Abstract
Increased life expectancy of persons living with HIV infection receiving antiretroviral therapy heightens the importance of preventing and treating chronic comorbidities such as cardiovascular disease. While guidelines have increasingly advocated more aggressive use of statins for low-density lipoprotein (LDL) cholesterol reduction, it is unclear whether people with HIV, especially women, are receiving statins when indicated, and whether their HIV disease is a factor in access. We assessed the cumulative incidence of statin use after an indication in the Women's Interagency HIV Study (WIHS), from 2000 to 2014. Additionally, we used weighted proportional hazards regression to estimate the effect of HIV serostatus on the time to initiation of a statin after an indication. Cumulative incidence of statin use 5 years after an indication was low: 38% in HIV-seropositive women and 30% in HIV-seronegative women. Compared to HIV-seronegative women, the weighted hazard ratio for initiation of a statin for HIV-seropositive women over 5 years was 0.94 [95% confidence interval (CI) 0.62, 1.43]. Applying the American College of Cardiology and the American Heart Association (ACC/AHA) guidelines increased the proportion of HIV-seropositive women with a statin indication from 16% to 45%. Clinicians treating HIV-seropositive women should consider more aggressive management of the dyslipidemia often found in this population.
Keywords: : human immunodeficiency virus, lipids, statins, hydroxymethylglutaryl-CoA reductase inhibitors, women's health, cardiovascular disease
Introduction
With the provision of effective antiretroviral therapy (ART), the management of HIV has shifted in part from preventing and treating opportunistic illnesses, to preventing and treating non-HIV-associated diseases associated with aging.1–12 Such non-HIV associated conditions have emerged as the leading causes of mortality among persons living with HIV, and among these, cardiovascular disease (CVD) has become a major health threat.1–5,11–16 In addition to lifestyle risk reduction, a cornerstone of CVD prevention is the reduction in atherogenic lipids, chiefly low-density lipoprotein (LDL) cholesterol, and 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG)-reductase inhibitors (i.e., statins) are very effective in lowering LDL cholesterol.17
Use of statins by people with HIV (PWH) has increased;18–21 yet it remains unclear whether these medications are prescribed equally to those with and without HIV infection when indicated—particularly among minority women. It has been reported that PWH are less likely to be prescribed preventive healthcare such as statins and aspirin for CVD prevention,22–24 and procedures after an acute myocardial infarction,25 but published studies have included relatively few women. Current guidelines do not differentiate indications for statin use by HIV status;17,26 yet HIV is recognized as a risk factor for CVD.27 To what extent women with HIV (WWH) experience barriers to preventive care for CVD is not well understood. Furthermore, despite more recent advances in simplifying ART regimens, PWH are often prescribed complex medication regimens, including multiple drugs for their HIV disease as well as any comorbid conditions.28–30 Polypharmacy in this population may be a concern for treating physicians, and may result in undertreatment of existing comorbid conditions such as dyslipidemia. Yet, very little research currently exists on the use of statins when indicated among WWH. Using data on 4607 women followed with semiannual visits for up to 14 years in the Women's Interagency HIV Study (WIHS), we evaluated the effect of HIV status on the use of statins among women with an indication for their use. We also described the use of statin therapy when indicated in this vulnerable population, and investigated the impact of the recent changes in national guidelines for statin initiation among WWH.
Materials and Methods
The WIHS is a multicenter, prospective cohort of WWH infection, and HIV-seronegative controls.31 Based in multiple urban sites across the United States, the WIHS began recruiting in 1994, and enrolled patients in three waves (1994–1995, 2001–2002, 2011–2012). Participants complete semiannual study visits, including an extensive interview on medication history, behavioral risk factors, and HIV-related outcomes. As part of a detailed medication history, participants are asked to bring in all prescribed medications, including concomitant medications such as statins, as well as report on all medications used since their last semiannual study visit. Blood has been drawn for HIV and non-HIV related lab values, including LDL cholesterol, since 2000. Our study period includes WIHS participants between 2000 and 2014. Institutional review boards at participating WIHS sites approve informed consent forms and study protocols, and all participants give written informed consent.
The population of interest included women without prior statin use who were followed for at least 1 year between October 2000 and December 2014, and who had an indication for the use of statin therapy based on the National Cholesterol Education Program's Adult Treatment Panel (ATP) III guidelines, and who had no prior reported history of statin use. Published in 2001 and last updated in 2004, the ATP III guidelines focus on risk factors for coronary heart disease and expanded the indication for statin initiation with an aim to lower LDL cholesterol values. The ATP III guidelines recommend statin use based on the presence of coronary heart disease or other atherosclerotic disease, diabetes mellitus, the Framingham 10-year risk score, and the current LDL cholesterol value. In 2013 a new set of guidelines for statin initiation was proposed by the American College of Cardiology and the American Heart Association (ACC/AHA).26 These new guidelines, which further expand the indication for statin initiation from ATP III, have been met with controversy.32
In the primary analysis, we used the ATP III guidelines to identify WIHS women with an indication for statin initiation, as these were the guidelines in use for the majority of the study period. However, we also applied the 2013 ACC/AHA guidelines to estimate the number of women who would have had an indication for statins if these guidelines had been in place during the study period. Our outcome of interest was the time to initiation of a statin after an indication according to ATP III guidelines. The exposure of interest was HIV serostatus, measured at each WIHS visit for HIV-seronegative participants. We excluded women who died or were lost to follow-up before October 2000, or were missing information on necessary covariates to determine an indication for a statin. We also excluded women without a full year of follow-up from the initial indication, women with reported statin use before the first known indication, and women who acquired HIV (due to small numbers) during the course of the study.
We calculated the unadjusted cumulative incidence of women in the cohort ever having an indication for a statin use, using an age time scale, when applying both the ATP III and the ACC/AHA treatment guidelines. Among women for whom statin therapy was indicated, we estimated the effect of HIV serostatus on the risk of statin initiation. We adjusted for confounding and loss to follow-up through the use of inverse probability weights, including weights for the probability of exposure and the probability of drop out.33 The final weights are a product of the exposure and drop out weights. We included age, race, education, smoking, hypertension, diabetes mellitus, baseline total cholesterol, baseline LDL cholesterol, baseline high-density lipoprotein (HDL) cholesterol, Framingham 10-year risk score, and insurance status as covariates. Cumulative incidence estimates were computed over the time since the statin indication.34 We used Cox proportional hazards regression to estimate hazard ratios and 95% confidence intervals (CIs). We report unadjusted models and models using inverse probability weights to account for baseline (measured at the indication) covariates and drop out from the cohort.34 We report 1-, 2-, and 5-year risk differences and risk ratios using the inverse-probability weighted cumulative mortality curves, and generated CIs using 200 bootstrapped resamples. To maintain flexibility in modeling nonlinear effects of continuous variables, we used restricted quadratic splines, with knots at the 5th, 35th, 65th, and 95th percentiles of the covariate distribution.35,36 Treatment effect heterogeneity by the Framingham risk score was explored through stratification of main effects. To identify characteristics of patients who did not receive statins when indicated, we also used conditional logistic regression to estimate odds ratios of not receiving a statin, using the same adjustment set as described above. We conducted all analyses using SAS Version 9.3 (Cary, NC).
Results
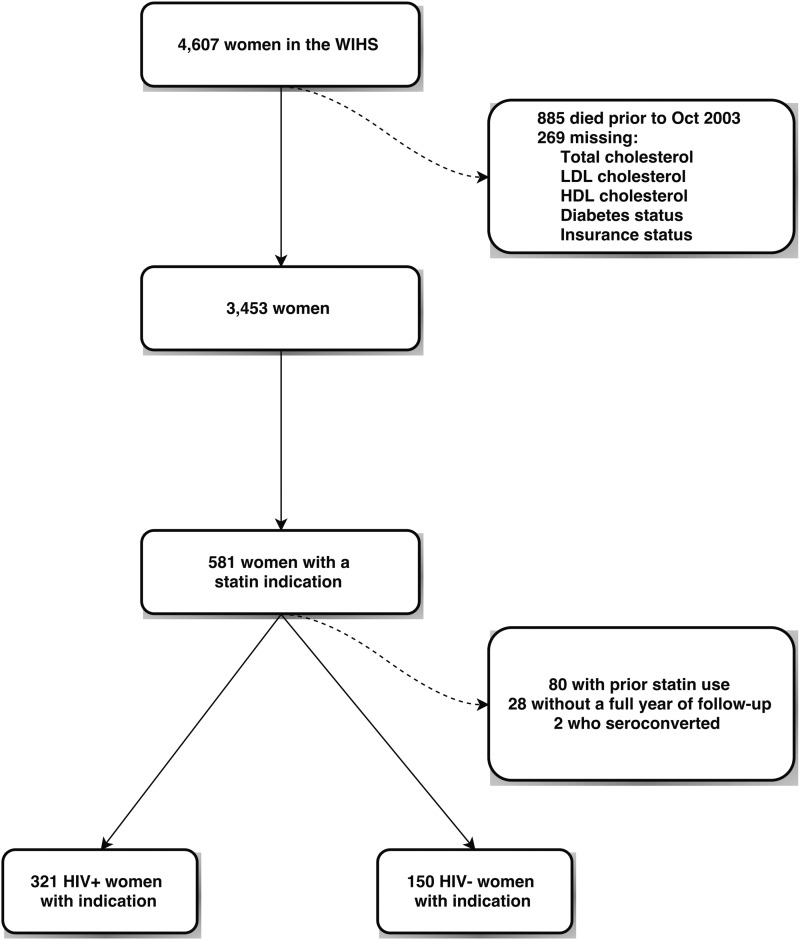
The final analysis cohort included 471 women with an indication for a statin; its construction is detailed in Fig. 1. From 4607 women in the WIHS cohort, we excluded 885 women who died or were lost to follow up before the start of the follow up period in October 2000. Two hundred sixty-nine women were missing information on total cholesterol, HDL cholesterol, LDL cholesterol, diabetes status, or insurance status and were excluded, leaving 3453 women. We then determined the number of women who had an indication for statin initiation using the ATP III guidelines; 581 had at least one indication. We excluded 28 women without a full year of follow-up, 2 women who seroconverted during the study, and 80 women who used a statin before their indication. Characteristics for these 471 women over a total of 1628 person-years are shown in Table 1. WWH comprised 68% of the women in our cohort. HIV-seropositive women were older on average and more likely to be white. Educational attainment was similar between the groups. LDL and HDL cholesterol were also relatively similar between the two groups. The median LDL cholesterol was 165 mg/dL among HIV-seropositive women and 160 mg/dL among HIV-seronegative women. Most (86%) WWH used ART, the median CD4 cell count was 490 cells/μL, and the median log10 HIV RNA viral load was 2.3 copies/mL, corresponding to an undetectable viral load.
FIG. 1.
Cohort flow diagram.
Table 1.
Characteristics of Women at the Indication for a Statin
| HIV-seropositive (N = 321) | HIV-seronegative (N = 150) | |||
|---|---|---|---|---|
| Characteristic | Median (IQR) | n (%) | Median (IQR) | n (%) |
| Age (years) | 45 (39, 50) | 43 (35, 48) | ||
| Race | ||||
| Black | 187 (58) | 92 (61) | ||
| White | 83 (26) | 23 (15) | ||
| Other | 51 (16) | 35 (23) | ||
| Education | ||||
| <12 years | 111 (35) | 55 (37) | ||
| 12 years | 94 (29) | 47 (31) | ||
| >12 years | 116 (36) | 48 (32) | ||
| Smoking | 142 (44) | 95 (63) | ||
| Hypertension | 156 (49) | 71 (47) | ||
| LDL cholesterol (mg/dL) | 165 (143, 190) | 160 (142, 178) | ||
| HDL cholesterol (mg/dL) | 45 (36, 55) | 46 (39, 55) | ||
| Triglycerides (mg/dL) | 160 (113, 224) | 130 (96, 177) | ||
| CD4 cell count | 490 (326, 682) | |||
| Log10 HIV RNA | 2.3 (2.3, 3.2) | |||
| ART use | 275 (86) | |||
| AIDS diagnosis | 140 (44) | |||
ART, antiretroviral therapy; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; RNA, ribonucleic acid.
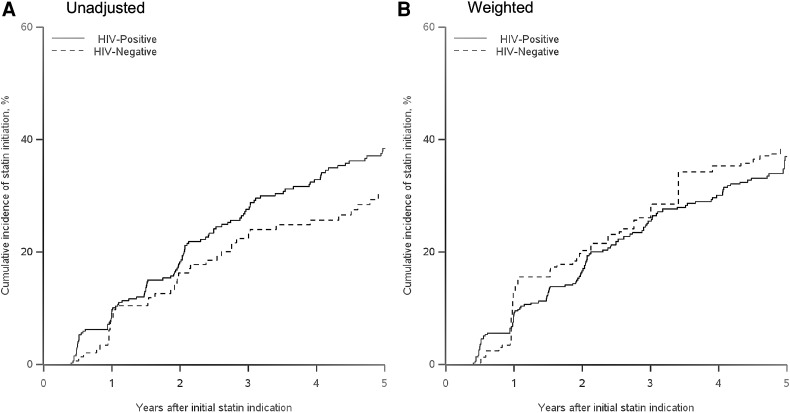
Within 5 years after an indication, 38% of WWH reported at least one episode of statin use, while 30% of HIV-seronegative women reported use. The unadjusted and inverse probability-weighted results for the effect of HIV status on time to statin initiation after an indication for statins are presented in Table 2. The unadjusted hazard ratio for HIV-seropositive women compared to HIV-seronegative women was 1.37 (95% CI 0.95, 1.97). After accounting for imbalances in baseline covariates and loss to follow-up, we observed essentially no difference in the uptake of statins, with a weighted hazard ratio of 0.94 (95% CI 0.62, 1.43). The weighted 1-year risk ratio was 0.73 (0.37, 1.76), and the 2-year risk ratio was 0.82 (0.49, 1.34). We also stratified by categories of the Framingham risk score (<10, ≥10 units) to examine heterogeneity in the effect of HIV on receipt of a statin. We again found little difference in the uptake of statins among WWH when stratified by high and low baseline Framingham risk score, although the precision of our results was limited by the small numbers of women with a Framingham score of at least 10 at baseline. We report unadjusted and inverse probability-weighted cumulative incidence of statin initiation curves in Fig. 2. While in the unadjusted curves there is an indication of an increase in statin initiation for HIV-seropositive women, in comparison to HIV-seronegative women, the weighted curves showed no difference between the two groups. There were two characteristics associated with a higher odds of not receiving a statin when indicated; women with total cholesterol less than 250 mg/dL and women younger than 40, with odds ratios of 3.02 (95% CI 1.65, 5.53) and 1.75 (95% CI 0.99, 3.11), respectively.
Table 2.
Effects of HIV on Statin Initiation Among Women with an Indication for Statin Use
| Indication | Statin initiators | Risk | RD (95% CI) | RR (95% CI) | HR (95% CI) | |
|---|---|---|---|---|---|---|
| 1 year risk | ||||||
| Unadjusted | ||||||
| HIV-seronegative | 150 | 12 | 0.08 | 0 | 1 | |
| HIV-seropositive | 321 | 31 | 0.10 | 0.01 (−0.04, 0.08) | 1.18 (0.66, 2.38) | |
| Weighteda | ||||||
| HIV-seronegative | 152 | 19 | 0.13 | 0 | 1 | |
| HIV-seropositive | 326 | 30 | 0.09 | −0.03 (−0.12, 0.05) | 0.73 (0.37, 1.76) | |
| 2 year risk | ||||||
| Unadjusted | ||||||
| HIV-seronegative | 150 | 23 | 0.16 | 0 | 1 | |
| HIV-seropositive | 321 | 56 | 0.18 | 0.02 (−0.07, 0.08) | 1.12 (0.68, 1.68) | |
| Weighteda | ||||||
| HIV-seronegative | 152 | 29 | 0.20 | 0 | 1 | |
| HIV-seropositive | 326 | 52 | 0.17 | −0.04 (−0.14, 0.05) | 0.82 (0.49, 1.34) | |
| 5 year risk | ||||||
| Unadjusted | ||||||
| HIV-seronegative | 150 | 40 | 0.30 | 0 | 1 | 1 |
| HIV-seropositive | 321 | 110 | 0.38 | 0.08 (−0.01, 0.17) | 1.27 (0.97, 1.74) | 1.37 (0.95, 1.97) |
| Weighteda | ||||||
| HIV-seronegative | 152 | 52 | 0.38 | 0 | 1 | 1 |
| HIV-seropositive | 326 | 104 | 0.37 | −0.01 (−0.14, 0.14) | 0.97 (0.72, 1.54) | 0.94 (0.62, 1.43) |
| 5 year risk, Stratified by Framingham Risk Scoreb | ||||||
| <10 | ||||||
| Unadjusted | ||||||
| HIV-seronegative | 112 | 27 | 0.28 | 0 | 1 | |
| HIV-seropositive | 253 | 87 | 0.38 | 0.10 (0.00, 0.20) | 1.36 (0.99, 2.02) | |
| Weighteda | ||||||
| HIV-seronegative | 106 | 31 | 0.33 | 0 | 1 | |
| HIV-seropositive | 249 | 79 | 0.36 | 0.03 (−0.10, 0.18) | 1.08 (0.78, 1.75) | |
| ≥10 | ||||||
| Unadjusted | ||||||
| HIV-seronegative | 38 | 13 | 0.37 | 0 | 1 | |
| HIV-seropositive | 68 | 23 | 0.41 | 0.04 (−0.18, 0.24) | 1.12 (0.64, 1.95) | |
| Weighteda | ||||||
| HIV-seronegative | 46 | 21 | 0.48 | 0 | 1 | |
| HIV-seropositive | 77 | 25 | 0.41 | −0.07 (−0.30, 0.22) | 0.86 (0.48, 1.91) | |
Weighted results are adjusted for baseline variables recorded at the first visit with an indication for a statin, and include age, race, smoking, hypertension, diabetes mellitus status, Framingham Risk Score category (<10 vs. ≥10), insurance status, total cholesterol, HDL cholesterol, LDL cholesterol, and education.
Results are stratified by the Framingham Risk Score (<10 vs. ≥10) at the initial statin indication.
CI, confidence interval; HR, hazard ratio; RD, risk difference; RR, risk ratio.
FIG. 2.
Cumulative incidence of statin initiation after an indication for statin use. (A) Unadjusted. (B) Weighted.
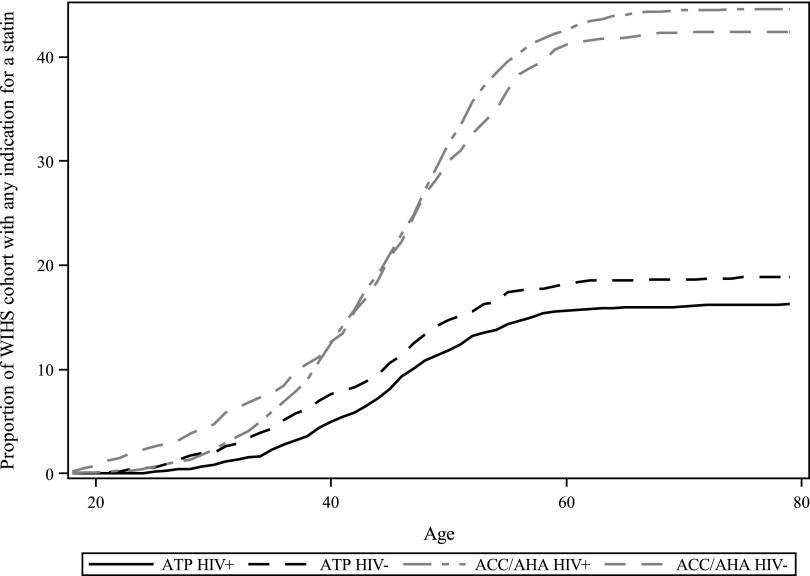
Applying the ACC/AHA guidelines for statin initiation to our population more than doubled the number of women with an indication for a statin. While 581 women had an indication with the ATP III guidelines (including women with less than a year of follow-up for actually initiating a statin), 1508 women had an indication with the ACC/AHA guidelines. The prevalence of HIV-seropositive women indicated for statin use by the ATP III guidelines was 16%, and increased to 45% with the ACC/AHA guidelines. Similar trends were seen among HIV-seronegative women. Figure 3 displays the proportion of women with an indication, as a function of age, when applying each set of guidelines. For both HIV-seropositive and seronegative women, the ACC/AHA guidelines dramatically increased the proportion of women with a statin indication.
FIG. 3.
Statin indication by age and changing guidelines.
Discussion
Among women with an indication for statin use, there was no difference in the uptake of statins among HIV-seropositive women compared to HIV-seronegative women, after adjustment for confounding by measured covariates. However, it is notable that the unadjusted cumulative incidence of reported statin use 5 years after an indication was relatively low: 38% in HIV-seropositive women and 30% in HIV-seronegative women. We also found a substantial increase in the proportion of women with a statin indication when applying the recent 2013 ACC/AHA guidelines, in comparison to the 2001 ATP III guidelines, with both HIV-seropositive (16% ATP III to 45% ACC/AHA) and HIV-seronegative (19–42%) women in our cohort.
Disparities in access to cardiovascular care may contribute to the observed underutilization of statins in our cohort, and more broadly, in cardiovascular care for WWH. Previous studies have identified PWH as being less likely to receive aspirin,22 lipid lowering therapy,23 and acute care procedures after an acute myocardial infarction.25 However, current reports on drug utilization are cross-sectional in nature and include populations that are overwhelmingly male. A strength of our contribution is the longitudinal nature of our design and follow-up, as well as a cohort containing exclusively women—a chronically understudied population in HIV research.
Statin use has continued to increase in the general US population, consistent with revised guidelines expanding their indications. Using data from the National Health and Nutrition Examination Survey from 2005 to 2010, the proportion of US adults eligible for statin treatment rose from 38%, using the ATP III guidelines, to 49% with the ACC/AHA guidelines.37 In the US Medicare population, statin use rose from 4% in 1992, to 41% in 2008.38 However, even in the general population, statins are prescribed less than would be expected based on the national guidelines. In a nationwide study of veterans with coronary heart disease in the Veterans Health Administration, only 58% of women and 65% of men were prescribed a statin, despite their recommended use in such patients under both sets of guidelines.39 Our results, in both the HIV-seropositive and seronegative groups, indicate a much lower uptake of statins, and a potential need for intervention in this population. The results in the HIV-seronegative group suggest that socioeconomic factors may also contribute to the lack of use of statins in this population.
Two recent reports highlight the potential for the ACC/AHA guidelines to underestimate atherosclerotic CVD risk in PWH.40,41 While Zanni et al. found a 2.5-fold increase in the proportion of patients indicated for a statin when applying the ACC/AHA guidelines versus the ATP III guidelines, they also reported that 74% of patients with high risk morphology coronary plaques would not be indicated for statin use in their cohort, suggesting that current recommendations may be missing an important group of patients with clinically significant lesions. We found similar results in our national multicenter cohort of women; the increase was even higher (∼2.8-fold increase in the proportion indicated for a statin) in HIV-seropositive women in our cohort. Our results, in the context of similar results from Zanni et al., suggest that the implementation of new guidance will likely result in a substantially more aggressive treatment paradigm for dyslipidemia—but that a substantial proportion of women at risk may remain untreated.
HIV specialists are increasingly responsible for management of chronic comorbidities such as CVD in their patients. In one recent study, there was no difference in the proportion of patients with access to lipid screening between providers with more experience treating PWH and those with less experience.42 Issues of increased cardiovascular risk among HIV patients, furthermore, are not restricted to the United States or resource-rich countries, but are also increasingly important in other contexts, including sub-Saharan Africa.43
Risk assessment for CVD drives much of the use of statins, but in the setting of HIV infection, there is also increasing interest in the use of these agents for their potential anti-inflammatory properties, given reports of heightened immune activation, inflammation,44 and rates of CVD in those living with HIV.1–5,11–16 As additional evidence for the benefits of statins accumulates, it can be expected that their use in PWH will grow. While the potential for these benefits will likely continue to drive increased statin use in WWH, treatment decisions must also take into account the importance of maintaining adherence to ART in an aging HIV-seropositive population likely to have continued exposure to increasing numbers of medications.28–30 Balancing these competing considerations will be essential for clinicians deciding on treatment plans for their patients.
Our study has limitations. First, statin use was self-reported; we did not have access to claims data to verify prescriptions, or biomarkers to verify use. It is possible that with access to electronic medical records or prescription claims data, the estimated incidence of new statin use could have been higher. Second, statin indications are not entirely fixed covariates; it is possible that patients may lower their cardiovascular risk through behaviors such as changes in diet and smoking habits, or HIV-specific factors such as ART. Third, the determination of an indication for a statin requires information on many different conditions, including smoking, diabetes mellitus, the presence of coronary heart disease, and several other factors. Misclassification of any of these conditions may affect the construction of our indication, and thus our analysis cohort. However, because any one condition only has a small effect on determining the indication, we expect this issue to be of limited importance. Finally, there was limited precision in our estimates of the effect of HIV status on statin use among those with an indication. However, the completeness of WIHS data collection, including ascertainment of comorbidities and comorbid medication use, is a strength.
In conclusion, in a cohort with extensive longitudinal clinical information and laboratory values necessary to construct an indication for statin initiation, statin use among women meeting criteria for this intervention was low with less than 40% reporting use of statins within 5 years of an indication. There was no difference in the uptake of statins among HIV-seropositive women in comparison to HIV-seronegative women. These findings indicate a possible target for intervention in this vulnerable population, and highlight the need for the development and implementation of strategies to optimize preventive healthcare for women, both with and without HIV, like those participating in the WIHS. Clinicians treating WWH should consider more aggressive management of the dyslipidemia often found in this population. For HIV-seropositive women, who may be at heightened risk for CVD, the stakes may be higher, and approaches to applying the newer ACC/AHA guidelines that expand the indication for statin therapy, should be integrated into their primary and HIV care.
Acknowledgments
Data in this article were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (M.C. and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (A.A.), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (S.G. and Elizabeth Golub), U01-AI-042590; Southern California WIHS (J.M.), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). This work was supported by R01AI100654 and P30AI050410 (CFAR) from the National Institutes of Health. These grants are located in Chapel Hill, NC.
Author Disclosure Statement
M.J.F. is a member of the Scientific Steering Committee (SSC) for a postapproval safety study funded by GlaxoSmithKline, and receives salary support through a contract with AstraZeneca. G.B. has research support from Amgen, Inc., Bristol-Myers Squibb, and has consulted for Definicare, LLC. All other authors have no potential conflicts of interest to disclose.
References
- 1.Dubé MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: Recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult. Clin Infect Dis 2003;37:613–627 [DOI] [PubMed] [Google Scholar]
- 2.Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep 2012;9:206–217 [DOI] [PubMed] [Google Scholar]
- 3.Wohl DA, McComsey G, Tebas P, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis 2006;43:645–653 [DOI] [PubMed] [Google Scholar]
- 4.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: Time for a better understanding. J Am Coll Cardiol 2013;61:511–523 [DOI] [PubMed] [Google Scholar]
- 5.O'Halloran JA, Satchell CS, Mallon PWG. Dyslipidemia, atherosclerosis and cardiovascular disease: An increasingly important triad in an aging population living with HIV. Future Virol 2013;8:1021–1034 [Google Scholar]
- 6.Behrens G, Maserati R, Rieger A, et al. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: Effect on lipid profiles. Antivir Ther 2012;17:1011–1020 [DOI] [PubMed] [Google Scholar]
- 7.Stein JH, Komarow L, Cotter BR, et al. Lipoprotein changes in HIV-infected antiretroviral-naïve individuals after starting antiretroviral therapy: ACTG Study A5152s. J Clin Lipidol 2008;2:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddler SA, Li X, Chu H, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med 2007;8:280–287 [DOI] [PubMed] [Google Scholar]
- 9.Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 2011;25:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—Association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–1193 [DOI] [PubMed] [Google Scholar]
- 11.Hanna DB, Jung M, Xue X, et al. Trends in Nonlipid Cardiovascular Disease Risk Factor Management in the Women's Interagency HIV Study and Association with Adherence to Antiretroviral Therapy. AIDS Patient Care STDS 2016;30:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015;61:640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 2006;145:397–406 [DOI] [PubMed] [Google Scholar]
- 14.The Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010;50:1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24:1537–1548 [DOI] [PubMed] [Google Scholar]
- 16.CASCADE Collaboration. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS 2006;20:741–749 [DOI] [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 18.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 2002;16:569–577 [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg DA. Cholesterol lowering in the management of coronary artery disease: The clinical implications of recent trials. Am J Med 1998;104:2S–5S [DOI] [PubMed] [Google Scholar]
- 20.Penzak SR, Chuck SK, Stajich GV. Safety and efficacy of HMG-CoA reductase inhibitors for treatment of hyperlipidemia in patients with HIV infection. Pharmacotherapy 2000;20:1066–1071 [DOI] [PubMed] [Google Scholar]
- 21.Henry K, Melroe H, Huebesch J, et al. Atorvastatin and gemfibrozil for protease-inhibitor-related lipid abnormalities. Lancet 1998;352:1031–1032 [DOI] [PubMed] [Google Scholar]
- 22.Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis 2012;55:1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freiberg MS, Leaf DA, Goulet JL, et al. The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med 2009;24:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park TE, Yusuff J, Sharma R. Use of aspirin and statins for the primary prevention of myocardial infarction and stroke in patients with human immunodeficiency virus infection. Int J STD AIDS 2016;27:447–452 [DOI] [PubMed] [Google Scholar]
- 25.Pearce D, Ani C, Espinosa-Silva Y, et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol 2012;110:1078–1084 [DOI] [PubMed] [Google Scholar]
- 26.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;June 24;129(25 Suppl 2):S1–S45 [DOI] [PubMed] [Google Scholar]
- 27.Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 2. J Clin Lipidol 2015;9:S1–S122 [DOI] [PubMed] [Google Scholar]
- 28.Tseng A, Szadkowski L, Walmsley S, et al. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother 2013;47:1429–1439 [DOI] [PubMed] [Google Scholar]
- 29.Holtzman C, Armon C, Tedaldi E, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013;28:1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelman EJ, Gordon KS, Glover J, et al. The next therapeutic challenge in HIV: Polypharmacy. Drugs Aging 2013;30:613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Agostino RB, Ansell BJ, Mora S, et al. The guidelines battle on starting statins. N Engl J Med 2014;370:1652–1658 [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–686 [DOI] [PubMed] [Google Scholar]
- 34.Cole SR, Hudgens MG, Brookhart MA, et al. Risk. Am J Epidemiol 2015;181:246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe CJ, Cole SR, Westreich DJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology 2011;22:874–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrell FE., Jr DASPLINE Macro. 2015. Available at: http://biostat.mc.vanderbilt.edu/twiki/pub/Main/SasMacros/survrisk.txt (Last accessed October1, 2016)
- 37.Pencina MJ, Navar-Boggan AM, D'Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–1431 [DOI] [PubMed] [Google Scholar]
- 38.Fang MC, Coca Perraillon M, Ghosh K, et al. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. Am J Med 2014;127:608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol 2015;115:21–26 [DOI] [PubMed] [Google Scholar]
- 40.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS 2014;28:2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clement ME, Park LP, Navar AM, et al. Statin utilization and recommendations among HIV- and HCV-infected veterans: A cohort study. Clin Infect Dis 2016;63:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landovitz RJ, Desmond KA, Gildner JL, Leibowitz AA. Quality of care for HIV/AIDS and for primary prevention by HIV specialists and nonspecialists. AIDS Patient Care STDS 2016;30:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in Southwestern Uganda. AIDS Patient Care STDS 2015;30:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longenecker CT, Hileman CO, Funderburg NT, et al. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: The SATURN-HIV trial. Clin Infect Dis 2014;59:1148–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]