased calcium influx. Subsequently, high sodium and calcium ion concentrations in neurons trigger the secretion of glutamate from nerve terminals. Increased synaptic glutamate leads to prolonged excitability in the postsynaptic neurons, driving eventual neuronal edema and death.
Riluzole is a benzothiazole (molecular weight of 234.2 Da) which inhibits voltage-gated sodium channels and glutamate release, thereby mitigating excitotoxicity. Riluzole has been reported to slow the progression of amyotrophic lateral sclerosis (ALS)—a progressive motor neuron disease—and currently is the only US Food and Drug Administration (FDA)-approved drug for ALS. In addition, riluzole has been shown to have neuroprotective potential in animal models of Parkinson’s disease 32 and Huntington’s disease 33 and currently is being used in clinical studies of mild Alzheimer’s disease (ClinicalTrials.gov identifier NCT01703117). Importantly, riluzole was also shown to suppress spasticity 34, a frequent co-morbidity in patients with SCI, and to promote neural preservation in rats with high cervical spinal hemisection injury 35.
Capitalizing on the preclinical success of riluzole, a phase I/IIA clinical trial was launched in April 2010 to assess the safety and pharmacokinetics of riluzole in patients with acute traumatic SCI (ClinicalTrials.gov identifier NCT00876889). In this trial, 36 patients with SCI (28 cervical and eight thoracic SCI) received riluzole (50 mg) orally every 12 hours for 28 doses. A control group consisting of 36 patients with SCI—matched for neurological impairment, gender, and age—received the standard of care but no riluzole. Patients who received riluzole showed statistically significant improvement compared with the control group ( P = 0.021). In particular, patients with incomplete cervical injury—American Spinal Injury Association (ASIA) Impairment Scale B—showed the highest improvement in the International Standards for Neurologic Classification of Spinal Cord Injury (ISNCSCI) motor score ( P = 0.037). In addition to being efficacious, riluzole was shown to be safe for this patient cohort 36. Interestingly, there was no difference within the thoracic injury group, as patient numbers were small, patients had more severe injuries, and the ISNCSCI motor scoring is less sensitive to thoracic recovery 37.
Based on these results, a phase IIB/III was launched in 2013 to evaluate the efficacy and safety of riluzole in patients with cervical traumatic SCI, entitled “Riluzole in Acute SCI Study” (RISCIS) (estimated enrollment: 351 patients, ClinicalTrials.gov identifier NCT01597518). In this multicenter, randomized, placebo-controlled, double-blinded trial, riluzole (100 mg, twice daily) is administered orally to patients within 24 hours from injury, followed by two 50 mg daily doses for 14 days after SCI. A capsule identical in shape and size to riluzole is administered to patients in the control group. The primary outcome of the study is improvement in ISNCSCI motor scores at 180 days after injury. The study is estimated to be completed by 2021 37.
Therapeutic hypothermia
In response to trauma, increased metabolic rate can lead to excitotoxicity and cell death. Local or systemic cooling following insult has been shown to reduce the metabolic demand, thereby limiting cell death. Moreover, therapeutic hypothermia has been shown to reduce inflammatory cell infiltration, myeloperoxidase activity, and vasogenic edema and stabilize the blood-brain barrier 38. Despite these benefits, systemic hypothermia may have some serious side effects, including bradycardia, respiratory infections, and deep vein thrombosis. While local cooling of the spinal cord circumvents many of these concomitant issues, randomized controlled trials are still needed to prove the efficacy of local hypothermia in neurological recovery after SCI. However, in one study, acute (within 8 hours of injury) local hypothermia was shown to improve recovery among cervical and thoracic populations (n = 12 out of 14 cervical SCI, n = 4 out of 6 thoracic SCI) when compared with historical controls 39. Similarly, a pilot study of systemic hypothermia in patients with cervical complete SCI (n = 14) demonstrated fewer adverse effects and a trend toward improved recovery compared with age- and injury level-matched historical controls when systemic hypothermia was induced within 9 hours of trauma 40. Furthermore, a follow-up randomized controlled trial assessing the efficacy of intravascularly delivered systemic hypothermia in acute cervical SCI commenced in May 2017 (estimated enrollment: 120 patients, ClinicalTrials.gov identifier NCT02991690). Although previous multicenter randomized clinical trials found hypothermia to be ineffective in adults with traumatic brain injury 41, expectations for SCI remain hopeful.
Glibenclamide (Glyburide, DiaBeta)
Capillary fragmentation following SCI contributes to hemorrhage. This process is initiated in the capillary-rich gray matter of the injury epicenter and expands rostro-caudally, leading to progressive tissue necrosis, cavitation, and neurological dysfunction. In a rat model of unilateral cervical SCI, Simard et al. found that sulfonylurea receptor 1 (SUR1)-regulated Ca 2+-activated [ATP] i-sensitive non-specific cation (NC Ca-ATP) channels of the capillary endothelium in the spinal cord are key to capillary fragmentation following SCI 42. By blocking NC Ca-ATP channels with the FDA-approved anti-diabetic drug glibenclamide (Glyburide), Simard et al. observed decreased lesion volumes and significant white matter preservation coupled with improved neurobehavioral outcomes 42. Recently, a phase I/II clinical trial was initiated to assess the safety and neuroprotective effectiveness of Glyburide (DiaBeta) in patients with acute traumatic cervical SCI (estimated enrollment: 10 patients, ClinicalTrials.gov identifier NCT02524379), with an estimated completion date in early 2020.
Granulocyte colony-stimulating factor
Initially overlooked for its potential in the central nervous system (CNS), granulocyte colony-stimulating factor (G-CSF) has shown positive preclinical results for SCI ( Table 1). In response to ischemia and CNS injury, G-CSF and its receptor (CD114; G-CSFR) are upregulated in neurons and endogenous stem cells, initiating a compensatory neuroprotective mechanism. By binding to its cognate receptor, G-CSF counteracts programmed cell death in mature neurons, induces neurogenesis, and promotes neuronal differentiation of adult neural stem cells 43. Moreover, angiogenesis 44 and reduced inflammation 45 have been attributed to the protective actions of G-CSF. Kamiya et al. administered G-CSF for 5 consecutive days after cervical SCI and assessed ASIA motor scores 3 months later 46. The improvements were significant compared with historical controls of patients with cervical SCI receiving high-dose MPSS 46. In a study by Inada et al., patients with cervical SCI who received G-CSF demonstrated improved recovery compared with a non-treated group 47. However, the treatment was administered in an open-label and non-randomized fashion 47. Interestingly, in a study by Saberi et al., in which G-CSF was administered in patients with chronic SCI, significant motor and sensory recovery was demonstrated, particularly in patients with incomplete cervical SCI 48. Despite these promising effects, a true double-blinded randomized control clinical trial for G-CSF has yet to be developed.
Minocycline
Inflammatory cytokines produced by resident microglia and astrocytes following trauma attract peripheral immune cells to the spinal cord. Neutrophils and monocytes are the first blood-derived cells to enter the injured parenchyma. While these cells are crucial in cleaning up the cellular debris, they produce inflammatory cytokines, such as tumor necrosis factor-alpha and interferon-gamma, as well as toxic by-products that exacerbate damage.
Minocycline is a tetracycline antibiotic with neuroprotective and anti-inflammatory properties. A single-center, placebo-controlled, double-blinded phase I/II clinical trial was initiated in 2004 to evaluate the efficacy and safety of intravenous minocycline within 12 hours of injury for 7 days. The study, which was completed in 2010 (27 patients received minocycline and 25 received placebo), showed a trend toward improved motor scores in incomplete cervical SCI cases in the absence of any serious adverse effects ( P = 0.05) but no improvement in thoracic SCI 49 (ClinicalTrials.gov identifier NCT00559494). Based on these results, a phase III clinical trial, titled “Minocycline in Acute Spinal Cord Injury (MASC)”, was initiated in 2013 and is expected to finish by 2018 (estimated enrollment: 248 patients, ClinicalTrials.gov identifier NCT01828203). Interestingly, a clinical trial evaluating the efficacy of minocycline in reducing neuropathic pain has been successfully completed, but the results have yet to be published (ClinicalTrials.gov identifier NCT01869907). Given that neuropathic pain is a common and debilitating co-morbidity in patients with SCI, the study results will be of significant interest to the field.
Cethrin (VX-210)
The injured spinal cord niche contains growth-inhibitory molecules, such as myelin debris and chondroitin sulfate proteoglycans, that lead to neuron growth cone collapse, thereby inhibiting regeneration. These molecules bind to respective receptors on regenerating neurons, where they initiate a phosphorylation cascade. At the converging point of this cascade are Rho GTPases, a family of intracellular enzymes that regulate cytoskeletal mechanisms and cellular mobility. Cethrin (VX-210) is a recombinant deactivator of RhoA (a member of the Rho family) with dura and cell membrane penetrance. An open-label uncontrolled phase I/IIa clinical trial showed significant neurological improvement in patients with SCI who received Cethrin (48 patients, ClinicalTrials.gov identifier NCT00500812). Benefits were particularly enhanced in patients with cervical SCI compared with their thoracic counterparts 50, a finding that incentivized the initiation of larger controlled double-blinded clinical trials for patients with cervical SCI. This phase IIb/III clinical trial will evaluate the safety and efficacy of two doses of VX-210 (formerly known as Cethrin) compared with placebo (a fibrin sealant) when applied extradurally at the site of injury, acutely after cervical SCI (estimated enrollment: 150 patients, ClinicalTrials.gov identifier NCT02669849).
Anti-Nogo-A antibody (ATI-355)
Anti-Nogo-A antibody is a monoclonal antibody against Nogo-A, a protein inhibitor of neurite growth found on adult CNS myelin. Widely assessed at the thoracic level in rodents 51– 53, in addition to several studies in primate models of cervical SCI 54, 55, anti-Nogo-A antibody has been shown to promote axonal sprouting and improve functional recovery following injury. A non-randomized, open-label phase I clinical trial of humanized anti-Nogo-A antibody (ATI-355; Novartis Pharmaceuticals) was initiated to assess the feasibility, tolerability, and safety of either repeated intrathecal bolus injections of AT1-355 or continuous intrathecal delivery in acute SCI (4–14 days after injury). In total, 52 cervical and thoracic patients with traumas between C5 and T12 level were recruited in the study, and results are pending dissemination (ClinicalTrials.gov identifier NCT00406016). A phase IIb trial, led by Armin Curt, is expected to begin in Europe shortly.
Emerging neuroprotective approaches
Intravenous immunoglobulin G
Intravenous immunoglobulin G (IVIG) consists of serum immunoglobulin G (IgG) pooled from thousands of healthy donors. Independent laboratory studies in cervical and thoracic models of SCI have shown that IVIG improves recovery by targeting the detrimental inflammatory response in the spinal cord after trauma 56– 58. While the efficacy of IVIG for SCI has not been assessed in clinical trials, the exciting preclinical results coupled with IVIG’s long-term clinical use for the treatment of autoimmune and immunodeficiency conditions make it a promising candidate for SCI clinical trials.
Cell therapies
With cell transplantation as an attractive treatment approach for SCI, a diverse range of cells has been evaluated in preclinical studies, resulting in a plethora of potential mechanisms. In short, transplanted cells have been used for immune modulation, trophic support, scaffolding, re-myelination, and cell replacement 59. Yet, predominantly applied in the subacute and chronic phases of injury, only a few cell transplantation strategies are thought to have neuroprotective potential for the acutely injured spinal cord.
Mesenchymal stem/stromal cells. Mesenchymal stem/stromal cells (MSCs) are multipotent mesodermal progenitors defined by their in vitro adhesion to plastic and their cell surface antigen profile 60. Readily accessible from various adult tissues such as bone marrow, cartilage, and fat, MSCs are among the most commonly studied cells in regenerative medicine. This popularity has led to significant heterogeneity in MSC isolation, cultivation, and purification procedures, further resulting in mixed therapeutic efficacy among preclinical studies and the increasing number of clinical studies 61. In SCI, MSCs have been reported to dampen inflammation, modulate the immune response, and secrete neuroprotective factors 59. A 2013 systematic meta-analysis of preclinical studies, involving intrathecal, intraparenchymal, and intravenous infusion of MSCs in various models of cervical and thoracic SCI, determined that the cells, overall, result in improved functional recovery after injury 62. While this is encouraging, a lot remains to be understood about the identity and function of MSCs. Furthermore, additional mechanistic studies are needed to effectively tailor their therapeutic application for SCI and identify differences in efficacy between anatomical levels of injury.
Final thoughts
Spinal cord level-dependent differences in vertebral structure, anatomy, and peripheral immune organ innervation may affect SCI pathophysiology. Though largely overlooked in preclinical studies, post hoc subgroup analysis from seminal large-scale clinical trials has been indicative of varying treatment efficacy between different anatomical levels of SCI 50, 63. As a result, the stratification of patients into injury-level subpopulations is being increasingly adopted in trial design, and the aforementioned RISCIS trial is a leading example 37.
Notwithstanding its strengths, this approach has considerable challenges. Firstly, given the clinical heterogeneity of SCI pathophysiology, even within the same level of injury, the recruitment of adequate patient numbers to reach statistical power may prove substantially difficult, especially for acute and infrequent injuries. Although multicenter trials may circumvent this issue, they require tremendous coordination, collaboration, and resources. However, a recently published assessment of patient recruitment for acute SCI trials determined that such multicenter Canadian trials are feasible with careful a priori planning and registry support 64. Secondly, stratified analysis is susceptible to confounding effects. For instance, treatment efficacy may be directly affected by patient age or injury causation rather than the anatomical level of injury 3. In addition, stratification according to injury level alone is unlikely to account for the significant variability in injury severity, presentation, and patient characteristics. Finally, a greater understanding of the impact of trauma-related mechanisms on the impact and outcomes of SCI is also required.
Therefore, it is increasingly recognized that optimal patient recovery will stem from a combinatorial treatment regimen of integrated pharmacological and rehabilitation-based strategies that will be personalized to their SCI signature (age, medical history, level, completeness, and mechanism of injury). For this reason, translational laboratory studies need to compare neuroprotective efficacy, as well as combinatorial approaches, between different anatomical levels of injury and severity. Moreover, advances in imaging and biochemical biomarkers are needed to help tailor trials within a heterogeneous SCI patient population, narrowing the inclusion window and increasing study power. These approaches can be further applied to better assess treatment efficacy, specifically beyond basic neurological recovery. Apart from outcomes, treatment protocols should ensure sufficient drug delivery to target sites, especially for systemically administered neuroprotective agents with CNS-specific effects 65. Moreover, coordinated efforts should be made by leading SCI care units to standardize early medical management 66, 67 and monitoring practices 68 in order to maximize the efficacy of randomized controlled trials. Lastly, with the increasing incidence of traumatic SCI in the elderly 3, it is important that more emphasis be placed on optimizing such practices to address the specific needs of this growing demographic.
In conclusion, neuroprotection has the potential to improve recovery of motor, sensory, and autonomic function following SCI. Significant strides in our understanding of SCI pathophysiology, patient presentation, and biomarkers will further align preclinical research with clinical reality, yielding translatable solutions that can benefit patients.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Marios Papadopoulos, Academic Neurosurgery Unit, St. George's, University of London, London, UK
Patrick Freund, Spinal Cord Injury Center Balgrist, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Neurology, Wellcome Trust Center for Neuroimaging, University College London , London, UK
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Blesch A, Tuszynski MH: Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32(1):41–7. 10.1016/j.tins.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 2. Kwon BK, Hillyer J, Tetzlaff W: Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 2010;27(1):21–33. 10.1089/neu.2009.1048 [DOI] [PubMed] [Google Scholar]
- 3. Singh A, Tetreault L, Kalsi-Ryan S, et al. : Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31. 10.2147/CLEP.S68889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kameyama T, Hashizume Y, Sobue G: Morphologic features of the normal human cadaveric spinal cord. Spine (Phila Pa 1976). 1996;21(11):1285–90. 10.1097/00007632-199606010-00001 [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Lucas-Osma AM, Black S, et al. : Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med. 2017;23(6):733–41. 10.1038/nm.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Winkler EA, Sengillo JD, Bell RD, et al. : Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab. 2012;32(10):1841–52. 10.1038/jcbfm.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKinley W, Santos K, Meade M, et al. : Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30(3):215–24. 10.1080/10790268.2007.11753929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nouri A, Tetreault L, Singh A, et al. : Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675–93. 10.1097/BRS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 9. Brommer B, Engel O, Kopp MA, et al. : Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain. 2016;139(Pt 3):692–707. 10.1093/brain/awv375 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Lucin KM, Sanders VM, Jones TB, et al. : Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207(1):75–84. 10.1016/j.expneurol.2007.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibarra A, Jiménez A, Cortes C, et al. : Influence of the intensity, level and phase of spinal cord injury on the proliferation of T cells and T-cell-dependent antibody reactions in rats. Spinal Cord. 2007;45(5):380–6. 10.1038/sj.sc.3101972 [DOI] [PubMed] [Google Scholar]
- 12. Ulndreaj A, Tzekou A, Mothe AJ, et al. : Characterization of the Antibody Response after Cervical Spinal Cord Injury. J Neurotrauma. 2017;34(6):1209–26. 10.1089/neu.2016.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Batchelor PE, Wills TE, Skeers P, et al. : Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. 2013;8(8):e72659. 10.1371/journal.pone.0072659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fehlings MG, Perrin RG: The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(11 Suppl):S28–35; discussion S36. 10.1097/01.brs.0000217973.11402.7f [DOI] [PubMed] [Google Scholar]
- 15. Fehlings MG, Vaccaro A, Wilson JR, et al. : Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012;7(2):e32037. 10.1371/journal.pone.0032037 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Furlan JC, Noonan V, Cadotte DW, et al. : Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28(8):1371–99. 10.1089/neu.2009.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalan D, Saini N, Zaidi M, et al. : Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J Neurosurg Spine. 2017;26(1):62–75. 10.3171/2016.6.SPINE16343 [DOI] [PubMed] [Google Scholar]
- 18. Smith JS, Anderson R, Pham T, et al. : Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J Bone Joint Surg Am. 2010;92(5):1206–14. 10.2106/JBJS.I.00740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fehlings MG, Rabin D, Sears W, et al. : Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976). 2010;35(21 Suppl):S166–73. 10.1097/BRS.0b013e3181f386f6 [DOI] [PubMed] [Google Scholar]
- 20. Wilson JR, Tetreault LA, Kwon BK, et al. : Timing of Decompression in Patients With Acute Spinal Cord Injury: A Systematic Review. Global Spine Journal. 2017;7(3 Suppl):95S–115. 10.1177/2192568217701716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Resnick DK: Updated Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury. Neurosurgery. 2013;72 Suppl 2:1. 10.1227/NEU.0b013e318276ee7e [DOI] [PubMed] [Google Scholar]
- 22. Fehlings MG, Wilson JR, Tetreault LA, et al. : A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Global Spine Journal. 2017;7(3 Suppl):203S–11S. 10.1177/2192568217703085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samuel AM, Bohl DD, Basques BA, et al. : Analysis of Delays to Surgery for Cervical Spinal Cord Injuries. Spine (Phila Pa 1976). 2015;40(13):992–1000. 10.1097/BRS.0000000000000883 [DOI] [PubMed] [Google Scholar]
- 24. Levi L, Wolf A, Belzberg H: Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery. 1993;33(6):1007–16; discussion 1016–7. [PubMed] [Google Scholar]
- 25. Ryken TC, Hurlbert RJ, Hadley MN, et al. : The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery. 2013;72 Suppl 2:84–92. 10.1227/NEU.0b013e318276ee16 [DOI] [PubMed] [Google Scholar]
- 26. Vale FL, Burns J, Jackson AB, et al. : Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87(2):239–46. 10.3171/jns.1997.87.2.0239 [DOI] [PubMed] [Google Scholar]
- 27. Hawryluk G, Whetstone W, Saigal R, et al. : Mean Arterial Blood Pressure Correlates with Neurological Recovery after Human Spinal Cord Injury: Analysis of High Frequency Physiologic Data. J Neurotrauma. 2015;32(24):1958–67. 10.1089/neu.2014.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Chen K, Marsh BC, Cowan M, et al. : Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol. 2017;292:135–44. 10.1016/j.expneurol.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 29. Saadoun S, Chen S, Papadopoulos MC: Intraspinal pressure and spinal cord perfusion pressure predict neurological outcome after traumatic spinal cord injury. J Neurol Neurosurg Psychiatry. 2017;88(5):452–3. 10.1136/jnnp-2016-314600 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Evaniew N, Belley-Côté EP, Fallah N, et al. : Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Systematic Review and Meta-Analysis. J Neurotrauma. 2016;33(5):468–81. 10.1089/neu.2015.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Evaniew N, Noonan VK, Fallah N, et al. : Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Propensity Score-Matched Cohort Study from a Canadian Multi-Center Spinal Cord Injury Registry. J Neurotrauma. 2015;32(21):1674–83. 10.1089/neu.2015.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Fernandez-Espejo E: Pathogenesis of Parkinson's disease: prospects of neuroprotective and restorative therapies. Mol Neurobiol. 2004;29(1):15–30. 10.1385/MN:29:1:15 [DOI] [PubMed] [Google Scholar]
- 33. Bonelli RM, Wenning GK, Kapfhammer HP: Huntington's disease: present treatments and future therapeutic modalities. Int Clin Psychopharmacol. 2004;19(2):51–62. 10.1097/00004850-200403000-00001 [DOI] [PubMed] [Google Scholar]
- 34. Kitzman PH: Effectiveness of riluzole in suppressing spasticity in the spinal cord injured rat. Neurosci Lett. 2009;455(2):150–3. 10.1016/j.neulet.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 35. Satkunendrarajah K, Nassiri F, Karadimas SK, et al. : Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016;276:59–71. 10.1016/j.expneurol.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 36. Grossman RG, Fehlings MG, Frankowski RF, et al. : A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31(3):239–55. 10.1089/neu.2013.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fehlings MG, Nakashima H, Nagoshi N, et al. : Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54(1):8–15. 10.1038/sc.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martirosyan NL, Patel AA, Carotenuto A, et al. : The role of therapeutic hypothermia in the management of acute spinal cord injury. Clin Neurol Neurosurg. 2017;154:79–88. 10.1016/j.clineuro.2017.01.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Hansebout RR, Hansebout CR: Local cooling for traumatic spinal cord injury: outcomes in 20 patients and review of the literature. J Neurosurg Spine. 2014;20(5):550–61. 10.3171/2014.2.SPINE13318 [DOI] [PubMed] [Google Scholar]
- 40. Levi AD, Casella G, Green BA, et al. : Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670–7. 10.1227/01.NEU.0000367557.77973.5F [DOI] [PubMed] [Google Scholar]
- 41. Clifton GL, Miller ER, Choi SC, et al. : Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–63. 10.1056/NEJM200102223440803 [DOI] [PubMed] [Google Scholar]
- 42. Simard JM, Tsymbalyuk O, Ivanov A, et al. : Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117(8):2105–13. 10.1172/JCI32041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Schneider A, Krüger C, Steigleder T, et al. : The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–98. 10.1172/JCI23559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawabe J, Koda M, Hashimoto M, et al. : Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats: laboratory investigation. J Neurosurg Spine. 2011;15(4):414–21. 10.3171/2011.5.SPINE10421 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Hartung T: Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5(3):221–5. [DOI] [PubMed] [Google Scholar]
- 46. Kamiya K, Koda M, Furuya T, et al. : Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J. 2015;24(5):963–7. 10.1007/s00586-014-3373-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Inada T, Takahashi H, Yamazaki M, et al. : Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: analyses of follow-up cases after at least 1 year. Spine (Phila Pa 1976). 2014;39(3):213–9. 10.1097/BRS.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 48. Saberi H, Derakhshanrad N, Yekaninejad MS: Comparison of neurological and functional outcomes after administration of granulocyte-colony-stimulating factor in motor-complete versus motor-incomplete postrehabilitated, chronic spinal cord injuries: a phase I/II study. Cell Transplant. 2014;23 Suppl 1:S19–23. 10.3727/096368914X684943 [DOI] [PubMed] [Google Scholar]
- 49. Casha S, Zygun D, McGowan MD, et al. : Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135(Pt 4):1224–36. 10.1093/brain/aws072 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Fehlings MG, Theodore N, Harrop J, et al. : A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011;28(5):787–96. 10.1089/neu.2011.1765 [DOI] [PubMed] [Google Scholar]
- 51. Bregman BS, Kunkel-Bagden E, Schnell L, et al. : Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378(6556):498–501. 10.1038/378498a0 [DOI] [PubMed] [Google Scholar]
- 52. Merkler D, Metz GA, Raineteau O, et al. : Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21(10):3665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schnell L, Schwab ME: Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343(6255):269–72. 10.1038/343269a0 [DOI] [PubMed] [Google Scholar]
- 54. Freund P, Schmidlin E, Wannier T, et al. : Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12(7):790–2. 10.1038/nm1436 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Freund P, Wannier T, Schmidlin E, et al. : Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol. 2007;502(4):644–59. 10.1002/cne.21321 [DOI] [PubMed] [Google Scholar]
- 56. Brennan FH, Kurniawan ND, Vukovic J, et al. : IVIg attenuates complement and improves spinal cord injury outcomes in mice. Ann Clin Transl Neurol. 2016;3(7):495–511. 10.1002/acn3.318 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Gok B, Sciubba DM, Okutan O, et al. : Immunomodulation of acute experimental spinal cord injury with human immunoglobulin G. J Clin Neurosci. 2009;16(4):549–53. 10.1016/j.jocn.2008.04.024 [DOI] [PubMed] [Google Scholar]
- 58. Nguyen DH, Cho N, Satkunendrarajah K, et al. : Immunoglobulin G (IgG) attenuates neuroinflammation and improves neurobehavioral recovery after cervical spinal cord injury. J Neuroinflammation. 2012;9:224. 10.1186/1742-2094-9-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Badner A, Siddiqui AM, Fehlings MG: Spinal cord injuries: how could cell therapy help? Expert Opin Biol Ther. 2017;17(5):529–41. 10.1080/14712598.2017.1308481 [DOI] [PubMed] [Google Scholar]
- 60. Dominici M, Le Blanc K, Mueller I, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Squillaro T, Peluso G, Galderisi U: Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25(5):829–48. 10.3727/096368915X689622 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Oliveri RS, Bello S, Biering-Sørensen F: Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis. 2014;62:338–53. 10.1016/j.nbd.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 63. Rahimi-Movaghar V: Clinical trials for the treatment of spinal cord injury: cervical and lumbar enlargements versus thoracic area. Brain. 2009;132(Pt 7):e115; author reply e116. 10.1093/brain/awn282 [DOI] [PubMed] [Google Scholar]
- 64. Thibault-Halman G, Rivers CS, Bailey CS, et al. : Predicting Recruitment Feasibility for Acute Spinal Cord Injury Clinical Trials in Canada Using National Registry Data. J Neurotrauma. 2017;34(3):599–606. 10.1089/neu.2016.4568 [DOI] [PubMed] [Google Scholar]
- 65. Phang I, Zoumprouli A, Papadopoulos MC, et al. : Microdialysis to Optimize Cord Perfusion and Drug Delivery in Spinal Cord Injury. Ann Neurol. 2016;80(4):522–31. 10.1002/ana.24750 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Fehlings MG, Cadotte DW, Fehlings LN: A series of systematic reviews on the treatment of acute spinal cord injury: a foundation for best medical practice. J Neurotrauma. 2011;28(8):1329–33. 10.1089/neu.2011.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fransen BL, Hosman AJ, van Middendorp JJ, et al. : Pre-hospital and acute management of traumatic spinal cord injury in the Netherlands: survey results urge the need for standardisation. Spinal Cord. 2016;54(1):34–8. 10.1038/sc.2015.111 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Saadoun S, Papadopoulos MC: Spinal cord injury: is monitoring from the injury site the future? Crit Care. 2016;20(1):308. 10.1186/s13054-016-1490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Ates O, Cayli SR, Gurses I, et al. : Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci. 2007;14(7):658–65. 10.1016/j.jocn.2006.03.023 [DOI] [PubMed] [Google Scholar]
- 70. Hachem LD, Mothe AJ, Tator CH: Evaluation of the effects of riluzole on adult spinal cord-derived neural stem/progenitor cells in vitro and in vivo. Int J Dev Neurosci. 2015;47(Pt B):140–6. 10.1016/j.ijdevneu.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 71. Hama A, Sagen J: Antinociceptive effect of riluzole in rats with neuropathic spinal cord injury pain. J Neurotrauma. 2011;28(1):127–34. 10.1089/neu.2010.1539 [DOI] [PubMed] [Google Scholar]
- 72. Mu X, Azbill RD, Springer JE: Riluzole improves measures of oxidative stress following traumatic spinal cord injury. Brain Res. 2000;870(1–2):66–72. 10.1016/S0006-8993(00)02402-1 [DOI] [PubMed] [Google Scholar]
- 73. Stutzmann JM, Pratt J, Boraud T, et al. : The effect of riluzole on post-traumatic spinal cord injury in the rat. Neuroreport. 1996;7(2):387–92. 10.1097/00001756-199601310-00003 [DOI] [PubMed] [Google Scholar]
- 74. Vasconcelos NL, Gomes ED, Oliveira EP, et al. : Combining neuroprotective agents: effect of riluzole and magnesium in a rat model of thoracic spinal cord injury. Spine J. 2016;16(8):1015–24. 10.1016/j.spinee.2016.04.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Hosier H, Peterson D, Tsymbalyuk O, et al. : A Direct Comparison of Three Clinically Relevant Treatments in a Rat Model of Cervical Spinal Cord Injury. J Neurotrauma. 2015;32(21):1633–44. 10.1089/neu.2015.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Schwartz G, Fehlings MG: Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg. 2001;94(2 Suppl):245–56. 10.3171/spi.2001.94.2.0245 [DOI] [PubMed] [Google Scholar]
- 77. Simard JM, Tsymbalyuk O, Keledjian K, et al. : Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp Neurol. 2012;233(1):566–74. 10.1016/j.expneurol.2011.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu Y, Satkundrarajah K, Teng Y, et al. : Evaluation of the sodium-glutamate blocker riluzole in a preclinical model of ervical spinal cord injury. Evid Based Spine Care J. 2010;1(2):71–2. 10.1055/s-0030-1267047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu Y, Satkunendrarajah K, Teng Y, et al. : Delayed post-injury administration of riluzole is neuroprotective in a preclinical rodent model of cervical spinal cord injury. J Neurotrauma. 2013;30(6):441–52. 10.1089/neu.2012.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Batchelor PE, Kerr NF, Gatt AM, et al. : Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. J Neurotrauma. 2010;27(8):1357–68. 10.1089/neu.2010.1360 [DOI] [PubMed] [Google Scholar]
- 81. Farooque M, Hillered L, Holtz A, et al. : Effects of moderate hypothermia on extracellular lactic acid and amino acids after severe compression injury of rat spinal cord. J Neurotrauma. 1997;14(1):63–9. 10.1089/neu.1997.14.63 [DOI] [PubMed] [Google Scholar]
- 82. Grulova I, Slovinska L, Nagyova M, et al. : The effect of hypothermia on sensory-motor function and tissue sparing after spinal cord injury. Spine J. 2013;13(12):1881–91. 10.1016/j.spinee.2013.06.073 [DOI] [PubMed] [Google Scholar]
- 83. Morino T, Ogata T, Takeba J, et al. : Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord. 2008;46(6):425–31. 10.1038/sj.sc.3102163 [DOI] [PubMed] [Google Scholar]
- 84. Seo JY, Kim YH, Kim JW, et al. : Effects of Therapeutic Hypothermia on Apoptosis and Autophagy After Spinal Cord Injury in Rats. Spine (Phila Pa 1976). 2015;40(12):883–90. 10.1097/BRS.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 85. Yu CG, Jimenez O, Marcillo AE, et al. : Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. J Neurosurg. 2000;93(1 Suppl):85–93. 10.3171/spi.2000.93.1.0085 [DOI] [PubMed] [Google Scholar]
- 86. Lo TP, Jr, Cho KS, Garg MS, et al. : Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol. 2009;514(5):433–48. 10.1002/cne.22014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Redondo-Castro E, Hernández J, Mahy N, et al. : Phagocytic microglial phenotype induced by glibenclamide improves functional recovery but worsens hyperalgesia after spinal cord injury in adult rats. Eur J Neurosci. 2013;38(12):3786–98. 10.1111/ejn.12382 [DOI] [PubMed] [Google Scholar]
- 88. Simard JM, Woo SK, Norenberg MD, et al. : Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci Transl Med. 2010;2(28):28ra29. 10.1126/scitranslmed.3000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Popovich PG, Lemeshow S, Gensel JC, et al. : Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp Neurol. 2012;233(2):615–22. 10.1016/j.expneurol.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Simard JM, Popovich PG, Tsymbalyuk O, et al. : Spinal cord injury with unilateral versus bilateral primary hemorrhage--effects of glibenclamide. Exp Neurol. 2012;233(2):829–35. 10.1016/j.expneurol.2011.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simard JM, Popovich PG, Tsymbalyuk O, et al. : MRI evidence that glibenclamide reduces acute lesion expansion in a rat model of spinal cord injury. Spinal Cord. 2013;51(11):823–7. 10.1038/sc.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen WF, Chen CH, Chen NF, et al. : Neuroprotective Effects of Direct Intrathecal Administration of Granulocyte Colony-Stimulating Factor in Rats with Spinal Cord Injury. CNS Neurosci Ther. 2015;21(9):698–707. 10.1111/cns.12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chung J, Kim MH, Yoon YJ, et al. : Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on glial scar formation after spinal cord injury in rats. J Neurosurg Spine. 2014;21(6):966–73. 10.3171/2014.8.SPINE131090 [DOI] [PubMed] [Google Scholar]
- 94. Dittgen T, Pitzer C, Plaas C, et al. : Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS One. 2012;7(1):e29880. 10.1371/journal.pone.0029880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guo Y, Liu S, Zhang X, et al. : G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice. Lab Invest. 2015;95(12):1439–49. 10.1038/labinvest.2015.120 [DOI] [PubMed] [Google Scholar]
- 96. Kadota R, Koda M, Kawabe J, et al. : Granulocyte colony-stimulating factor (G-CSF) protects oligodendrocyte and promotes hindlimb functional recovery after spinal cord injury in rats. PLoS One. 2012;7(11):e50391. 10.1371/journal.pone.0050391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kato K, Koda M, Takahashi H, et al. : Granulocyte colony-stimulating factor attenuates spinal cord injury-induced mechanical allodynia in adult rats. J Neurol Sci. 2015;355(1–2):79–83. 10.1016/j.jns.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 98. Koda M, Nishio Y, Kamada T, et al. : Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–31. 10.1016/j.brainres.2007.02.058 [DOI] [PubMed] [Google Scholar]
- 99. Nishio Y, Koda M, Kamada T, et al. : Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007;66(8):724–31. 10.1097/nen.0b013e3181257176 [DOI] [PubMed] [Google Scholar]
- 100. Pitzer C, Klussmann S, Krüger C, et al. : The hematopoietic factor granulocyte-colony stimulating factor improves outcome in experimental spinal cord injury. J Neurochem. 2010;113(4):930–42. 10.1111/j.1471-4159.2010.06659.x [DOI] [PubMed] [Google Scholar]
- 101. Sanli AM, Serbes G, Calişkan M, et al. : Effect of granulocyte-colony stimulating factor on spinal cord tissue after experimental contusion injury. J Clin Neurosci. 2010;17(12):1548–52. 10.1016/j.jocn.2010.03.043 [DOI] [PubMed] [Google Scholar]
- 102. Urdzíková L, Jendelová P, Glogarová K, et al. : Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23(9):1379–91. 10.1089/neu.2006.23.1379 [DOI] [PubMed] [Google Scholar]
- 103. Ahmad M, Zakaria A, Almutairi KM: Effectiveness of minocycline and FK506 alone and in combination on enhanced behavioral and biochemical recovery from spinal cord injury in rats. Pharmacol Biochem Behav. 2016;145:45–54. 10.1016/j.pbb.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 104. Aras M, Altas M, Motor S, et al. : Protective effects of minocycline on experimental spinal cord injury in rats. Injury. 2015;46(8):1471–4. 10.1016/j.injury.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 105. Arnold SA, Hagg T: Anti-inflammatory treatments during the chronic phase of spinal cord injury improve locomotor function in adult mice. J Neurotrauma. 2011;28(9):1995–2002. 10.1089/neu.2011.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Festoff BW, Ameenuddin S, Arnold PM, et al. : Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97(5):1314–26. 10.1111/j.1471-4159.2006.03799.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Lee SM, Yune TY, Kim SJ, et al. : Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20(10):1017–27. 10.1089/089771503770195867 [DOI] [PubMed] [Google Scholar]
- 108. Marchand F, Tsantoulas C, Singh D, et al. : Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13(7):673–81. 10.1016/j.ejpain.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 109. Sonmez E, Kabatas S, Ozen O, et al. : Minocycline treatment inhibits lipid peroxidation, preserves spinal cord ultrastructure, and improves functional outcome after traumatic spinal cord injury in the rat. Spine (Phila Pa 1976). 2013;38(15):1253–9. 10.1097/BRS.0b013e3182895587 [DOI] [PubMed] [Google Scholar]
- 110. Tan AM, Zhao P, Waxman SG, et al. : Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury. J Rehabil Res Dev. 2009;46(1):123–33. 10.1682/JRRD.2008.03.0048 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Teng YD, Choi H, Onario RC, et al. : Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101(9):3071–6. 10.1073/pnas.0306239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yune TY, Lee JY, Jung GY, et al. : Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27(29):7751–61. 10.1523/JNEUROSCI.1661-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stirling DP, Khodarahmi K, Liu J, et al. : Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24(9):2182–90. 10.1523/JNEUROSCI.5275-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Z, Nong J, Shultz RB, et al. : Local delivery of minocycline from metal ion-assisted self-assembled complexes promotes neuroprotection and functional recovery after spinal cord injury. Biomaterials. 2017;112:62–71. 10.1016/j.biomaterials.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Wells JE, Hurlbert RJ, Fehlings MG, et al. : Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126(Pt 7):1628–37. 10.1093/brain/awg178 [DOI] [PubMed] [Google Scholar]
- 116. Boato F, Hendrix S, Huelsenbeck SC, et al. : C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci. 2010;123(Pt 10):1652–62. 10.1242/jcs.066050 [DOI] [PubMed] [Google Scholar]
- 117. Dergham P, Ellezam B, Essagian C, et al. : Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22(15):6570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fournier AE, Takizawa BT, Strittmatter SM: Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23(4):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sung JK, Miao L, Calvert JW, et al. : A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res. 2003;959(1):29–38. 10.1016/S0006-8993(02)03717-4 [DOI] [PubMed] [Google Scholar]
- 120. Atalay B, Bavbek M, Cekinmez M, et al. : Antibodies neutralizing Nogo-A increase pan-cadherin expression and motor recovery following spinal cord injury in rats. Spinal Cord. 2007;45(12):780–6. 10.1038/sj.sc.3102113 [DOI] [PubMed] [Google Scholar]
- 121. Brösamle C, Huber AB, Fiedler M, et al. : Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20(21):8061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen S, Smielewski P, Czosnyka M, et al. : Continuous Monitoring and Visualization of Optimum Spinal Cord Perfusion Pressure in Patients with Acute Cord Injury. J Neurotrauma. 2017;34(21):2941–2949. 10.1089/neu.2017.4982 [DOI] [PubMed] [Google Scholar]
- 123. Fouad K, Klusman I, Schwab ME: Regenerating corticospinal fibers in the Marmoset ( Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci. 2004;20(9):2479–82. 10.1111/j.1460-9568.2004.03716.x [DOI] [PubMed] [Google Scholar]
- 124. Gonzenbach RR, Zoerner B, Schnell L, et al. : Delayed anti-nogo-a antibody application after spinal cord injury shows progressive loss of responsiveness. J Neurotrauma. 2012;29(3):567–78. 10.1089/neu.2011.1752 [DOI] [PubMed] [Google Scholar]
- 125. Liebscher T, Schnell L, Schnell D, et al. : Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58(5):706–19. 10.1002/ana.20627 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 126. Maier IC, Ichiyama RM, Courtine G, et al. : Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132(Pt 6):1426–40. 10.1093/brain/awp085 [DOI] [PubMed] [Google Scholar]
- 127. Zörner B, Schwab ME: Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci. 2010;1198 Suppl 1:E22–34. 10.1111/j.1749-6632.2010.05566.x [DOI] [PubMed] [Google Scholar]
- 128. Fawcett JW, Curt A, Steeves JD, et al. : Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190–205. 10.1038/sj.sc.3102007 [DOI] [PubMed] [Google Scholar]