Abstract
Using an innovative, tissue-independent approach to decellularized tissue processing and biomaterial fabrication, the development of a series of “tissue papers” derived from native porcine tissues/organs (heart, kidney, liver, muscle), native bovine tissue/organ (ovary and uterus), and purified bovine Achilles tendon collagen as a control from decellularized extracellular matrix particle ink suspensions cast into molds is described. Each tissue paper type has distinct microstructural characteristics as well as physical and mechanical properties, is capable of absorbing up to 300% of its own weight in liquid, and remains mechanically robust (E = 1–18 MPa) when hydrated; permitting it to be cut, rolled, folded, and sutured, as needed. In vitro characterization with human mesenchymal stem cells reveals that all tissue paper types support cell adhesion, viability, and proliferation over four weeks. Ovarian tissue papers support mouse ovarian follicle adhesion, viability, and health in vitro, as well as support, and maintain the viability and hormonal function of nonhuman primate and human follicle-containing, live ovarian cortical tissues ex vivo for eight weeks postmortem. “Tissue papers” can be further augmented with additional synthetic and natural biomaterials, as well as integrated with recently developed, advanced 3D-printable biomaterials, providing a versatile platform for future multi-biomaterial construct manufacturing.
Keywords: biomaterials, decellularized extracellular matrices, ovary tissue engineering, tissue engineering
1. Introduction
Extracellular matrix (ECM)-based biomaterials, derived from decellularized tissues, have been the subject of substantial research and development in recent years.[1] Stripped of cells, decellularized extracellular matrix (dECM) is primarily comprised of nonwater soluble structural proteins (collagens, laminins, elastins, etc.),[2] but can also contain nontrivial concentrations of endogenous bioactive agents including growth factors.[1,3–6] Because of their composition and inherent ability to induce new tissue formation and regeneration, dECMs derived from human skin,[7] amnion,[8] nerve,[9] and demineralized bone[10] have been in successful clinical use for many years. This long track record and acceptance by clinicians is indicative of the promise of new ECM-based biomaterials that can be applied to repair or regenerate damaged, missing, or dysfunctional tissues and organs without activating host immune response,[1] or even serve as model materials for in vitro healthy or diseased tissue modeling.[11,12] Despite dECM’s demonstrated advantages as a raw material and promise for use in an extensive range of tissue and organ targets including but not limited to: heart,[13] muscle,[1,14] nerve,[15] liver,[3,16,17] lung,[18,19] kidney,[4,20] bone,[21] skin,[22] bladder,[23] ovary,[24] and penis,[25] the existing processing routes for creating diverse sets of clinically practical, tissue-specific dECM-based biomaterials remain limited.
There are two general approaches for isolating dECM and utilizing it as a biomaterial. The first approach involves whole organ decellularization and recellularization[4,5,17,19,26] prior to implantation. Although promising, this approach still suffers from a variety of technical and practical deficiencies including 1) the inability to fully recellularize the organs with the variety of distinct cell types in the required locations within the organ to make them functional, and 2) the need for whole, viable organs to be available for initial decellularization. An alternative approach to whole organ decellularization and recellularization, and the one taken in this work, is to isolate and process tissue and organ-specific dECM into a separate, distinct, biomaterial form prior to utilization. In addition to direct use of decellularized tissue pieces and sections,[3,4,24] these forms have included dECM containing suspensions and soft and rigid gels for injection,[27,28] coatings,[29] polymer scaffold infusions,[3,30] and 3D-printing.[31–33] These previous works have demonstrated that dECM is a promising class of raw biomaterials and can be processed into many physical forms to suit a variety of in vitro and in vivo applications.
Here, we introduce a highly versatile process for creating an array of tissue and organ-specific dECM biomaterials: “tissue papers” (TPs). These TPs can be rapidly fabricated from cast, dECM particle-suspension “inks,” which are synthesized via simple, room-temperature mixing of components and do not rely on any chemical reactions, nor acid, basic, or enzymatic solubilization of the raw dECM. This approach results in a collection of tissue-specific “tissue papers,” so named due to their handling and textural characteristics which we have observed to be reminiscent of common paper. The process outlined in this work is an extension of a methodology we have previously demonstrated for creating 3D-inks for 3D-printing osteogenic,[34] neurogenic,[35] and mixed-functionality synthetic biomaterials,[36] as well as metals,[24,37] extraterrestrial soil simulants, and ceramics.[38] We evaluated, in parallel, the microstructural, mechanical, physical, and in vitro biological characteristics of TPs fabricated from decellularized porcine heart (HTP), kidney (KTP), liver (LTP), muscle (MTP), bovine ovary (OTP) and uterus (UTP), and purified collagen (CTP) as a control material.
Additionally, although there are numerous potential applications for the range of TPs presented here, we elected to further investigate ovary specific applications of OTP. This is due in part because there is a relative deficiency in biomaterials intended for specific use in reproductive medicine and engineering. Moreover, the properties of the OTP align well with current clinical research protocols for preserving ovarian tissue as ovarian cortical strips that contain the ovarian reserve of potential egg cells, oocytes. Supportive OTP scaffolds integrated with ovarian tissue or containing isolated ovarian follicles (hormone cells with potential egg cells), could provide a source of hormones for pubertal progression and/or restore fertility in female cancer survivors who develop premature ovarian failure, for example. The results described here demonstrate that OTP supported the viability and function of isolated mouse ovarian follicles, as well as ex vivo function of human and rhesus macaque follicles within ovarian cortical tissues that secreted estradiol in long-term (eight week) cultures.
2. Results and Discussion
2.1. Tissue and Organ Processing
Tissue papers are comprised of tissue-specific dECM and poly-lactic-co-glycolic acid (PLGA), a commonly utilized biomedical, biodegradable elastomer. As a composite of elastomer and dECM, TPs are distinct from previously reported dECM composites[3,28,30] in that they are primarily comprised of the natural bioactive component, tissue-specific decellularized extracellular matrix (65 vol%), rather than a polymer (35 vol%), which is primarily present to aid in processing and handling. Additionally, previously reported dECM biomaterials and composites require that the dECM be processed under acid or basic conditions and/or undergo enzymatic digestion (for solubilization purposes) prior to being processed into the final biomaterial state.[3,39] These additional solubilization steps are not only time consuming and tissue/organ specific, but are biased toward retaining certain dECM components over others, resulting in the final dECM biomaterials being further removed in both composition and structure from the native tissue/organ extracellular matrices from which they are derived.[40] The process we present here is entirely physical in nature, does not require acid, basic, or enzymatic digestion of the dECM prior to use, and thus is primarily independent of tissue and organ type, composition, and source, while simultaneously retaining much of the composition and structure of the native dECM.
The primary component of the TPs, dECM powders, was obtained by dicing, decellularizing, washing, lyophilizing, and mechanically milling porcine and bovine tissues and organs. Specifically, porcine whole organs (heart, kidney, liver) and muscle sections from the psoas major (tenderloin), and bovine whole ovaries and uterine horns were utilized. With the exception of the bovine ovaries, which were decellularized as whole organs and large tissue sections, according to a previously described process,[24] porcine tissues and organs were cut into small pieces no greater than 5 mm on any single side to expedite the decellularization process (times for each tissue given in the summary, Table 1). Any fascia and large vessels were removed from the tissues or whole organs and discarded prior to decellularization. After being rinsed multiple times in water or holding medium to remove excess blood and plasma, tissues were decellularized in 0.5% sodium dodecyl sulfate (SDS), washed in H2O, lyophilized, and cryomilled to produce dECM powders.
Table 1.
Summary of tissue decellularization times and mechanical and physical properties of resulting tissue papers. * denotes that ovarian and uterine tissues were decellularized as whole or near whole organs, rather than cut fragments (see the Experimental Section). Δ denotes indirectly calculated porosity based on average tissue paper, (max – min)/2 thickness. E = Elastic modulus; UTS = Ultimate tensile strength; F% = Tensile strain to failure; Absorb. = maximum aqueous media absorbency. Bolded values for E, UTS, and F% indicate as-hydrated properties, while nonbold indicate as-dried properties.
| CTP | HTP | KTP | LTP | MTP | OTP | UTP | |
|---|---|---|---|---|---|---|---|
| Decell’ time | N/A | 4 d | 3 d | 3 d | 5 d | 28 d* | 28 d* |
| PorosityΔ [%] | 61.7 ± 3.0 | 88.0 ± 0.6 | 85.5 ± 1.8 | 84.7 ± 2.7 | 76.7 ± 3.5 | 82.6 ± 2.1 | 80.6 ± 1.9 |
| E [MPa] | 26.1 ± 7.0 11.3 ± 1.1a) | 2.9 ± 0.9 1.3 ± 0.1a) | 2.4 ± 0.8 1.3 ± 0.7a) | 2.3 ± 0.1 2.1 ± 0.2a) | 28.7 ± 3.5 18.0 ± 0.9a) | 0.9 ± 0.1 0.9 ± 0.3a) | 5.2 ± 1.7 1.4 ± 0.2a) |
| UTS [kPa] | 983 ± 345 650 ± 183a) | 148 ± 5 126 ± 35a) | 176 ± 72 134 ± 55a) | 376 ± 51 202 ± 69a) | 1300 ± 199 1140 ± 80a) | 79 ± 17 69 ± 14a) | 193 ± 65 121 ± 39a) |
| F% | 4.7 ± 1.0 11.8 ± 5.1b) | 6.1 ± 0.6 12.5 ± 3.1b) | 8.1 ± 3.1 11.3 ± 4.5b) | 10.2 ± 0.8 16.2 ± 7.9b) | 14.8 ± 1.0 18.0 ± 3.3b) | 6.3 ± 2.1 10.5 ± 3.5b) | 4.7 ± 0.6 10.3 ± 0.7b) |
| Absorb. [%] | 149 ± 19 | 300 ± 19 | 177 ± 34 | 153 ± 44 | 77 ± 42 | 299 ± 18 | 246 ± 21 |
Refers to reduction of property value in the hydrated state relative to dry state;
Refers to increase of property value in the hydrated state relative to the dry state.
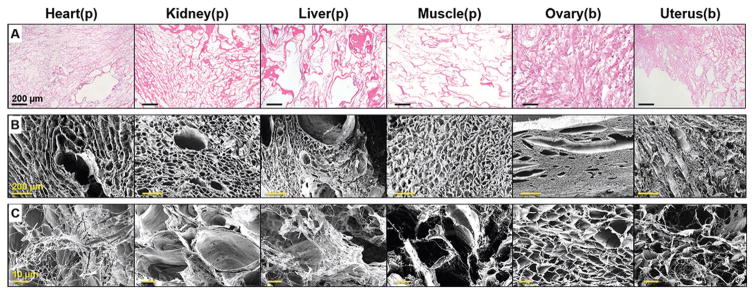
Cell nuclei were not observed to be present in histological sections of the decellularized tissues prior to cryomilling (hematoxylin and eosin stain; Figure 1A). Scanning electron microscopy (SEM) revealed the conserved and varied structures of the six tissue extracellular matrices (Figure 1B). The decellularized tissue fragments were rinsed for ≈24 h in sterile water to remove residual SDS prior to being lyophilized. The resulting anhydrous dECM materials were mechanically milled into powders (≤200 μm particles), which remained large enough to maintain some of the native structure of the original decellularized tissue (Figure 1C). Decellularization was further validated on the milled collagen and dECM materials using DNA quantification, which revealed that all seven powdered materials contained less than 1 ng double stranded DNA (dsDNA) per 1 mg material (Figure S1, Supporting Information); well-below the 50 ng mg−1 standard proposed by Crapo et al.[41]
Figure 1.
Ultrastructure of decellularized tissues and resulting dECM powders. A) Optical images of H&E stained decellularized tissues sections from porcine (p) and bovine (b) sources. Remaining (decellularized) extracellular matrix appears pink/red, illustrating that ECM remains after the removal of cellular material from each tissue type. B) Scanning electron images of each decellularized tissue type prior to milling, highlighting the distinct, porous, and fibrous structures. C) Scanning electron images of some of the larger dECM powder pieces that result from milling and which are used for the tissue paper fabrication.
2.2. Tissue Paper Fabrication
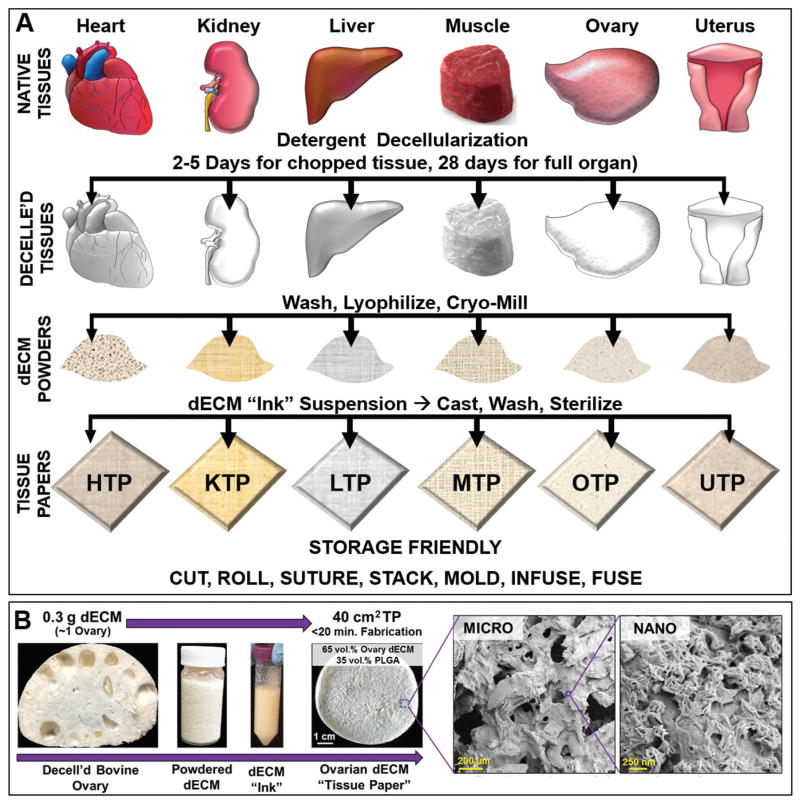
Six distinct TP types were fabricated from six tissue- and organ-specific dECM powders according to the scheme illustrated in Figure 2A. This process-conserved approach to biomaterial fabrication resulted in HTP, KTP, LTP, MTP, OTP, and UTP. As a control, commercially available purified bovine collagen flakes (Type I from Achilles tendon) were milled and also transformed into CTP. dECM powders (65 vol% by solids content) derived from these specific tissues and organs were suspended in a trisolvent mixture containing dichloromethane (DCM), 2-butoxyethanol (surfactant), and dibutyl phthalate (plasticizer) in addition to solubilized PLGA (82:18 lactic acid to glycolic acid; 35 vol% by solids), to create dECM “inks.” This procedure, along with the importance and function of each solvent, is an extension of a process we have previously described for synthesizing 3D-printable particle-laden inks,[34–38,42] thus the reasoning for referring to the resulting dECM suspensions as inks.
Figure 2.
Schematic representation of the tissue paper fabrication process. A) Fresh organs and tissues are collected, minced, decellularized, lyophilized, and milled to obtain dry tissue and organ-specific decellularized extracellular matrix (dECM) powders. In this particular case, whole bovine ovaries and uterus, rather than pieces, were decellularized following previously established protocols for these specific organs[24] prior to lyophilization and milling. The resulting dry powders are then used to manufacture dECM suspensions. The suspensions are cast into molds and allowed to dry for several minutes to yield tissue-specific dECM tissue papers: HTP = Cardiac (heart) tissue paper; KTP = Kidney tissue paper; LTP = Liver tissue paper; MTP = Muscle tissue paper; OTP = Ovary tissue paper; UTP = Uterus tissue paper. Other than decellularization time, the TP fabrication process is fully conserved across tissue and organ types. B) Representative example of the TP fabrication process using decellularized bovine ovaries. 300 mg of dECM yields enough powder to create a dECM ink capable of being cast into 40 cm2 worth of tissue paper. The associated images illustrate the micro- and nanostructures (scanning electron images) of this OTP.
The resulting dECM inks were immediately cast into flat-bottom containers and allowed to dry under ambient conditions (room temperature and atmospheric pressure). After ≈15 min, the DCM has fully evaporated, yielding ≈40 cm2 of porous, highly micro- and nanotextured tissue paper per 0.3 g of dECM (Figure 2B). Residual solvents are removed by washing the TPs three times in 70% ethanol for up to 1 h each followed by multiple rinses in sterile water prior to use. We have previously demonstrated that this washing process removes solvents, while simultaneously sterilizing materials comprised of powder, PLGA, and trisolvent mixture.[34] The ethanol rinse has the added benefit of solubilizing and removing any residual SDS from the material that may have carried over from the initial decellularization process.[43] Residual double stranded DNA and SDS quantifications were performed on the final, washed, and dried TPs before their use (Figure S1, Supporting Information). As expected, there was no significant change in dsDNA content between the dECM powders and the resulting, corresponding tissue papers. Additionally, SDS could not be detected in any measurable quantity in any of the seven tissue papers when compared against a standard curve of known SDS concentrations. We found that TPs could be utilized for in vitro use upward of one year (longest length of time tested) after initial fabrication, without observable changes in physical handling or cell response if stored in dry, cool environments (−20 °C).
2.3. Tissue Paper Microstructure and Topography
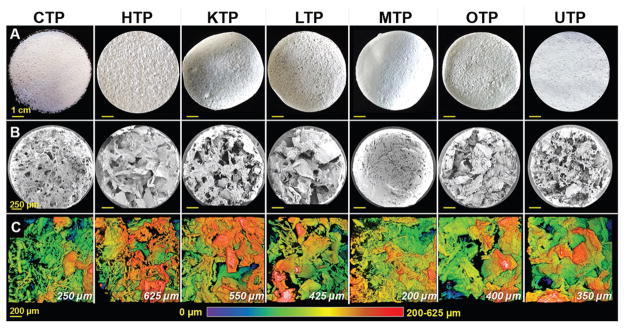
Despite the compositional (65% dECM and 35% PLGA by solids volume) and fabrication process uniformity, the resulting TPs exhibit distinct microstructures and topographies (Figure 3) and range in thickness from 200 to 625 μm (Figure 3C and Figure S2 (Supporting Information)). Macroscopically (Figure 3A), each TP type, including CTP, has unique tactile and topological characteristics. Closer examination of the tissue paper surfaces (Figure 3B and Figure S2 (Supporting Information)) reveal highly porous, nonplanar environments in each TP type. There are large variations and inhomogeneities of open versus solid structure that characterize the porosity of each TP type and ranges from <100 nm (Figure 2B) to several hundred micrometers (Figure 3B). Estimated porosity is calculated based on estimated theoretical solid density (based on PLGA and dECM content, which is dominated by structural proteins, such as collagens) and sample volume total porosities range from 61.7 ± 3.0% for CTP up to 88.0 ± 0.6% for HTP (Table 1). We hypothesize that the origin of this porosity range is derived from differences in tissue-specific dECM particle packing, which is not only influenced by dECM particle morphology, size, and size distribution, but also by differences in the degree of Coulombic attraction, or “self-adhesion” of dECM particles to each other. For example, it was observed that dECM powders derived from milled collagen and muscle tissues tended to aggregate much more (resulting in lower porosity) than dECM powders derived from the other tissues.
Figure 3.
Tissue paper microstructure and topography. A) Representative photographs of 7 cm diameter sheets of the eight types of TPs utilized in this work, including collagen tissue paper (CTP), derived from commercially available collagen powder. B) SEM images of the top surfaces of 2 mm diameter sections cut from each TP in (A), illustrating the distinct surface topographies of each tissue paper. C) Top-down view, topographical maps based on volumetric reconstructions of collagen distributions in each TP type obtained via confocal scanning laser microscopy and collagen’s inherent autofluorescence. The PLGA component of the TPs is not visible in the topographical maps. Topographical maps range in height from 0–200 to 0–625 μm depending on the tissue paper type (maximum height given in lower right of each map).
In addition to the structural characteristics being potentially advantageous for in vitro and in vivo use, the tissue papers, as mentioned previously, are comprised primarily of dECM (65 vol% by solids). Because it is difficult to fully distinguish dECM from PLGA using macroscopic imaging and even nano-to-micrometer scale scanning electron images (Figure 3B and Figure S2 (Supporting Information)), innate second harmonic autofluorescence generated by collagen[44] was utilized to volumetrically map the topography and structure of each tissue paper (Figure 3C). Figure 3C shows collagen, due to its autofluorescence, and does not depict the interconnected PLGA network, which does not substantially autofluoresce. These data illustrate the unique topographical characteristics of each TP type. Although photographs, scanning electron microscopy, or topographical fluorescence mapping reveal the micro- and mesostructures of the tissue papers, they do not elucidate the interaction between the dECM powder particles and the PLGA; we hypothesize, based on our previous work with related ink and 3D-printed systems,[24,34–38] that the PLGA forms a continuous matrix throughout the TP materials, physically encapsulating dECM particles, but not covalently bonding with them.
2.4. Mechanical and Physical Properties
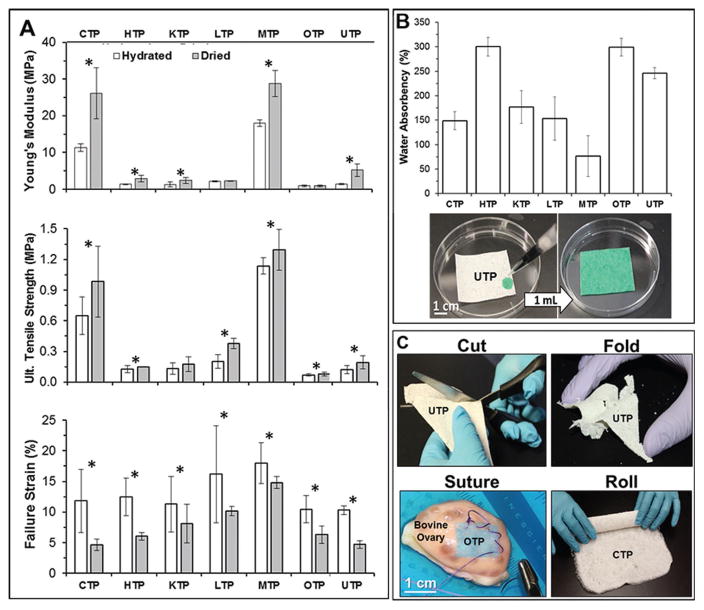
Although the tissue papers are composed primarily of spatially discrete dECM particles, they are mechanically robust, and exhibit mechanical characteristics that are comparatively analogous to the original tissue or organ types from which they are derived (i.e., liver is much less stiff than muscle). Under tensile loading (Figure 4A and Figure S3 (Supporting Information)), dry and hydrated TPs exhibit a range of elastic moduli (E), ultimate tensile strengths (UTS), and failure strains (F%), which are summarized for both dry and hydrated conditions in Table 1.
Figure 4.
Mechanical, physical, and handling properties of TPs. A) Tensile mechanical properties of hydrated and dry tissue papers. B) Percent water absorbency of each TP determined by normalized change in mass from dry mass and photographs illustrating example of liquid absorbance of UTP with green, aqueous food coloring. C) Photographs of tissue paper physical manipulations relevant to surgical implementation, including cutting, folding, rolling, and suturing. Tissue paper types are indicated in each photo. OTP was stained with alamar Blue prior to suturing to bovine ovary for the purposes of visual clarity. Bars represent mean ± standard deviation. * indicates p ≤ 0.05 significance between hydrated and dry tissue paper of the same type.
Elastic moduli of the dry TPs range from 0.93 ± 0.04 MPa for OTP up to 28.7 ± 3.5 MPa for MTP; UTS from 0.08 MPa (80 kPa) for OTP up to 1.3 MPa for MTP; and F% from 4.7 ± 1.0% for CTP up to 14.8 ± 1% for MTP. These moduli are several orders of magnitude greater than native soft tissues,[45] but fall within the same range of 100–101 MPa exhibited by previously described 3D-printed hydroxyapatite-, graphene-, metal-and metal oxide-, and Lunar- and Martian-regolith-simulant PLGA material systems produced using this general ink approach,[35,36,38,42] indicating that the mechanical load in the TPs is primarily carried by the elastomeric polymer matrix, PLGA. The variation in mechanical properties among TPs, however, is likely due to the mesostructural variation in porosity and dECM-particle/PLGA-matrix distribution within individual tissue paper types, rather than variations in the mechanical properties of the dECM itself, which do not play a significant role in carrying or transmitting mechanical loads.
TPs are able to rapidly absorb substantial volumes of liquid (Figure 4B), which is likely due to their porosity (Figure 3) and composition dominated by dECM (polar and charged). This ranges from 76 ± 40% for MTP up to ≈300% for both HTP and OTP. The ability to absorb large volumes of surrounding liquid media, while also maintaining structural integrity and mechanical properties, is an ideal biomaterial characteristic.[33,34] This not only indicates that the TPs could be pretreated with and serve as carriers for bioactive molecules such as growth factors, small molecules, and/or antibiotics prior to use, but that, once implanted, the materials will rapidly absorb and interact with native cells, plasma, and factors. When fully hydrated, which represents likely in vitro and in vivo environmental conditions, TPs experience a decrease in elastic moduli and UTS, and increase in F% (not statistically significantly different across TP types, except between UTP and MTP), but ultimately maintain their robust mechanical properties (Figure 4A and Table 1). Elastic moduli of hydrated TPs range from 0.90 ± 0.09 MPa for OTP up to 18.0 ± 1 MPa for MTP; UTS from 70 kPa for OTP up to 1130 kPa for MTP; and F% from 10.3 ± 0.7% for UTP up to 18.0 ± 3.3% for MTP. The elastic moduli and UTS of tissue papers are comparable with previously measured and reported mechanical properties of decellularized tissue and organs by Schleifenbaum et al.[46]
Due to the extensive variability of individual patients, their wounds, and their needs, surgical implementation of biomaterials of any kind, often requires that they be modified in some way immediately prior to or during surgery.[33–35] Like standard paper, the mechanical properties of the TPs permit them to be manipulated in numerous ways (Video S1 (Supporting Information) and Figure 4C), including cutting, folding, rolling, and suturing (in hydrated state).
2.5. In Vitro Evaluation with Human Mesenchymal Stem Cells
To probe the ability of human cells to adhere to, survive, and proliferate on the TPs, composed of porcine or bovine dECM, human mesenchymal stem cells (hMSCs; female) were seeded onto dry, 4-mm-diameter TP disks (punched from larger tissue paper pieces) and cultured in standard Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum for up to 28 d. Over the course of this experiment, there was no observable degradation or dimensional reduction in the tissue paper disks, which remained mechanically robust and easy to handle following the 28 d culture. This is in stark contrast to ECM-based hydrogels and films encapsulated or top-seeded with cells, which often undergo substantial contraction and “balling-up” due to the mechanical stresses from the cells overcoming the mechanical properties of the gels (moduli no greater than several kPa).[47] We hypothesize that despite intimate cellular interaction with dECM particles, the cellular contractile forces do not surpass the continuous, load bearing, and relatively stiff PLGA matrix, which also acts to mechanically separate individual dECM particles.
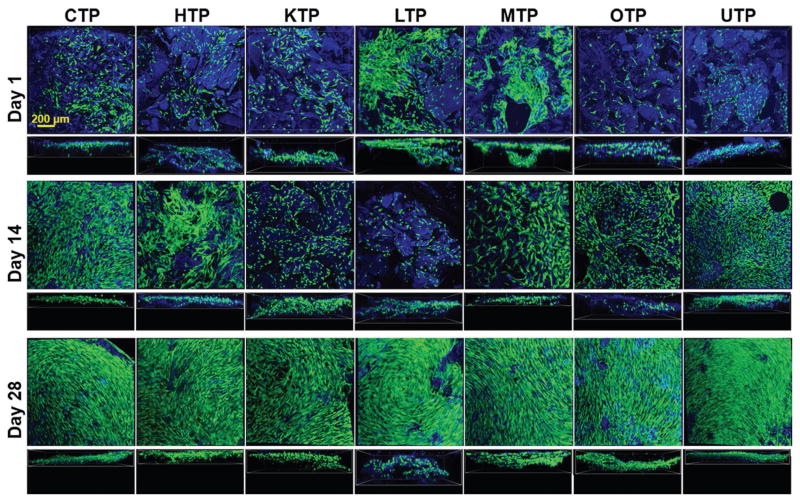
As can be seen in Figure 5, the hMSCs adhered to all TPs (blue = collagen autofluorescence) after initial seeding (day 1) and were viable (green). Distinct variations in cell morphologies across the tissue paper types became apparent after 14 d of culture on TPs (Figure 6 and Figure S4 (Supporting Information)). This indicates that the tissue-specific nature of the TPs is having an impact on cell morphology and behavior, which is in accordance with previously described observations of the effects of tissue-specific dECMs on cell behavior.[11] However, further comprehensive investigations will need to be conducted to determine the exact nature and influence of TP type on the phenotype of hMSCs as well as tissue specific cell types. By day 28 (Figure 5 and Figure S5 (Supporting Information)), the cells on all TPs had proliferated to confluently cover both the top seeded and underside of the TPs. These results indicate that the TPs support cell adhesion, proliferation, and migration over an extended period without contracting or degrading the TPs and that individual types of TPs appear to influence adult stem cell morphology and multicellular organization (Figure 5, day 14; Figure 6). Based on these results as well on previous in vivo results with analogous biomaterial systems, hyperelastic bone and 3D-graphene,[35] as well as ovarian dECM for inducing puberty in mice,[24] there is reason to believe that tissue papers will not elicit negative in vivo responses. However, future biocompatibility as well as indication-tissue-paper-specific studies, will need to be performed to validate this hypothesis.
Figure 5.
hMSC culture on TPs. hMSC adheres, proliferates, and infiltrates TPs. For each row, the top figures represent surface images of the cells cultured on the different TPs and the bottom (smaller) figures show stacked images displaying cellular infiltration into the materials. All images are fluorescent confocal reconstructions of live–dead stained hMSCs on tissue papers at 1, 14, and 28 d after initial seeding. Green, red, and blue represent live cells, dead cells, and tissue papers (collagen autofluorescence), respectively.
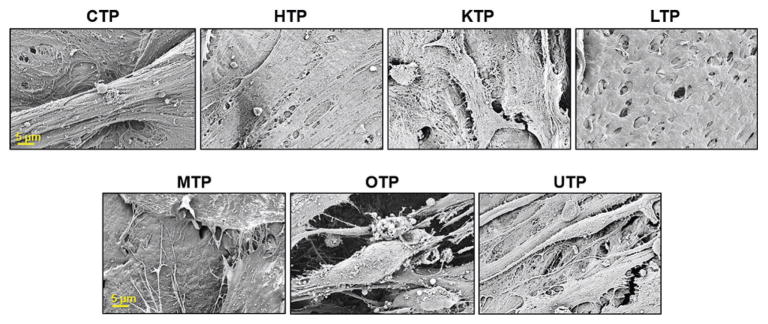
Figure 6.
Representative scanning electron images of hMSCs on tissue papers 14 d after initial seeding. Scale bars are consistent across the respective rows.
2.6. Ovarian Cortical Strip Culture
Although there are numerous applications for the range of TPs presented here, we elected to perform additional studies on OTP because its properties and characteristics align well with current clinical ovarian cortical strip preservation and have potential uses for subsequent transplantation of isolated follicles or strips.[48,49] These ovarian cortical strips can be isolated at all patient ages and contain the ovarian follicle reserve. Ovarian follicles require that the oocyte and granulosa cells remain connected through intracellular connections, as a spherical cell aggregate, in order to survive and function through folliculogenesis.[50] Because OTP contains pores, we sought to determine if the 3D nature of the pores would support the 3D structure of ovarian follicles in culture. Secondary mouse follicles were isolated and pipetted onto OTP in transwells. We identified that OTP can support mouse ovarian follicle attachment, survival, and maintain the spherical cell aggregate for at least 4 d (Figure S6, Supporting Information). These initial in vitro results are promising and require additional experimentation. However, follicles from human ovarian cortical tissue are challenging to isolate.[48] Additionally, several clinics offer ovarian tissue cryopreservation (tissue pieces that contain ovarian follicles), and autografts of this tissue into patients has led to over 60 births.[49] Surgical methods used in these cases include insertion of cortical strips in ovarian pockets and adherence of strips on the surface of the ovary. The OTP developed here could build on these methods to surgically implant individual follicles or can act as a patch or membrane that can secure cortical strips or a completely engineered organ transplant to any site. Therefore, we chose to continue investigating the ability of OTP to support follicles using whole human and rhesus macaque ovarian cortical strips instead of isolated follicles.
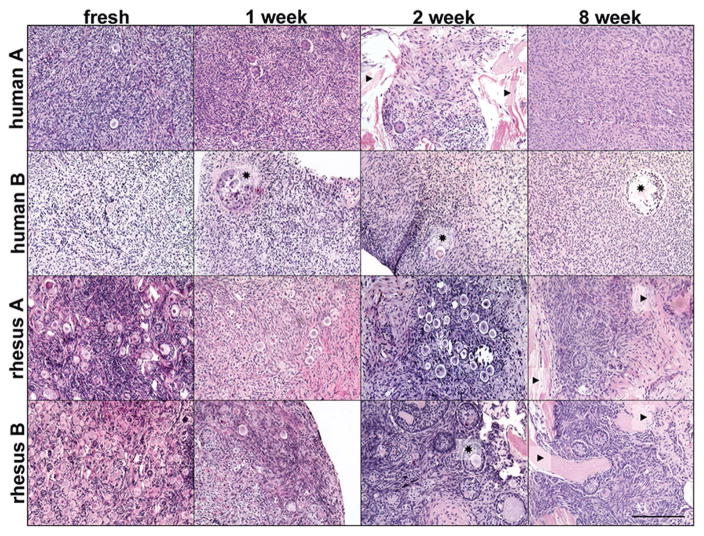
Previous experiments using human ovarian cortical strips were conducted by encapsulating the tissue in alginate.[48] The number of tissue pieces that contained live follicular cells and/or stromal cells declined over the duration of the culture beginning at two weeks (35 pieces containing cells out of 41 cultured) and ending at six weeks (7 pieces containing cells out of 15 cultured).[48] The experiments described here attempted to build on this analysis by culturing ovarian cortical strips with a nonencapsulating biomaterial and to determine if the length of in situ culture could be extended beyond six weeks. Ovarian cortex pieces from two human and two rhesus macaque tissues were processed into 0.5 mm tissue strips (Table S1, Supporting Information). Ovarian cortical strips were sliced and cut into ≈3–5 mm square pieces, similar to what is done for cryopreservation of ovarian cortical tissue.[51] The pieces were cultured on top of biopsy-punched OTP, which were then placed on a transwell and cultured for up to eight weeks (Figure S8A, Supporting Information). Histological analysis with hematoxylin and eosin (H&E) stained sections revealed healthy follicles, with centralized oocytes surrounded by granulosa cells, within healthy dense stroma after as long as eight weeks in culture and for all four organ samples (Figure 7 and Figure S8B (Supporting Information)). Oocytes were confirmed in the fresh or one, two, and eight week-cultured tissue by immunohistochemistry against the gamete-specific DEAD (Asp–Glu–Ala–Asp) box polypeptide 4 (DDX4, Figure S7, Supporting Information). Several sections also had ovarian tissue within the OTP and a few cells appear along strips of exposed OTP (Figures 7 and 8), indicating a potential interaction and integration of the ovarian cortical strip with the OTP. Primordial and small growing follicles were present in several sections after eight weeks of culture (Figure 8C,D) and each cortical strip cultured for eight weeks contained healthy cells (Figure S8B, Supporting Information), which supersedes our previous results of 46.7% tissue survival at six weeks in culture.[48] Additionally, the histological analyses at eight weeks demonstrate the presence of healthy follicles in tissue pieces from each of the four samples that could influence the longevity of a transplanted graft.
Figure 7.
Histological analysis of rhesus macaque and human ovarian cortical strip tissue cultured on OTP. Hematoxylin and eosin (H&E) stained sections of ovarian cortical tissue from two human and two rhesus macaque ovaries revealed multiple follicles within fresh tissue pieces and pieces cultured for one, two, and eight weeks. ★ = examples of multilayered growing follicles. ▶ = examples of OTP visible within tissue slice. Scale bar, 200 μm.
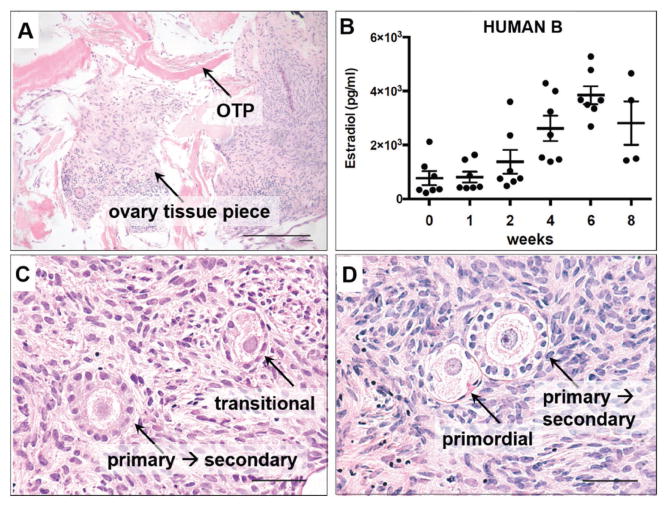
Figure 8.
Histological and secreted hormone analysis of human ovarian cortical strip tissue cultured on OTP. A) H&E stained sections of human ovarian cortical tissue cultured for two weeks on ovary tissue paper. Cells from the ovarian cortical tissue appear within the OTP pieces. B) The cortical strip pieces from human B were cultured on OTP for up to eight weeks. Estradiol was produced and secreted into the media over the course of this culture. Bars represent mean +/− SEM, n = 4 or 7 samples. C,D) H&E stained sections of human participant ovarian cortical tissue cultured for eight weeks. Ovarian follicles from multiple stages of differentiation are visible. Scale bar, (A,C,D) 50 μm.
Healthy growing ovarian follicles produce estradiol by a two-step process from cholesterol in the media. One step is performed by the theca cell, or outer follicle cell layer, that produces androstenedione. This steroid hormone is aromatized by granulosa cells, the cells that surround the oocyte, to produce estradiol, which is secreted and detectable in the media. Estradiol was detected in all media samples collected from four to seven separate ovarian cortical pieces on OTP from human B samples. Additionally, estradiol increased between one and six weeks in culture. There were fewer samples to analyze at the later time points as tissue was collected for histological analysis through the duration of the culture.
2.7. Scalability and Additional Versatility
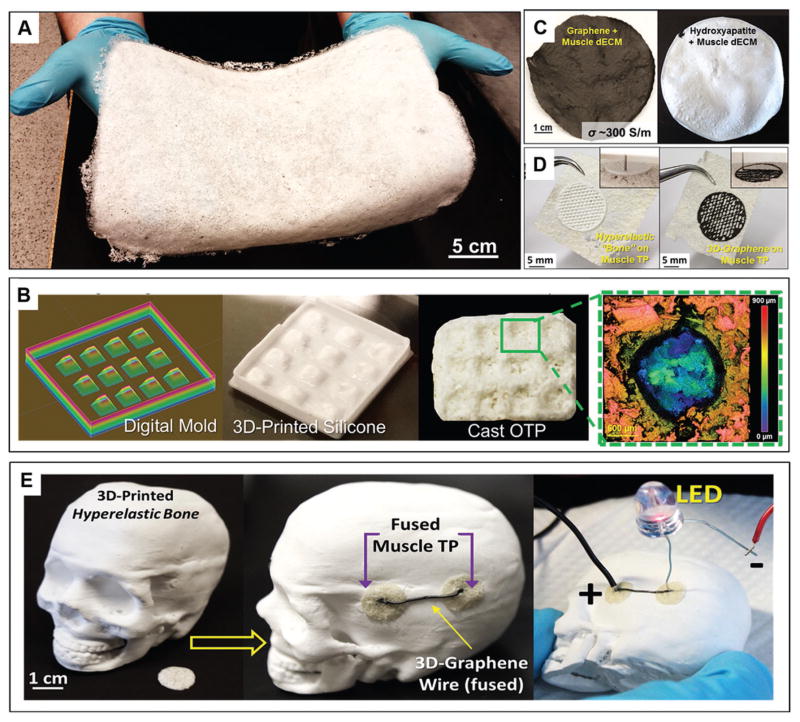
Due to the fact that TPs are fabricated using purely physical means through simple mixing, casting, and drying of components, the process is highly scalable, permitting large area sheets (Figure 9A; ≈800 cm2) to be rapidly fabricated. Additionally, dECM inks can be cast into molds to give the resulting tissue paper user-defined contours and geometric features (Figure 9B). dECM inks may also be modified or augmented with other natural or synthetic powders, such as hydroxyapatite or graphene, which we have previously demonstrated using this approach to create osteogenic[34] and neurogenic[35] biomaterials, respectively (Figure 9C). Finally, due to the compositional similarities between the TPs and previously described 3D-printable inks,[34–36] TPs can serve as 3D-printing substrates, resulting in complex biomaterial structures (Figure 9D and Video S2 (Supporting Information)). Extending this substrate approach, TPs can also be manually grafted to and integrated with 3D-printed materials derived from similar base ink formulations (Figure 9E), including hyperelastic “bone”[34,36] and 3D-Graphene,[35,36] enabling the ability to design and fabricate complex, multifunctional biomaterial constructs.
Figure 9.
Additional scalability and versatility of tissue papers. A) Photograph of 36 × 22 cm CTP sheet. B) Work flow for creating textured and nonplanar tissue papers via design and 3D-printing of silicone molds used for TP ink casting. This particular example illustrates the ability to create ovarian tissue papers with individual, cylindrical wells that are ≈1 mm deep (green inset of collagen topography map) for individual follicle culture. C) Photographs of hybrid, natural-synthetic TPs comprised of 1:1 by volume graphene and muscle dECM and 1:1 by volume hydroxyapatite and muscle dECM. D) Photographs of osteogenic hyperelastic bone[34] and neurogenic 3D-graphene[35] 3D-printed onto and fused to MTP pieces. Inset shows the 3D-printing in process. E) Photograph of a scaled down 3D-printed hyperelastic bone[34,36] human skull (from CT data) illustrating the ability to fuse TPs onto 3D-printed structures, and add additional materials, such as syringed extruded, conductive 3D-graphene[35,36] onto fused tissue papers.
2.8. Limitations
Although highly versatile, tissue papers do have a number of known limitations. These limitations include the inability to directly encapsulate cells into the materials during fabrication. This is due to the fact that tissue papers are prepared via an organic solvent route rather than an aqueous route, like many hydrogels. Additionally, because tissue papers solidify via evaporation mechanisms, dECM constructs greater than several millimeters thick could likely not be directly synthesized using this process; however, it would be possible to stack tissue paper sheets to create thicker constructs. Finally, like all dECM derived biomaterials, batch-to-batch variability must always be considered as tissue composition, structure, and quality vary from animal to animal and human to human.
3. Conclusions
We have illustrated here that this new, highly versatile approach for utilizing dECM can yield scalable, highly porous, mechanically robust, absorbent, pliable, and bioactive tissue- and organ-specific biomaterials. Specifically, we have demonstrated the ability to fabricate tissue papers from heart, kidney, liver, muscle, ovary, and uterus tissues, as well as from generic, purified collagen using a modified approach that we have previously demonstrated for creating a wide range of 3D-printable materials. These materials differed in porosity, topography, and mechanical properties, which were primarily maintained under hydrated conditions, as well as their impact on human mesenchymal stem cell morphology when cultured in vitro over the course of 28 d.
We further investigated an organ-specific, clinical application utilizing OTP to culture nonhuman primate and human ovarian cortical strips over eight weeks. The ovarian cortical strips could potentially be cultured on OTP in vitro to grow follicles into fertilizable eggs or used as a mechanism for transplanting the strips onto ovarian tissue, or orthotopic locations such as along the abdominal wall or subcutaneously in the forearm.[52] This success with relatively sensitive nonhuman primate and human ovarian follicles and tissues with OTP indicates promise for other, distinct TPs in tissue-specific functional applications.
The demonstrated success of this process to yield TPs across multiple tissue types, from two distinct species (porcine and bovine sources), combined with the tissue-specific success of the OTP culture with rhesus macaque and human ovarian tissues, strongly indicates that the tissue paper fabrication process could be extended to tissues and organs from other species, as well as tissues and organs not examined in this work, such as lung, pancreas, testes, prostate, bladder, peripheral and central nerves. Additionally, the process could likely be used to create tissue papers derived from specific organ regions, such as cortices or medullae, as well as diseased or cancerous tissues, or even mixed tissues. Moreover, TPs could potentially be augmented with antibiotics, growth factors, and other functional agents, or be extended toward creating acellular, 3D-printable constructs for complex tissue repair and regeneration and 3D in vitro culture models.
4. Experimental Section
Acquisition and Preparation of Native Tissues for Decellularization
Porcine kidney, liver, and heart organs and muscle tissue (tenderloin) were obtained fresh from a local, wholesale butcher (<12 h postmortem). Fascia, large vessels, fat, and fibrous connective tissues within the organs were removed using scalpels and discarded. Remaining tissues were cut using razor blades and scalpels into pieces no greater than 5 mm on any single side. Sectioned tissues were rinsed in water under constant agitation (stir plate) for up to 24 h to remove excess blood, with fresh water exchanges at 2–4 h intervals. Once no additional blood was observable in the water surrounding the tissues, the tissue pieces were placed in 1 L of 0.5% by weight SDS and agitated on a magnetic stir plate at room temperature. Every 3–4 h, the SDS solution was replaced with a fresh solution. This process continued until all tissue pieces had become translucent (1–2 d for kidney and liver, 4–5 d for heart and muscle). The tissue pieces were then rinsed in sterile water for up to 24 h under magnetic stir plate agitation to remove residual SDS. The water was replaced every 2–4 h for a total of three washes and time overnight. Several pieces of washed tissues were reserved for histology and scanning electron microscopy. Remaining tissue was lyophilized for 3 d (Labconco lyophilizer, USA).
Bovine ovaries and uterine horns were collected from Aurora Packing Company (Aurora, IL, USA) from young cows and transported to the lab in BoviPro Oocyte Holding Medium (1182/1210 USA). Upon arrival, the tissues were rinsed in fresh medium and the excess fat was removed. Whole ovaries and uterine pieces were incubated in 0.1% SDS for two to four weeks with agitation at 4 °C with frequent SDS solution changes, and monitoring until the material appeared white and the tissue overall appeared transparent. The tissues were rinsed in running reverse osmosis (RO) water and washed in water at 4 °C overnight with agitation, prior to lyophilization and milling into powders for use in ovary and uterus tissue paper fabrication.
Preparation of Decellularized Extracellular Matrix Powders
Lyophilized tissues were flash frozen in liquid nitrogen and mechanically milled until the resulting dECM powder fitted through a 200 μm mesh. These powders were stored at −20 °C until needed. Collagen flakes from bovine Achilles tendon (Sigma, USA) were milled and stored in a similar manner.
Tissue Paper Fabrication
Tissue papers were fabricated based on previously reported methods for making 3D-printable inks[24,34–38] and an estimated specific gravity of collagen (≈1.05 g cm−3), the primary component in the tissues. dECM powder was suspended in 2-butoxyethanol (Sigma, USA; 0.3 g per 0.35 g dECM), dibutyl phthalate (Sigma, USA; 0.15 g per 0.35 g dECM), and dichloromethane (Sigma, USA; 3 mL for every 0.35 g of dECM powder). Separately, PLGA (Evonik LLC, Germany; 82:18 poly-lacide to poly-glycolide; 0.3 g per 0.35 g dECM powder) was dissolved in dichloromethane (4 mL per 0.3 g PLGA). Once dissolved, the PLGA–DCM solution was combined with the dECM particle suspension and vortexed for several minutes to ensure complete mixing. The resulting ≈8 mL total volume dECM ink was rapidly poured into 7-cm-diameter aluminum, high-walled dishes and permitted to dry overnight in a fume hood. Large tissue papers were fabricated using the same process, but scaling the components and casting vessel accordingly. The resulting tissue papers were rinsed in ethanol for ≈2 h at 1 h wash intervals, followed by three to four 5 min rinses in sterile water. All washed tissue papers were permitted to dry overnight in a sterile cell culture hood prior to further analysis or use.
Porosity Estimation
Percent porosity for each tissue paper was determined by normalizing the calculated density with the theoretical solid density of the PLGA–dECM composites. Theoretical solid density was calculated based on 35 vol% PLGA (ρ = 1.15 g cm−3) and 65 vol% dECM (ρ = 1.05 g cm−3; estimation based on the specific gravity of collagen), and the estimated volume of the tissue paper sample. Estimated volume was based on the diameter of the samples (5 mm punches from larger 7 cm diameter TP sheets) and half-max-height thickness, due to the large variability in thickness across a single piece of TP (not flat). Samples (n = 5) of each TP type were massed. This measured mass was normalized by the theoretical solid mass (based on theoretical solid density and TP sample volume), multiplied by 100, and subtracted from unity.
Mechanical Testing and Analyses
A 20-mm-gauge-length tensile specimen, stainless steel stamp was used to cut tensile specimens out of the 7-cm-diameter circles. TPs were mechanically tested under tensile conditions in both dry and hydrated (soaked in water for 5 min, excess water shaken off prior to testing) forms (n = 4 for both dry and hydrated for each tissue paper) using a LF Plus Mechanical Tester (Lloyed Instruments LLC, USA), at a displacement rate of 2 mm min−1 until fracture. Elastic moduli were determined using the standard 0.2% offset approach. Ultimate tensile strength was determined as the highest stress value achieved for each samples. Failure strain was determined as the strain at which the sample fractured. All results were averaged and two-tailed, equal variance t-tests were used to determine significance between hydrated and dry mechanical properties for a given TP type.
Human Mesenchymal Stem Cell Seeding and Culture
Passage 2 female human bone marrow derived mesenchymal stem cells (Lonza, Walkersville, MD USA) were expanded up to passage 5 using MSC basal medium and proliferation kit (Lonza) according to the manufacturer’s instructions. Previously washed and dried 4-mm-diameter TP disks (n = 12 for each of seven TP types) were individually placed into wells in 48-well plates and each statically seeded with 15 000 hMSCs suspended in 5 μL media (1 × low glucose DMEM modified with 10% fetal bone serum), HEPES buffer, L-glutamine, and ten units antibiotic antimyotic (Invitrogen, USA). 200 μL of this medium was added to each well 30 min after initial seeding. All cell seeded samples were incubated at 37 °C in 5% CO2. Four samples from each TP type were removed at designated time points (1, 14, 28 d) for imaging.
Live–Dead Confocal Imaging
Samples reserved for confocal fluorescence imaging were first rinsed in sterile phosphate buffered saline (PBS) and incubated in a PBS solution of 5 × 10−3 M calcein AM and 5 × 10−3 M ethidium bromide homodimer (Invitrogen, USA) for 30 min prior to imaging. A Nikon C2+ fluorescent scanning confocal microscope (Nikon, USA) was used to obtain a series of images through the top ≈500 μm of each sample. Images were reconstructed into 3D representations using ImageJ software (NIH).
Collagen Topographical Mapping
4-mm-diameter tissue paper disks were submerged in PBS and kept at 4 °C overnight. A Nikon C2+ fluorescent scanning confocal microscope (Nikon, USA), with DAPI (4′,6-diamidino-2-phenylindole; 358 nm wavelength) laser intensity turned up to maximum, was used to obtain a series of images at 5-μm-depth intervals that were reconstructed into color-coded topographical maps using Nikon NIS Elements software.
Scanning Electron Microscopy
Samples without cells were prepared for scanning electron microscopy (SEM; LEO Gemini 1525) by freezing samples at −80 °C and sectioned using a scalpel followed by coating with 15 nm osmium metal via osmium plasma (Osmium Coater, SPI Supplies). Samples from in vitro studies were prepared directly from samples imaged with Live/Dead staining. Immediately after confocal fluorescence imaging, samples were fixed in a solution of 3 wt% sucrose (Sigma) and 2 wt% glutaraldehyde (Sigma) in sterile nanofiltered water for 30 min followed by graded ethanol washes up to 100%, after which the samples were critically point dried (Critical Point Dryer, Tousimis Samdri). Samples were then coated with 15 nm osmium prior to imaging (LEO Gemini 1525).
Ovarian Cortical Tissue Culture
Adult human ovarian tissue was obtained through the University of Pittsburgh Health Sciences Tissue Bank and the Center for Organ Recovery and Education (CORE) under the University of Pittsburgh IRB #0506140. Following the removal of the tissue, it was transported to the laboratory on ice in lactated Ringer’s solution. Rhesus macaque ovarian tissue from subjects KR99 and KI60 were received from the Tulane National Primate Center. The subjects were used for a low pathogenic shigella study. Human and rhesus ovaries were kept at 4 °C in sterile specimen cups (Covidien, 8889207026, USA) with Oocyte Handling Medium (Mofa, 19982/1210, USA) until used the next day and as performed previously.[1] Human and rhesus ovary culture media and short-term culture media were prepared in a sterile hood and filtered with a vacuum flask (Nalgene, 168-0045). Ovary culture media consisted of Waymouth’s medium (Gibco, 1120-035, USA) supplemented with 3 mg mL−1 human serum albumin (HSA, SAGE ART-3003), 0.5 mg mL−1 bovine fetuin (Sigma-Aldrich, F3385, USA), 5 μg mL−1 insulin, 5 μg mL−1 transferrin, 5 ng mL−1 selenium (ITS; Sigma, I1884-1vl), 64 μg mL−1 ascorbic acid (Sigma-Aldrich, 1043003, USA), and 10 mIU mL−1 follicle stimulating hormone (Abcam, ab51888, USA). Short-term culture media (dissection media to be used outside of the incubator) consisted of L15 (Gibco, 11415-064, USA) and 1% HSA. 24-well plates (Corning, 353047, USA) with 400 μL of ovary culture media in each well were prepared. Any empty wells were filled with 400 μL of Dulbecco’s PBS (DPBS; ThermoFisher Scientific, 14190-250, USA). These plates were equilibrated in the incubator while the tissue was processed. Ovarian tissue paper made on 10-31-15 was processed into culture pieces with 6-mm-diameter biopsy punches (Henry Schein, 900-4870, USA) and placed on 12 mm transwells (Millipore, PICM01250, USA) in the wells with media.
Each human and rhesus ovary was halved through the coronal plane and then cut into thirds. These pieces were held in Oocyte Handling Medium, flattened, and sliced. A Stadie Riggs tissue slicer was used to make 0.5 mm slices from the cortex as done previously.[1] The resultant cortex pieces were placed in dissection media for holding. After slicing the cortex pieces from the ovary, they were cut with a #10 scalpel (BD Bard-Parker, 371610, USA) into ≈4 mm × 4 mm pieces under the stereomicroscope. These pieces were placed into a 60 mm dish (Corning, 351007, USA) with dissection media. The pieces were observed under the light microscope, and those identified as containing follicles, were placed onto OTP in the 24-well plate prepared above. Follicles were identified as a “clearing” spot within the tissue that was perfectly circular and ranged from 150–200 mm.[1] Then, the pieces were transferred with forceps placed lightly on the corner edge of the tissue. 4 × 4 mm cortex pieces were fixed for the day 0 time point in 10% neutral buffered formalin (VWR, 95042-908, USA) rotating at 4 °C. Half of the culture medium (200 mL) was collected every other day and kept frozen at −20 °C.
Estradiol ELISAs
Estradiol enzyme-linked immunosorbent assay (ELISA) was used to measure secreted estradiol levels in the culture media. Representative media samples from each time point (week 0, 1, 2, 8) were analyzed with an Estradiol ELISA (Calbiotech, ES180S, USA, detectable range 10–1000 pg mL−1) and anti-Müllerian Hormone ELISA (Ansh Labs, AL-105, USA). Data were analyzed using Graphpad Prism 6 software and expressed as individual points with bars representing the average ± SEM. Data were not statistical significant when analyzed using an ordinary one-way analysis of variance (ANOVA) with α = 0.05.
Histological Analysis of OTP and Nonhuman Primate and Human Cortical Strip Cultures
After fixing cortex pieces overnight in 10% formalin, samples were dehydrated with an ethanol gradient of 50%, 60%, then 70% for 15 min each while rotating at room temperature. Serial sections of 5-μm-thickness and H&E staining were prepared by the Reproductive Science Histology Core (Northwestern University, USA). Every 20th section was stained with H&E using a Leica Autostainer XL (Leica Microsystems, Germany), and the health of each tissue piece was assessed at each time point (week 0, 1, 2, 8) using a Nikon E600 Microscope (USA).
Supplementary Material
Acknowledgments
The authors would like to acknowledge Kathrin Gassei (Magee-Womens Research Institute, University of Pittsburgh School of Medicine) for coordinating ovary tissue acquisition and distribution, which is funded by the Departments of Urology and of Obstetrics, Gynecology & Reproductive Sciences at the University of Pittsburgh School of Medicine (K.E.O.), and Michael McRaven and Thomas Hope for acquisition of rhesus macaque ovary tissue. The majority of experiments were performed at the Simpson Querrey Institute for BioNanotechnology at the Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Material Command, and the Northwestern University provided funding to develop this facility. The Northwestern University Center for Advanced Microscopy generously supported by the NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. The authors made use of the EPIC facility (NUANCE Center—Northwestern University) supported by the NSFDMR-1121262 and EEC-0118025|003; the Northwestern University Mouse Histology and Phenotyping Laboratory and Cancer Center supported by the NCI CA060553; the Office of Naval Research MURI Program (N00014-11-1-0690). Additional funding support was provided by a gift from Google (R.N.S.). This work was also supported by the Watkins Chair of Obstetrics and Gynecology (T.K.W.), and by the Center for Reproductive Health after Disease (P50 HD076188-02) from the National Centers for Translational Research in Reproduction and Infertility (NCTRI) (T.K.W.), A.E.J. was supported by a postdoctoral fellowship from The Hartwell Foundation. M.M.L. was supported by the Burroughs Wellcome Fund Career Award at the Scientific Interface.
Footnotes
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adfm.201700992.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Adam E. Jakus, Department of Materials Science and Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL 60208, USA. Simpson Querrey Institute for BioNanotechnology, Northwestern University, Chicago, IL 60611, USA
Prof. Monica M. Laronda, Division of Reproductive Science in Medicine, Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
Alexandra S. Rashedi, Division of Reproductive Science in Medicine, Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
Christina M. Robinson, Department of Materials Science and Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL 60208, USA. Simpson Querrey Institute for BioNanotechnology, Northwestern University, Chicago, IL 60611, USA
Chris Lee, Department of Materials Science and Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL 60208, USA. Simpson Querrey Institute for BioNanotechnology, Northwestern University, Chicago, IL 60611, USA.
Dr. Sumanas W. Jordan, Division of Plastic and Reconstructive Surgery, Department of Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Prof. Kyle E. Orwig, Department of Obstetrics, Gynecology and Reproductive Sciences and Magee-Womens Research Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA
Prof. Teresa K. Woodruff, Division of Reproductive Science in Medicine, Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
Prof. Ramille N. Shah, Department of Materials Science and Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL 60208, USA. Simpson Querrey Institute for BioNanotechnology, Northwestern University, Chicago, IL 60611, USA. Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL 60208, USA. Divsion of Organ Transplantation, Comprehensive Transplant Center, Department of Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
References
- 1.Swinehart IT, Badylak SF. Dev Dyn. 2016;245:351. doi: 10.1002/dvdy.24379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Adv Drug Delivery Rev. 2016;97:4. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Jakus AE, Baptista PM, Soker S, Soto-Gutierrez A, Abecassis MM, Shah RN, Wertheim JA. Stem Cells Transl Med. 2016;5:1257. doi: 10.5966/sctm.2015-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uzarski JS, Su J, Xie Y, Zhang ZJ, Ward HH, Wandinger-Ness A, Miller WM, Wertheim JA. J Visualized Exp. 2015;2015:1. doi: 10.3791/53271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, Soker S. Stem Cell Res Ther. 2015;6 doi: 10.1186/s13287-015-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teodori L, Costa A, Marzio R, Perniconi B, Coletti D, Adamo S, Gupta B, Tarnok A. Front Physiol. 2014;5:218. doi: 10.3389/fphys.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Aldekhayel SA, Sinno H, Gilardino MS. Plast Reconstr Surg. 2012;130:177. doi: 10.1097/PRS.0b013e318254b2dc. [DOI] [PubMed] [Google Scholar]; b) Chang M, Ahn SE, Baek S. J Cranio-Maxillofacial Surg. 2014;42:695. doi: 10.1016/j.jcms.2013.10.002. [DOI] [PubMed] [Google Scholar]; c) Agir H, Eren GG, Yasar EK. J Craniofacial Surg. 2015;26:1517. doi: 10.1097/SCS.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 8.Zelen CM, Serena TE, Denoziere G, Fetterolf DE. Int Wound J. 2013;10:502. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Muscle Nerve. 2009;39:787. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]; b) Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz E, Wisotsky SM, Cho MS, Wilson C, Cooper EO, Ingari JV, Safa B, Parrett BM, Buncke GM. Microsurgery. 2012;32:1. doi: 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- 10.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Adv Drug Delivery Rev. 2012;64:1063. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshiba T, Chen GP, Endo C, Maruyama H, Wakui M, Nemoto E, Kawazoe N, Tanaka M. Stem Cells Int. 2016;2016 doi: 10.1155/2016/6397820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshiba T, Tanaka M. Biochim Biophys Acta. 2016;1863:2749. doi: 10.1016/j.bbamcr.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 13.a) Kitahara H, Yagi H, Tajima K, Okamoto K, Yoshitake A, Aeba R, Kudo M, Kashima I, Kawaguchi S, Hirano A, Kasai M, Akamatsu Y, Oka H, Kitagawa Y, Shimizu H. Interact Cardiovasc Thorac Surg. 2016;22:571. doi: 10.1093/icvts/ivw022. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Biomaterials. 2009;30:5409. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner NJ, Badylak SF. Cell Tissue Res. 2012;347:759. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 15.a) Carriel V, Alaminos M, Garzon I, Campos A, Cornelissen M. Expert Rev Neurother. 2014;14:301. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]; b) Medberry CJ, Crapo PM, Siu BF, Carruthers CA, Wolf MT, Nagarkar SP, Agrawal V, Jones KE, Kelly J, Johnson SA, Velankar SS, Watkins SC, Modo M, Badylak SF. Biomaterials. 2013;34:1033. doi: 10.1016/j.biomaterials.2012.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Sudo R. Organogenesis. 2014;10:216. doi: 10.4161/org.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhou PC, Huang Y, Guo YB, Wang L, Ling CC, Guo QS, Wang Y, Zhu SJ, Fan XJ, Zhu MY, Huang H, Lu YH, Wang ZW. Artif Organs. 2016;40:E25. doi: 10.1111/aor.12645. [DOI] [PubMed] [Google Scholar]; c) Lewis PL, Shah RN. Curr Transplant Rep. 2016;3:100. [Google Scholar]
- 17.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang HB, Reing J, Gramignoli R, Komori J, Ross M, Nagaya M, Lagasse E, Stolz D, Strom SC, Fox IJ, Badylak SF. Tissue Eng, Part C. 2011;17:677. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Organogenesis. 2014;10:196. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, Hoffman AM, Weiss DJ. Respirology. 2013;18:895. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Stabler CT, Lecht S, Mondrinos MJ, Goulart E, Lazarovici P, Lelkes PI. Am J Physiol: Lung Cell Mol Physiol. 2015;309:L1273. doi: 10.1152/ajplung.00237.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AP, Godin LM, Domek A, Cotter T, D’Cunha J, Taylor DA, Panoskaltsis-Mortari A. Tissue Eng, Part C. 2015;21:94. doi: 10.1089/ten.tec.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Petrosyan A, Zanusso I, Lavarreda-Pearce M, Leslie S, Sedrakyan S, De Filippo RE, Orlando G, Da Sacco S, Perin L. Tissue Eng, Part B. 2016;22:183. doi: 10.1089/ten.TEB.2015.0368. [DOI] [PubMed] [Google Scholar]; b) Yamanaka S, Yokoo T. Stem Cells Int. 2015;2015 doi: 10.1155/2015/724047. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Abolbashari M, Agcaoili SM, Lee MK, Ko IK, Aboushwareb T, Jackson JD, Yoo JJ, Atala A. Acta Biomater. 2016;29:52. doi: 10.1016/j.actbio.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 21.a) Cheng CW, Solorio LD, Alsberg E. Biotechnol Adv. 2014;32:462. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Papadimitropoulos A, Scotti C, Bourgine P, Scherberich A, Martin I. Bone. 2015;70:66. doi: 10.1016/j.bone.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 22.a) Eweida AM, Marei MK. Biomed Res Int. 2015;2015 doi: 10.1155/2015/839694. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D’Amore A, Nagarkar SR, Velankar SS, Badylak SF. Biomaterials. 2012;33:7028. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aitken KJ, Bagli DJ. Nat Rev Urol. 2009;6:612. doi: 10.1038/nrurol.2009.202. [DOI] [PubMed] [Google Scholar]
- 24.Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Biomaterials. 2015;50:20. doi: 10.1016/j.biomaterials.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Filippo RE, Kornitzer BS, Yoo JJ, Atala A. J Tissue Eng Regener Med. 2015;9:257. doi: 10.1002/term.1647. [DOI] [PubMed] [Google Scholar]
- 26.Yagi H, Soto-Gutierrez A, Kitagawa Y. Surg Today. 2013;43:587. doi: 10.1007/s00595-012-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Grover GN, Rao N, Christman KL. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/1/014011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK. Prog Polym Sci. 2014;39:1973. [Google Scholar]; c) Koshy ST, Desai RM, Joly P, Li JY, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ. Adv Healthcare Mater. 2016;5:541. doi: 10.1002/adhm.201500757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beenken-Rothkopf LN, Karfeld-Sulzer LS, Davis NE, Forster R, Barron AE, Fontaine MJ. Ann Clin Lab Sci. 2013;43:111. [PubMed] [Google Scholar]
- 29.a) De Laporte L, Yan AL, Shea LD. Biomaterials. 2009;30:2361. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Faulk DM, Londono R, Wolf MT, Ranallo CA, Carruthers CA, Wildemann JD, Dearth CL, Badylak SF. Biomaterials. 2014;35:8585. doi: 10.1016/j.biomaterials.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pati F, Jang J, Ha DH, Kim SW, Rhie JW, Shim JH, Kim DH, Cho DW. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanton MM, Samitier J, Sanchez S. Lab Chip. 2015;15:3111. doi: 10.1039/c5lc90069g. [DOI] [PubMed] [Google Scholar]
- 32.Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN. Adv Mater. 2015;27:1607. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakus AE, Rutz AL, Shah RN. Biomed Mater. 2016;11 doi: 10.1088/1748-6041/11/1/014102. [DOI] [PubMed] [Google Scholar]
- 34.Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell S, Yun C, Koube KD, Yoo SC, Whiteley HE, Richter CP, Galiano RD, Hsu WK, Stock SR, Hsu EL, Shah RN. Sci Transl Med. 2016;8:358ra127. doi: 10.1126/scitranslmed.aaf7704. [DOI] [PubMed] [Google Scholar]
- 35.Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC, Shah RN. ACS Nano. 2015;9:4636. doi: 10.1021/acsnano.5b01179. [DOI] [PubMed] [Google Scholar]
- 36.Jakus AE, Shah RN. J Biomed Mater Res, Part A. 2017;105A:274. doi: 10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SL, Jakus AE, Shah RN, Dunand DC. Adv Eng Mater. 2017 [Google Scholar]
- 38.Jakus AE, Koube KD, Geisendorfer NR, Shah RN. Sci Rep. 2017;7:44931. doi: 10.1038/srep44931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badylak SF, Freytes DO, Gilbert TW. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Acta Biomater. 2017;49:1. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crapo PM, Gilbert TW, Badylak SF. Biomaterials. 2011;32:3233. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakus AE, Taylor SL, Geisendorfer NR, Dunand DC, Shah RN. Adv Funct Mater. 2015;25:6985. [Google Scholar]
- 43.Gratzer PF, Harrison RD, Woods T. Tissue Eng. 2006;12:2975. doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 44.Kruger A, Hovakimyan M, Ramirez DF, Stachs O, Lubatschowski H, Wree A, Guthoff R, Heisterkamp A. Klin Monatsblat Augenheilkd. 2009;226:970. doi: 10.1055/s-0028-1109918. [DOI] [PubMed] [Google Scholar]
- 45.Discher DE, Mooney DJ, Zandstra PW. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleifenbaum S, Prietzel T, Aust G, Boldt A, Fritsch S, Keil I, Koch H, Möbius R, Scheidt HA, Wagner MFX, Hammer N. PLoS One. 2016;11:e0151223. doi: 10.1371/journal.pone.0151223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smithmyer ME, Sawicki LA, Kloxin AM. Biomater Sci. 2014;2:634. doi: 10.1039/c3bm60319a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. J Assisted Reprod Genet. 2014;31:1013. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.a) Donnez J, Dolmans MM. J Assisted Reprod Genet. 2015;32:1167. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Salama M, Woodruff TK. Cancer Metastasis Rev. 2015;34:807. doi: 10.1007/s10555-015-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.a) Anderson E, Albertini DF. J Cell Biol. 1976;71:680. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Simon AM, Goodenough DA, Li E, Paul DL. Nature. 1997;385:525. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 51.a) Silber S, Kagawa N, Kuwayama M, Gosden R. Fertil Steril. 2010;94:2191. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]; b) Ting AY, Yeoman RR, Lawson MS, Zelinski MB. Hum Reprod. 2011;26:2461. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.a) Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. J Am Med Assoc. 2001;286:1490. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]; b) Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. Fertil Steril. 2003;80:193. doi: 10.1016/s0015-0282(03)00568-5. [DOI] [PubMed] [Google Scholar]; c von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Andersen CY. Eur J Cancer. 2009;45:1547. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.