Abstract
Background:
Bone is one of the most common sites of metastases, with bone metastases-related pain representing a significant source of morbidity among patients with cancer. Magnetic resonance–guided focused ultrasound is a noninvasive, outpatient modality with the potential for treating painful bone metastases. The aim of this study is to report our initial experience with magnetic resonance–guided focused ultrasound in the treatment of bone metastases and our preliminary analysis of urinary cytokine levels after therapy.
Methods:
This was a single-center pilot study of 10 patients with metastatic cancer to investigate the feasibility of magnetic resonance–guided focused ultrasound for primary pain control in device-accessible skeletal metastases. Treatments were performed on a clinical magnetic resonance–guided focused ultrasound system using a volumetric ablation technique. Primary efficacy was assessed using Brief Pain Inventory scores and morphine equivalent daily dose intake at 3 time points: before, day 14, and day 30 after the magnetic resonance–guided focused ultrasound treatment. Urine cytokines were measured 3 days before treatment and 2 days after the treatment.
Results:
Of the 10 patients, 8 were followed up 14 days and 6 were followed up 30 days after the treatment. At day 14, 3 patients (37.5%) exhibited partial pain response and 4 patients (50%) exhibited an indeterminate response, and at day 30 after the treatment, 5 patients (83%) exhibited partial pain response. No treatment-related adverse events were recorded. Of the urine cytokines measured, only Transforming growth factor alpha (TGFα) demonstrated an overall decrease, with a trend toward statistical significance (P = .078).
Conclusion:
Our study corroborates magnetic resonance–guided focused ultrasound as a feasible and safe modality as a primary, palliative treatment for painful bone metastases and contributes to the limited body of literature using magnetic resonance–guided focused ultrasound for this clinical indication.
Keywords: bone metastases, magnetic resonance–guided focused ultrasound, high-intensity-focused ultrasound, magnetic resonance thermometry, Brief Pain Inventory
Introduction
Bone is one of the most common sites of metastases for all cancers, with the incidence of bone metastases as high as 75% in patients with breast and prostate cancer with advanced metastatic disease and as high as 40% in patients with lung cancer with advanced disease.1,2 Not only do bone metastases indicate a poorer prognosis, but these patients often present with significant pain, which severely impacts their quality of life.3,4 Other complications include pathologic fractures, spinal cord compression, and hypercalcemia. As increasingly effective systemic therapies continue to lengthen the survival of patients with metastases , the burden of disease has considerably increased, which necessitated novel therapies for bone metastases–related pain to improve the quality of life.5 Previous studies suggest that the pain related to bone metastases is currently undertreated.5–8
Currently, the standard of care for patients with painful localized bone metastases is external beam radiotherapy (EBRT) in conjunction with other systemic therapies and analgesics. However, up to 30% of patients treated with radiotherapy do not respond to therapy, and in 30% of responders the pain recurs at some point after treatment.9–13 In such cases, further treatment options are limited but include reirradiation, surgical intervention, and percutaneous cryoablation.14–16 Retreatment with radiotherapy is also limited by cumulative doses delivered to critical structures, while ablative techniques such as cryotherapy and percutaneous radiofrequency ablation are invasive procedures with associated risks and complications.
Magnetic resonance–guided focused ultrasound (MRgFUS) is a noninvasive, outpatient modality with the potential for treating painful bone metastases. In high-intensity-focused ultrasound (HIFU) therapy, a high-energy ultrasound transducer is used to deliver acoustic energy into a target site, with the goal of heating lesions. Ablation is achieved when target the tissue temperatures reach more than 57°C. Thermal periosteal denervation of the bone is thought to be the mechanism of action for pain relief. A secondary mechanism may be due to the thermal ablation of the tumor mass that decreases pressure on the surrounding tissue.17–19 Tumor response and its ongoing effects may be due to a decrease in circulating immunosuppressive cytokines.20 One advantage of MR-HIFU is the ability for repeated treatments in the setting of recurrence as there is no dose limit for focused ultrasound energy. Another major advantage is that MR image (MRI) guidance allows for precise control of the tumor tissue thermal ablation with real-time thermal mapping.
Preliminary clinical studies on the use of HIFU for palliation of painful bone metastases demonstrated excellent response rates and safety in heterogeneous groups of patients with painful bone metastases.17–19,21–31 The aim of this study is to report our initial experience with MRgFUS for the treatment of bone metastases with respect to its efficacy in improving pain and functional scores as measured on the Brief Pain Inventory (BPI) and our preliminary analysis of urinary cytokines levels after therapy.
Materials and Methods
Study Design and Population
This trial was approved by the local institutional ethics review board. Informed consent was obtained from all patients prior to ablation.
This study was a single-center pilot study to investigate the feasibility of MRgFUS for primary pain control in device-accessible skeletal metastases. Patients were aged at least 18 years with a pain score related to the target lesion of 4 or greater on a 0- to 10-point scale, irrespective of medications. Bone metastases had to be uncomplicated and visible by noncontract MR imaging and be at least 1 cm from the skin, nerve bundles, bladder, and bowel. Table 1 outlines the demographic and tumor characteristics of patients.
Table 1.
Demographic and Tumour Characteristics of Patients.
| ID | Age | Gender | Primary Cancer Site | Target Site | Cortex |
|---|---|---|---|---|---|
| 1 | 61 | F | Breast | Left scapula | Partially intact |
| 2 | 77 | M | Prostate | Left iliac crest | Partially intact |
| 3 | 45 | F | Breast | Right iliac crest | Intact |
| 4 | 72 | M | Neuroendocrine NOS | Right femur | Intact |
| 5 | 68 | F | Liver | Left hip | Intact |
| 6 | 78 | M | Esophagus | Left hip | Intact |
| 7 | 69 | F | Pancreas | Right iliac crest | Intact |
| 8 | 42 | M | Lung | Left ribs | Intact |
| 9a | 62 | M | Orbit | Right iliac crest | Baseline CT scan not available |
| 10 | 71 | M | Prostate | Right scapula | Intact |
Abbreviations: CT, computed tomography; F, female; M, male; NOS, not otherwise specified.
aPatient dropped out of the study after the treatment and was lost to follow up.
Patients were excluded from the study if they had any prior local therapy to the target lesion (including radiotherapy, surgery, ablative therapy, etc), the lesion was complicated (fracture, spinal cord compression, soft tissue component, etc), the target lesion was located in the skull, spine (excluding the sacrum), or sternum, there was a scar or orthopedic implants along the proposed MRgFUS path, the patient had an active infection or other serious systemic disease, the patient was unable to tolerate the required stationary position during treatment, or the patient had contraindications to MRI or gadolinium (Gd)-based contrast agents.
Treatment Procedure
On the treatment day, the hair on the skin close to the lesion was shaved. A gel pad (Aquaflex; Parker Laboratories, Fairfield, New Jersey) was used between the transducer membrane and the skin to provide a good acoustic coupling. Patients were given conscious sedation according to the institutional guidelines. Patients received midazolam intravenously for sedation and fentanyl intravenously for pain control. Each patient was administered with enough fentanyl to proceed pain free with HIFU treatments and sufficient midazolam to remain alert during treatments to indicate if they were having any pain. Patients were instructed to terminate the sonication with a button controller when the pain was intolerable.
Previously acquired bone scans and computed tomography (CT) scans were used to identify the location of the lesion that caused the pain and confirmed by pretreatment MR scans. The lesions in the bone were located as low-intensity signals (dark areas) in T1-weighted MRIs (gradient echo sequence, TR = 3.5 ms, TE = 2.3 ms, and slice thickness 2.5 mm).
For MR thermometry, fat-saturated echo-planar imaging sequence (TE = 16 ms and temporal resolution 4.1 s) was applied at 4 planes: 3 orthogonal planes across the focus along the acoustic axes and 1 freely oriented plane, normally positioned at the muscle layer behind the subcutaneous fat to monitor the skin heating. After the treatment, Gd-enhanced (Magnevist, 0.2 mL/kg; Bayer, Toronto, Canada) T1-weighted images (gradient echo sequence, TR = 7 ms and TE = 3.5 ms) were acquired to verify the treatment effect. Features that indicated a positive treatment effect included assessment of the nonperfused volume at the bone–muscle interface and contrast extravasation, a marker of a heat-induced increase in vascular permeability.
Treatments were performed on a clinical MRgFUS system (Philips Sonalleve; Philips Healthcare, Vantaa, Finland) by a team that included a radiation oncologist (G.C.), nursing support as well as an HIFU technical team (Y.H. and C.M.). The system consists of a 256-channel FUS phased array of 12 cm in diameter and 12 cm radius of curvature, operating at 1.2 or 1.44 MHz under the 3-Tesla MR scanner (Achieva; Philips Healthcare). At 1.2 or 1.44 MHz, the size of the natural focus was less than 2 mm in diameter in the lateral dimension. To enlarge the treatment volume, the system electronically steered the ultrasound beam in the lateral plane in a concentric order, which is known as volumetric ablation. Based on the diameter of the steering, the system defined the size of the treatment cells as 4, 8, 12, and 16 mm. Using the ablation approaches described by Huisman et al, a direct approach was used in 6 patients, whereas a combination of a near-field and direct approach was used in 4 patients.21 At the time patients 1 to 4 were treated, the system was configured with only 4-mm cells available and using the near-field approach was assumed with this design. However, in practice, it was difficult to estimate the necessary compensation of power levels using the near-field approach within a reasonable safety margin (up to 100 W). Since the direct approach had poor ability to measure heating on MR thermometry with 4-mm cells due to the small heating volume at the bone–muscle interface, larger cells (8 mm and 12 mm) were enabled for patients 5 to 10 to allow the direct approach, which was considered the best way to induce a large heating volume at a relatively low-power level (10-30 W). The change from a near approach to a direct approach was part of the evolving technical development in collaboration with Philips Healthcare.
Efficacy and Safety End points
Primary efficacy was assessed at 2 time points: day 14 and day 30 after the MRgFUS treatment. Primary end points included BPI scores and morphine equivalent daily dose intake, which were collected at baseline and each of these time points and compared according to the previously established standards and end points as published by the updated international consensus on palliative radiotherapy end points for future clinical trials in bone metastases (Table 2).32,33 The BPI includes pain scores as well as assessment of functional interference related to pain.34 In addition, urine cytokines—including interleukin 1β (IL-1β), IL-6, tumor necrosis factor α (TNF-α), IL-4, IL-10, and TGFα—were measured 3 days before treatment and 2 days after treatment. A paired t test was used to compare the urine cytokine levels measured 3 days prior to treatment with levels measured 2 days after treatment. Computed tomography scans were obtained at day 30 to assess imaging response to treatment. Treatment-related adverse events (AEs) were monitored during the treatment and at predefined end points according to the study protocol—days 0, 2, and 7, and 14, 30, and 90.
Table 2.
Updated International Consensus on Palliative Radiotherapy End Points for Future Clinical Trials in Bone Metastases.
| Response Categories | |
|---|---|
| Complete response | A pain score of 0 at the treated site with no concomitant increase in analgesic intake (stable or reducing analgesics in daily oral morphine equivalent). |
| Partial response | Pain reduction of 2 or more at the treated site on a scale of 0 to 10 without analgesic increase, or analgesic reduction of 25% or more from baseline without an increase in pain. |
| Pain progression | Increase in pain score of 2 or more above baseline at the treated site with stable daily oral morphine equivalent, or an increase of 25% or more in daily oral morphine equivalent compared with baseline with the pain score stable or 1 point above baseline. |
| Indeterminate response | Any response that is not captured by the complete response, partial response, or pain progression definitions. |
Results
From February 2011 to March 2012, a total of 10 patients (4 women and 6 men; median age [range], 68.5 [42-78] years) with painful bone metastases from primary tumors of the breast (n = 2), prostate (n = 2), lung (n = 1), liver (n = 1), pancreas (n = 1), esophagus (n = 1), neuroendocrine not otherwise specified (n = 1), and orbit (n = 1) were enrolled and treated with MRgFUS at our department (Table 1).
Treatment Procedure
Of the 10 patients who underwent the MRgFUS treatment, 8 patients (80%) were followed up 14 days after treatment, 6 patients (60%) were followed up 30 days after treatment, and 1 patient (10%) was dropped out of the study and was lost to follow up after the treatment. All but 1 patient presented with multiple bone metastases.
The diameter of the target bone metastases ranged from 13 to 74 mm (mean, 32 mm); they were predominantly mixed lesions (9/10; 90%) and only 1 (10%) of 10 were osteolytic. Table 1 outlines the location of lesions.
The mean (standard deviation [SD]) number of sonication was 24 (12; range, 8-46) and the total room time varied between 115 and 230 minutes. The mean (SD) of the maximum sonication energy used per sonication was 976 J (478; range, 400-1920 J). Table 3 provides further details of the treatment parameters.
Table 3.
Treatment Parameters.
| ID | Duration, min | N, Treatment cells (n, Sonication) | Treatment Cell Diameter, mm | Acoustic Power, W (Mean, Range) | Sonication Energy, kJ; Mean | Total Delivered Energy, kJ | Ablation Approach |
|---|---|---|---|---|---|---|---|
| 1 | 70 | 30 (32) | 4 | 49 (30-50) | 1.0 | 30.1 | Direct/near fielda |
| 2 | 124 | 51 (51) | 2 and 4 | 65 (25-100) | 1.3 | 66.3 | Direct/near fielda |
| 3 | 83 | 19 (25) | 4 | 35 (20-40) | 0.7 | 14.6 | Direct/near fielda |
| 4 | 115 | 35 (42) | 4 | 54 (30-80) | 1.1 | 42.2 | Direct/near fielda |
| 5 | 80 | 26 (40) | 4, 8, and 12 | 12 (10-20) | 0.3 | 10.5 | Direct |
| 6 | 56 | 11 (16) | 4 and 12 | 22 (15-30) | 0.6 | 8.2 | Direct |
| 7 | 49 | 14 (18) | 4 and 12 | 38 (30-50) | 1.4 | 20.3 | Direct |
| 8 | 103 | 26 (29) | 4 | 18 (5-30) | 0.4 | 9.2 | Direct |
| 9 | 112 | 27 (33) | 12 | 21 (15-25) | 1.0 | 28.6 | Direct |
| 10 | 32 | 11 (12) | 4 and 8 | 27 (20-40) | 0.7 | 7.4 | Direct |
aDirect and near-field ablation approaches were used in the different sonication in these patients.
In 8 of the 10 treatments, patients pressed the stop button due to pain, including 1 time in 1 treatment, 2 times in 1 treatment, 3 times in 2 treatments, 4 times in 1 treatment, 5 times in 1 treatment, 6 times in 1 treatment, and 15 times in 1 treatment.
Treatment Efficacy
While 8 of 10 patients did experience enough pain during the procedure to require the stop button, no treatment-related AEs (including skin ulceration or burns) were recorded immediately after the treatment or during the 30-day follow-up period at physical examination or at patient interview. At day 14, 3 (37.5%) out of 8 patients exhibited partial pain response, 4 (50%) out of 8 patients exhibited an indeterminate response, and 1 (12.5%) out of 8 patients exhibited pain progression. At day 30, 5 (83%) out of 6 patients exhibited partial pain response, while 1 (17%) out of 6 patients exhibited a complete response.
Among the partial pain responders and indeterminate responders, the mean values of worst pain, average pain, and current pain at day 14 and day 30 were decreased from baseline. This trend is demonstrated in Figure 1. Similarly, Figure 2 shows the trend of the BPI functional interference items after MRgFUS at different follow-up intervals. All functional scores were decreased at day 14 and day 30 from baseline. This indicates better function and less pain related to the target lesion at follow-up.
Figure 1.
Pain response over time among partial and indeterminate responders.
Figure 2.
Brief Pain Inventory functional score changes over time among partial and indeterminate responders.
None of the urine cytokines were significantly different between 3 days prior to treatment and 2 days after treatment, although TGFα did demonstrate an overall decrease with a trend toward statistical significance (P = .078).
Only 3 of 10 patients had CT scans available at follow-up day 30. Lesion sizes were similar at day 30 compared to baseline. All 3 lesions demonstrated increased CT density at day 30 compared to baseline (Figure 3).
Figure 3.
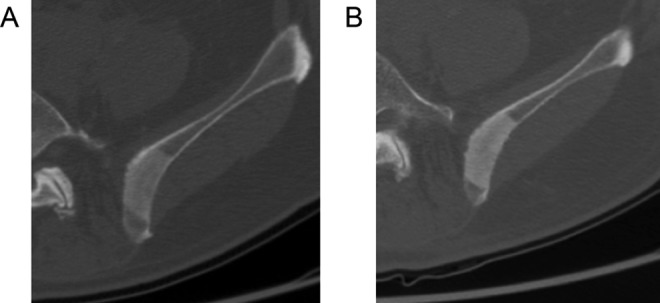
A 77-year-old man with metastatic prostate cancer to the left iliac crest. Axial computed tomography (CT) images at baseline (A) and 30-day posttreatment (B) demonstrating an increased density. The average CT number at baseline was 296 HU compared with 408 HU after treatment.
Discussion
Recently, the first study using volumetric MRgFUS for ablation of painful bone metastases was reported.21 In contrast to point-by-point ablation involving multiple single-focal point sonication, volumetric ablation involves electronic steering of the focal spot along multiple concentric circles of increasing diameter in order to increase the volume of tissue ablated per sonication. In the treatment of uterine fibroids, the volumetric sonication method improves the energy efficiency and treatment speed of MRgFUS.35–37 Huisman et al showed a treatment response in 6 of their 9 patients remaining at 1-month follow-up.21
Throughout this pilot study, we also employed a volumetric ablation technique using the Philips Sonalleve system. Treatment parameters were modified prospectively in an effort to optimize therapy as we gained more experience. The first 4 patients were treated with a 4-mm diameter treatment cell that produced thermometric changes beyond the resolution of the MR scanner. In contrast, the subsequent 6 patients were treated with larger 12-mm diameter treatment cells with improved detection of temperature changes using MR thermometry. Furthermore, the latter patients were all treated with a direct approach to focus ablation of the periosteum. This approach is presumed to improve pain control since the periosteum is the most highly innervated component of mature bone tissue. In our study, 3 out of 8 patients demonstrated partial pain response by day 14; while at day 30, 5 out of 6 patients demonstrated partial pain response. No serious adverse treatment effects were observed. While our study is not novel, our results are consistent with previous studies demonstrating the safety and efficacy of MRgFUS as a treatment for metastatic bone pain.17–19,21–24 Together, our results and the findings of Huisman et al emphasize the need for further research aimed at optimizing and standardizing volumetric ablation in the palliation of bony metastases.
Of the cytokines measured, TGFα was the only cytokine to demonstrate an overall decrease with a trend toward statistical significance. The work done by Zhou et al demonstrated that serum immunosuppressive cytokines—such as Vascular endothelial growth factor (VEGF) and TGFα—decreased after HIFU treatment in patients with solid tumors.20 Nevertheless, our study has a very limited sample size, and our future analyses will characterize the significance of cytokines in this setting.
In the 3 patients with CT examinations available at follow-up day 30, the treated metastases demonstrated increased CT density compared with baseline, which likely represents sclerosis in the bone related to the treatment effect. While the patient numbers are low, our findings are in agreement with treatment responses reported in previous studies, which demonstrated either increase or no change in bone density in the majority of patients.18,19,23
Hurwitz et al recently reported the first completed phase III randomized trial, studying MRgFUS for patients with painful bone metastases.22 These patients had radiation refractory metastases or were not candidates for EBRT. They demonstrated a response rate of 64.3% in the MRgFUS arm compared with 20.0% in the placebo arm (P < .001) at 3 months. Our study demonstrated a higher response rate, albeit at a shorter end point of 1 month. One explanation for this difference may be that we accepted patients without prior radiation therapy to the targeted lesion, whereas 43.8% of the patients in the aforementioned study had a history of prior radiation to the site. Napoli et al also demonstrated a slightly higher response rate 88.9%, which includes complete responders (72.2%) and partial responders (16.7%) at a 3-month time point.23 In the current literature, an international consensus statement recognizes MRgFUS as a safe and effective secondary treatment option in radiation-refractory painful bone metastases outside the spine.38 The MRgFUS may be considered in the setting where primary therapeutic modalities—namely, radiotherapy—are contraindicated or refused by the patient. Nonetheless, further evidence from large randomized control studies is needed to establish MRgFUS as an option for the primary treatment and palliation of bone metastases among other available treatment options.
Our study has several limitations. The most notable is a small, heterogeneous patient sample with a relatively short follow-up period. Furthermore, many of our study patients were extremely ill with widespread metastatic disease. This resulted in a high number lost to follow up. While it is not uncommon to lose patients to follow up with a significant disease burden who are approaching the end of life, this does introduces a source of bias. In addition, while MR thermometry did confirm that therapeutic temperatures were achieved in approximately half of the sonications, the remaining sonication suffered from motion artifact and tissue inhomogeneity, which made temperature confirmation difficult. Therefore, we were unable to confirm whether therapeutic temperatures were achieved in a subset of cases. Accurate temperature measurements are crucial for treatment monitoring and interpretation of treatment outcomes since lethal cell damage occurs when temperatures >55°C are maintained for longer than a second.39,40 Lam et al reported that MR thermometry is susceptible to a range of artifacts, including magnetic field inhomogeneities related to respiratory and nonrespiratory factors, as well as patient motion and arterial ghosting.41
In conclusion, our study corroborates MRgFUS as a feasible and safe modality as a primary, palliative treatment for painful bone metastases and contributes to the limited body of literature using MRgFUS for this clinical indication. Future research should include large cohort and randomized control studies with longer follow-up periods to establish the efficacy of MRgFUS as a primary treatment modality or in comparative studies with radiation therapy and other targeted agents or used in combined treatment regimens.
Abbreviations
- BPI
Brief Pain Inventory
- CT
computed tomography
- EBRT
external beam radiotherapy
- HIFU
high-intensity-focused ultrasound
- MRgFUS
magnetic resonance–guided focused ultrasound
- SD
standard deviation
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Charles Mougenot is employed by Philips Healthcare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by the Ontario Research Fund.
References
- 1. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56(3):365–378. [DOI] [PubMed] [Google Scholar]
- 2. Brodowicz T, O’Byrne K, Manegold C. Bone matters in lung cancer. Ann Oncol. 2012;23(9):2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509–524. [DOI] [PubMed] [Google Scholar]
- 5. von Moos R, Sternberg C, Body JJ, Bokemeyer C. Reducing the burden of bone metastases: current concepts and treatment options. Support Care Cancer. 2013;21(6):1773–1783. [DOI] [PubMed] [Google Scholar]
- 6. Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. [DOI] [PubMed] [Google Scholar]
- 7. Kirou-Mauro AM, Hird A, Wong J, et al. Has pain management in cancer patients with bone metastases improved? A seven-year review at an outpatient palliative radiotherapy clinic. J Pain Symptom Manage. 2009;37(1):77–84. [DOI] [PubMed] [Google Scholar]
- 8. Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer. 2004;90(12):2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. [DOI] [PubMed] [Google Scholar]
- 10. Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol). 2012;24(2):112–124. [DOI] [PubMed] [Google Scholar]
- 11. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. [DOI] [PubMed] [Google Scholar]
- 12. Saarto T, Janes R, Tenhunen M, Kouri M. Palliative radiotherapy in the treatment of skeletal metastases. Eur J Pain. 2002;6(5):323–330. [DOI] [PubMed] [Google Scholar]
- 13. Poulsen HS, Nielsen OS, Klee M, Rorth M. Palliative irradiation of bone metastases. Cancer Treat Rev. 1989;16(1):41–48. [DOI] [PubMed] [Google Scholar]
- 14. Huisman M, van den Bosch MA, Wijlemans JW, et al. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2012;84(1):8–14. [DOI] [PubMed] [Google Scholar]
- 15. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: scandinavian sarcoma group skeletal metastasis registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22(2):132–138. [DOI] [PubMed] [Google Scholar]
- 16. Callstrom MR, Dupuy DE, Solomon SB, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(5):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases–preliminary clinical experience. Ann Oncol. 2007;18(1):163–167. [DOI] [PubMed] [Google Scholar]
- 18. Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology. 2008;249(1):355–363. [DOI] [PubMed] [Google Scholar]
- 19. Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16(1):140–146. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Q, Zhu XQ, Zhang J, et al. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34(1):81–87. [DOI] [PubMed] [Google Scholar]
- 21. Huisman M, Lam MK, Bartels LW, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound. 2014;2:16–5736- 2–16. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurwitz MD, Ghanouni P, Kanaev SV, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5):10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Napoli A, Anzidei M, Marincola BC, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol. 2013;48(6):351–358. [DOI] [PubMed] [Google Scholar]
- 24. Joo B, Park MS, Lee SH, et al. Pain palliation in patients with bone metastases using magnetic resonance-guided focused ultrasound with conformal bone system: a preliminary report. Yonsei Med J. 2015;56(2):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Catane R, Gianfelice D, Kawasaki M, et al. Pain palliation of bone metastases using magnetic resonance guided focused ultrasound—multi-center multi-trial results. Poster presented at: ESMO Congress 2012; October 1, 2012; Vienna, Austria. [Google Scholar]
- 26. Turkevich VG, Savelyeva VV, Kanaev SV, Dunaevsky IV, Krzhivitsky PI. Treatment of painful bone metastases with magnetic resonance guided focused ultrasound. Eur J Cancer. 2011;47(suppl 1): S227–S228. [Google Scholar]
- 27. Kawasaki M, Nanba H, Kato T, Tani T, Ushida T. Efficacy of magnetic resonance-guided focused ultrasound surgery for bone metastases pain palliation. In: Matsumoto Y, crum LA, TerHaar GR, eds. 10th Int Symp Therapeut Ultrasound 2011:488–492. Web site http://scitation.aip.org/search?value1=ultrasound+surgery+bone&option1=all&option912=resultCategory&value912=ResearchPublicationContent&operator8=AND&option8=pub_serialIdent&value8=aip%2Fproceeding%2Faipcp&qs=truePublisher-AIP Scitation [Google Scholar]
- 28. Orgera G, Monfardini L, Della Vigna P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med. 2011;116(5):734–748. [DOI] [PubMed] [Google Scholar]
- 29. Carteret T, Trillaud H, Maire F, et al. MR-guided high intensity focused ultrasound for palliation of bone metastasis. Cardiovasc Intervent Radiol. 2011;34(3):539. [Google Scholar]
- 30. Orgera G, Fanelli F, Hatzidakis A, et al. US-guided high-intensity focused ultrasound of bone and soft tissues metastases: early clinical experience in palliation of chronic pain. Cardiovasc Intervent Radiol. 2010;33(2):274–275. [Google Scholar]
- 31. Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010;116(16):3934–3942. [DOI] [PubMed] [Google Scholar]
- 32. Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730–1737. [DOI] [PubMed] [Google Scholar]
- 33. Chow E, Wu JS, Hoskin P, et al. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64(3):275–280. [DOI] [PubMed] [Google Scholar]
- 34. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 35. Park MJ, Kim YS, Keserci B, Rhim H, Lim HK. Volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids: treatment speed and factors influencing speed. Eur Radiol. 2013;23(4):943–950. [DOI] [PubMed] [Google Scholar]
- 36. Kim YS, Keserci B, Partanen A, et al. Volumetric MR-HIFU ablation of uterine fibroids: role of treatment cell size in the improvement of energy efficiency. Eur J Radiol. 2012;81(11):3652–3659. [DOI] [PubMed] [Google Scholar]
- 37. Kohler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–3535. [DOI] [PubMed] [Google Scholar]
- 38. Huisman M, Ter Haar G, Napoli A, et al. International consensus on use of focused ultrasound for painful bone metastases: current status and future directions. Int J Hyperthermia. 2015;31(3):251–259. [DOI] [PubMed] [Google Scholar]
- 39. Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. [DOI] [PubMed] [Google Scholar]
- 40. Ter Haar G. Principles of high-intensity focused ultrasound In: Mueller PR, Adams A, eds. Interventional Oncology: A Practical Guide for the Interventional Radiologist. New York, NY: Springer Science + Business Media LLC; 2012: 51–63. [Google Scholar]
- 41. Lam MK, Huisman M, Nijenhuis RJ, et al. Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases. J Ther Ultrasound. 2015;3:5 DOI:10.1186/s40349-015-0026-7. Web site http://jtultrasound.biomedcentral.com/articles/10.1186/s40349-015-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




