Abstract
Background
Actigraphy is commonly used to measure sleep outcomes so sleep can be measured conveniently at home over multiple nights. Validity of actigraphy has been demonstrated in people with sleep disturbances; however, validity of scoring settings in people with chronic medical illnesses such as chronic obstructive pulmonary disease remains unclear. The purpose of this secondary analysis was to compare actigraphy customized scoring settings with polysomnography for measurement of sleep outcomes in people with chronic obstructive pulmonary disease who have insomnia.
Methods
Participants underwent overnight sleep assessment simultaneously by polysomnography and actigraphy at the University of Illinois of Chicago Sleep Science Center. Fifty participants (35 men, 15 women) with mild to severe chronic obstructive pulmonary disease and insomnia were included in the analysis. Sleep onset latency, total sleep time, wake after sleep onset, and sleep efficiency were calculated independently from data derived from polysomnography and from actigraphy. Actigraphy sleep outcome scores obtained at the default setting and several customized actigraphy settings were compared to the scored polysomnography results.
Results
Although no single setting was optimal for all sleep outcomes, the combination of 10 consecutive immobile minutes for sleep onset or end and activity threshold of 10 worked well. Actigraphy overestimated TST and SE and underestimated WASO but there was no difference in variance between PSG and actigraphy in TST and SE when the 10*10 combination was used. As the average TST and SE increased, the agreement between PSG and actigraphy appeared to increase and as the average WASO decreased, the agreement between PSG and actigraphy appeared to increase.
Conclusion
Results support the conclusion that the default actigraphy settings may not be optimal for people with chronic obstructive pulmonary disease and insomnia.
Keywords: sleep disturbance, chronic obstructive pulmonary disease, polysomnography, WASO, emphysema, chronic bronchitis, insomnia
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic illness worldwide[1]. It is estimated that more than half of people with COPD (emphysema or chronic bronchitis) report insomnia[2,3], and the prevalence of insomnia in COPD is increasing[4]. People with COPD commonly experience fatigue, anxiety, and depression that interfere with daily activities and could be related to disturbed sleep[5]. Accordingly, studies are urgently needed of sleep disturbance in COPD using valid measures for sleep. Overnight polysomnography (PSG) is the gold standard for measuring sleep outcomes, but it is expensive and takes place in a laboratory, not in the patient’s home, and lasts one or two nights.
Actigraphy is widely used for assessment of sleep outcomes at home and over longer periods of time. A number of studies have demonstrated validity of actigraphy for measuring sleep using selected scoring settings in adults[6–9]. Actigraph scoring settings can be adjusted, but also include default settings that vary from actigraph to actigraph. Default actigraphy scoring settings are commonly used in research of adults with sleep disturbances, but recent studies have provided evidence challenging the use of default settings in other populations such as Parkinson’s disease[6] and children[7]. In addition to sleep disturbances, people with chronic illnesses may have disease-related characteristics that affect actigraphy scoring. Thus, the optimal actigraphy scoring settings for sleep in people with chronic illnesses such as COPD is unclear. Therefore, the purpose of this study was to compare actigraphy customized scoring settings with polysomnography for measurement of sleep outcomes in people with COPD and co-existing sleep disturbance.
2. Methods
2.1. Study participants and design
This secondary analysis included 50 participants recruited from the Chicago area. Participants with mild to severe COPD according to the GOLD criteria[8] and no other major health problems were recruited through print advertisements and word of mouth as part of two of our studies on insomnia therapy in COPD[10,11]. The first study was a randomized, controlled pilot study examining feasibility and preliminary effects of cognitive behavioral therapy for insomnia (CBT-I) and COPD education on outcomes in people with co-existing insomnia and COPD. The second is a randomized, controlled study of efficacy and mechanisms of components of insomnia therapy on outcomes for people with COPD. For the current analysis we combined data from screening for sleep disorders performed as part of the two studies.
The inclusion criteria were diagnosis of COPD; abnormal FEV1/FVC ratio < 70%; age ≥ 45 years; and stable clinical condition (no major exacerbation of COPD within the previous month). Participants underwent pulmonary function testing (PFT) on the VMAX Encore 22 (Viasys Healthcare, Inc., Yorba Linda, CA) according to established standards (Miller et al., 2005). Presence of insomnia was indicated by a score of ≥ 10 on the Insomnia Severity Index (ISI)[12]. Participants were excluded if they showed evidence of other current major medical illness, major depression indicated by a Hospital Anxiety and Depression Scale (HADS) score >11[13], other sleep disorders or were currently participating in pulmonary rehabilitation. Full details of the research protocols are described elsewhere[10,11].
2.2. Measures
A standard PSG montage was used. The polysomnography included electroencephalogram (EEG; C4-M1, C3-M2, O4-M1, O1-M2), electrooculogram (EOG), electromyogram (EMG; chin and lower extremities), respiratory (c-flow, chest, and abdominal effort piezo bands), electrocardiogram, pulse oximetry, and body position. PSG data were collected and stored using the ALICE3 digital system (Respironics). Sleep stages were scored by an experienced technician according to standardized criteria[14]. Sleep outcome variables derived from the PSG data included sleep onset latency (SOL; minutes between lights off and first sleep episode), time in bed (TIB; minutes between lights on and lights off), total sleep time (TST; minutes between sleep onset and wake time), wake after sleep onset (WASO; minutes awake between sleep onset and wake time), and sleep efficiency (SE; percentage of time asleep from lights off to lights on). All PSG scores were reviewed by a board-certified sleep physician.
Actigraphy data were collected simultaneously with PSG data. Actigraphic sleep variables were assessed using the AW-2 Actiwatch (Minimitter, Philips Respironics, Andover MA). It was programmed for start time and data collection interval, and data were retrieved for analysis via a PC interface using scoring software (Actiware 6.0.8) provided with the accelerometer. The accelerometer was worn on the non-dominant wrist while undergoing polysomnography. The actigraph recorded data in 30 second epochs, the same epoch length as the PSG. The lights off to lights on times recorded by the PSG technician were used to define the rest interval during scoring of the actigraphy. AW-2 data were scored as wake or sleep using a validated algorithm that codes epochs as sleep or wake by examining activity counts for two minutes surrounding the epoch. The AW-2 includes a range of potential activity count settings: low (20), medium (40), and high (80). Customized settings are also available. Various threshold activity count settings (0, 5, 10, 20 and 40) were tested in this study. Scores were derived from the actigraphy for the same sleep outcomes as from the PSG (SOL, TIB, TST, WASO, and SE). The Actiware software defined sleep onset or end by finding consecutive immobile minutes for sleep onset and consecutive active minutes for sleep end. In this study we tested 3 different immobility settings – 5 minutes, 10 minutes and 15 minutes for sleep onset and end.
2.3. Procedures
The research was approved by the UIC Institutional Review Board, and all participants provided written, informed consent to participate. People who agreed to participate performed PFTs and completed the ISI and HADS at the first screening visit. Participants who met initial inclusion criteria underwent overnight sleep assessment simultaneously by PSG and actigraphy at the University of Illinois of Chicago (UIC) Sleep Science Center at the second screening visit in order to be screened for other sleep disorders. Sleep variable data were calculated independently from data derived from PSG and from actigraphy. Actigraphy sleep scores obtained at the default setting and several customized actigraphy settings were compared to the scored PSG results.
2.4. Analysis
Data were analyzed with descriptive statistics, paired t tests, Bland-Altman concordance plots, and Pitman’s Test of difference in variance using Stata 13.0 (StataCorp LP, College Station, Texas) and SPSS 23.0 (SPSS Inc., Chicago, IL). A two-tailed p value < 0.05 was considered statistically significant for all analyses. Data were collected on 53 subjects, but 3 subjects were excluded due to PSG TST < 100 min.
3. Results
Table 1 shows characteristics for study participants. Participants (35 men, 15 women) had mild to severe COPD according to the GOLD standard[8] and moderate insomnia according to the ISI[12]. On average, they smoked cigarettes for over 35 years, were of normal weight for their age, and had a high school education.
Table 1.
Participant Characteristics (n = 50)
| Characteristics | |
|---|---|
| Age (years) | 63.2 ± 8.4 |
| Male | 35 (70.0) |
| Race | |
| White | 28 (56.0) |
| Black | 20 (40.0) |
| Asian | 1 (2.0) |
| American Indian or Alaskan | 1 (2.0) |
| Education (years) | 13.8 ± 2.4 |
| Characteristics | |
| BMI | 26.7 ± 6.6 |
| Smoking (pack/year) | 35.2 ± 25.2 |
| FEV1/FVC | 51.8 ± 10.4 |
| FEV1, % predicted | 63.1 ± 22.4 |
| PSG Apnea Hypopnea Index | 6.4 ± 9.6 |
| PSG mean SaO2 | 93.9 ± 2.5 |
| PSG minimum SaO2 | 86.0 ± 6.6 |
| Insomnia Severity Index | 17.8 ± 4.1 |
Data are presented as N (%) or mean ± standard deviation.
BMI, body mass index; FEV1/FVC, Forced expiratory volume in one second/forced vital capacity; PSG, polysomnography
Tables 2, 3, and 4 depict comparisons of sleep outcomes assessed by PSG and actigraphy at selected settings. Of the selected settings, a customized actigraphy setting of 5 consecutive immobile minutes for sleep onset and an activity threshold of 5 yielded the best approximated mean values determined by PSG for TST and SE (N = 50), with differences of 1.9 ± 71.4 minutes for TST, and 0.5 ± 16.9% for SE. When actigraphy was set to 5 consecutive immobile minutes for sleep onset or end, and activity thresholds of 10, actigraphy produced the best approximated values determined by PSG for WASO (N = 50), with differences of 0.3 ± 47.2 minutes. When actigraphy was set to 10 consecutive immobile minutes for sleep onset or end, and activity threshold of 10, actigraphy produced closely approximated values determined by PSG for TST, SE and WASO.
Table 2.
Comparison of Sleep Parameters Assessed by PSG and Actigraphy (x̄ ± SD): 5 Immobile or Mobile Minutes Required for Sleep Onset or End (n = 50)
| Parameters | PSG | Actigraphy | Difference | P value |
|---|---|---|---|---|
| TST (min) | 267.5 ± 71.4 | |||
| 0 | 178.8 ± 81.7 | −88.7 | < 0.001 | |
| 5 | 265.6 ± 71.4 | −1.9 | 0.818 | |
| 10 | 289.1 ± 67.8 | 21.6 | 0.009 | |
| 20 | 312.7 ± 64.0 | 45.2 | < 0.001 | |
| 40 | 333.4 ± 59.5 | 65.9 | < 0.001 | |
| SE (%) | 65.6 ± 16.9 | |||
| 0 | 43.8 ± 19.7 | −21.8 | < 0.001 | |
| 5 | 65.1 ± 16.9 | −0.5 | 0.797 | |
| 10 | 70.8 ± 15.7 | 5.2 | 0.009 | |
| 20 | 76.6 ± 14.3 | 11.0 | < 0.001 | |
| 40 | 81.7 ± 12.9 | 16.1 | < 0.001 | |
| WASO (min) | 96.6 ± 59.2 | |||
| 0 | 207.2 ± 73.0 | 110.6 | < 0.001 | |
| 5 | 120.4 ± 54.0 | 23.7 | < 0.001 | |
| 10 | 96.9 ± 47.2 | 0.3 | 0.975 | |
| 20 | 73.3 ± 38.9 | −23.3 | 0.004 | |
| 40 | 52.5 ± 31.2 | −44.1 | < 0.001 |
Parameters in italic: various actigraphy thresholds. Values in bold are closest to PSG values. TST: total sleep time; SE: sleep efficiency; WASO: wake after sleep onset.
Table 3.
Comparison of Sleep Parameters Assessed by PSG and Actigraphy (x̄ ± SD): 10 Immobile or Mobile Minutes Required for Sleep Onset or End (n = 50)
| Parameters | PSG | Actigraphy | Difference | P value |
|---|---|---|---|---|
| TST (min) | 267.5 ± 71.4 | |||
| 0 | 176.8 ± 82.8 | −90.7 | < 0.001 | |
| 5 | 257.3 ± 75.2 | −10.2 | 0.247 | |
| 10 | 278.4 ± 72.4 | 10.9 | 0.208 | |
| 20 | 299.0 ± 69.6 | 31.5 | < 0.001 | |
| 40 | 317.4 ± 65.6 | 49.9 | < 0.001 | |
| SE (%) | 65.6 ± 16.9 | |||
| 0 | 43.3 ± 20.0 | −22.3 | < 0.001 | |
| 5 | 63.0 ± 17.9 | −2.6 | 0.235 | |
| 10 | 68.2 ± 17.0 | 2.6 | 0.215 | |
| 20 | 73.3 ± 15.9 | 7.7 | < 0.001 | |
| 40 | 77.7 ± 14.7 | 12.1 | < 0.001 | |
| WASO (min) | 96.6 ± 59.2 | |||
| 0 | 188.0 ± 64.9 | 91.4 | < 0.001 | |
| 5 | 107.5 ± 48.6 | 10.9 | 0.152 | |
| 10 | 86.4 ± 43.0 | −10.2 | 0.160 | |
| 20 | 65.7 ± 35.5 | −30.9 | < 0.001 | |
| 40 | 47.4 ± 29.3 | −49.2 | < 0.001 |
Values in italic: various actigraphy thresholds. Values in bold are closest to PSG values. TST: total sleep time; SE: sleep efficiency; WASO: wake after sleep onset.
Table 4.
Comparison of Sleep Parameters Assessed by PSG and Actigraphy (x̄ ± SD): 15 Immobile or Mobile Minutes Required for Sleep Onset or End (n = 49***)
| Parameters | PSG | Actigraphy | Difference | P value |
|---|---|---|---|---|
| TST (min) | 268.5 ± 71.8 | |||
| 0 | 174.6 ± 84.0 | −93.9 | < 0.001 | |
| 5 | 246.4 ± 82.0 | −22.1 | 0.029 | |
| 10 | 264.0 ± 81.9 | −4.5 | 0.644 | |
| 20 | 281.0 ± 80.6 | 12.5 | 0.219 | |
| 40 | 296.0 ± 78.4 | 27.5 | 0.009 | |
| SE (%) | 65.9 ± 17.0 | |||
| 0 | 42.8 ± 20.3 | −23.1 | < 0.001 | |
| 5 | 60.5 ± 19.8 | −5.4 | 0.029 | |
| 10 | 64.7 ± 19.6 | −1.2 | 0.641 | |
| 20 | 68.9 ± 19.1 | 3.0 | 0.220 | |
| 40 | 72.6 ± 18.5 | 6.7 | 0.009 | |
| WASO (min) | 95.3 ± 59.1 | |||
| 0 | 153.8 ± 49.6 | 70.2 | < 0.001 | |
| 5 | 81.9 ± 34.0 | −1.7 | 0.826 | |
| 10 | 64.7 ± 30.4 | −18.9 | 0.009 | |
| 20 | 48.2 ± 26.8 | −35.4 | < 0.001 | |
| 40 | 34.0 ± 23.1 | −49.6 | < 0.001 |
Values in italic: various actigraphy thresholds. Values in bold are closest to PSG values. TST: total sleep time; SE: sleep efficiency; WASO: wake after sleep onset.
One subject does not have data for 15 immobile minutes. Thus, the paired t-test can only be done in 49 subjects.
Comparison of SOL assessed by PSG and actigraphy at 10 immobile or mobile minutes for sleep onset or end, with an activity threshold of 10 yielded a mean value for SOL of 29.0 ± 29.1 that was not significantly different from the PSG SOL value of 34.5 ± 31.4 (p = 0.239).
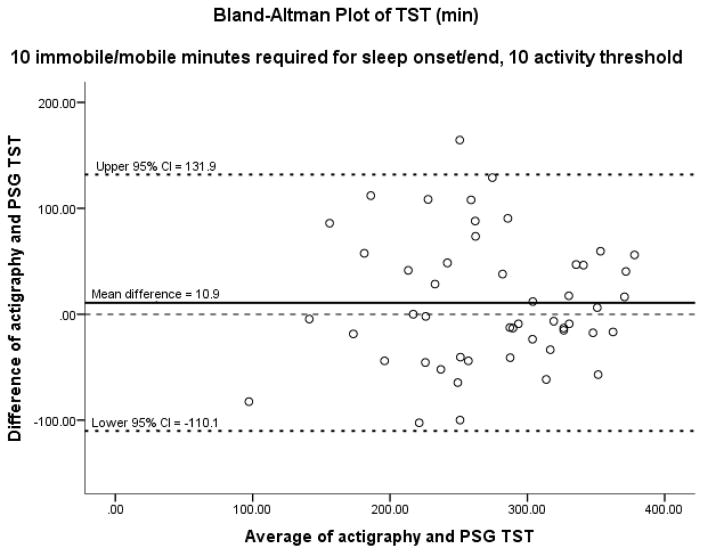
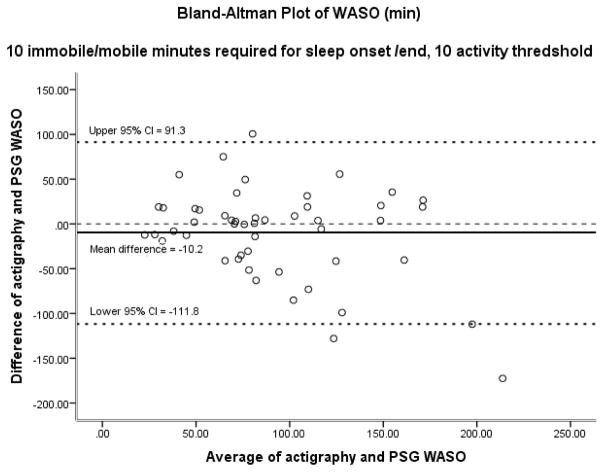
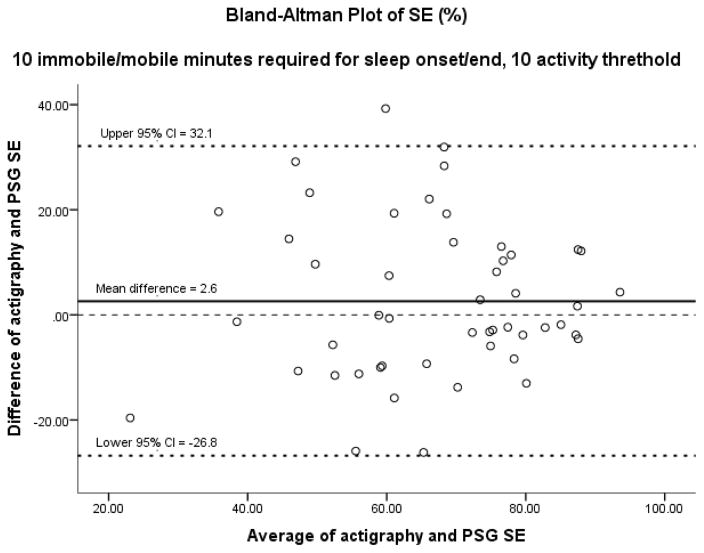
Figures 1–3 display results of the Bland-Altman analysis for the best settings for the three sleep outcomes taken as a whole: 10 immobile or mobile minutes for sleep onset or end with an activity threshold of 10. On average, actigraphy overestimated TST by 10.9 minutes and SE by 2.6%. Actigraphy underestimated WASO by 10.2 minutes. Results of Bland-Altman analysis and Pitman’s Test of difference in variance revealed no difference between PSG and actigraphy in TST and SE, but a significant difference in WASO. However, the difference of 10 minutes in WASO is likely not clinically significant. In visual inspection of the Bland-Altman plots, it is noted that as the average TST and SE increased, the agreement between PSG and actigraphy appeared to increase. As the average WASO decreased, the agreement between PSG and actigraphy appeared to increase. These findings suggest less agreement between PSG and actigraphy in people with more severe insomnia.
Figure 1.
Using 10 and 10 combination. TST: total sleep time; Pitman’s Test of difference in variance: r = 0.018, n = 50, p = 0.902.
Figure 3.
Using 10 and 10 combination. WASO: wake after sleep onset; Pitman’s test of difference in variance: r = −0.362, n = 50, p = 0.010
4. Discussion
Our results support the use of actigraphy for assessment of TST, SE, and WASO in people with COPD who have insomnia. However, the default actigraphy settings of 10 immobile or mobile minutes required for sleep onset or end and activity threshold of 40 may not yield the optimal result for the sleep variable of interest. The default settings overestimated TST by 50 minutes and SE by 12%, and underestimated WASO by 49 minutes when compared to PSG. No single actigraphy setting was optimal for all sleep outcomes. We demonstrated that a customized actigraphy setting of 5 consecutive immobile minutes for sleep onset and an activity threshold of 5 yielded the best approximated mean values determined by PSG for TST and SE. For WASO, 5 or 15 consecutive immobile minutes for sleep onset or end, and activity thresholds of 5 or 10 produced the best approximated values determined by PSG. We then provided evidence to support the conclusion that the 10*10 setting is a respectable combination to look at the sleep outcomes of interest in people who have COPD and insomnia. We showed that although actigraphy overestimated TST and SE and underestimated WASO, there was no difference in variance between PSG and actigraphy in TST and SE when the 10*10 combination was used, and the differences are not clinically meaningful. We found that as the average TST and SE increased, the agreement between PSG and actigraphy appeared to increase and as the average WASO decreased, the agreement between PSG and actigraphy appeared to increase. Collectively, this evidence supports the overall concept that reliability and validity of actigraphy settings may vary in different populations such as those with chronic illnesses and insomnia.
Our findings are in line with those of researchers examining actigraphy sleep scoring settings in adult populations. Recent studies demonstrated that lower actigraphy activity thresholds had closer TST and SE agreement rates to PSG than higher activity thresholds[15–17]. In the only other study we found that examined actigraphy scoring settings in people with a chronic medical illness, Maglione and colleagues[6] reported that the actigraphy setting of 5 consecutive immobile minutes for sleep onset and an activity threshold of 10 yielded the best approximated mean values determined by PSG for TST, SE and WASO in people with Parkinson’s disease.
Actigraphy overestimated TST and SE and underestimated WASO in our study. This is in accordance with previous studies in diverse populations in which actigraphy overestimated sleep and underestimated wake. Kushida and colleagues[16] demonstrated that actigraphy overestimated sleep (TST and SE) in people with sleep disordered breathing, subsequent researchers showed that actigraphy overestimated sleep in older adults with insomnia [17,18] and Maglione and colleagues[6] reported that actigraphy overestimated sleep in people with Parkinson’s disease. Our results are similar to others that found that actigraphy underestimated wake time. Sivertsen and colleagues showed that total wake time and sleep latency was underestimated by actigraphy in older adults with primary insomnia[18] and Taibi et al. demonstrated similar results in older women with insomnia[17].
As the average TST and SE increased, and as the average WASO decreased, the agreement between PSG and actigraphy appeared to increase. This is in agreement with Chae and colleagues who found that there were greater deviations from PSGs with lower TST in people with obstructive sleep apnea[15]. McCall et al reported that as WASO increased, the discrepancy versus PSG increased in people with depression and insomnia[19]. Taibi and colleagues demonstrated that reduced SE was associated with reduced accuracy of actigraphy in women with insomnia.
There are a number of considerations regarding individualizing scoring parameters in the population of interest. On the positive side, ensuring more precise and accurate scoring of sleep would be beneficial in research that includes pre-post measurement of sleep, as well as measuring the effects of therapy on individuals in clinical practice. However, on the negative side, such individualization of scoring parameters would be cumbersome compared to using the default settings. It would make it challenging to compare the results of studies in people with various medical illnesses. Researchers and clinicians should make scoring parameter decisions based on their particular sleep outcome measurement goals.
To our knowledge, our study is the first to compare actigraphy scoring settings for sleep outcome measures to PSG in people with COPD. A strength of our study is our sample which included diverse participants with COPD and insomnia. Limitations of this study include that our sample was mostly men, however, our sample includes 30% women, which is more women than in many studies of people with COPD. Additionally, in this study we used PSG parameters to set the rest intervals for the analyses, which is different than how rest intervals are usually set when using actigraphy in clinical research and practice. In general, changes in activity, light levels, event markers and sleep diaries are used to set the rest intervals. For this study sleep diary data were not available because many participants did not complete the diary. These limitations will limit generalizability of our study results. We also did not conduct an epoch to epoch direct comparison of actigraphy settings to PSG data, which would have allowed a more direct comparison of the scoring parameters. Despite limitations, this study provides valuable information about actigraphy scoring for sleep in adults with COPD and insomnia and adds to the body of knowledge that emphasizes the opportunity to identify and use the optimal settings for actigraphy scoring in the population of interest. Future studiess of actigraphy scoring in people with other chronic illnesses will be useful to identify optimal actigraphy scoring settings for people with other chronic medical illnesses.
Figure 2.
Using 10 and 10 combination. SE: sleep efficiency; Pitman’s test of difference in variance: r = 0.009, n = 50, p = 0.953
Highlights.
Actigraphy sleep outcome scores at the default setting and several customized actigraphy settings were compared with polysomnography.
No single setting was optimal for all sleep outcomes, but the combination of 10 consecutive immobile minutes for sleep onset or end and activity threshold of 10 worked well.
Actigraphy overestimated TST and SE and underestimated WASO.
The default actigraphy settings may not be optimal for people with chronic obstructive pulmonary disease and insomnia.
Acknowledgments
This research was supported by the National Institute of Nursing Research of the National Institutes of Health R01NR013937 and KO1 NR010749. The authors thank Kevin Grandfield, Publication Manager for the UIC Department of Biobehavioral Health Science, for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta–analysis. J Glob Health. n.d;5 doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellia V, Catalano F, Scichilone N, Incalzi RA, Spatafora M, Vergani C, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26:318–23. doi: 10.1093/sleep/26.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest. 1987;91:540–6. doi: 10.1378/chest.91.4.540. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372–8. doi: 10.1016/j.sleep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res. 2006;55:10–7. doi: 10.1097/00006199-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maglione JE, Liu L, Neikrug AB, Poon T, Natarajan L, Calderon J, et al. Actigraphy for the assessment of sleep measures in Parkinson’s disease. Sleep. 2013;36:1209–17. doi: 10.5665/sleep.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer LJ, Walsh CM, Peightal AA. Comparison of actigraphy immobility rules with polysomnographic sleep onset latency in children and adolescents. Sleep Breath Schlaf Atm. 2015;19:1415–23. doi: 10.1007/s11325-015-1138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [accessed December 8, 2016];Global Strategy for Diagnosis, Management, and Prevention of COPD - 2016 - Global Initiative for Chronic Obstructive Lung Disease - GOLD. n.d http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 9.GOLD C. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2010) [Google Scholar]
- 10.Kapella MC, Herdegen JJ, Perlis ML, Shaver JL, Larson JL, Law JA, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis. 2011;6:625–35. doi: 10.2147/COPD.S24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapella MC, Herdegen JJ, Laghi F, Steffen AD, Carley DW. Efficacy and mechanisms of behavioral therapy components for insomnia coexisting with chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. Trials. 2016;17:258. doi: 10.1186/s13063-016-1334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Iber C. American Academy of Sleep Medicine. 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 15.Chae KY, Kripke DF, Poceta JS, Shadan F, Jamil SM, Cronin JW, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–5. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 17.Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2013;9:217–25. doi: 10.5664/jcsm.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivertsen B, Omvik S, Havik OE, Pallesen S, Bjorvatn B, Nielsen GH, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–8. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 19.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21:122–7. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]