Abstract
Epigenetic modifications are involved in the pathogenesis of cancer, and histone deacetylase inhibitors are considered potential therapeutic agents. Histone tails undergo acetylation at lysine residues, which is associated with transcriptional activation. However, previous studies indicated that as histone deacetylase inhibitors, both (−)-epigallocatechin-3-gallate and valproic acid presented the effects of downregulation of amyloid precursor protein expression, which resulted in the induction of apoptosis. The downregulation of amyloid precursor protein, instead of conventionally activating gene expression as histone deacetylase inhibitor, was attractive. However, there was no relevant report on the correlation of the expression of amyloid precursor protein and histone deacetylase 1 in cancer. In the present study, we detected the expression of amyloid precursor protein and histone deacetylase 1 in hepatocellular carcinoma and adjacent tissues, as well as the correlations among histone deacetylase 1, amyloid precursor protein, and tumor stage. The results showed that the expressions of amyloid precursor protein and histone deacetylase 1 were significantly higher in hepatocellular carcinoma tissues than that in adjacent tissues (P < .05), however, there was no statistical difference between amyloid precursor protein and histone deacetylase 1 with tumor stages. The present findings provided more foundation for the study on amyloid precursor protein metabolism in cancer, especially on the regulation of amyloid precursor protein by histone deacetylases.
Keywords: (−)-epigallocatechin-3-gallate, Alzheimer disease, β-amyloid, neprilysin, histone deacetylase inhibitor
Introduction
In human cancers, aberrant epigenomics is known to contribute to neoplastic development, which includes DNA methylation, histone modifications, and noncoding RNAs.1 Histone acetyltransferases and histone deacetylases (HDACs) are known to play an important role in gene expression.2 Histone deacetylases have been shown to be commonly associated with many types of cancers and to affect cancer development. Consequently, numerous HDAC inhibitors (HDACis) have been identified and used in clinical trials for the treatment of cancer.3,4 Histone deacetylase inhibitors were mainly thought to act by modulating the gene expression patterns, including genes associated with cell cycle arrest and apoptosis, by inhibiting the activity of HDAC.5 In particular, HDAC1 is believed to be crucial in controlling cellular apoptosis.6
Vivek et al indicated that histone deacetylase inhibitor valproic acid (HDACIVA) inhibited cancer cell proliferation via downregulation of amyloid precursor protein (APP). Amyloid precursor protein is a highly conserved type 1 transmembrane glycoprotein with a receptor-like structure and consists of a heterogeneous group of proteins migrating between 110 and 135 kDa.7–9 As a growth factor, APP has received considerable attention in the oncology field, where patients with increased APP levels have a significantly lower survival rate, and therefore, it has been suggested as a potential biomarker to evaluate cancer prognosis.7,10 Khue indicated a role of epigenetic mechanisms in APP pre-messenger RNA (mRNA) splicing such as DNA methylation, chromatin structure, and histone modifications.11,12 Previously, we demonstrated that as a HDACi, (−)-epigallocatechin-3-gallate (EGCG) induces cancer cell apoptosis via epigenetic modification of APP. Moreover, when specific silenced the expression of HDAC1 we found it leaded to APP downregulation, this also showed some connections between APP and HDAC1. As HDACis, different from conventionally activating gene expression, both EGCG and valproic acid (VA) led to APP downregulation, indicating acetylation could directly modulate effectors of apoptotic/cell survival. Hence, the APP expression is possibly associated with HDAC1 regulation.
In order to search for a link between APP and HDAC1, we have examined the expression of HDAC1 and APP in hepatocellular carcinoma (HCC) and adjacent normal tissue samples, as well as the correlation of APP and HDAC1 in the present study. Moreover, the relationship with tumor stage was also investigated.
Materials and Methods
Specimen
Twenty-seven human hepatic carcinoma samples with 24 paired adjacent normal tissue samples were obtained from patients undergoing elective surgery for hepatic resections with written informed consent after approval from the Medical Research Human Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University, between the ages of 19 and 70 years from January 2012 to November 2014. Tumor stage was defined by the seventh edition of the tumor–node–metastasis classification, according to the American Joint Committee on Cancer Staging and Union for International Cancer Control classification guidelines (2010).
Immunohistochemical Staining
The tissues were fixed in 4% paraformaldehyde solution, and then the samples were embedded in paraffin for immunohistochemical analysis. The sections were subsequently deparaffinized and rehydrated by xylene and graded alcohol for dewaxing and hydration and then washed with phosphate-buffered saline. The immunohistochemical staining procedure was performed according to the manufacturer’s protocol (Tiangen Biotech, Beijing, China). Briefly, slides were blocked with 3% hydrogen peroxide to avoid the endogenous peroxidase activity. The primary antibody against APP (1:100; Bioss Biotechnology, Beijing, China) and HDAC1 (1:100; Cell Signaling Technology, Beverly, Massachusetts) was incubated overnight at 4°C. Then the slides were incubated with a secondary antibody (Bioss Biotechnology) for 20 minutes at room temperature, followed by 3,3-diaminobenzidine (Tiangen Biotech) and used hematoxylin to counterstain sections. In the negative control staining, we omitted the primary antibodies.
Image Acquisition and Analysis
All the slides were scanned under a light microscope (BX71; Olympus, Japan). The images were collected using the image analysis system under the same parameters at an absolute magnification of 200×. Five fields of view for each section were randomly selected. For quantitative analyses, the Image-Pro Plus 6.0 software (Media Cybernetics) was used to determine the average optical density. Measurement parameters are area, mean density, and integrated option density (IOD). First, we wrote a microfile, for example, (1) setting macrofile name and shortcut keys; (2) opened a picture, loading the measurement parameter file (data. env); (3) selected colors, loading the options file (rgb24. Rge); (4) counted and selected the output data (export data) in the results column (Statistics) to; (5) the programming end. Then opened pictures, using the macro file shortcuts estimates, data is automatically input Excel. The data of immunohistochemical staining were collected and assessed by 2 researchers using a double-blind protocol.
Statistical Analysis
Excel libraries were used to perform statistical analysis, where all the results are named img with arabic numbers and then 1-way analysis of variance and Spearman analysis with the Statistical Package for the Social Sciences 17.0 were performed (SPSS Inc, Chicago, Illinois). P < .05 was considered statistically significant.
Results
Patients’ Basic Information
Twenty-seven cases of HCC were included in the present study, 24 of them had paired adjacent tissues as the control group. Three adjacent tissues (00361, 00549, 00645) were missing, so the statistical analysis was taken by the 24 paired specimens. The general information is shown as follows (Table 1).
Table 1.
The Basic Information of Patients.
| HCC Stage | N | Gender | Mean Age | |
|---|---|---|---|---|
| Male | Female | |||
| I | 23 | 21 | 2 | 50.5 |
| II | 1 | 1 | 32 | |
| III | 2 | 2 | 42.5 | |
| IV | 1 | 1 | 44 | |
Abbreviation: HCC, hepatocellular carcinoma.
The Expression of HDAC1 Was UpRegulated in HCC Cancer Cells
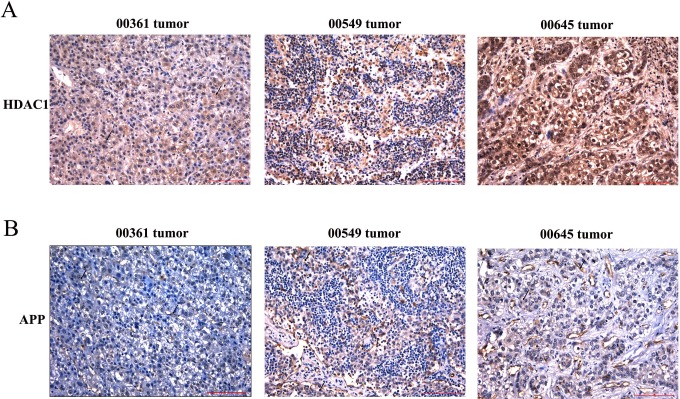
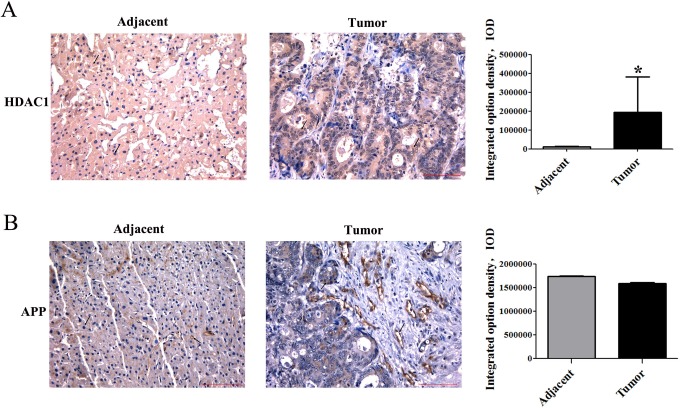
Previous publications showed that class 1 HDACs are highly enriched in many cancer cell lines. We first examined HDAC1 in 27 cases of HCC. Our immunohistochemical staining results showed that HDAC1 expression was significantly higher in HCC tissues than that in adjacent tissues (Figure 1). The relative quantification analysis was evaluated by IOD using IPP 6.0 software and is shown in Table 2, which excluded 00361, 00549, and 00645 tumor tissues (Figure 2).
Figure 1.
Immunohistochemical staining of HDAC1 in HCC and the adjacent tissues. Immunohistochemical staining of paraffin-embedded tissue serial sections from 27 HCC specimens and 24 paired adjacent specimens with antibodies against HDAC1. Immunoreactivity is indicated by brown staining. Magnification 200×, scale bars represent 100 μm. Statistics of HDAC1 expression in HCC tissues and the adjacent tissues (*P < 0.05).
Table 2.
The Expression of APP in Hepatocellular Carcinoma and Adjacent Tissues.
| Tests of Between-Patients Effects | |||||
|---|---|---|---|---|---|
| Dependent Variable: IOD | |||||
| Source | Type III Sum of Squares | df | Mean Square | F | Significance |
| Corrected model | 1.160×1013 | 24 | 4.86×1011 | 26.729 | .000 |
| Intercept | 3.568×1012 | 1 | 3.57×1012 | 196.203 | .000 |
| ID | 1.148×1013 | 23 | 4.99×1011 | 27.458 | .000 |
| Organization | 8.13×1010 | 1 | 1.81×1011 | 9.971 | .004 |
| Error | 4.18E×1011 | 23 | 1.818×1010 | ||
| Total | 1.57×1013 | 48 | |||
| Corrected total | 1.21×1013 | 47 | |||
Abbreviations: APP, amyloid precursor protein; IOD, integrated option density.
aR2 = .965 (adjusted R2 = .929).
Figure 2.
Immunohistochemical staining APP and HDAC1. A, B, C positive staining APP at 00361, 00549, 00645 specimen. (A-B) Immunohistochemical analysis of HDAC1 and APP in the adjacent tissues and tumor tissues at 00361, 0054 and 00645 specimens.
The High-Level Expressions of APP in HCC Tissues
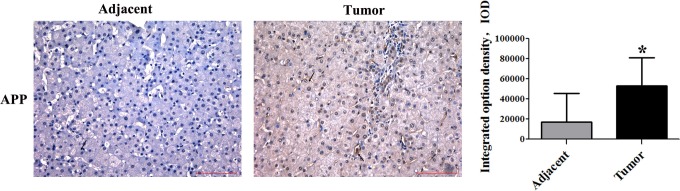
Studies have shown that APP was pathophysiologically upregulated in various cancer types, including melanoma, lung cancer, gastric and prostate cancer, pancreatic cancer, and oral squamous cell carcinoma.13–16 However, its expression in HCC remains unknown. The clinicopathological findings by immunohistochemical analyses are shown in Figure 3. The levels of APP in tumor tissues and adjacent tissues were significantly different. The staining of APP appeared not only in the cell membrane but also in the cytoplasm. Results of the IOD analysis showed that APP expression was obviously higher in HCC tissue than in adjacent tissue (Table 3), which also excluded 00361, 00549, and 00645 tumor tissues (Figure 2).
Figure 3.
Immunohistochemical stainning of APP in HCC and the adjacent tissues. Immunohistochemical staining of paraffin-embedded tissue serial sections from 27 HCC specimens and 24 paired adjacent specimens with antibodies against APP. Immunoreactivity is indicated by brown staining. Magnification 200×, scale bars represent 100 μm. Statistics of APP expression in HCC tissues and the adjacent tissues (*P < 0.05).
Table 3.
The Expression of HDAC1 in Hepatocellular Carcinoma and Adjacent Tissues.
| Tests of Between-Patients Effects | |||||
|---|---|---|---|---|---|
| Dependent Variable: IOD | |||||
| Source | Type III Sum of Squares | df | Mean Square | F | Significance |
| Corrected model | 1.58×1012 | 2 | 7.88×1011 | 2.224 | .12 |
| Intercept | 4.33×1012 | 1 | 4.33×1012 | 12.227 | .001 |
| ID | 1.89×1010 | 1 | 1.89×1010 | 0.053 | .818 |
| Organization | 1.56×1012 | 1 | 1.56×1012 | 4.396 | .042 |
| Error | 1.60×1013 | 45 | 3.54×1011 | ||
| Total | 2.17×1013 | 48 | |||
| Corrected total | 1.75×1013 | 47 | |||
Abbreviations: HDAC1, histone deacetylase 1; IOD, integrated option density.
aR2 = .090 (adjusted R2 = .050).
Relationship Among APP, HDAC1, and the HCC Stage
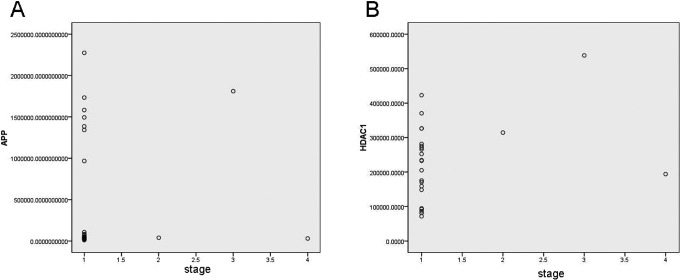
To further evaluate the correlation of APP and HDAC1, spearman analysis was conducted, and the results showed that there was no statistical difference between APP and HDAC1 expression in HCC (P = .558, Table 4). Moreover, the correlations of APP or HDAC1 with HCC stage were also analyzed, HDAC1 Spearman ρ = 0.268, P = .206 (Table 5 and Figure 4A) and APP Spearman ρ = −0.159, P = .458 (Table 6 and Figure 4B), and these results indicated that there was no significant difference in the HCC stage with HDAC1 or APP expression.
Table 4.
The Relationship Between APP and HDAC1.
| Correlations | ||
|---|---|---|
| Control Variables: Stage | HDAC1 | APP |
| HDAC1 | ||
| Correlation | 1.000 | −0.129 |
| Significance (2 tailed) | - | 0.558 |
| df | 0 | 21 |
| APP | ||
| Correlation | −0.129 | 1.000 |
| Significance (2 tailed) | .558 | - |
| df | 21 | 0 |
Abbreviations: APP, amyloid precursor protein; HDAC1, histone deacetylase 1; IOD, integrated option density.
Table 5.
The Correlation of APP With the Tumor Stage.
| Correlations | ||
|---|---|---|
| Spearman’s ρ | APP | Stage |
| APP | ||
| Correlation coefficient | 1.000 | −0.159 |
| Significance (2 tailed) | - | .458 |
| N | 24 | 24 |
| Stage | ||
| Correlation coefficient | −0.159 | 1.000 |
| Significance (2 tailed) | .458 | - |
| N | 24 | 24 |
Abbreviation: APP, amyloid precursor protein.
Figure 4.
Figure of spearman's correlation of APP or HDAC1 with HCC stage. (A) The figure of spearman's correlation of APP HCC stage, spearman's rho = −0.159, P = 0.458; (B) The figure of spearman's correlation of APP or HDAC1 with HCC stage, spearman's rho = 0.268, P = 0.206.
Table 6.
The Correlation of HDAC1 With the Tumor Stage.
| Correlations | ||
|---|---|---|
| Spearman’s ρ | HDAC1 | Stage |
| HDAC1 | ||
| Correlation coefficient | 1.000 | 0.268 |
| Significance (2 tailed) | - | .206 |
| N | 24 | 24 |
| Stage | ||
| Correlation coefficient | 0.268 | 1.000 |
| Significance (2 tailed) | .206 | - |
| N | 24 | 24 |
Abbreviation: HDAC1, histone deacetylase 1.
The Special Expression in HCC Tissues and Adjacent Tissues
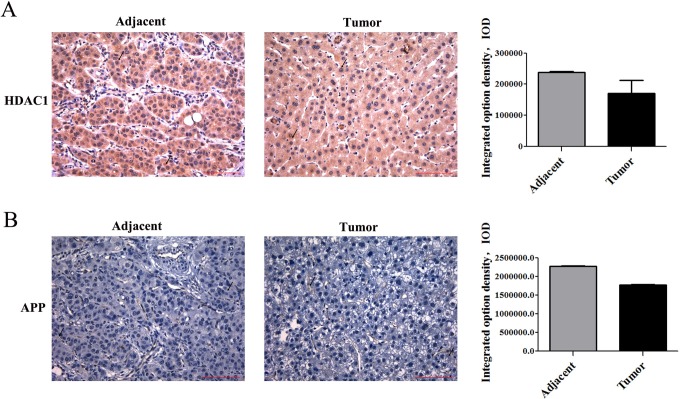
In most specimens, we found that the higher expression of APP was consistent with higher expression of HDAC1 in HCC tissues than in adjacent tissue. However, in the 00021 specimen, the expression of HDAC1 was higher in adjacent tissues than that in tumor tissues, so does APP (Figure 5).
Figure 5.
Immunohistochemical stainning of APP and HDAC1 at 00021 HCC patient. (A-B) Immunohistochemical analysis of HDAC1 and APP in the adjacent tissues and tumor tissues at 00021 specimen. Magnification 200×, scale bars represent 100 μm. Statistics of APP and HDAC1 expression in HCC tissues and the adjacent tissues.
We also found another interesting phenomenon in specimen 00467, that is, the expression of APP and HDAC1 presented a negative correlation (Figure 6), in which HDAC1 expression was higher in tumor tissue, whereas there was no significant difference in APP expression between tumor tissue and adjacent tissue.
Figure 6.
Immunohistochemical stainning of APP and HDAC1 at 00467 HCC patient. (A-B) Immunohistochemical analysis of HDAC1 and APP in the adjacent tissues and tumor tissues at 00467 specimen. Magnification 200×, scale bars represent 100 μm. (A) Statistics of HDAC1 expression in HCC tissues and the adjacent tissues (*P < 0.05). (B) Statistics of APP expression in HCC tissues and the adjacent tissues.
Discussion
Recent evidence supports the observation of an inverse link between cancer and Alzheimer disease (AD).13 It meant that having one of the disease may reduce the risk of the other diseases, suggesting a common mechanism linking between AD and cancer.13 Nevertheless, cancer and AD had been demonstrated to be a common manifestation of both genetic and epigenetic modifications.14–16 Studies showed epigenetic linkage of aging, cancer, and nutrition.17,18 Histone deacetylase inhibitors have been proven to be important key targets in cancer therapy and also have attracted significant attention to treating neurodegenerative disorders recently.18–22 Our previous studies found EGCG as an HDACi could inhibit the growth of adrenal pheochromocytoma, meanwhile, its neuroprotective effect was also confirmed in the AD model both in vivo and in vitro.22
The cross talk of genes in different diseases has attracted increasing attention recently. We also find that the disorder of APP occurs in both AD and cancer. The widely studied in detail mainly because of APP its vital pathophysiological functions in AD, mutations cause the accumulation of neurotoxic β-amyloid.23,24 However, studies have shown that APP was also upregulated pathophysiologically in various cancer types, including melanoma, lung cancer, gastric and prostate cancer, pancreatic cancer, and oral squamous cell carcinoma and indicated that the patients with higher APP levels have a significantly lower survival rate.25–28 Our results showed that the expression of APP was also in HCC.
A number of well-characterized epigenetic modifications are linked to aberrant gene functions and altered patterns of gene expression that play critical roles in the pathobiology of cancer.29 Our previous study demonstrates that EGCG inhibits HDAC1 activity and downregulates APP, which results in the induction of apoptosis in cancer cells and suppresses tumor growth. Moreover, Vivek et al indicated that HDACIVA inhibits cancer cell proliferation and this process is achieved by downregulating APP. These findings strongly suggested that there was a correlation between APP and HDACs.
Histone acetylation is often associated with the transcription of genes characteristic in a differentiated state. The acetylation at lysine residues is one of the most thoroughly studied modifications of histone tails, which is associated with transcriptional activation.30 By contrast, histone deacetylation correlates with transcriptional silencing, specifically with the downregulation of the expression of proapoptotic genes in cancer cells.31,32 However, our previous study found that EGCG promoted the downregulation of APP, instead of conventionally activating gene expression as HDACi. Recently, it has been proposed that it would not be the only mechanism responsible for the antiproliferative/proapoptotic action of these drugs by HDACi-inducing chromatin remodeling. Acetylation can directly modulate effectors of apoptotic/cell survival mechanisms.33 Our immunohistochemical staining results showed that HDAC1 expression was significantly higher in HCC tissues than that in adjacent tissues. So far, there was no relevant report on the correlation of the expression of APP and HDAC1 in cancer. As HDACi, EGCG directly induced the apoptosis of cancer cells, consistent with the increase in acetylation. Moreover, we found that specific knockdown of HDAC1 lead to the downregulation of APP and resulted in executor protein caspase-7 cleavage, ultimately inducing the apoptosis, which highlighted the role of APP as a critical player in the effect of EGCG as a HDACi on proapoptotic activity. In the present study, most specimens were found that the higher expression of APP was consistent with higher expression of HDAC1 in HCC tissues. It was in a good agreement with our previous study and other studies. Interestingly, there were 2 special specimens (00021 and 00467) present higher APP expression and HDAC1 expression in adjacent tissues than that in tumor tissues. APP expression and HDAC1 expression in adjacent tissues than that in tumor tissues. We suspected that the tumor cells maybe have diffused and invaded the adjacent tissues in this patient. It indicated that the changes in APP and HDAC1 expression might have occurred before other clinical characteristics. However, 00467 showed that HDAC1 expression was higher in tumor tissue, while there was no significant difference in APP expression between tumor tissue and adjacent tissue. Even though APP presented a slightly higher trend in adjacent tissues than that in the HCC tissue, the APP metabolism process is in close contact with the tumor. Our previous studies have shown that APP metabolism in tumor and in AD model was different. As HDAC1I, EGCG downregulated the total APP expression both in tumor and AD. However, the product of APP proteolysis, the non-amyloidogenic a-secretase activation of APP thereby secretes soluble Amyloid precusor protein (sAPPa), was upregulated in AD, whereas it was downregulated in tumor. These results indicated that APP metabolism was complicated and varied in different situations and was under epigenetic modifications, so that the 00467 specimen will be further explored in the APP proteolysis process, as well as the other clinical characteristics.
We also analyzed the link between APP and HDAC1 expression in HCC. Moreover, the correlations of APP or HDAC1 with HCC stage were also analyzed, and the results indicated that there was no significant difference in the HCC stage with HDAC1 or APP expression. However, there was a trend that in stages 2 and 3, the staining of APP was relatively stronger than that in stage 1. However, there was no statistical significance between APP or HDAC1 expression and HCC stage. We speculated that it might be due to the little sample amount and only 24 paired specimens were involved in the analysis.
Khue suggested the epigenetic regulation in APP. The real profile of APP isoforms accounted for epigenetic mechanisms in the regulation of alternative APP pre-mRNA splicing from normal patients as well as from patients with Lesch-Nyhan syndrome (LNS) and Lesch-Nyhan variants (LNV). It suggests a role for epigenetic mechanisms and implications for the uncovered role of epigenetic components, such as DNA methylation, chromatin remodeling, histone modifications, small interfering RNA, and so on, in the regulation of alternative pre-mRNA splicing.30 Previously, we found that the HDACi, EGCG, induces cancer cell apoptosis via epigenetic modification of APP. Moreover, specific silenced HDAC1 led to APP downregulation. As HDACis, both EGCG and VA are not conventionally activating gene expression but led to APP downregulation, indicating acetylation could directly modulate effectors of apoptotic/cell survival. Hence, the APP expression is possibly associated with HDAC1 regulation. However, the underlying mechanism is still elusive. The present findings provided more foundation for our further study on APP metabolism in cancer, especially on the regulation of APP by HDACs. We intend to fully explore the epigenetic mechanism of HDACs on APP regulation in a future study. Moreover, the special specimens such as 00021 and 00467 need further study.
Abbreviations
- ACE
angiotensin-converting enzyme
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- DAPI
4′,6-diamino-2-phenylindole
- ECE
endothelin-converting enzyme
- EDU
5-ethynyl-2′-deoxyuridine
- EGCG
(−)-epigallocatechin-3-gallate
- FBS
fetal bovine serum
- HDAC
histone deacetylase
- IDE
insulin-degrading enzyme
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- NEP
neprilysin
- PS1
presenilin 1
- TUNEL
terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine, 5′-triphosphate (dUTP) nick-end labeling.
Footnotes
Author’s Note: Luguang Zhao and Dan He contributed equally to this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Nature Science Foundation of China (Grant Numbers: 81403144, 81473740, and 81273817), the Program for Doctoral Station in University, China (Grant Numbers: 20124425120016 and 20134425110003), the Major Science and Technology Projects of Guangdong Province, China (Grant Number: 2012A080202017), the Medical Research Foundation of Guangdong Province, China (Grant Number: B2012151), Characteristic Key Discipline Construction Fund of Chinese Internal Medicine of Guangzhou University of Chinese Medicine, and South China Chinese Medicine Collaborative Innovation Center (No. A1-AFD01514A05).
References
- 1. Hu Q, Chang X, Yan R, et al. (-)-Epigallocatechin-3-gallate induces cancer cell apoptosis via acetylation of amyloid precursor protein. Med Oncol. 2015;32(1):390. [DOI] [PubMed] [Google Scholar]
- 2. Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108(4):475–487. [DOI] [PubMed] [Google Scholar]
- 3. Emanuele S, Lauricella M, Tesoriere G. Histone deacetylase inhibitors: apoptotic effects and clinical implications (Review). Int J Oncol. 2008;33(4):637–646. [PubMed] [Google Scholar]
- 4. Jazirehi AR. Regulation of apoptosis-associated genes by histone deacetylase inhibitors: implications in cancer therapy. Anticancer Drugs. 2010;21(9):805–813. [DOI] [PubMed] [Google Scholar]
- 5. Hoshino I, Matsubara H. Recent advances in histone deacetylase targeted cancer therapy. Surg Today. 2010;40(9):809–815. [DOI] [PubMed] [Google Scholar]
- 6. Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia. 2005;7(7):646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venkataramani V, Rossner C, Iffland L, et al. Histone deacetylase inhibitor valproic acid inhibits cancer cell proliferation via downregulation of the Alzheimer amyloid precursor protein. J Biol Chem. 2010;285(14):10678–10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selkoe DJ, Podlisny MB, Joachim CL, et al. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988;85(19):7341–7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ko SY, Lin SC, Chang KW, et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer. 2004;111(5):727–732. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen KV. Epigenetic regulation in amyloid precursor protein and the Lesch-Nyhan syndrome. Biochem Biophys Res Commun. 2014;446(4):1091–1095. [DOI] [PubMed] [Google Scholar]
- 12. Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. [DOI] [PubMed] [Google Scholar]
- 15. Bradley-Whitman MA, Lovell MA. Epigenetic changes in the progression of Alzheimer’s disease. Mech Ageing Dev. 2013;134(10):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic mechanisms in Alzheimer’s disease. Neurobiol Aging. 2011;32(7):1161–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniel M, Tollefsbol TO. Epigenetic linkage of aging, cancer and nutrition. J Exp Biol. 2015;218(pt 1):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang X, Rong C, Chen Y, et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimers disease model mice by upregulating neprilysin expression. Exp Cell Res. 2015;334(1):136–145. [DOI] [PubMed] [Google Scholar]
- 19. Nalivaeva NN, Belyaev ND, Turner AJ. Sodium valproate: an old drug with new roles. Trends Pharmacol Sci. 2009;30(10):509–514. [DOI] [PubMed] [Google Scholar]
- 20. Fischer A. HDAC inhibitors as therapy for neural disorders. Discovery of a new therapy. Pharm Unserer Zeit. 2010;39(3):204–209. [DOI] [PubMed] [Google Scholar]
- 21. Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31(12):605–617. [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Fang H, Xu W. Strategies in developing promising histone deacetylase inhibitors. Med Res Rev. 2010;30(4):585–602. [DOI] [PubMed] [Google Scholar]
- 23. Murrell JR, Hake AM, Quaid KA, Farlow MR, Ghetti B. Early-onset Alzheimer disease caused by a new mutation (V717L) in the amyloid precursor protein gene. Arch Neurol. 2000;57(6):885–887. [DOI] [PubMed] [Google Scholar]
- 24. Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254(5028):97–99. [DOI] [PubMed] [Google Scholar]
- 25. Botelho MG, Wang X, Arndt-Jovin DJ, Becker D, Jovin TM. Induction of terminal differentiation in melanoma cells on downregulation of beta-amyloid precursor protein. J Invest Dermatol. 2010;130(5):1400–1410. [DOI] [PubMed] [Google Scholar]
- 26. Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63(21):7032–7037. [PubMed] [Google Scholar]
- 27. Meng JY, Kataoka H, Itoh H, Koono M. Amyloid beta protein precursor is involved in the growth of human colon carcinoma cell in vitro and in vivo. Int J Cancer. 2001;92(1):31–39. [PubMed] [Google Scholar]
- 28. Takayama K, Tsutsumi S, Suzuki T, et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009;69(1):137–142. [DOI] [PubMed] [Google Scholar]
- 29. Kanwal R, Gupta S. Epigenetics and cancer. J Appl Physiol (1985). 2010;109(2):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;8(11):1056–1072. [DOI] [PubMed] [Google Scholar]
- 31. Enokida H, Nakagawa M. Epigenetics in bladder cancer. Int J Clin Oncol. 2008;13(4):298–307. [DOI] [PubMed] [Google Scholar]
- 32. Kanao K, Mikami S, Mizuno R, Shinojima T, Murai M, Oya M. Decreased acetylation of histone H3 in renal cell carcinoma: a potential target of histone deacetylase inhibitors. J Urol. 2008;180(3):1131–1136. [DOI] [PubMed] [Google Scholar]
- 33. Lin HY, Chen CS, Lin SP, Weng JR, Chen CS. Targeting histone deacetylase in cancer therapy. Med Res Rev. 2006;26(4):397–413. [DOI] [PubMed] [Google Scholar]