Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency worldwide. Detection of heterozygously deficient females can be difficult as residual activity in G6PD-sufficient red blood cells (RBCs) can mask deficiency. In this study, we compared accuracy of 4 methods for detection of G6PD deficiency in females. Blood samples from females more than 3 months of age were used for spectrophotometric measurement of G6PD activity and for determination of the percentage G6PD-negative RBCs by cytofluorometry. An additional sample from females suspected to have G6PD deficiency based on the spectrophotometric G6PD activity was used for measuring chromate inhibition and sequencing of the G6PD gene. Of 165 included females, 114 were suspected to have heterozygous deficiency. From 75 females, an extra sample was obtained. In this group, mutation analysis detected 27 heterozygously deficient females. The sensitivity of spectrophotometry, cytofluorometry, and chromate inhibition was calculated to be 0.52 (confidence interval [CI]: 0.32–0.71), 0.85 (CI: 0.66–0.96), and 0.96 (CI: 0.71–1.00, respectively, and the specificity was 1.00 (CI: 0.93–1.00), 0.88 (CI: 0.75–0.95), and 0.98 (CI: 0.89–1.00), respectively. Heterozygously G6PD-deficient females with a larger percentage of G6PD-sufficient RBCs are missed by routine methods measuring total G6PD activity. However, the majority of these females can be detected with both chromate inhibition and cytofluorometry.
Keywords: chrome inhibition, cytofluorometry, females, gene sequencing, glucose-6-phosphate dehydrogenase deficiency, spectrophotometry
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) is the key enzyme in the oxidative pentose phosphate pathway. In this pathway, NADP+ is converted into its reduced form NADPH, which is essential for protection against reactive oxygen species in red blood cells (RBCs).1 G6PD deficiency is the most common enzyme deficiency, and worldwide an estimated 300 to 400 million people carry at least one deficient G6PD gene.2,3 G6PD deficiency has been suggested to protect against severe malaria infection4–6 and is mainly found in Africa, Oceania, Asia, and Mediterranean Europe, areas where malaria is, or has been, endemic.2,7
G6PD deficiency can lead to acute hemolytic anemia, favism, hemolysis, and hyperbilirubinemia resulting in neonatal kernicterus.8 In daily life, G6PD deficiency is usually clinically silent because only 1–2% of the total NADPH production capacity is needed in RBCs.9 G6PD deficiency can cause acute hemolytic anemia when stress is induced by medications such as ciprofloxacin, glibenclamide, and nitrofurantoin, consumption of fava beans, or during infection.10,11
G6PD deficiency is linked to the X-chromosome. By random inactivation of one of the two X-chromosomes in heterozygote females, any ratio of G6PD-sufficient and G6PD-deficient RBCs can be produced (lyonization). In clinical terms, the phenotype is more severe with a larger fraction of G6PD-deficient cells.12 Diagnosing G6PD deficiency in heterozygous women with methods based on measuring the total RBC G6PD activity lacks sensitivity for females with only a small fraction of G6PD-deficient RBCs.13,14
Some diagnostic laboratories use a test based on the inhibitory effect of chromate on glutathione reductase (GR) activity. Inhibition only occurs in the presence of NADPH. As G6PD-deficient RBCs produce less NADPH, the loss of inhibition of GR activity by chromate is a measure for the amount of G6PD-deficient cells. The sensitivity for detecting heterozygously deficient females with chromate inhibition is superior to measuring total G6PD activity. However, the test cannot be used to detect heterozygous G6PD deficiency in females with a small population of G6PD-deficient RBCs.15 These women are less at risk for clinically relevant hemolysis, but they may pass the defect to their children.
Until recently, the methods that most reliably identify heterozygously deficient females were the cytochemical assay16 and mutation analysis.14 Despite major improvements in labor and costs of mutation analysis, it is still a demanding procedure for most laboratories as more than 140 mutations have been identified.7 The cytochemical assay is time-consuming and technically difficult. The assay can also miss females with a small population of G6PD-deficient cells as it is based on measurement of G6PD enzyme activity in individual RBCs.17,18 Shah et al. recently developed a relatively simple cytofluorometric assay that appeared to have a high sensitivity for G6PD deficiency and especially for detecting heterozygous females.19
In the present study, we compare the test characteristics of the cytofluorometric assay with the spectrophotometric enzyme activity assay, with and without addition of chromate, and sequencing of the G6PD gene for the detection of heterozygously G6PD-deficient females.
Materials and Methods
Sample Collection
The Laboratory for Red Blood Cell Diagnostics at Sanquin routinely determines G6PD activity in RBCs of patients suspected to have G6PD deficiency. From March 1, 2015 until September 30, 2016, all blood samples from female patients more than 3 months of age that were screened for heterozygous G6PD deficiency were used in parallel to validate the cytofluorometric assay as developed by Shah et al.19 Patients younger than 3 months were excluded as these patients have other reference intervals for detection of G6PD deficiency. Venous samples were drawn at least 3 months after a suspected episode of hemolysis. The blood was collected in vacuum tubes coated with EDTA and sent to the laboratory at 4C where the RBCs were washed 3 times and stored in saline containing adenine, glucose, and mannitol until analysis. Analyses were performed once a week and included spectrophotometric measurement of RBC enzyme activities, chromate inhibition test, cytofluorometry, and G6PD gene sequencing. Red cell indices and reticulocyte counts were not available for this study.
Spectrophotometric Measurement of RBC Enzyme Activities
The activities of G6PD, pyruvate kinase (PK), and GR were measured spectrophotometrically in hemolysates according to the method of Zurcher.20 A detailed description has been published earlier by our group.15 In short, RBCs were washed with 154 mmol/l NaCl. The equivalent of 1 μl packed cells were added to a microtiter plate containing 50 μl buffer consisting of 48 mmol/l Tris-HCl, 17 mmol/l MgCl2, 6.7 mmol/l EDTA, 80 mmol/l KC1, and 0.02% (w/v) saponin 137, pH 7.5. The reaction to determine G6PD activity was started by the addition of 50 μl 5.3 mmol/l glucose-6-phosphate (G6P; Roche, Basel, Switzerland) and 50 μl 0.35 mmol/l NADP+ (Roche). The reaction to determine GR activity was started by the addition of 50 μl 5.3 mmol/l oxidized glutathione (GSSG; Sigma-Aldrich, St. Louis, Missouri) and 50 μl 0.4 mmol/l NADPH (Roche). The change in absorbance of the reaction mixtures was measured at 340 nm on an EON microtiter plate spectrophotometer (BioTek, Winooksi, Vermont) during a 30 min incubation period at 30C. Afterward, the absorbance at 540 nm was read to determine the hemoglobin concentration. Activities of G6PD and GR were expressed in international units per gram hemoglobin (IU/g Hb). The reference interval for G6PD activity for individuals older than 3 months was 3.8 to 5.9 IU/g Hb. The reference interval for the G6PD:GR activity ratio was 0.9 to 1.31. A G6PD:GR activity ratio of 0.46 to 0.9 was considered to be an indication of heterozygous G6PD deficiency, and a ratio of <0.46 was interpreted as G6PD deficiency (Table 1). Patients were considered to be G6PD deficient when both G6PD activity and G6PD:GR activity ratio were below the reference threshold.
Table 1.
Baseline Characteristics and Test Result of Patients Categorized on the Basis of the G6PD:GR Ratio.
| Females |
||||
|---|---|---|---|---|
| Ratio <0.46 mean (SD) |
Ratio 0.46–0.9 mean (SD) |
Ratio >0.9 mean (SD) |
Cutoff Points for Normal Values | |
| Number | 3 | 62 | 10 | |
| Age (years) | 58 (12) | 41 (27) | 46 (25) | |
| G6PD activity (IU/g Hb) | 2.23 (0.38) | 4.39 (0.73) | 4.80 (0.49) | >3.8 IU/g Hb |
| Inhibited GR activity in the presence of chromate (IU/g Hb) | 4.30 (0.61) | 1.74 (1.37) | 0.71 (0.28) | <1.7 IU/g Hb |
| G6PD:GR ratio | 0.42 (0.04) | 0.74 (0.12) | 1.07 (0.20) | ≥0.9 |
| Cytofluorometry G6PD-negative cells (%) | 68.2 (10.9) | 15.2 (18.7) | 1.5 (1.6) | <10.15 |
A ratio >0.9 indicates no G6PD deficiency, a ratio <0.46 indicates G6PD deficiency. Females with a ratio between 0.46 and 0.9 are suspected to have heterozygous G6PD deficiency. In the last column, the reference values for spectrophotometry, spectrophotometry with chromate inhibition, and cytofluorometry are given. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; IU g/Hb, international units per gram hemoglobin; GR, glutathione reductase.
Chromate Inhibition Test
When the ratio G6PD:GR activity was ≤0.9 for females, a second blood sample was requested for the chromate inhibition test. The blood was mixed 20:1 with 0.1 mol/l Na2CrO4. G6PD and GR activity was determined as described in the previous section (reference interval, GR < 1.7 IU/g Hb; Table 1).
Cytofluorometric Assay
We used the protocol of Shah et al. to determine G6PD activity per individual RBC.19 First, all hemoglobin was converted to methemoglobin. To this end, 10 μl RBC suspension (approximately 50% hematocrit) was suspended in 90 μl PBS (Sigma) and 100 μl 0.125 mmol/l sodium nitrite (Sigma). The sample was incubated at room temperature for 20 min. Samples were then washed 3 times with PBS, with a centrifugation step of 300 relative centrifugal force (RCF) for 1 min, and resuspended in 1 ml PBS. After washing, the RBCs were incubated to allow methemoglobin reduction in 100 μl PBS, 18 μl 0.28 mmol/l glucose (Sigma, St. Louis, Missouri), and 6 μl 0.3 mmol/l Nile Blue sulphate (Sigma-Aldrich, St. Louis, Missouri). The samples were incubated at 37C for 120 min with the tube lids removed. After completion of methemoglobin reduction, 2.5 μl 0.4 M KCN (Sigma) was added to all samples followed by incubation at room temperature for 5 min. Five μl of each sample was then added to 100 μl 3 wt% H2O2 (Sigma) in PBS and incubated for 3 min. After 2 final washing steps (PBS; 800 RCF for 3 min at room temperature), the samples were analyzed in duplicate using a LSRII flow cytometer (BD, Franklin Lakes, New Jersey). Samples were excited using a blue laser (488 nm), and fluorescence emission was measured in the Alexa 488 (530 nm) channel.
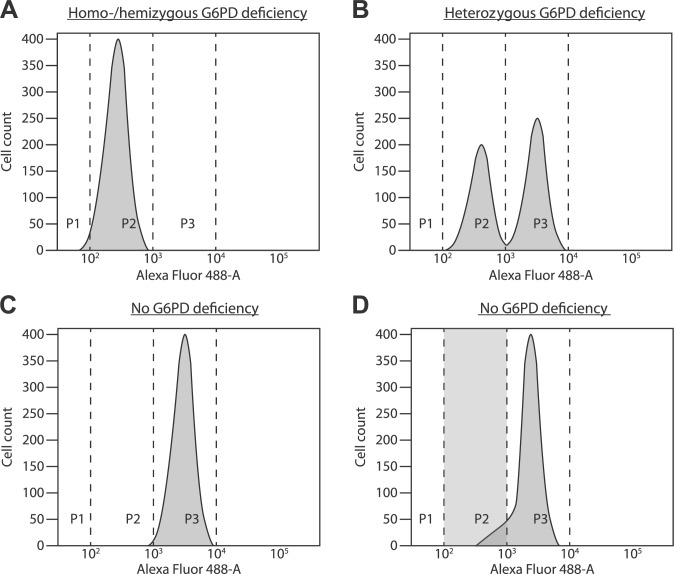
We used untreated RBCs as negative control and RBCs of healthy volunteers as positive control. All fluorescence plots of all tests were carefully inspected for test quality. Only if the plots showed a clear separation between G6PD-sufficient and G6PD-deficient cells, the test was considered to detect G6PD deficiency. The assay had to be repeated with a new sample when the positive population was asymmetrical and extended more than healthy controls into the area of negative cells (>10%), without the appearance of a distinct negative peak. Plots with this “shoulder” of G6PD-negative cells were considered to be false positive (Fig. 1).
Figure 1.
(A) Fluorescence distribution patterns after cytofluorometric analysis of G6PD activity in RBCs of a homozygous or hemizygous G6PD-deficient individual. All RBCs have a low fluorescence intensity (region P2). (B) Heterozygous G6PD deficiency. One population of RBCs has a low fluorescence intensity (region P2), and another one has a high fluorescence intensity (region P3). (C) Healthy individual without G6PD deficiency. The majority of RBCs have a high fluorescence intensity (region P3). Only a few old RBCs with low G6PD activity are found in region P2. (D) Healthy individual with an asymmetric population of RBCs with a high fluorescence intensity (intensity in region P3 and a shoulder in region P2). A substantial amount of RBCs with low fluorescence intensity are localized in region P2. Without proper visual inspection of the figure, this patient is falsely classified as heterozygously G6PD deficient. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; RBCs, red blood cells.
The percentage of fluorescence-negative cells in healthy volunteers has defined the sensitivity of the cytofluorometric method. We used the mean percentage negative cells +/-3 SD. Females with a number exceeding this threshold value were classified as G6PD deficient.
Gene Sequencing
DNA was extracted from peripheral blood. An Ampliseq custom panel (Thermo Fisher Scientific, Waltham, Massachusetts) was used to sequence the coding exons, intron-exon boundaries, and the promotor region of the G6PD gene. Library preparation was performed according to manufacturer protocols, and sequencing was performed on an Ion Torrent Personal Genome Machine or S5 system (Thermo Fisher Scientific).
Statistical Analysis
Sequencing of the G6PD gene was used as the reference standard. Sequencing results were compared with results of spectrophotometric measurements of G6PD activity in relation to GR activity, with or without additional chromate inhibition, and with the percentages of G6PD-deficient cells as evaluated by cytofluorometry. Patients can be grouped as “intermediate activity” in the chromate inhibition assay and G6PD:GR ratio test. To calculate sensitivity and specificity for these assays, the intermediate group and the deficient group were combined (Table 2).
Table 2.
Characteristics of 75 Female Patients Suspected to Have Heterozygous G6PD Deficiency as Detected by Genotyping, Related to G6PD Activity, G6PD:GR Activity Ratio, Chromate Inhibition, and Cytofluorometry.
| No Deficiency Number of Patients (%) | Heterozygous Deficiency Number of Patients (%) | Total Number of Patients (%) | Sensitivity (95% Confidence Interval) | Specificity (95% Confidence Interval) | |
|---|---|---|---|---|---|
| Spectrophotometry | |||||
| G6PD < 3.8 IU/g Hb (deficient) | 0 (0.0) | 14 (18.7) | 14 (18.7) | 0.52 [0.32, 0.71] | 1.00 [0.93, 1.00] |
| G6PD ≥ 3.8 IU/g Hb (normal) | 48 (64.0) | 13 (17.3) | 61 (81.3) | ||
| G6PD:GR < 0.46 (deficient) | 0 (0.0) | 3 (4.0) | 3 (4.0) | 0.96 [0.81, 1.00] | 0.19 [0.09, 0.33] |
| G6PD:GR 0.46 – 0.9 (intermediate) | 39 (52) | 23 (30.7) | 62 (82.7) | ||
| G6PD:GR > 0.9 (normal) | 9 (12.0) | 1 (1.3) | 10 (13.3) | ||
| Chromate inhibition | |||||
| Chromate > 2.0 IU/g Hb (deficient) | 1 (1.3) | 25 (33.3) | 26 (34.7) | 0.96 [0.71, 1.00] | 0.98 [0.89, 1.00] |
| Chromate 1.7 – 2.0 IU/g Hb (intermediate) | 0 (0.0) | 1 (1.3) | 1 (1.3) | ||
| Chromate < 1.7 IU/g Hb (normal) | 47 (62.7) | 1 (1.3) | 48 (64.0) | ||
| Cytofluorometry | |||||
| Cytofluorometry > 10.15% (deficient) | 6 (8.0) | 23 (30.7) | 29 (38.7) | 0.85 [0.66, 0.96] | 0.88 [0.75, 0.95] |
| Cytofluorometry < 10.15% (normal) | 42 (56) | 4 (5.3) | 46 (61.3) | ||
Sensitivity of chromate inhibition and G6PD:GR ratio was calculated after combination of the intermediate group and the deficient group as one group. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; GR, glutathione reductase; IU g/Hb, international units per gram hemoglobin.
We performed a power calculation on the number of false negative–tested patients. In a previous study, chromate inhibition was compared with the cytochemical method.15 In this study, the cytochemical method was assumed to be 100% specific and sensitive, and chromate inhibition testing and spectrophotometric measurement of G6PD activity resulted in 26% and 51% false negatives, respectively, for detection of heterozygously G6PD-deficient females. We assumed that cytofluorometry has a similar sensitivity as chromate testing and calculated that we needed at least 78 inclusions to obtain a power of 90% and α of 0.05 to validate the hypothesis that chromate inhibition or cytofluorometry have better accuracy for detection of heterozygous G6PD deficiency than spectrophotometry without chromate inhibition. All data were inspected for normal distribution. Normal distributed data were expressed as mean ± SD. Non-normal data were expressed as median with inter-quartile range.
Test results of chromate inhibition and cytofluorometry were compared as paired nominal data with the McNemar’s Exact test to investigate whether one of these test has superior sensitivity or specificity. A p<0.05 was considered statistically significant. All statistical analyses were conducted with R version 3.1.2 (R Core Team, 2015, Vienna, Austria).
Results
Patients
From March 2015 until September 2016, we assayed blood samples of 165 females more than 3 months of age for G6PD deficiency. All samples were from patients who were suspected to be G6PD deficient by their physician or who were screened because G6PD deficiency was detected in a family member. Test results, grouped according to the G6PD:GR activity ratio, are presented in Table 1.
Determination of the Cutoff of the Cytofluorometric Assay for G6PD Deficiency
Healthy volunteers (n=72) expressed on average 2.27 ± 2.62% G6PD-negative cells in the cytofluorometric assay. We used 10.15% negative cells (mean + 3 SD) as the threshold value.
Spectrophotometric Measurement of G6PD Activity and Additional Chromate Inhibition
Of the 165 included females, we identified 10 females with G6PD activity below 3.8 IU/g Hb and a G6PD:GR activity ratio below 0.46 by spectrophotometric measurement. On the basis of G6PD activity <3.8 IU/g Hb alone, 14 females were considered to be G6PD deficient. In 114 females, a G6PD:GR ratio in the range of 0.46–0.9 was measured, which, in our laboratory, mandates additional investigation with chromate inhibition to detect G6PD deficiency. These 114 females were referred to our laboratory for collection of an extra blood sample for detection of G6PD deficiency with G6PD gene sequencing, spectrophotometric determination of G6PD activity after chromate inhibition, cytofluorometry, and regular spectrophotometric determination of G6PD activity. A total of 78 females returned a sample for chromate inhibition. Three females were excluded from our analysis because of missing test data. Therefore, the study group in which all parameters were available comprised 75 individuals.
Comparison of Spectrophotometry, Chromate Inhibition, or Cytofluorometry and Mutation Analysis
A total of 27 females were heterozygously G6PD deficient (1 G6PD Aures (WHO class 2, 48 Ile–>Thr), 8 G6PD A- (WHO class 3, 68 Val–>Met; 126 Asn–>Asp), 1 G6PD Chatham (WHO class 2, 335 Ala–>Thr), 2 G6PD Gaohe (WHO class 2, 32 His–>Arg), 1 G6PD Modena (WHO class 3, 282 Asp–>His), 5 G6PD Nefza (WHO class 3, 323 Leu–>Pro), 9 G6PD Sassari/Cagliari (WHO class 2, 188 Ser–>Phe).
Of these females, 25 had residual GR activity >2.0 IU/g Hb, which indicates G6PD deficiency. In total, 48 females presented full inhibition of GR activity, <1.7 IU/g Hb, which indicates the presence of mainly G6PD-sufficient cells. However, one of these females tested false negatively. One patient had an intermediate chromate-inhibited GR activity between 1.7 and 2.0 IU/g Hb.
With cytofluorometry and with chromate inhibition, we identified 23 and 25 heterozygously G6PD-deficient females, respectively, whereas the spectrophotometric assay detected 14 heterozygous females (Table 2, Figs. 2A–C and 3). Only 3 patients had a ratio G6PD:GR <0.46, which indicates heterozygous G6PD deficiency. One patient sample had a false positive result after chromate inhibition testing. In 6 patients with a normal G6PD genotype, the cytofluorometric analysis resulted in a significant fraction of G6PD-negative cells. However, in all 6 patients, there was no distinct negative cell population visible as occurs with heterozygous G6PD deficiency (Fig. 1). In these patients, the flow-cytometric data image showed a “foot” or “shoulder” as illustrated in Fig. 1D. With chromate inhibition, 1 patient was false negative. In 4 heterozygously G6PD-deficient females, without a substantial fraction of G6PD-negative RBCs, cytofluorometry produced false negative results.
Figure 2.
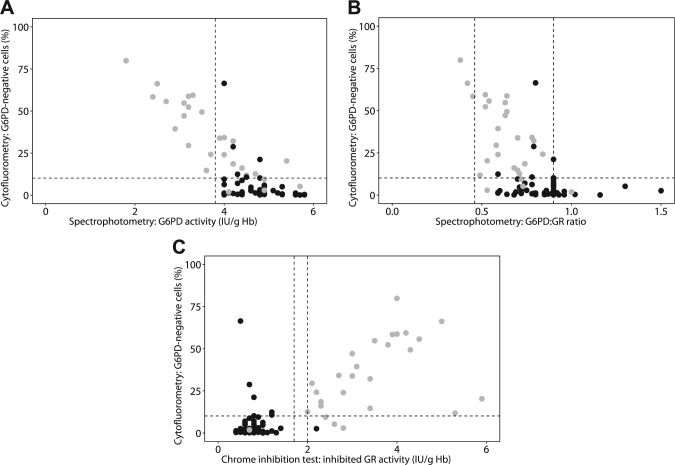
(A) Relationship between spectrophotometrically detected G6PD activity in RBC lysates and the percentage negative cells as determined with the cytofluorometric assay. The dashed horizontal line indicates the threshold for the percentage G6PD-negative cells in healthy controls (10.15%). The dashed vertical line indicates the lower threshold for G6PD activity (3.8 IU g/Hb) in healthy controls. Black dots represent patients without G6PD deficiency, and gray dots represent patients with heterozygous G6PD deficiency as determined by genetic analysis. Note that 4 females with normal G6PD activity and without mutations in G6PD showed a significant number of negative RBCs as measured by flow cytometry. In all these cases, the negative population appeared as a shoulder of the positive populations and should be interpreted as inconclusive. (B) Relationship between G6PD:GR ratio and the percentage negative cells as determined with the cytofluorometric assay. Additional testing for heterozygous G6PD deficiency was recommended for females with a G6PD:GR ratio between 0.46 and 0.9 (dashed vertical lines). The dashed horizontal line indicates the threshold for the percentage G6PD-negative cells in healthy controls (10.15%). Black dots represent patients without G6PD deficiency, and gray dots represent patients with G6PD deficiency as determined by the reference standard, genetic analysis. (C) Relationship between chromate-inhibited spectrophotometric determination of GR activity and the percentage negative cells as determined with cytofluorometry. The dashed horizontal line indicates the threshold for the percentage G6PD-negative cells in healthy controls (10.15%). The dashed vertical lines indicate the range from 1.7 to 2.0, which was considered to represent an inconclusive result of chromate inhibition. Chromate-inhibited activity <1.7 was considered to indicate absence of G6PD deficiency. Chromate-inhibited activity >2.0 was considered to indicate G6PD deficiency. Black dots represent patients without G6PD deficiency, and gray dots represent patients with G6PD deficiency as determined by genetic analysis. In all 4 females without a mutation in G6PD that showed a significant number of negative RBCs as measured by flow cytometry, the negative population appeared as a shoulder of the positive peak and should be interpreted as inconclusive. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; RBC, red blood cell; IU g/Hb, international units per gram hemoglobin; GR, glutathione reductase.
Figure 3.
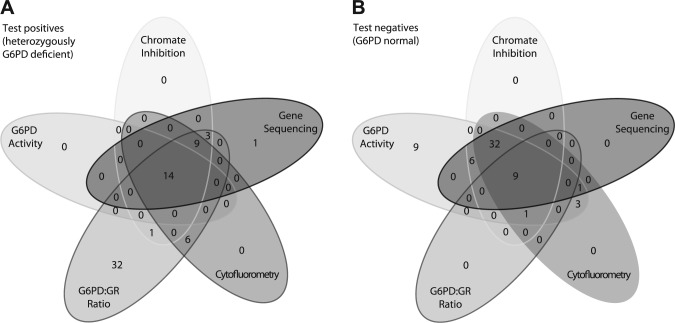
(A) Results of G6PD gene sequencing, chromate inhibition, cytofluorometry, and spectrophotometric determination of G6PD activity and the G6PD:GR activity ratio to detect heterozygous G6PD deficiency. The Venn diagram indicates the number of females with a positive result in the tests, which implies G6PD deficiency. For example, 14 females had a positive result in all tests whereas 31 patients had a positive result of the G6PD:GR ratio determination only. (B) Results of G6PD gene sequencing, chromate inhibition, cytofluorometry, and spectrophotometric determination of G6PD activity and the G6PD:GR ratio to detect heterozygous G6PD deficiency. The Venn diagram indicates the number of females with a negative result in the tests, which implies normal G6PD function. For example, 9 females had a negative result in all tests whereas 9 patients only had a negative result in the G6PD activity determination. Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; GR, glutathione reductase.
Table 2 presents estimates of sensitivity and specificity in detecting G6PD deficiency in females by spectrophotometry, chromate inhibition, and cytofluorometry, using mutation analysis as the reference standard. The sensitivity for the chromate inhibition test is 0.96 (95% CI: 0.71–1.00) and the specificity 0.98 (95% CI: 0.89–1.00). For the cytofluorometric test, the sensitivity is 0.85 (95% CI: 0.66–0.96) and the specificity 0.88 (95% CI: 0.75-0.95). We compared the sensitivity and specificity of spectrophotometry and chromate inhibition with McNemar’s Exact test and found that chromate inhibition has a superior sensitivity for detection of G6PD deficiency (p=0.0005 for sensitivity and p=1 for specificity). The sensitivity and specificity of cytofluorometry and chromate inhibition were also compared with McNemar’s Exact test and had a similar test accuracy (p=0.25 for sensitivity and p=0.13 for specificity).
Figure 3 illustrates the overlap between the positive and negative test results of all assays used in this study. Figure 3A shows the number of positive test results. In this Venn diagram, all positive results that are not also positive in the oval of G6PD sequencing are considered false positive. Figure 3B shows the negative test results. All negative results that are not also negative in the oval of G6PD sequencing are false negative.
Discussion
We investigated the accuracy of the cytofluorometric assay as developed by Shah et al.19 to detect heterozygously G6PD-deficient females. Based on our results, we conclude that (1) measuring the presence or absence of a significant G6PD-negative population by flow cytometry or chromate inhibition is a far more sensitive method for detecting heterozygote G6PD deficiency than measuring the total G6PD activity in cell lysates by spectrophotometry; (2) the test characteristics of the cytofluorometric test and chromate inhibition test are comparable; (3) the flow cytometry data of the cytofluorometric assay have to be inspected precisely to judge the test quality as this test can result in false positive detection of G6PD deficiency.
Various studies have shown that quantitative assays are unable to detect all heterozygously G6PD-deficient females.13–16,21–23 However, until recently, no (relatively) easy and cost-effective test was available to detect these females. Shah et al. developed a new cytofluorometric assay and reported that this assay was more reliable to detect heterozygous females as compared with spectrophotometry.19 Our findings confirm these data: we identified 14 of 27 heterozygously G6PD-deficient females with spectrophotometry, whereas 23 of 27 females were detected with cytofluorometry. Chromate inhibition was even more reliable and identified 26 of 27 females.
During our study, it became apparent that several of the cytofluorometric analyses did not result in a symmetric population of positive (fluorescent) cells. In these fluorograms, the peak of fluorescent cells extended to the area where G6PD-deficient RBCs normally appear (Fig. 1). It is, therefore, paramount that all flow cytometry data are inspected carefully to judge the test quality. The assay has to be repeated with a new sample when the positive population is asymmetrical and extends more than healthy controls into the area of negative cells (>10%), without the appearance of a distinct negative peak. Negative peak tailing can be induced by storage of blood but is sometimes present in freshly drawn samples as well. It cannot be excluded that, even without a mutation in the gene, G6PD activity is affected by unknown modifier genes. The peak may also have been caused by senescent RBCs with decreased G6PD activity. The phenomenon of negative peak tailing is a subject for further investigations. For now, we have excluded these samples from our analysis, which has increased specificity. A limitation of this assay is that patients with a population of G6PD-deficient erythrocytes smaller than the current cutoff of 10.15% cannot be detected.
It could be debated whether it is essential to develop a test to detect all heterozygous G6PD-deficient females. G6PD deficiency is mainly found in individuals originating from Africa, Asia, Oceania, and Mediterranean Europe, areas where malaria is endemic or has been endemic in the past.2 Both G6PD-deficient females and males have a reduced risk for lethal Plasmodium infection, although, in contrast with older studies, recent evidence suggests that malaria protection is mainly exerted in heterozygous females.6,24–26 The protective effect of G6PD deficiency has not yet been fully elucidated. Evidence on whether both homozygotes/hemizygotes and heterozygotes are protected from severe malaria is conflicting. Moreover, deficiency may not protect against all clinical expressions of malaria infection. For example, G6PD deficiency protects against cerebral malaria whereas it increases the risk for severe anemia.4,5,27,28
G6PD deficiency may cause hemolysis during illness, after consumption of fava beans or after ingestion of certain drugs.4,27,28 Luzzatto et al. state that
When a heterozygote female tests as G6PD deficient, in practice her acute haemolytic anaemia is likely to be just as severe as in a G6PD-deficient male, whereas a normal G6PD activity indicates that she is likely not to develop clinically significant haemolysis, and a result in the middle range indicates that her haemolysis is likely to be mild.10
Moreover, Reclos et al. already recommended in 2000 that females with a low G6PD activity should be advised to take preventive measures against acute hemolytic crises (avoid certain drugs, fava beans, etc.),29 thus, staying “on the safe side” with heterozygotes. Our study shows that females with significant numbers of G6PD-negative cells can still have a normal G6PD activity (Fig. 2). This may partly be caused by enhanced erythropoiesis after a self-limited hemolytic episode and high numbers of young RBCs with relative high G6PD activity.
However, even if detection of mild G6PD deficiency does not directly benefit patients with high residual G6PD activity, it is important that a heterozygously deficient female is informed about the implications of G6PD deficiency. Heterozygotes pass the deficiency to half of her children and during pregnancy, she should avoid drugs that may cause intra-uterine hemolysis. Also, post-natal jaundice can be treated with the knowledge that the mother is G6PD deficient. Therefore, development and validation of faster, reliable tests to detect heterozygous G6PD deficiency is essential.
With this study, we are the first to compare standard spectrophotometry, chromate inhibition, and the relatively new cytofluorometric assay with G6PD sequencing as a reference standard. Our study is limited by the relative small number of inclusions. Although rare in the Northern European population, G6PD deficiency is the most common enzyme deficiency worldwide. In the area of our laboratory, our inclusions are mainly dependent on immigrants from Africa, Asia, and the Mediterranean that have been referred for diagnostics.3 Our results for detecting heterozygously deficient females indicate good test accuracy for both the chromate inhibition test and the cytofluorometric assay. However, additional inclusions are needed to narrow the confidence intervals. Moreover, conclusions cannot be drawn on test characteristics for the general population as we only included females suspected of being G6PD deficient. Future studies with more inclusions may investigate which of the methods to detect G6PD deficiency are most reliable for detection of heterozygotes that may develop clinically significant hemolysis.
We used G6PD gene testing as the gold standard but it should be stressed that, even in extensive sequence-based studies, there is often a subpopulation of individuals that has partial/full enzyme deficiency but is genetically normal. This suggests that other variants, possibly local non-coding or epigenetic modifiers, exert a role in modifying G6PD activity. Therefore, we cannot exclude that in our study, a test based on G6PD phenotype has been scored false positive.
As the cytofluorometric assay can be performed on anticoagulated blood that is collected without special treatment, in contrast with the dedicated tube and procedure that is needed for the chromate inhibition test, we recommend it to be used as a screening test for females suspected for G6PD deficiency. However, it should be realized that the assay, as most assays that depend on enzyme activity, is less reliable shortly after a period of hemolysis as young RBCs do not yet express a G6PD-deficient phenotype.16 In such cases, comparing the ratio of G6PD activity with the activity of another RBC enzyme (e.g., GR) may help to select suspicious cases as candidates for genotyping. Heterozygously G6PD-deficient females with a relative high percentage of G6PD-sufficient RBCs are missed by regular methods measuring total G6PD activity. However, the majority of these females can be detected with both chromate inhibition and cytofluorometry.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ALP, APJV, RB, DK, CJFN, and RZ designed the study. MV, KL, and RZ obtained and analyzed the samples. ALP, MV, KL, PMMB, CJFN, and RZ analyzed the data. ALP, CJFN, and RZ wrote the article. All authors read and corrected the original and revised version of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: R Taurino  https://orcid.org/0000-0001-9772-7377
https://orcid.org/0000-0001-9772-7377
Availability of Data and Material: All data are available. No material is available as all material has been used for this study.
Ethics Approval and Consent to Participate: No additional material was obtained from included patients, and all included material was anonymized. Ethics approval was, therefore, waived, and consent to participate was not necessary.
Contributor Information
Anna L. Peters, Department of Intensive Care, Academic Medical Centre, Amsterdam, The Netherlands
Martijn Veldthuis, Department of Blood Cell Research, Sanquin Amsterdam, Amsterdam, The Netherlands.
Karin van Leeuwen, Department of Blood Cell Research, Sanquin Amsterdam, Amsterdam, The Netherlands.
Patrick M.M. Bossuyt, Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Academic Medical Centre, Amsterdam, The Netherlands
Alexander P.J. Vlaar, Department of Intensive Care, Academic Medical Centre, Amsterdam, The Netherlands
Robin van Bruggen, Department of Blood Cell Research, Sanquin Amsterdam, Amsterdam, The Netherlands.
Dirk de Korte, Department of Blood Cell Research, Sanquin Amsterdam, Amsterdam, The Netherlands.
Cornelis J.F. Van Noorden, Department of Medical Biology, Academic Medical Centre, Amsterdam, The Netherlands.
Rob van Zwieten, Department of Blood Cell Research, Sanquin Amsterdam, Amsterdam, The Netherlands.
Literature Cited
- 1. van Zwieten R, Verhoeven AJ, Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic Biol Med. 2014;67:377–86. [DOI] [PubMed] [Google Scholar]
- 2. Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, Hogg MM, Battle KE, Padilla CD, Baird JK, Hay SI. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42:267–78. [DOI] [PubMed] [Google Scholar]
- 4. Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 2007;4:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah SS, Rockett KA, Jallow M, Sisay-Joof F, Bojang KA, Pinder M, Jeffreys A, Craik R, Hubbart C, Wellems TE, Kwiatkowski DP. Heterogeneous alleles comprising G6PD deficiency trait in West Africa exert contrasting effects on two major clinical presentations of severe malaria. Malar J. 2016;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uyoga S, Ndila CM, Macharia AW, Nyutu G, Shah S, Peshu N, Clarke GM, Kwiatkowski DP, Rockett KA, Williams TN. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case-control and a cohort study. Lancet Haematol. 2015;2:e437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters AL, Van Noorden CJ. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem. 2009;57:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: from genotype to phenotype. Haematologica. 2006;91:1303–6. [PubMed] [Google Scholar]
- 9. Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67:601–11. [PMC free article] [PubMed] [Google Scholar]
- 10. Luzzatto L, Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol. 2014;164:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beutler E. G6PD deficiency. Blood. 1994;84:3613–36. [PubMed] [Google Scholar]
- 12. Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961;190:372–3. [DOI] [PubMed] [Google Scholar]
- 13. LaRue N, Kahn M, Murray M, Leader BT, Bansil P, McGray S, Kalnoky M, Zhang H, Huang H, Jiang H, Domingo GJ. Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am J Trop Med Hyg. 2014;91:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nantakomol D, Paul R, Palasuwan A, Day NP, White NJ, Imwong M. Evaluation of the phenotypic test and genetic analysis in the detection of glucose-6-phosphate dehydrogenase deficiency. Malar J. 2013;12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jonges GN, Hagen H, Van Noorden CJ, Weening RS, Roos D. Comparison between the chromate inhibition test and a cytochemical method for the determination of glucose-6-phosphate dehydrogenase deficiency in erythrocytes. Clin Chim Acta. 1989;181:135–41. [DOI] [PubMed] [Google Scholar]
- 16. Van Noorden CJ, Vogels IM, James J, Tas J. A. sensitive cytochemical staining method for glucose-6-phosphate dehydrogenase activity in individual erythrocytes. I. Optimalization of the staining procedure. Histochemistry. 1982;75:493–506. [DOI] [PubMed] [Google Scholar]
- 17. Roos D, van Zwieten R, Wijnen JT, Gómez-Gallego F, de Boer M, Stevens D, Pronk-Admiraal CJ, de Rijk T, van Noorden CJ, Weening RS, Vulliamy TJ, Ploem JE, Mason PJ, Bautista JM, Khan PM, Beutler E. Molecular basis and enzymatic properties of glucose 6-phosphate dehydrogenase volendam, leading to chronic nonspherocytic anemia, granulocyte dysfunction, and increased susceptibility to infections. Blood. 1999;94:2955–62. [PubMed] [Google Scholar]
- 18. Peters AL, Van Noorden CJ. Single cell cytochemistry illustrated by the demonstration of glucose-6-phosphate dehydrogenase deficiency in erythrocytes. In: Pellicciari C, Biggiogera M. editors. Histochemistry of single molecules: methods and protocols. Springer, New York; 2017;3-13. [DOI] [PubMed] [Google Scholar]
- 19. Shah SS, Diakite SA, Traore K, Diakite M, Kwiatkowski DP, Rockett KA, Wellems TE, Fairhurst RM. A novel cytofluorometric assay for the detection and quantification of glucose-6-phosphate dehydrogenase deficiency. Sci Rep. 2012;2:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zurcher C. Enige onderzoekingen betreffende de reactie van normale en abnormale erytrhocyten met chromaat [Some investigations on the reaction of normal and abnormal erythrycotes with chromate]. Medicin. Vol PhD. Amsterdam: University of Amsterdam; 1976. [Google Scholar]
- 21. Nadarajan V, Shanmugam H, Sthaneshwar P, Jayaranee S, Sultan KS, Ang C, Arumugam S. Modification to reporting of qualitative fluorescent spot test results improves detection of glucose-6-phosphate dehydrogenase (G6PD)-deficient heterozygote female newborns. Int J Lab Hematol. 2011;33:463–70.21501392 [Google Scholar]
- 22. Van Noorden CJ, Dolbeare F, Aten J. Flow cytofluorometric analysis of enzyme reactions based on quenching of fluorescence by the final reaction product: detection of glucose-6-phosphate dehydrogenase deficiency in human erythrocytes. J Histochem Cytochem. 1989;37:1313–8. [DOI] [PubMed] [Google Scholar]
- 23. Kosaryan M, Mahdavi MR, Jalali H, Roshan P. Why does the Iranian national program of screening newborns for G6PD enzyme deficiency miss a large number of affected infants? Pediatr Hematol Oncol. 2014;31:95–100. [DOI] [PubMed] [Google Scholar]
- 24. Luzzatto L, Bienzle U. The malaria/G.-6-P.D. hypothesis. Lancet. 1979;1:1183–4. [DOI] [PubMed] [Google Scholar]
- 25. Tripathy V, Reddy BM. Present status of understanding on the G6PD deficiency and natural selection. J Postgrad Med. 2007;53:193–202. [DOI] [PubMed] [Google Scholar]
- 26. Luzzatto L. G6PD deficiency: a polymorphism balanced by heterozygote advantage against malaria. Lancet Haematol. 2015;2:e400–1. [DOI] [PubMed] [Google Scholar]
- 27. Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376:246–9. [DOI] [PubMed] [Google Scholar]
- 28. Mockenhaupt FP, Mandelkow J, Till H, Ehrhardt S, Eggelte TA, Bienzle U. Reduced prevalence of Plasmodium falciparum infection and of concomitant anaemia in pregnant women with heterozygous G6PD deficiency. Trop Med Int Health. 2003;8:118–24. [DOI] [PubMed] [Google Scholar]
- 29. Reclos GJ, Hatzidakis CJ, Schulpis KH. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J Med Screen. 2000;7:46–51. [DOI] [PubMed] [Google Scholar]