Abstract
The microcirculation describes the smallest elements of the cardiovascular conducting system and is pivotal in the maintenance of homeostasis. Microcirculatory dysfunction is present early in the pathophysiology of sepsis, with the extent of microcirculatory derangement relating to disease severity and prognosis in ICU patients. However, at present microcirculatory function is not routinely monitored at the bedside. This article describes the pathophysiology of microcirculatory derangements in sepsis, methods of its measurement and evidence to support their clinical use.
Keywords: Microcirculation, sepsis, spectroscopy, near-infrared, infrared rays, microscopy, video
The microcirculation
The microcirculation comprises the smallest elements of the circulatory system, a dense network of arterioles, capillaries and venules whose diameter is less than 150 µm.1 It is present in all organs and is integral to the global functions of the cardiovascular system, which are the supply of nutrients and oxygen for aerobic metabolism, and removal of cellular waste products. The microcirculation also plays a pivotal role in the maintenance of homeostasis throughout the body including the autoregulation of blood flow to all organ systems, and temperature regulation by control of cutaneous blood flow.
Under physiological conditions, microcirculatory flow is controlled by both systemic and local mechanisms. Systemic mechanisms that coordinate circulatory function include the autonomic nervous system, the renin-angiotensin system and the vasopressin pathway (neuro-humoral mechanisms). These act by controlling both basal tone of the cardiovascular system and circulating plasma volume, responding in changes in both to maintain homeostasis. Local mechanisms regulating microcirculatory flow describe those molecules acting directly on local vascular smooth muscle. Vasoactive molecules released directly from the endothelium include eicosanoids (prostaglandins and thromboxane), nitric oxide (NO) and endothelin, released in response to shear stress acting on vessel walls. Release of NO can be stimulated by other vasoactive peptides including ADP, bradykinin, substance P and histamine. Vasodilator metabolites, (adenosine, hydrogen ions, potassium ions, CO2 and oxygen tensions) are also important in the local control of microcirculatory flow.
Sepsis and the microcirculation
Sepsis affects all elements of the microcirculation. It is associated with a decrease in capillary density and increased heterogeneity of perfusion caused by inappropriate vasodilatation and vasoconstriction, leading to decreased oxygen delivery, tissue hypoxia and organ dysfunction.2 Mechanisms of microcirculatory dysfunction in sepsis include arteriolar hyporesponsiveness and capillary dysfunction, leading to extravasation of fluid protein and neutrophils.
Mechanisms of microcirculatory dysfunction in sepsis
Nitric oxide dependent
Nitric oxide (NO) is thought to play a key role in the development of arteriolar hypo-responsiveness to vasoactive agents. Nitric oxide is generated from L-arginine via the enzyme nitric oxide synthase (NOS), of which there are three isoenzymes; nNOS (NOS1), iNOS (NOS2) and eNOS (NOS3). Vasomotor tone is maintained via continuous constitutive eNOS activity. NO binds to and activates guanylate cyclase in smooth muscle cells, catalysing the dephosphorylation of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP).
cGMP induces smooth muscle relaxation via a number of mechanisms:3
Inhibition of Ca2+ entry in to the cell and decrease in intracellular Ca2+ concentration via Ca2+ ATPase.
Activation of cell membrane K+ channels leading to hyperpolarisation and vascular smooth muscle relaxation.
Upregulation of cGMP-dependent protein kinases.
In sepsis, the inducible isoenzyme (iNOS) is activated by various cytokines and endotoxin via a calcium-independent process, generating large amounts of NO.4 This leads to arteriolar vasodilatation and micro/macrocirculatory dysfunction.
Capillary surface area
Capillary surface area and diffusion distances are major determinants of oxygen flux into cells. Under physiological conditions, in response to increasing oxygen demand, blood flow in capillaries becomes homogenous and plentiful, providing a significant safety margin against tissue hypoxia. In sepsis, however, capillary density is decreased and heterogeneous because of occlusion from non-deformable erythrocytes, neutrophil adhesion and a procoagulant state,4 leading to tissue hypoxia.
Reactive oxygen species
Production of ATP via the electron transport chain (oxidative phosphorylation) leads to the physiological generation of reactive oxygen species (ROS), including superoxide. Reactive oxygen species act as strong oxidising agents, reacting readily with other molecules leading to oxidative damage. Their activity is normally tightly regulated by the antioxidant protective mechanisms; however, sepsis causes an imbalance between generation and inactivation leading to accumulation of ROS, a process known as oxidative stress.5 Superoxide reacts with NO to form peroxynitrite, which is thought to be responsible for many of the microcirculatory abnormalities associated with sepsis,6 including endothelial dysfunction and capillary/venular fluid and protein leak.
The importance of microcirculatory assessment in sepsis
Several studies have demonstrated that: (a) improvements in the microcirculatory function in sepsis after early resuscitation are associated with a decreased incidence of organ dysfunction7 and (b) persistent microcirculatory dysfunction after resuscitation is associated with worse outcomes.8,9 However, the microcirculation is difficult to monitor in practice and so current resuscitation goals rely on the monitoring and restoration of macro-haemodynamic values (such as systemic arterial pressure, cardiac output, heart rate), along with restoration of organ perfusion (inferred from normalisation of serum lactate and ScVO2).10 Moreover, restoration of macro-haemodynamic variables such as arterial pressure, especially with vasoactive agents such as noradrenaline, does not guarantee improvements in microcirculatory flow; in fact, noradrenaline can inhibit microcirculatory function irrespective of the presence of hypotension.11
Assessment of the microcirculation
Currently, there are no well-established means of detecting and monitoring microcirculatory dysfunction in clinical practice; available methods can be divided into indirect, direct and dynamic techniques (Table 1).
Table 1.
Methods of microcirculatory assessment.
| Indirect methods |
| Measuring elements of tissue oxygenation |
| Transcutaneous tissue PO2 |
| SvO2 |
| Tissue CO2 (gastric tonometry, transcutaneous CO2) |
| Lactate physiology |
| Near-infrared spectroscopy (NIRS) |
| Direct methods |
| Measuring microcirculatory perfusion |
| Laser Doppler |
| Videomicroscopic techniques |
| Orthogonal polarisation spectral (OPS) imaging |
| Sidestream dark-field (SDF) imaging |
| Incident dark-field (IDF) imaging – CytoCam |
| Dynamic methods |
| Vascular occlusion tests (VOT) |
Indirect measures
Indirect measures can be broadly defined as those which assess tissue oxygenation as a surrogate for microcirculatory function and include SvO2, transcutaneous PO2, tissue CO2 (gastric tonometry and transcutaneous CO2 – not discussed further here), near-infrared spectroscopy and measures of cellular anaerobic metabolism such as serum lactate.
Lactate physiology as a measure of microcirculatory function
Under aerobic conditions, pyruvate is converted to acetyl coenzyme A (acetyl CoA) via the enzyme pyruvate dehydrogenase. Acetyl CoA enters Krebs cycle leading to the formation of multiple cofactors. Cofactors undergo oxidative phosphorylation, with a net generation of 36 molecules of ATP. Under anaerobic conditions, pyruvate is converted to lactic acid via the enzyme lactate dehydrogenase, regenerating NAD+ and allowing ongoing glycolysis and ATP generation. In aqueous solution and at physiological pH, lactic acid is almost completely dissociated in to lactate and hydrogen ions.12
Several studies have demonstrated the relationship between tissue hypoxia and lactate production,13 with a sharp rise in lactate concentration when oxygen consumption becomes limited by oxygen delivery.14 Delivery of oxygen to tissues takes place via the microcirculation and, therefore, microcirculatory dysfunction can be inferred from the hyperlactataemia that occurs with tissue hypoxia. Measurement of serum lactate is integral in the diagnosis and management of patients with sepsis, but its use as a specific measure of tissue hypoxia has problems. Serum lactate concentrations depend on the balance between production and clearance. Stimulation of aerobic glycolysis for any reason increases lactate without microcirculatory dysfunction, a process which can occur in the early phases of sepsis due to increased endogenous or exogenous catecholamine concentrations. Long-term beta-blocker therapy has been shown to decrease blood lactate levels in patients presenting with severe sepsis.15 Decreased hepatic metabolism and decreased renal clearance will also lead to increased lactate concentrations irrespective of microcirculatory dysfunction.
Near-infrared spectroscopy
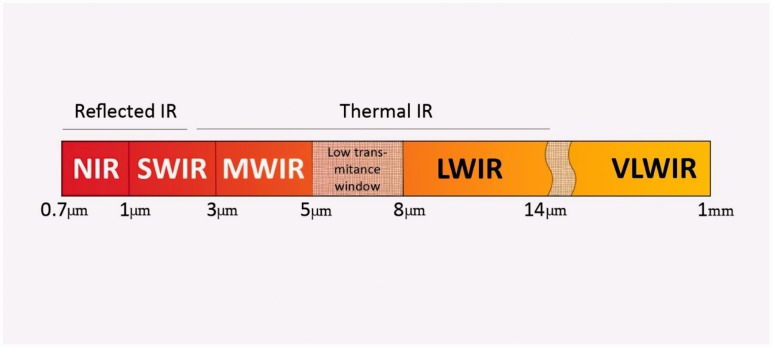
Near-infrared spectroscopy (NIRS) utilises the attenuation of non-visible wavelengths of light by oxygenated and de-oxygenated haemoglobin. The near-infrared (NIR) spectrum lies between the wavelengths of 700–1000 nm (Figure 1), with the clinically utilised wavelengths between 700 and 850 nm. Unlike plethysmographic pulse oximetry, NIRS uses the attenuation of both infrared and NIR light by oxygenated and deoxygenated haemoglobin. The attenuation of light by a chromophore is proportional to its absorption coefficient and the path-length of the light, as determined by the Beer–Lambert laws16 (Figure 2). If all other components of the law are known, then the concentration of the chromophores in question can be determined. NIR provides a non-invasive means of continually assessing tissue oxygen saturation (StO2) and, therefore, indirectly microcirculatory function.
Figure 1.
Infrared electromagnetic spectrum. N: near; SW: short wave; MW: mid wave; LW: long wave; VLW: very long wave.
Figure 2.
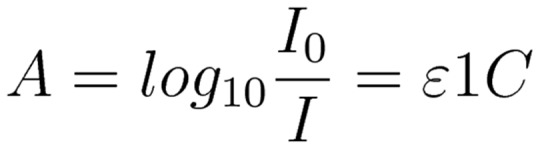
Beer–Lambert Law. A: absorbance; I: intensity of light; ɛ: molar absorptivity; l: length of solution the light passes through; c: concentration of solution.
Bazerbashi et al.17 demonstrated that a spot/static (single time-point) StO2 value of <70% at presentation to the Emergency Department was associated with a 2.87 times increase in ICU admission compared to those with StO2 of >70%. Data from our own group demonstrated that in patients presenting to the Emergency Department, spot StO2 values did not improve following resuscitation with iv fluids in non-survivors, and mortality was also increased two-fold in patients with an absolute StO2 value <75% after resuscitation.18 Conversely, a number of studies have demonstrated an overlap in static StO2 measurements between those patients with sepsis (of varying severity) and healthy volunteers.19,20 Doerschug et al.21 compared StO2 in 24 patients with severe sepsis and 15 healthy volunteers, demonstrating similar baseline StO2 values of 82% and 84% respectively.
Direct measures
Most direct measures of the microcirculation encompass techniques involving highly sensitive video microscopes. Video microscopic techniques provide in vivo visualisation of the microcirculation, allowing direct measurement of capillary density, perfusion and flow dynamics. Abnormalities of the microcirculation detected by video microscopy have been associated with an increased in hospital mortality in unselected ICU patients.22 However, until recently these techniques, which included orthogonal polarisation spectral (OPS) and sidestream dark-field (SDF) imaging, have been clinically inaccessible owing to their large size, operator-dependent output and requirement for time-consuming offline analysis to generate data.
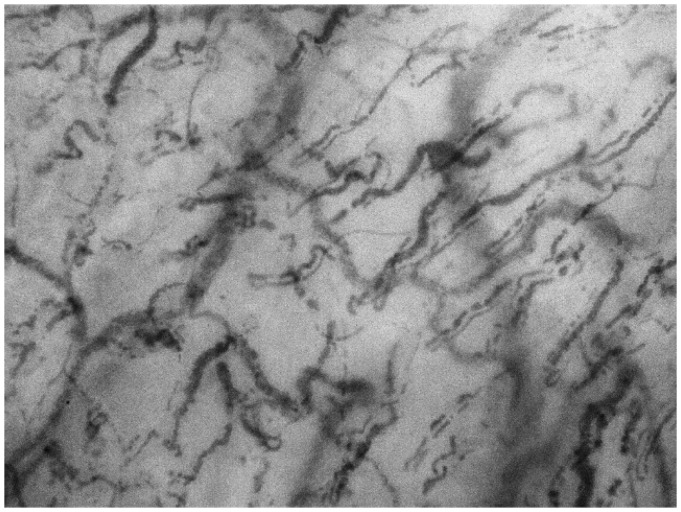
A third-generation handheld microscope has recently been developed (CytoCam-IDF, Braedius Medical, NL), utilising incident dark-field (IDF) illumination and real-time automated digital image analysis (Figure 3). Dark-field microscopy allows visualisation of the microcirculation by means of epi-illumination, without the requirement for illumination from below the tissue (as would be required in standard bright field microscopy). This new device employs a revolutionised hardware platform incorporating a high-resolution sensor displaying an image area of 1.55 × 1.16 mm2, almost twice the size of earlier devices, alongside a vastly improved resolution and a stepping motor for automatic focusing to within 2 µm of the target.23 IDF-based microscopy has been shown to correlate well with the established techniques;24 however, there are no data as to its usefulness as a diagnostic instrument, nor regarding what values of microcirculatory function are considered ‘normal.’
Figure 3.
IDF image of the sublingual microcirculation.
Analysis of the sublingual microcirculation
A round table discussion in 2006 by experts in the field sought to clarify the features that should be included in microcirculatory analysis, concluding that assessment of the microcirculation should include measures of vascular density, assessment of capillary perfusion and a heterogeneity index25 (in vessels of <20 µm diameter) (Table 2). In sepsis, perfused vessel density (PVD), proportion of perfused vessels (PPV), microvascular flow index (MFI), are all decreased and heterogeneity index (HI) increased, irrespective of the macro-haemodynamic condition.26 Total capillary density appears to be unaffected by sepsis. These findings were corroborated by the International Study on Microcirculatory Shock Occurrence in Acutely Ill Patients (microSOAP).27
Table 2.
Microcirculatory variables.
| Microcirculation variable | Information provided | Measurement |
|---|---|---|
| Microvascular flow index (MFI) | Perfusion quality | Image divided in to quadrants. A number is assigned to each quadrant according to predominant flow type (0 = no flow, 1 = intermittent, 2 = sluggish, 3 = continuous). The MFI results from the mean of the four values. |
| Total vessel density (TVD) | Vessel density | The total length of the vessels divided by the total surface area of the analysed area. |
| Perfused vessel density (PVD) | Functional vessel density | Total length of perfused vessels (MFI score 2/3) divided by the analysed area. |
| Proportional of perfused vessels (PPV) | Perfusion quality | 100 * number of perfused vessels divided by the total number of vessels. |
| Heterogeneity index (HI) | Measure of the heterogeneity of flow between vessels. | Highest MFI – lowest MFI divided by mean MFI across all sites analysed. |
The microSOAP study is the largest trial to date investigating the significance of microcirculatory alterations in a heterogeneous ICU population (not just those with sepsis). This study found that of the 501 patients included for analysis, 86 (17%) had an abnormal MFI (defined a priori as <2.6). Of those with an abnormal MFI, the HI was increased with decreased PPV and PVD. Total vessel density was not affected. Abnormal MFI in conjunction with tachycardia was associated with an increased mortality (OR 3.24).
Dynamic methods of assessment
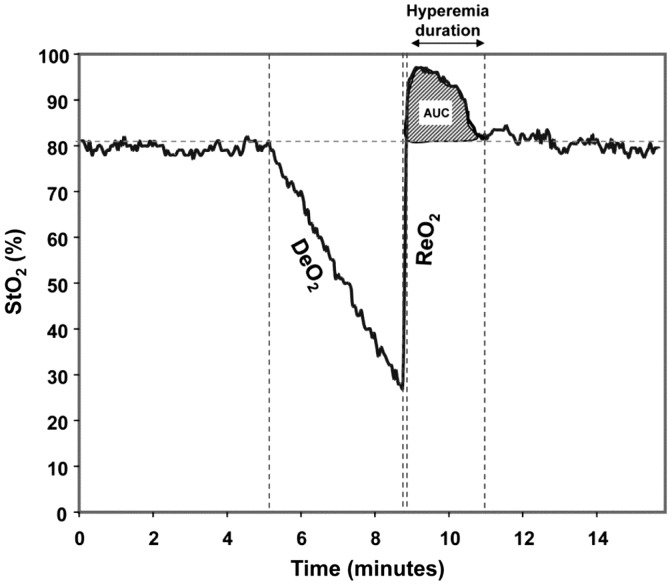
Along with continuous ‘static’ measurements of tissue oxygenation, ‘dynamic’ measures (although still indirect), can be obtained after brief periods of arterial occlusion by means of a blood pressure cuff inflated above systolic pressure (a vascular occlusion test (VOT)). Plotting StO2 against time allows the derivation of desaturation, re-saturation and hyperaemic slopes, correlating to oxygen consumption (VO2) and microvascular reactivity28 allowing us to interrogate the function of microcirculation in more detail (Figure 4).
Figure 4.
Desaturation and resaturation slopes after a VOT.29
Specific alterations in dynamic measurements occur in patients with sepsis, the most sensitive of which appears to be the resaturation slope after a period of stagnant hypoxia. Skarda et al.30 compared StO2 recovery slopes in age-matched healthy volunteers to patients with severe sepsis. StO2 recovery slope (%/second) was found to be 3.3 [0.7] in the volunteer group as compared to 2.3 [1.0] in the severe sepsis group (p = 0.05). A meta-analysis by Neto et al. demonstrated a decrease in the StO2 recovery slopes between healthy controls (245 patients), severe sepsis (160 patients) and septic shock (455 patients), these being 5.19 [2.86], 3.27 [0.71] and 2.45 [0.41] %/second, respectively (p = 0.008). Not only is this feature characteristic of sepsis, it correlates with outcome: the slope of the recovery phase was significantly reduced in non-survivors.31–33
Despite these studies providing evidence as to the usefulness of NIRS in measuring disease severity in sepsis, dynamic NIRS measurements have not yet been investigated as a potentially useful diagnostic tool in the early phases of sepsis. NIRS allows investigation of the microcirculation via non-invasive means, allowing potential use in patients who would otherwise be unsuitable for invasive monitoring in intensive care.
Clinical examination
Examination of the skin is done in clinical practice to evaluate the function of the microcirculation. Cool peripheries, delayed capillary refill time and skin mottling are used as indicators of reduced peripheral perfusion/circulatory failure. Mottling is an irregular patchy discolouration of the skin caused by heterogeneous blood flow and has been assessed as a potential prognostic feature in sepsis.
Two small studies by Ait-Oufella et al.34,35 investigated skin mottling over the anterior surface of the knee in patients with a clinical diagnosis of sepsis. Mottling was a frequent clinical finding in patients with septic shock (46% and 70% in these studies), and was associated with increased mortality. Coudroy et al.36 performed a prospective observational study of this clinical finding in intensive care patients: those with septic shock and mottling had an almost four-fold increase in ICU mortality. However, the assessment of mottling in these studies was entirely subjective and not investigated before admission to ICU. Mottling cannot always be seen in patients with darker skin pigmentation.
Skin temperature
Microcirculatory function is important in the maintenance and regulation of body temperature, with skin blood flow varying from 1 to 150 mL.100g−1.min−1, via local and systemic neurohumoral mechanisms.37 Microcirculatory dysfunction with loss of autoregulation can lead to core body temperature derangements during sepsis. Therefore, measurement of skin temperature may be a useful means of non-invasively assessing microcirculatory function, with infrared thermal imaging a potential method of quantifying this.
The potential role of infrared thermal imaging
Infrared thermal imaging describes the acquisition of passively emitted infrared radiation and its pictorial representation as an infrared image. Images are colour-coded to represent the relative intensity of radiated energy and therefore temperature according to the Stefan–Boltzmann laws. Thermal imaging provides a highly accurate (<0.02°C between pixels), two dimensional and non-contact means of demonstrating temperature distribution. Thermal imaging has been used in various areas of medicine including the diagnosis of breast cancer and a variety of rheumatological conditions, but there is little published evidence of its use in septic illness despite an obvious potential to monitor the cutaneous microcirculation. The sensitivity in thermal imaging cameras may allow quantification of cutaneous temperature gradients (and therefore the underlying microcirculation) before this becomes apparent clinically. This includes the ability to detect ‘thermal mottling’ before it is apparent to the clinician’s naked eye.
Summary
Microcirculatory derangement is common in patients with sepsis and cannot necessarily be predicted from macro-haemodynamic values. Improvement in macro-haemodynamic values in the critically ill does not imply improvement in microcirculatory flow and patients whose microcirculation fails to improve following resuscitation are at increased risk of mortality. Detection of microcirculatory dysfunction may aid diagnosis and risk stratification in patients with sepsis; restoration of the function of the microcirculation may be a useful therapeutic target for resuscitation but further data are needed.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Scheeren TW. Journal of Clinical Monitoring and Computing 2015 end of year summary: Tissue oxygenation and microcirculation. J Clin Monit Comput 2016; 30: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit Care Med 2013; 41: 791–799. [DOI] [PubMed] [Google Scholar]
- 3.Guix FX, Uribesalgo I, Coma M, et al. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 2005; 76: 126–152. [DOI] [PubMed] [Google Scholar]
- 4.Lush CW, Kvietys PR. Microvascular dysfunction in sepsis. Microcirculation 2000; 7: 83–101. [DOI] [PubMed] [Google Scholar]
- 5.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 2011; 107: 57–64. [DOI] [PubMed] [Google Scholar]
- 6.Sharawy N, Lehmann C. New directions for sepsis and septic shock research. J Surg Res 2014. [DOI] [PubMed] [Google Scholar]
- 7.Trzeciak S, McCoy JV, Phillip Dellinger R, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 2008; 34: 2210–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007; 49: 88–98, 98.e1-2. [DOI] [PubMed] [Google Scholar]
- 9.Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004; 32: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: A prospective study. Crit Care 2009; 13: R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phypers B, Pierce TJM. Lactate physiology in health and disease. Contin Educ Anaesth Crit Care Pain 2006; 6: 128–132. [Google Scholar]
- 13.Bakker J, Vincent JL. The oxygen supply dependency phenomenon is associated with increased blood lactate levels. J Crit Care 1991; 6: 152–159. [Google Scholar]
- 14.Ronco JJ, Fenwick JC, Tweeddale MG, et al. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 1993; 270: 1724–1730. [PubMed] [Google Scholar]
- 15.Contenti J, Occelli C, Corraze H, et al. Long-term beta-blocker therapy decreases blood lactate concentration in severely septic patients. Crit Care Med 2015; 43: 2616–2622. [DOI] [PubMed] [Google Scholar]
- 16.Umeyama S, Yamada T. New cross-talk measure of near-infrared spectroscopy and its application to wavelength combination optimization. J Biomed Opt 2009; 14: 034017. [DOI] [PubMed] [Google Scholar]
- 17.Bazerbashi H, Merriman KW, Toale KM, et al. Low tissue oxygen saturation at emergency center triage is predictive of intensive care unit admission. J Crit Care 2014; 29: 775–779. [DOI] [PubMed] [Google Scholar]
- 18.Vorwerk C, Coats TJ. The prognostic value of tissue oxygen saturation in emergency department patients with severe sepsis or septic shock. Emerg Med J 2012; 29: 699–703. [DOI] [PubMed] [Google Scholar]
- 19.Pareznik R, Knezevic R, Voga G, et al. Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med 2006; 32: 87–92. [DOI] [PubMed] [Google Scholar]
- 20.Nanas S, Gerovasili V, Renieris P, et al. Non-invasive assessment of the microcirculation in critically ill patients. Anaesth Intensive Care 2009; 37: 733–739. [DOI] [PubMed] [Google Scholar]
- 21.Doerschug KC, Delsing AS, Schmidt GA, et al. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol 2007; 293: H1065–1071. [DOI] [PubMed] [Google Scholar]
- 22.Vellinga NA, Boerma EC, Koopmans M, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med 2015; 43: 48–56. [DOI] [PubMed] [Google Scholar]
- 23.Braedius Medical. How it works/CytoCam-IDF, http://www.braedius.com/magnoliaPublic/braedius/products/cytoCam/how-it-works.html (accessed 11 May 2016).
- 24.Aykut G, Veenstra G, Scorcella C, et al. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp 2015; 3: 40-015-0040-7, Epub ahead of print 31 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: Report of a round table conference. Crit Care 2007; 11: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edul VS, Ince C, Navarro N, et al. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann Intensive Care 2014; 4: 39-014-0039-3, eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vellinga NA, Boerma EC, Koopmans M, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med 2015; 43: 48–56. [DOI] [PubMed] [Google Scholar]
- 28.Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care 2009; 13: S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez H, Mesquida J, Simon P, et al. Characterization of tissue oxygen saturation and the vascular occlusion test: Influence of measurement sites, probe sizes and deflation thresholds. Crit Care 2009; 13: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skarda DE, Mulier KE, Myers DE, et al. Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock 2007; 27: 348–353. [DOI] [PubMed] [Google Scholar]
- 31.Neto AS, Pereira VG, Manetta JA, et al. Association between static and dynamic thenar near-infrared spectroscopy and mortality in patients with sepsis: A systematic review and meta-analysis. J Trauma Acute Care Surg 2014; 76: 226–233. [DOI] [PubMed] [Google Scholar]
- 32.Payen D, Luengo C, Heyer L, et al. Is thenar tissue hemoglobin oxygen saturation in septic shock related to macrohemodynamic variables and outcome? Crit Care 2009; 13: S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro NI, Arnold R, Sherwin R, et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Crit Care 2011; 15: R223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ait-Oufella H, Joffre J, Boelle PY, et al. Knee area tissue oxygen saturation is predictive of 14-day mortality in septic shock. Intensive Care Med 2012; 38: 976–983. [DOI] [PubMed] [Google Scholar]
- 35.Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011; 37: 801–807. [DOI] [PubMed] [Google Scholar]
- 36.Coudroy R, Jamet A, Frat JP, et al. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med 2014. [DOI] [PubMed] [Google Scholar]
- 37.Venus M, Waterman J, McNab I. Basic physiology of the skin. Surgery (Oxford) 2010; 28: 469–472. [Google Scholar]