Abstract
Surviving Purkinje fibers in myocardial infarct are regarded as an important substrate in arrhythmogenesis. However, poorly understood are functional properties of Purkinje fibers in the infarcted heart. We sought to visualize intracellular Ca2+ ([Ca2+]i) dynamics of Purkinje fiber networks in the mouse myocardial infarct. Using 3- to 4-day-old or 7- to 9-day-old infarcted hearts after the left coronary-artery ligation corresponding, respectively, to acute or healing phase, we conducted rapid fluo4-fluorescence imaging on the endocardial surface of the left ventricular septum by macro-zoom fluorescence microscopy and rapid-scanning confocal microscopy. In contrast with the intact heart, where uniform Ca2+ transients propagated rapidly, the infarcted heart exhibited slow, non-uniform impulse propagations. On confocal microscopy, Purkinje fibers in the peri-infarct zone exhibited non-uniform [Ca2+]i dynamics: beat-to-beat alternans of the Ca2+ transient amplitude in and among the individual fibers, whereas the intact fibers exhibited uniform Ca2+ transients. Such non-uniform [Ca2+]i dynamics were more conspicuous in the acute infarcted hearts than in the healing ones. In accordance with [Ca2+]i dynamics, fixed fluo4-loaded heart preparations exhibited definitive connexin-40 plaques in the peri-infarct Purkinje fibers, whereas the subjacent myocardium presented coagulative necrosis and granulation tissues, respectively. The surviving Purkinje fibers in the peri-infarct zone exhibited non-uniform [Ca2+]i dynamics, which may lead to arrhythmogenesis.
Keywords: arrhythmia, alternans, calcium, heart, histopathology
Introduction
Cardiac Purkinje fibers, which are distributed widely throughout the ventricular endocardial surface, play essential roles in impulse conductions of the ventricles for coordinated contractile functions of the heart.1–3 Along with the subendocardial myocardium, Purkinje fibers are known to be spared from the ischemic necrosis even when the coronary arterial flow is totally occluded. Clinical and experimental studies have suggested that the surviving subendocardial layers of the infarcted myocardium including granulation tissue and fibrous scar are suggested to be an important origin of ventricular tachyarrhythmias.4–8 Electrophysiological studies of the canine infarcted myocardium further revealed that ventricular tachycardia was initiated at the subendocardial layers within 10 min after ligation of the left coronary artery.9 In addition, triggered activity and the following delayed after depolarizations were reportedly induced at the focal ischemic regions of the endocardial surface of canine myocardial infarct during the acute phase.8 However, such local electrical activities of the endocardial surface are insufficient to understand whether or not the surviving Purkinje fibers in the post-infarcted heart are responsible for genesis of arrhythmias.
Previously, Boyden et al.10 demonstrated that surviving Purkinje cell aggregates dispersed from the canine infarct border zone exhibit non-uniform Ca2+ transients, especially Ca2+ waves. Despite this landmark study, however, no information is available regarding the intracellular calcium ([Ca2+]i) dynamics of the surviving Purkinje fibers in the heart in situ. The advent of the fluorescent imaging technologies of the living heart tissue has enabled us to visualize spatiotemporally precise behaviors of the [Ca2+]i dynamics in the heart.11 Using this approach, we have successfully visualized spatiotemporally precise [Ca2+]i dynamics of Purkinje fibers and their interconnection with subjacent ventricular myocardium in excised rat hearts.12 Based on such background, we postulated that [Ca2+]i dynamics of the impaired ischemic Purkinje cells and relevant morphology of the myocardial infarct will provide important information for understanding the ventricular arrhythmogenesis in the infarcted heart. To address this hypothesis, we sought to visualize spatiotemporal patterns of conduction on the endocardial surface of the infarcted heart and their relevant cellular aspects of [Ca2+]i dynamics of Purkinje fiber networks in the mouse infarcted heart in association with the corresponding histology.
Materials and Methods
Animal Model of Myocardial Infarction (MI)
The animal experimental procedures in this study had been conducted from August 2014 to May 2015 in accordance with Guide for the Care and Use of Laboratory Animals (8th ed., National Academies Press, Washington, DC, 2011) under approval of the Animal Research Committee, Kyoto Prefectural University of Medicine (approval No: M25-145). Adult male mice (C57BL/6N) of 20–30 g body weight were used. Creation of MI of the mouse was essentially based on a previous report by Michael et al.13 In particular, we applied deep generalized anesthesia to the mouse with sodium pentobarbital (0.1 mg/g body weight) by intra-peritoneal injection. After establishment of sufficient general anesthesia confirmed by periodic prick of the abdominal wall, mice were placed in a supine position on the surgical table with paws taped. Under endotracheal intubation via a 23G polyethylene tube, the mouse was artificially ventilated with a rodent ventilator (Minivent Type 845, Harvard Apparatus, Holliston, Massachusetts) with a tidal volume of 160 μl at 150 cycles/min. After opening of the left chest by a lateral incision along the ribs extending approximately 10-mm length, the left anterior descending coronary artery was ligated with a 7–0 nylon thread connected with a U-shaped needle 1–3 mm from the lower border of the left atrial appendage. Thereafter, the chest was closed by a 5–0 nylon thread, and the mouse was allowed to recover by removal from the respirator and the endotracheal tube. During the postoperative recovery period, the mouse was kept warm by a heat lamp and allowed 100% oxygen via nasal cone with no specific analgesics added.
We examined the 3- to 4-day-old infarcted hearts (n=5) for early phase including coagulative necrosis, and the 7- to 9-day-old infarcted hearts (n=5) for healing phase including granulation tissues.14 For comparison, non-infarcted control hearts (n=5) were used.
Fluo4 Loading of the Heart
After confirmation of the mouse under terminal anesthesia by intra-peritoneal injection of pentobarbital sodium (0.25 mg/g body weight), the heart was quickly excised and perfused in a Langendorff manner. After washout of the blood with HEPES-buffered Tyrode’s solution consisting of (in mM) NaCl 145, KCl 5.4, MgCl2 1, CaCl2 1, HEPES 10, and glucose 10 (pH = 7.4 adjusted by NaOH) at 23–25C, Ca2+-indicator, fluo4-AM (8 μg/ml, Dojindo, Kumamoto, Japan) was loaded for 20 min. After loading of the heart with fluo4, the heart was perfused with the Tyrode’s solution at 37C for 15 min for de-esterification of the AM form of the probe with probenecid (0.1 mg/ml) added. During the experiments, the heart was under constant perfusion with Krebs-Henseleit solution containing (in mM) NaCl 115, NaHCO3 25, KCl 5.4, NaH2PO4 1.2, NaHCO3 25, MgSO4 1, CaCl2 1, and glucose 10, aerated with 95% O2 and 5% CO2 at room temperature (~25C). The mechanical motion of the heart was attenuated by perfusion with cytochalasin D (4 μM). For imaging of the endocardial layers of the left ventricular septum, the endocardial surface was totally exposed by incision of the ventricle along the longitudinal direction through the atrioventricular junction at the posterolateral wall.
Optical [Ca2+]i Imaging
Macroscopic [Ca2+]i imaging was performed on the mouse subendocardial layer with the use of a fluorescence imaging system consisting of a macro-zoom fluorescence microscope (MVX10 MacroView, Olympus, Tokyo, Japan) equipped with a high-speed complementary metal-oxide-semiconductor (CMOS) camera (188 × 160 pixels, MiCAM02-CMOS, Brainvision, Tokyo, Japan). Under stabilization of the heart on the stage of the microscope, the whole endocardial surface of the left ventricular septum was imaged by zooming out the objective lens (Olympus MV PLAPO 1×, N.A. = 0.25) to ×0.63 or ×0.8. The light-emitting diode light (490 nm) was directed onto the endocardial surface of the left ventricle to excite the fluo4. The emitted fluorescence signals (>670 nm) were detected with the CMOS camera at an acquisition rate of 500 frames/sec, and the data were transferred to a computer by means of the MiCAM02 system (Brainvision, Tokyo, Japan). The imaged region was 15.8 × 13.5 mm in size for ×0.63 zoom, and 12.5 × 10.7 mm for ×0.8 zoom, incorporating the entire area of interest for left ventricular excitation. After macroscopic imaging of the [Ca2+]i dynamics, the endocardial surface was imaged by rapid-scanning confocal microscopy equipped with the upright microscope (BX-50WI, Olympus, Tokyo, Japan) and a spinning disc-type confocal unit CSU-21 (Yokogawa, Tokyo, Japan) under placement with a glass coverslip (170-μm thickness) on the heart with consecutive 2-Hz pacing from the right atrial appendage. Although absence of superfusion under placement of the coverslip may have rendered the subendocardial layers ischemic, we regard the involvement of “coverslip ischemia” as minor, because we confirmed that under coronary perfusion, short-term observations for about 15 min with intermittent releases (within 5 min) of the coverslip placement preserve the [Ca2+]i dynamics unaltered. The emitted fluo4-fluorescence signals were detected through an image intensifier (C8600, Hamamatsu Photonics, Hamamatsu, Japan) by a charge-coupled device (CCD) camera (MiCAM02, Brainvision, Tokyo, Japan) with a pixel size of 384 × 256 (361 × 241 μm) via a 20× objective lens (UMPLan FI, NA = 0.5, Olympus, Tokyo, Japan).
Quantitative Data Analysis
The digitized fluorescence videos, captured by the rapid-scanning confocal microscope and subsequently converted to the sequential bitmap images, were analyzed with Image J software (National Institutes of Health, Bethesda, Maryland). To evaluate the spatiotemporal [Ca2+]i dynamics, the X-t images were obtained by plotting the fluorescent intensity that was scanned along the longitudinal axis of the Purkinje fibers. The velocity of the impulse propagation within the left ventricular endocardial surface was calculated by the macroscopic X-t image scanned along the direction of the impulse. The plot profiles of Ca2+ transients were obtained from X-t images as average fluorescent intensities along the scan line. The spatiotemporal variability of [Ca2+]i was evaluated by “alternans ratio,” which was obtained from the amplitudes of the two consecutive Ca2+ transients by dividing the smaller one over the larger one in the plot profiles. The quantitative data (mean ± SD) were statistically analyzed by unpaired t-test, and a value of p<0.05 calculated by R version 3.1.0 (The R Project) was considered significant.
Histological Observation
After the [Ca2+]i imaging, white-light images of the left ventricular endocardial surface were obtained by intravital microscopy with attached CCD camera (DS-SMc, Nikon, Tokyo, Japan). Subsequently, the heart was fixed with 2% paraformaldehyde for histological and histochemical analysis. For IHC, the hearts were immersed in the primary antibodies of connexin-40 (Rabbit, polyclonal, dilution 1:250, Fitzgerald, Acton, Massachusetts) for identification as Purkinje fibers and vimentin (Chicken, polyclonal, dilution 1:500, Novus Biologicals, Littleton, Colorado) for interstitial fibroblasts and labeled by Alexa Fluor 488 (dilution 1:250, Invitrogen, Tokyo, Japan) and Alexa Fluor 594 (dilution 1:250, Invitrogen, Tokyo, Japan), respectively. Confocal images of the fixed samples were obtained by FV-1000 confocal microscopy (Olympus, Tokyo, Japan). For conventional histology, the heart embedded in paraffin blocks was sectioned every 500 µm interval from the apex to the base of the left ventricle through the transverse section of both ventricles. The glass-mounted sections were stained with Masson’s trichrome for detection of the ischemic necrosis and granulation after MI. In addition, we visualized subcellular structure of the endocardial surface of the left ventricular septum by confocal fluorescence images of the heart after staining with di-4-ANEPPS (3.5 μg/ml, Wako Pure Chemicals, Osaka, Japan) for excitation and emission at 488 nm and >530 nm (n=3).
Results
[Ca2+]i Dynamics and Corresponding Histology of the Non-Infarcted Heart
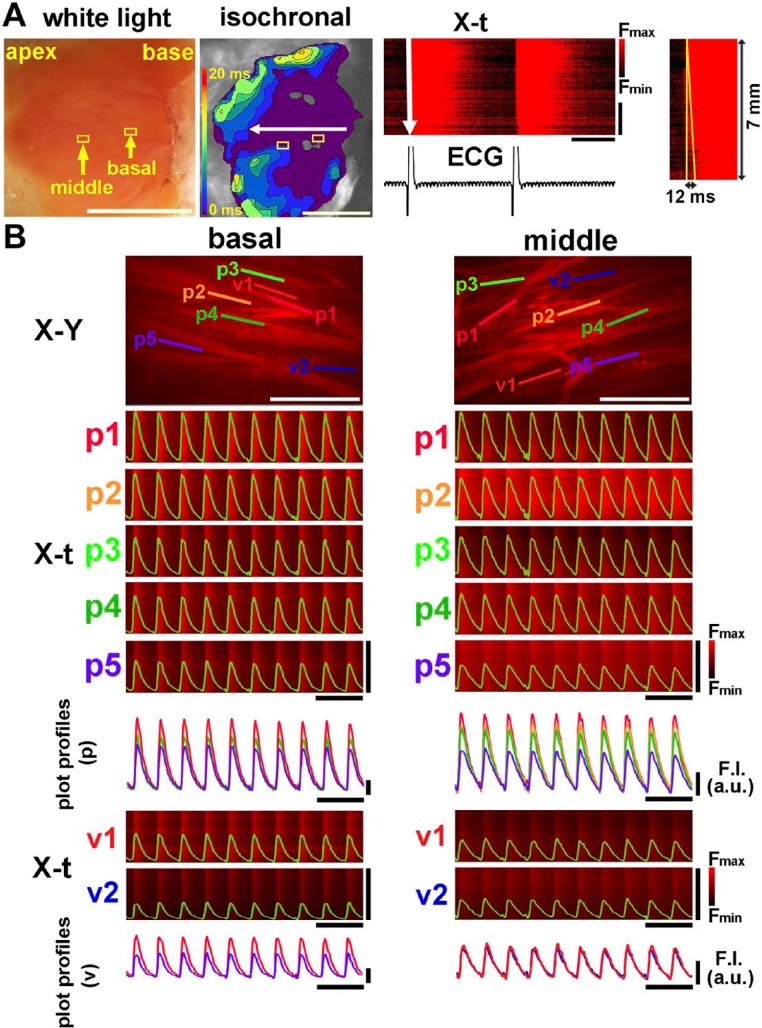
The intact, non-infarcted heart exhibited rapid impulse propagation within the ventricle. As shown in Fig. 1A and Supplemental Video 1, macroscopic images of the fluo4-fluorescence intensity revealed that the impulse propagates rapidly from the base to the apex on the endocardial surface of the left ventricle. The corresponding isochronal propagation map (middle panel) and X-t image (right panel) also revealed that the impulses showed quick, uniform propagations on the endocardial surface. In a total of 5 non-infarcted hearts, the propagation velocity was 42.2 ± 14.9 cm/sec, a value slightly lower than a previous data15 possibly because our experiments were conducted under lower temperatures. On the rapid-scanning confocal microscopy, the individual Purkinje fibers on the intact endocardial surface exhibited spatiotemporally uniform Ca2+ transient waveforms upon ventricular excitation as shown on the X-t and the corresponding plot profiles of the individual Ca2+ transients (Fig. 1B, also see Supplemental Video 2). These observations are in good agreement with our previous observations on the rat Purkinje fiber network.12 The Ca2+ transient durations of Purkinje fibers and ventricular myocytes appear as long as the cycle length, values longer than those in the previous data of the mouse heart16 possibly due to the relatively lower temperatures. The Ca2+ transients on the subjacent ventricular myocytes were also uniform (see X-t images and plot profiles for v1 and v2), although their fluorescence intensities were relatively lower than those of the Purkinje fiber layers due to the out-of-focus signals from the confocal plane. Thus, the non-infarcted heart exhibits uniform Ca2+ transients with rapid intraventricular impulse propagation.
Figure 1.
Intracellular Ca2+ ([Ca2+]i) dynamics of the mouse non-infarcted heart. (A) Mesoscopic [Ca2+]i dynamics of the endocardial surface of the left ventricular septum. Shown on the leftmost panel is the white-light image of the endocardial surface. The isochronal propagation map of the endocardial surface (middle-left panel) indicates rapid impulse propagation from the base to the apex. The corresponding X-t image along the direction of propagation (horizontal white arrow in the isochronal map) is shown in the middle-right panel. Shown on the rightmost panel is the corresponding X-t image of a Ca2+ transient in an expanded time scale. White scale bars = 5 mm, horizontal bars in X-t = 200 msec, and vertical bars in X-t = 5 mm. (B) [Ca2+]i dynamics of Purkinje fibers and subjacent ventricular myocytes viewed by rapid-scanning confocal microscopy at the basal and middle areas of the left ventricular septum depicted from the two boxes indicated in A. Five X-t images (p1 to p5) obtained from the 100-µm straight scan lines along the longitudinal direction of Purkinje fibers in the X-Y images overlaid with the corresponding plot profiles (upper panel). These plot profiles are superimposed below the X-t images. For the ventricular myocardium beneath the Purkinje fibers (v1 and v2), X-t images and the corresponding plot profiles are on the bottom. White scale bars in X-Y images = 200 µm, horizontal black bars = 1 sec, and vertical bars for X-t image = 100 µm. Abbreviation: ECG, electrocardiogram.
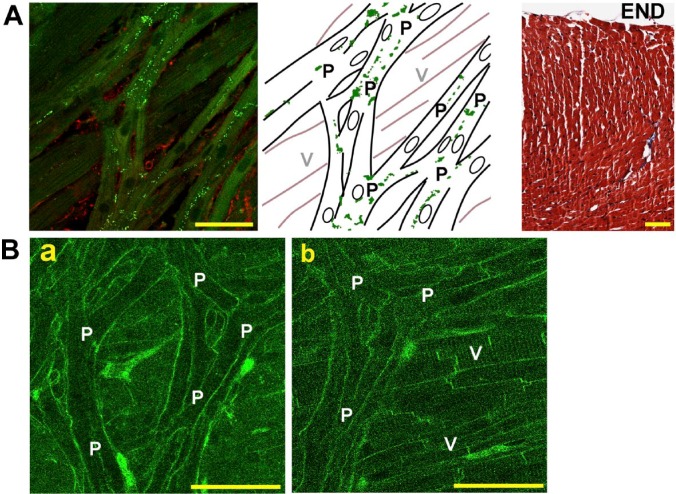
The IHC and histology revealed characteristics of the endocardial surface; the en face image of the endocardial surface provided us a clear distinction of Purkinje fibers from ventricular myocytes in the non-infarcted heart (Fig. 2A). Within the Purkinje fiber networks, connexin-40 (Cx40), a most reliable evidence for them as being Purkinje fibers (indicated by “P” in the figure)17 was abundantly identified not only transversely but also longitudinally along the direction of the fibers, due possibly to long finger-like projections with oblique running of Purkinje fiber bundles.18 In contrast, the subjacent ventricular myocytes (indicated by “V”) of regularly arranged, thick strands were devoid of Cx40. The subcellular structure of Purkinje fibers was also different from that of ventricular myocytes. On confocal fluorescence images of the di-4-ANEPPS-stained endocardial surface (Fig. 2B), Purkinje fibers have no discernible transverse tubules (T-tubules) (a), whereas the ventricular myocytes have fine striations of T-tubules (b).
Figure 2.
Representative histological images of Purkinje fibers in the non-infarcted heart. (A) The confocal fluorescence image of the endocardial surface of the left ventricular septum (left) and its corresponding schematic illustration (middle). The fluorescence image exhibits Purkinje fiber networks with definitive Cx40 plaques (green dots), whereas the ventricular myocardium running behind the Purkinje fibers is devoid of Cx40. The red signals below the Purkinje fibers denote vimentin. Green dots in the illustration indicate the plaques of Cx40. The rightmost panel shows a cross section stained with Masson’s trichrome of the left ventricular septum, indicating absence of infarct. (B) Confocal fluorescence images of the endocardial surface of the left ventricular septum stained with di-4-ANEPPS focused on Purkinje fibers (a) and on the subjacent ventricular myocardium (b). “P” and “V” denote Purkinje fibers and ventricular myocardium, respectively. “END” indicates the endocardial surface. Bars = 50 µm. Abbreviation: Cx40, connexin-40.
Non-Uniform [Ca2+]i Dynamics on the Subendocardial Layer of the Infarcted Heart
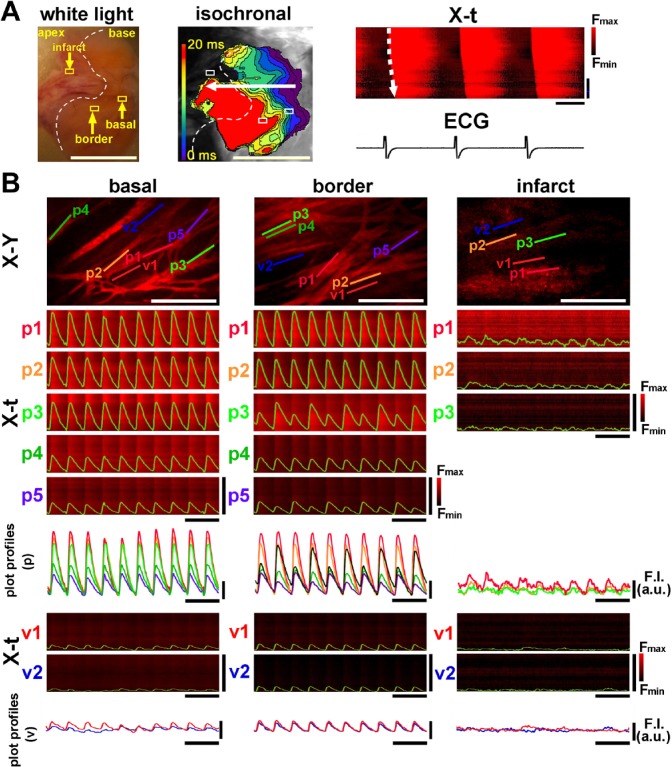
The spatiotemporal [Ca2+]i dynamics in the infarct border zone were quite distinct from those in the non-infarct heart. As shown in the white-light image of the 3-day-old infarct (left panel in Fig. 3A), the infarcted region of a pale wedge-shape was clearly discriminated from the non-infarcted region. The corresponding fluo4-fluorescence images of the infarcted region exhibited faint, inhomogeneous [Ca2+]i transients with a depressed impulse propagation with irregular wave fronts as compared with the basal zone (middle panel in Fig. 3A and Supplemental Video 3). The X-t image in the direction of the propagation also indicated slower conduction velocity in the border zone and its distal region than in the basal region (right panel in Fig. 3A). Quantitatively, the mean propagation velocity was 20.4 ± 5.3 cm/sec at 3- to 4-day-old myocardial infarct (n=5), a value significantly slower as compared with control (p=0.024). Confocal fluo4-fluorescence images of the infarct border zone revealed that the individual Purkinje fibers often exhibited non-uniform [Ca2+]i dynamics showing beat-to-beat alternans of the Ca2+ transient amplitude, especially in the middle portions of the left ventricle (Fig. 3B and Supplemental Video 4). The X-t images of the representative individual Purkinje fibers (1–5) also revealed beat-to-beat alternans of Ca2+ transients, whereas the basal region showed relatively uniform Ca2+ transients. In addition, the superimposed plot profiles of the corresponding X-t images clearly indicated variable [Ca2+]i dynamics in the border zone Purkinje fiber networks (bottom panels in Fig. 3B).
Figure 3.
[Ca2+]i dynamics of the 3-day-old infarcted heart. (A) A mesoscopic white-light image obtained from the endocardial surface of the left ventricular septum (left panel). A pale, tan-colored area bordered by the white dotted line denotes the infarcted lesion. The isochronal propagation map of the corresponding endocardial surface (middle) and the X-t images of [Ca2+]i dynamics with ECG trace (right) show slowing of impulse propagation on the infarct border zone. White scale bars = 5 mm, horizontal bars in X-t = 200 msec, and vertical bars in X-t = 5 mm. (B) [Ca2+]i dynamics of Purkinje fibers and subjacent ventricular myocytes viewed by rapid-scanning confocal microscopy. The fluo4-fluorescence X-Y images of the basal zone, infarct border zone (middle area), and infarct (apical area) and the corresponding X-t images of the five individual Purkinje fibers (p1–p5). As shown in the three X-t images on the infarct zone (p1–p3), ventricular myocytes (v1 and v2) subjacent to the Purkinje fiber networks show nearly indiscernible Ca2+ transients. White scale bars in X-Y images = 200 µm, horizontal black bars for X-t image = 1 sec, and vertical bars for X-t image = 100 µm. Abbreviation: ECG, electrocardiogram.
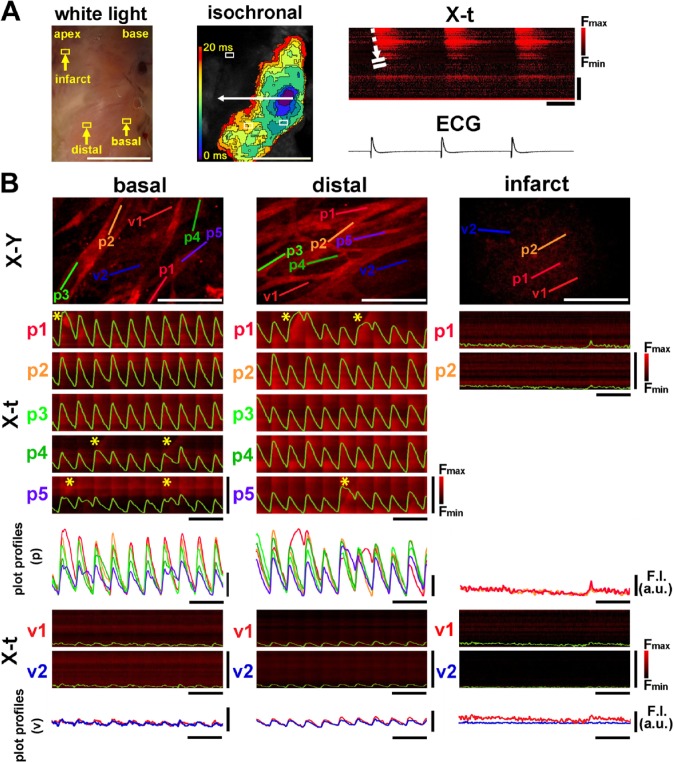
In the 7- to 9-day-old infarcted heart, the defective area of Ca2+ transients extended more widely (Fig. 4A and Supplemental Video 5) as compared with that of the 3- to 4-day-old infarcted heart. In addition, the patterns of impulse propagation were more obscured and irregular with slower propagation velocity of 9.7 ± 2.2 cm/min than that in 3- to 4-day-old infarct (n=5, p<0.01). In accordance with this, the white-light image of the infarcted area was blurred in its borders as compared with 3- to 4-day-old infarct. Furthermore, the non-uniformity in [Ca2+]i dynamics was evident at the cellular levels, that is, beat-to-beat alternans of Ca2+ transients amplitude in both the base and middle portions of the endocardial regions (Fig. 4B and Supplementary Video 6). In addition, we occasionally identified localized, wave-like [Ca2+]i rises on excitation (indicated by * in X-t images), an abnormal [Ca2+]i dynamics that arise under conditions of impaired Ca2+ release from the sarcoplasmic reticulum.19 Quantitatively, the beat-to-beat variability for the amplitude of Ca2+ transients was evident on the infarct border zone at 3–4 days after ligation as shown in the alternans ratio, which returned close to the control level at 7–9 days after ligation (Fig. 5).
Figure 4.
[Ca2+]i dynamics of the 7-day-old infarcted heart. (A) A mesoscopic white-light image of the endocardial surface is shown on the left panel. Note the poorly marginated, pale tan-colored area extending from the middle portion to the apex. The corresponding propagation map shows no discernible rise in [Ca2+]i signals in the infarcted area at the apex with marked slowing of the impulse propagation at the basal zone. The X-t image (right panel) scanned along the white dotted arrow (in the middle panel) indicates somewhat inhomogeneous, diffusely slowed impulse propagation. White scale bars = 5 mm, horizontal bars in X-t = 200 msec, and vertical bars in X-t = 5 mm. (B) [Ca2+]i dynamics of individual Purkinje fibers (p1–p5) and subjacent ventricular myocytes (v1 and v2) in three distinct regions of the endocardial surface (depicted from the boxes shown on the white-light image). The X-t images of Purkinje fibers (1–5) exhibit spatiotemporally non-uniform patterns on the basal and border zones. Asterisks (*) indicate the localized wave-like [Ca2+]i rises. White scale bars in X-Y images = 200 µm, horizontal black bars for X-t image = 1 sec, and vertical bars for X-t image = 100 µm. Abbreviation: ECG, electrocardiogram.
Figure 5.
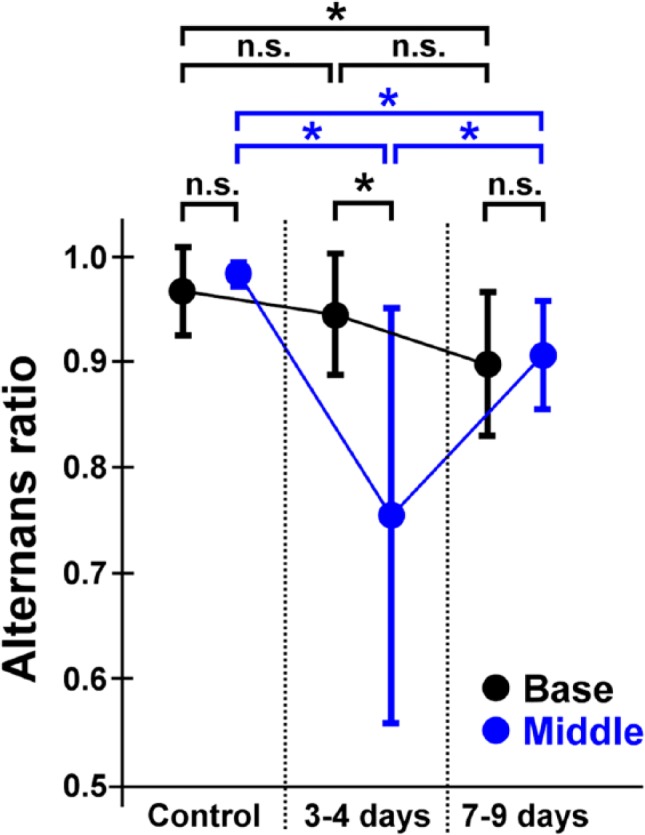
Quantitative comparisons of the beat-to-beat variability for the amplitude of Ca2+ transients (alternans ratio). Among the 3 groups of hearts, the beat-to-beat alternans is more remarkable on the infarct border zone at 3–4 days after the coronary-artery ligation than in the control and in the 7- to 9-day-old infarcted hearts as shown in the low alternans ratio. The alternans ratio is returned close to the control level at 7–9 days after ligation. *p<0.01. Abbreviation: NS, not significant.
Histology on the Subendocardial Layer of the Infarcted Heart
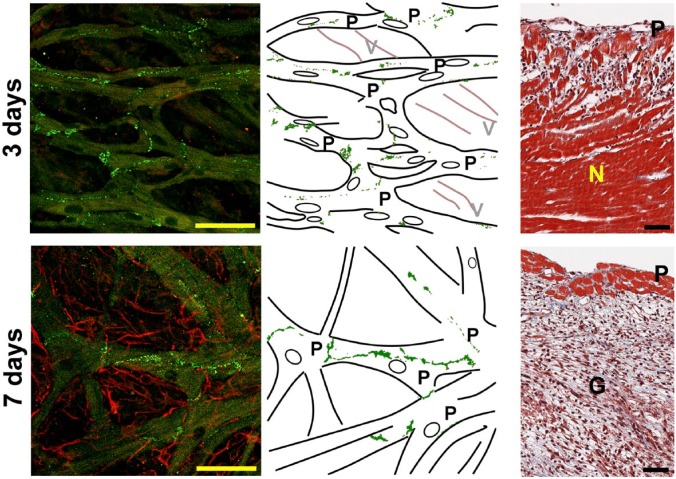
The IHC and histology of the infarcted hearts revealed that Cx40 plaques in Purkinje fiber networks were preserved 3–4 days and 7–9 days after coronary ligation, whereas the fluo4 fluorescence was remarkably lacking in subjacent ventricular layers as exemplified in Fig. 6. For 7- to 9-day-old infarct, the subjacent ventricular muscle layer was replaced by abundant vimentin-positive fiber-like structures, consistent with the formation of granulation tissue (“G” on the right panel) in the myocardial layer, while the Purkinje fiber layers (“P” on the right panel) were well preserved. This was consistent with the corresponding Masson’s trichrome stained histology of the cross section; the subendocardial layer was significantly devoid of ventricular muscles showing jeopardized distributions within the granulation tissue (right upper panel).
Figure 6.
Representative histological images of the endocardial surfaces and the cross sections of the 3-day-old and 7-day-old infarcted hearts. The confocal fluorescence images (left), their corresponding illustrations (middle), and the cross sections stained with Masson’s trichrome of the left ventricle (right). In the confocal fluorescence endocardial images, the Purkinje fiber network is positive for Cx40 (green), whereas the left ventricular myocardium running behind the Purkinje fibers shows no discernible Cx40 signals. On day 7, abundant reticular fibroblasts that are positive for vimentin (red) are observed beneath the endocardial surface, instead of the regular arrangements of the myocardium. The cross section shows massive coagulative necrosis (N) and granulation tissue (G) with apparently viable myocardium and Purkinje fibers (P) on the subendocardial layers. Granulation tissue, replaced by the necrotic myocardium, is identified in the cross section. Scale bars (black and yellow) = 50 µm. Abbreviations: V, ventricular myocardium; Cx40, connexin-40.
Discussion
Summary of the Present Study
In the present study, we demonstrated that Purkinje fibers in the infarct border zone exhibit spatiotemporally non-uniform [Ca2+]i dynamics in the mouse heart. Macroscopic images of [Ca2+]i dynamics revealed depressed, inhomogeneous intraventricular propagation with irregular, less dense wave fronts for the acute necrotic phase, and almost completely diminished Ca2+ transients in the infarct area at the late, healing phase. These observations were relevant to the corresponding histology and IHC of the endocardial surface, where necrotized myocytes were predominantly distributed with jeopardized distribution of surviving myocytes for the former phase, and for the latter, they were definitively replaced by granulation tissue.
Viability of Purkinje Fibers in the Infarct Border Zone
It has long been accepted that Purkinje fibers in the infarct border zone are viable owing to direct blood supply to the endocardial surface from the ventricular chamber20 and due to relatively lower oxygen demand as compared with ventricular myocytes21 together with ability to tolerate ischemia and hypoxia in the specialized conduction system.22 In accordance with this notion, we have confirmed that Purkinje fibers in the vicinity of the infarcted myocardium were viable by confocal [Ca2+]i imaging, where the fibers still exhibited Ca2+ transients, but spatiotemporally non-uniform [Ca2+]i dynamics in and among individual fibers. Previously, Boyden et al.10 observed spatiotemporally precise [Ca2+]i dynamics of Purkinje cell aggregates dispersed from the infarct border zone of the canine heart, for example, non-uniform [Ca2+]i transients and electrogenic Ca2+ waves; however, their observations were not necessarily identical to ours probably due to the observations on the enzymatically dispersed cells, which are free from the influence of the surrounding tissues, as will be described below. The present study is the first to demonstrate the altered [Ca2+]i dynamics of Purkinje fibers in the infarct border zone in situ.
Mechanisms of Non-Uniform [Ca2+]i Dynamics in the Infarct Border Zone Purkinje Fibers
The beat-to-beat non-uniformity and the wave-like [Ca2+]i rises in the infarct border zone Purkinje fibers might not be caused by [Ca2+]i overload. This is because we barely observed Ca2+ waves in the fiber network during diastole, a representation of [Ca2+]i overload,23 which was in stark contrast with our previous observations on subepicardial myocardium of the rat infarct border zone, where the Ca2+ waves emerged predominantly during diastole.24 Paucity of the diastolic “Ca2+-overloaded” waves is in good agreement with our previous observations on rat Purkinje fiber networks, which barely exhibit the “diastolic” Ca2+ waves, although the high-frequency, oscillatory “agonal” Ca2+ waves did emerge under irreversible Ca2+-overloaded conditions.12 One possible explanation for the rare occurrence of the diastolic waves would be the paucity of T-tubules in Purkinje fibers as demonstrated in this study (Fig. 2), which is analogous to those of the rat atrial myocytes.25 As compared with ventricular myocytes, the nominal distribution of T-tubules would impair efficient Ca2+ release from the sarcoplasmic reticulum (SR),26 and thereby, spatiotemporal uniformity of [Ca2+]i transients would easily be impaired, leading to non-uniform [Ca2+]i dynamics especially under ischemic conditions, where the Ca2+ release from and reuptake into the SR would further be suppressed. In accordance with this, ischemic insults could augment the non-uniformity of [Ca2+]i dynamics by impairment of Ca2+ release in surviving Purkinje fibers, whereas ventricular myocytes, even if they were not necrotized, would not have exhibited Ca2+ transients any longer due to their relatively higher oxygen demands.
The beat-to-beat alternans of Ca2+ transients occasionally accompanied non-uniform Ca2+ transients represented as Ca2+ wave-like propagations on systole. These atypical patterns of [Ca2+]i dynamics could be caused by depressed Ca2+ release from the SR as demonstrated in the previous studies on isolated atrial27,28 and ventricular myocytes.29 Similar wave-like [Ca2+]i dynamics evoked on systole were also observed by us in rat atria due to the nominal T-tubular distributions25 and in rat ventricles evoked under global, stop-flow ischemia,11 both of which led to beat-to-beat alternans of [Ca2+]i rises. Although the present study examined [Ca2+]i dynamics under relatively low-frequency ventricular excitations at only 2 Hz, it would be reasonable to assume that higher frequencies of excitation could augment the spatiotemporal non-uniformity due to the impaired Ca2+ release from the SR.
Regarding the precise mechanisms for the altered [Ca2+]i dynamics, undetermined is to what extent the Ca2+ is loaded in the SR in the infarct border zone of Purkinje fibers. This is because it is impossible to evaluate the contents of Ca2+ in the SR, for example, by rapid application of caffeine in our experimental setup, where no transient surge of Ca2+was observed, possibly due to the inability of burst activation of the ryanodine receptors. This is in agreement with Minamikawa et al.,30 who observed that 5 mM caffeine showed a gradual decline of the fluo3 fluorescence intensity of the ventricular myocardium in the perfused rat heart.
Comparison of Ca2+ Alternans Between 3–4 Days and 7–9 Days
We found that the beat-to-beat Ca2+ alternans was greater and more variable on acute, 3- to 4-day-old infarcted heart than on the 7- to 9-day-old, healing infarcted heart in terms of the “alternans ratio.” The reason for such sequential difference in the alternans ratio is uncertain. One possibility would be the influence on intercellular coupling. Given that the connexin-43 (Cx43) gap junctions are functionally depressed and altered in distribution in acute MI,31,32 intercellular communication via gap junctions may be impaired during acute necrotic phase, which could also augment beat-to-beat and cell-to-cell variability of Ca2+ transients. Thus, it is reasonable to consider that the variable [Ca2+]i dynamics at the early ischemic period might have been caused by impaired functions of Cx40, given that the Cx43 functions can be dramatically altered during myocardial ischemia.33 In addition, the myofibroblasts, a key constituent of the granulation tissue for replacement of the infarct myocardium, might have affected the functions of Purkinje fibers electrotonically in 7- to 9-day-old infarct, if heterocellular gap-junctional communications could be established during the healing phase of the MI.34 Although no studies have been reported on Purkinje fibers in their electrical coupling with (myo)fibroblasts, we assume that such heterocellular coupling, if it occurs, would modulate the electrical activities of Purkinje fibers, for example, depressed excitation and conduction, and prolonged action-potential duration as demonstrated by in vitro co-culture study using myocytes and fibroblasts35 and its computer simulation study,36 which would also lead to development of the non-uniform [Ca2+]i dynamics in Purkinje fibers. To further unveil the functional aspects of the Purkinje fiber network in the healing myocardial infarct, it would be required to examine the functional alterations of Cx40 gap junctions in between the Purkinje fibers and intercellular coupling with fibroblasts or surviving ventricular myocytes under ischemic conditions.
Limitations and Pathological Implications
We should note several limitations to this study. First, it is unclear whether the alternans phenomenon observed in this study can lead to genesis of ventricular arrhythmias in the human heart. We were unable to induce ventricular arrhythmias by fixed atrial pacing of the mouse infarcted hearts of small size, which might not have attained adequate conditions for the derangements of impulse propagation that lead to arrhythmias. Arrhythmogenic potentials of the post-MI mouse hearts have previously been published.37 Loss of arrhythmia generation in our study would be due to the experimental conditions, for example, excised heart perfused with blood-free solutions at relatively lower temperature under constant pacing at 2 Hz. It, therefore, remains to be determined whether and how Purkinje fibers in the infarct border zone lead to ventricular arrhythmogenesis in the post-MI mouse hearts. The non-physiological conditions, for example, under blood-free, ex-vivo, fluo4-loaded perfused hearts with incision of the ventricular free wall at room temperature, would also have influenced the outcome of this study. In addition, Purkinje fibers in the rodent hearts may be different from those in large mammals, where the fibers barely attain [Ca2+]i overload that could lead to Ca2+ oscillation for triggered activities. We should, therefore, note that our observations would not necessarily extrapolate to the hearts of large mammals including humans in that they possess relatively abundant T-tubules as compared with Purkinje fibers or atrial myocytes of rodents. Despite these limitations, however, it would be reasonable to surmise that infarct border zone Purkinje fibers are viable and can exhibit spatiotemporally non-uniform [Ca2+]i dynamics, the latter of which would be definitively distinct from the non-infarcted heart.
Finally, given that the beat-to-beat alternans of [Ca2+]i dynamics provide a close link to electrical alternans of action-potential waveforms38 and in clinical settings, and proarrhythmic index of T-wave alternans in electrocardiogram,39 our integrated study on both the [Ca2+]i dynamics and relevant histopathology in Purkinje fibers could indicate a condition that may constitute a substrate for generation of arrhythmias.
Supplementary Material
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TM developed the concept of this study, conducted experiments, analyzed the data, and wrote the manuscript. HT developed the concept of this study, integrated the data, and wrote the manuscript. H-IU and TT discussed the data and manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K08407 to HT and 16K08682 to TM).
Contributor Information
Taka-aki Matsuyama, Department of Pathology and Cell Regulation, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan.
Hideo Tanaka, Department of Pathology and Cell Regulation, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan.
Hatsue Ishibashi-Ueda, Department of Pathology, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan.
Tetsuro Takamatsu, Department of Medical Photonics, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Literature Cited
- 1. Tawara S. The conduction system of the mammalian heart: an anatomico-histological study of the atrioventricular bundle and the Purkinje fibers. London: Imperial College Press; 2000. [Google Scholar]
- 2. Miquerol L, Meysen S, Mangoni M, Bois P, van Rijen HV, Abran P, Jongsma H, Nargeot J, Gros D. Architectural and functional asymmetry of the His-Purkinje system of the murine heart. Cardiovasc Res. 2004;63:77–86. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka H, Hamamoto T, Takamatsu T. Toward an integrated understanding of the Purkinje fibers in the heart: the functional and morphological interconnection between the Purkinje fibers and ventricular muscle. Acta Histochem Cytochem. 2005;38:257–65. [Google Scholar]
- 4. Daniel TM, Boineau JP, Sabiston DC., Jr. Comparison of human ventricular activation with a canine model in chronic myocardial infarction. Circulation. 1971;44:74–89. [DOI] [PubMed] [Google Scholar]
- 5. Friedman PL, Stewart JR, Wit AL. Spontaneous and induced cardiac arrhythmias in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res. 1973;33:612–26. [DOI] [PubMed] [Google Scholar]
- 6. Horowitz LN, Spear JF, Moore EN. Subendocardial origin of ventricular arrhythmias in 24-hour-old experimental myocardial infarction. Circulation. 1976;53:56–63. [DOI] [PubMed] [Google Scholar]
- 7. Fenoglio JJ, Jr, Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL. Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation. 1983;68:518–33. [DOI] [PubMed] [Google Scholar]
- 8. Xing D, Martins JB. Triggered activity due to delayed afterdepolarizations in sites of focal origin of ischemic ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2004;287:H2078–84. [DOI] [PubMed] [Google Scholar]
- 9. Arnar DO, Bullinga JR, Martins JB. Role of the Purkinje system in spontaneous ventricular tachycardia during acute ischemia in a canine model. Circulation. 1997;96:2421–9. [DOI] [PubMed] [Google Scholar]
- 10. Boyden PA, Barbhaiya C, Lee T, ter Keurs HE. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka H, Takamatsu T. Spatiotemporal visualization of intracellular Ca2+ in living heart muscle cells viewed by confocal laser scanning microscopy. Acta Histochem Cytochem. 2003;36:193–204. [Google Scholar]
- 12. Hamamoto T, Tanaka H, Mani H, Tanabe T, Fujiwara K, Nakagami T, Horie M, Oyamada M, Takamatsu T. In situ Ca2+ dynamics of Purkinje fibers and its interconnection with subjacent ventricular myocytes. J Mol Cell Cardiol. 2005;38:561–9. [DOI] [PubMed] [Google Scholar]
- 13. Michael LH, Entman ML, Harley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol.1995;269:H2146–54. [DOI] [PubMed] [Google Scholar]
- 14. Salto-Tellez M, Yung Lim S, El-Oakley RM, Tang TP, ALmsherqi ZA, Lim SK. Myocardial infarction in the C57BL/6J mouse: a quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–7. [DOI] [PubMed] [Google Scholar]
- 15. Tamaddon HS, Vaidya D, Simon AM, Paul DL, Jalife J, Morley GE. High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ Res. 2000;87:929–36. [DOI] [PubMed] [Google Scholar]
- 16. Kim EE, Shekhar A, Lu Jia, Lin X, Liu F-Y, Zhang J, Delmar M, Fishman GI. PCP4 regulates Purkinje cell excitability and cardiac rhythmicity. J Clin Invest. 2014;124:5027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth Patricia, Thompson RP. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci. 1993;105:985–91. [DOI] [PubMed] [Google Scholar]
- 18. Di Malio A, Ter Keurs HE, Franzini Armstrong F. T-tubule profiles in Purkinje fibres of mammalian myocardium. J Muscle Res Cell Motil. 2007;28:115–21. [DOI] [PubMed] [Google Scholar]
- 19. Tanaka H, Matsuyama TA, Takamatsu T. Towards an integrated understanding of cardiac arrhythmogenesis—growing roles of experimental pathology. Pathol Int. 2017;67:8–16. [DOI] [PubMed] [Google Scholar]
- 20. Myers WW, Honig CR. Amount and distribution of Rb-86 transported into myocardium from ventricular lumen. Am J Physiol. 1966;211:739–45. [DOI] [PubMed] [Google Scholar]
- 21. Greenspan K, Cranefield PF. Influence of some factors on oxygen uptake of canine cardiac Purkinje fibers. Am J Physiol. 1963;204:5–8. [DOI] [PubMed] [Google Scholar]
- 22. Bagdonas AA, Stuckey JH, Piera J, Amer NS, Hoffman BF. Effects of ischemia and hypoxia on the specialized conducting system of the canine heart. Am Heart J. 1961;61:206–18. [DOI] [PubMed] [Google Scholar]
- 23. Kaneko T, Tanaka H, Oyamada M, Kawata S, Takamatsu T. Three distinct types of Ca2+ waves in Langendorff-perfused rat heart revealed by real-time confocal microscopy. Circ Res. 2000;86:1093–9. [DOI] [PubMed] [Google Scholar]
- 24. Tsujii E, Tanaka H, Oyamada M, Fujita K, Hamamoto T, Takamatsu T. In situ visualization of the intracellular Ca2+ dynamics at the border of the acute myocardial infarct. Mol Cell Biochem. 2003;248:135–9. [DOI] [PubMed] [Google Scholar]
- 25. Jiang Y, Tanaka H, Matsuyama TA, Yamaoka Y, Takamatsu T. Pacing-induced non-uniform Ca2+ dynamics in rat atria revealed by rapid-scanning confocal microscopy. Acta Histochem Cytochem. 2014;47:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JH. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol. 2001;531:301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kockskämper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius L. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Díaz ME, Eisner DA, O’Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–93. [DOI] [PubMed] [Google Scholar]
- 30. Minamikawa T, Cody SH, Williams DA. In situ visualization of spontaneous calcium waves within perfused whole rat heart by confocal imaging T. Am J Physiol Heart Circ Physiol. 1997;272:H236–43. [DOI] [PubMed] [Google Scholar]
- 31. Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–96. [DOI] [PubMed] [Google Scholar]
- 32. Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999;85:1046–55. [DOI] [PubMed] [Google Scholar]
- 33. Zeevi-Levin N, Barac YD, Reisner Y, Reiter I, Yaniv G, Meiry G, Abassi Z, Kostin S, Schaper J, Rosen MR, Resnick N, Binah O. Gap junctional remodeling by hypoxia in cultured neonatal rat ventricular myocytes. Cardiovasc Res. 2005;66:64–73. [DOI] [PubMed] [Google Scholar]
- 34. Takamatsu T. Arrhythmogenic substrates in myocardial infarct. Pathol Int. 2008;58:533–43. [DOI] [PubMed] [Google Scholar]
- 35. Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 2010;107:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Y, Garfinkel A, Camelliti P, Kohl P, Weiss JN, Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: a computational study. Heart Rhythm. 2009;6:1641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Betsuyaku T, Kanno S, Lerner DL, Schuessler RB, Saffitz JE, Yamada KA. Spontaneous and inducible ventricular arrhythmias after myocardial infarction in mice. Cardiovasc Pathol. 2004;13:156–64. [DOI] [PubMed] [Google Scholar]
- 38. Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res. 2015;116:846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanno K, Ryu S, Watanabe N, Minoura Y, Kawamura M, Asano T, Kobayashi Y, Katagiri T. Microvolt T-wave alternans as a predictor of ventricular tachyarrhythmias: a prospective study using atrial pacing. Circulation. 2004;109:1854–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.