Abstract
The purpose of this study was to examine the MAC30 expression in non–small cell lung cancer and to evaluate its prognostic value on therapeutic response in patients with non–small cell lung cancer receiving postoperative chemotherapy. Among a total of 218 retrospective Chinese patients with non–small cell lung cancer, 164 patients receiving adjuvant chemotherapy were enrolled in this study. Real-time polymerase chain reaction was performed to confirm the expression of MAC30 messenger RNA in 32 cases of non–small cell lung cancer tumors with the corresponding nontumor lung tissues. The MAC30 protein expression in all specimens was analyzed by immunohistochemical staining. Moreover, we assessed the correlation of MAC30 expression with clinicopathological features, therapeutic response, and survival of patients. Here, we observed the increased expression of MAC30 messenger RNA in patients with non–small cell lung cancer compared to those in control samples. The overexpression of MAC30 was strongly associated with poor tumor differentiation, high tumor–node–metastasis stage, and lymph node metastasis. In addition, we observed that patients with increased MAC30 expression showed gloomy overall survival and disease-free survival. A multivariate analysis explicated that higher MAC30 expression was a valuable independent prognostic factor of poorer tumor differentiation, shorter overall survival, and disease-free survival in patients receiving chemotherapy. MAC30 could be a useful biomarker of tumor differentiation and outcome of patients with non–small cell lung cancer. Overexpression of MAC30 predicts a worse tumor differentiated stage and prognosis in patients with non–small cell lung cancer receiving adjuvant chemotherapy.
Keywords: MAC30, NSCLC, chemotherapy, survival
Introduction
Although definitive improvements in diagnosis and therapy were achieved, lung cancer remains the most common cause of neoplasia-related death, leading to a 5-year overall survival (OS) of less than 10%.1 More than 80% of the total lung malignancies is non–small cell lung cancer (NSCLC),2 which always exhibits advanced local invasion and distant metastasis at diagnosis.3 In addition, classical therapeutic manners in clinical application, surgical resection, chemotherapy, and radiotherapy have been generally established with little improvement in long-term survival.4 Notwithstanding, adjuvant chemotherapy was accepted as the main choice for patients with advanced NSCLC.5,6 Recent research suggested that NSCLC with the similar clinicopathological characteristics always showed capricious sensitivity to chemotherapy.7 Furthermore, it was widely accepted that individual therapy with particular chemotherapeutic drugs for more sensitive patients seemed to receive expected treatment efficacy and minor side effects.2 So, valuable biomarkers for chemotherapeutic response prediction and prognosis are imperatively needed for further identification of treatable regimens in patients with NSCLC. Unfortunately, until now very few studies have confirmed the effective predictors for adjuvant chemotherapeutic response, which should be highly anticipated.8
Meningioma-associated protein (MAC30), transmembrane protein 97 (TMEM97) locating on 17q11.2, encodes a conserved integral membrane protein of 176 amino acids with 4 transmembrane domains.9 As a member of the insulin-like growth factor-binding protein family, MAC30 regulates insulin-like growth factor activity.9 Besides regulating cholesterol and lipid metabolism,10 MAC30 also plays an important role in live growth and differentiation.11 Indeed, MAC30 was originally confirmed as an elevated gene in human meningioma.9 Recent studies suggested that high levels of MAC30 were presented in breast, esophagus, stomach, and colon cancers in contrast to low levels in pancreatic and renal cancers.12–14 The distinct expression of MAC30 determines its potential roles in human malignancies. We believed that MAC30 plays as a suppressor or a promoter in different tumors with unknown clear functions. Although overexpression of MAC30 associated with short survival in NSCLC was shown,15 there’s no report to clarify the association of MAC30 with clinicopathological features and response of adjuvant chemotherapy in patients with NSCLC.
Thus, via the relationship of MAC30 expression with clinicopathological features, therapeutic response, and survival of patients with NSCLC, we investigate to evaluate whether MAC30 could be a predictive biomarker for the response of adjuvant chemotherapy and prognosis of patients with NSCLC.
Material and Methods
Patients and Tissue Samples
We conducted this retrospective study in a total of 218 Chinese patients diagnosed with NSCLC who underwent resection at Yixing People’s Hospital, Affiliated Jiangsu University between June 2004 and July 2014. This study was retrospectively performed and was approved by the institutional review board of the Faculty of Medicine, Jiangsu University. The informed consent obtained from all participants was confirmed. And all participants consent to participate in the study with their information letters. The lung specimens analyzed in this study were obtained from patients with NSCLC, who were diagnosed with stages IA to IIIB, according to the World Health Organization and International Union against Cancer Tumor–Node–Metastasis (TNM) staging system.16 None of the patients received adjuvant chemotherapy or radiotherapy before surgery. The age of patients ranged from 26 to 84 years, with a median age of 57 years. Two pathologists classified the tumor specimens independently and unanimous agreement was obtained. The histologic classification included squamous cell carcinomas, adenocarcinoma, and adenosquamous. Among all the patients, 164 patients receiving postoperative chemotherapy were selected for a retrospective analysis.
Quantitative Real-Time Polymerase Chain Reaction
TRIzol reagent (Life Technologies, Maryland) was used to collect total RNA from frozen tissues, according to the manufacturer’s instructions. Reverse transcription was performed on 1 μg of total RNA from each sample. The real-time polymerase chain reaction (RT-PCR) primer sequences were designed according to the human MAC30 and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene sequences reported in the GenBank: MAC30, sense: 5′-GGCAGCAGAGGAGTAGCTTGA-3′; antisense: 5′-GCTTGCTGGCGCTAAAAGG-3′. The reactions were carried out at 95°C for 30 seconds, then 35 cycles of 95°C for 20 seconds, 55°C for 15 seconds, and 72°C for 20 seconds, and a final extension at 72°C for 10 minutes. Real-time PCR was used to detect the specificity of the PCR via the dissociation reaction plot subsequent to examination. Data were normalized to GAPDH.
Immunohistochemistry Analysis
Three-micrometer-thick sections cut from paraffin-embedded specimens were used to confirm MAC30 protein expression by immunohistochemistry analysis. After being pretreated at 60°C for 1 hour, the sections were dewaxed in xylene, hydrated, and washed in phosphate-buffered saline Tween solution (PBST). The sections were treated with 3% H2O2 and then incubated with a polyclonal antibody against MAC30 (1:500, SC-1971; Santa Cruz, California) overnight at 4°C. After being washed by PBST 3 times with each 15 minutes, the sections were incubated with the corresponding second antibody at room temperature for 1 hour. The results were visualized with diaminobenzidine. In each immunohistochemistry run, the negative controls were stained without a primary antibody.
Two independent pathologists with particular experience in immunohistochemistry evaluated MAC30 staining in all sections. The expression of MAC30 was quantified using a visual grading system based on the percentage of stained cells out of the total number of tumor cells and divided from 0 to 3: 0 = negative, 1+, 1% to 30%; 2+, 31% to 60%; 3+, >60%. The intensity of staining was graded on a scale: 0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive. The sums of extend score and intensity score were used to define the MAC30 expression levels, which were graded into 2 groups: low-level MAC30 expression (with a score of ≤3) and high-level MAC30 expression (with a score of >3).
Statistical Analysis
The χ2 test was used to check the relationships between MAC30 expression and clinicopathological characteristics. And the relationships between MAC30 expression with OS and disease-free survival (DFS) were analyzed via Kaplan-Meier method. The relationship between MAC30 expression and tumor differentiation stages was assessed by the univariate and multivariate logistic regression. A cox regression model was conducted to identify the independent prognostic factors that influenced the OS or DFS. All statistical analyses were operated using SPSS version 13.0. A P value of <.05 was considered to be statistically significant.
Results
Overexpression of MAC30 Messenger RNA in NSCLC Tissues
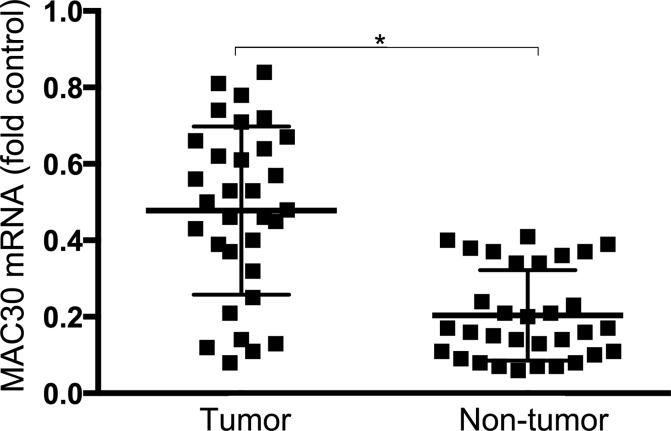
Quantitative real-time PCR was prepared to clarify the expression of MAC30 messenger RNA (mRNA) in 32 cases of patients with NSCLC and the corresponding adjacent nontumor tissues. As a result, increased expression of MAC30 mRNA in 24 of the 32 sections was significantly revealed in Figure 1. Moreover, statistical analysis confirmed the expression level of MAC30 mRNA in NSCLC was over 3.5-fold than that in the corresponding control samples (P < .05). So, MAC30 may exhibit important biological function in NSCLC.
Figure 1.
Expression of MAC30 mRNA in NSCLC specimens. MAC30 mRNA expression was confirmed in NSCLC tissues and adjacent normal tissues via quantitative real-time PCR and normalized to GAPDH. *P < .05. mRNA indicates messenger RNA; NSCLC, non–small cell lung cancer; PCR, polymerase chain reaction.
Relationship Between MAC30 Expression and Clinicopathological Parameters in NSCLC
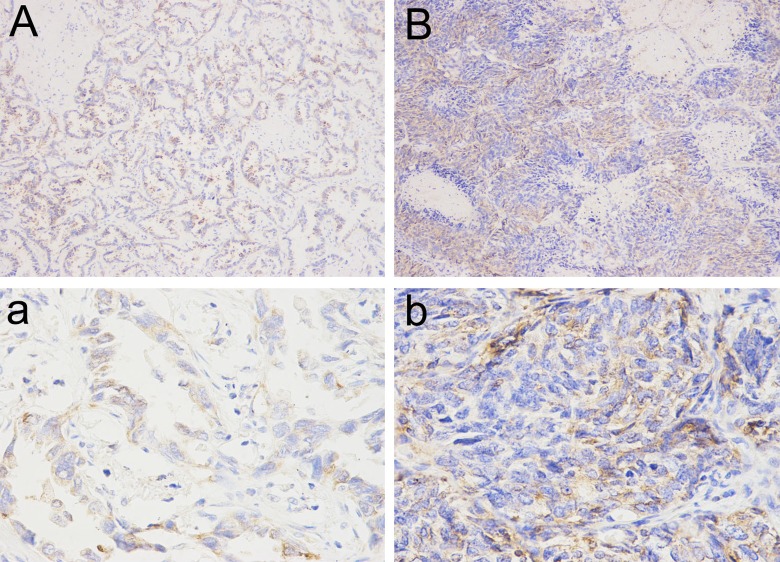
The expression of MAC30 was analyzed by immunohistochemistry in a total of 218 primary NSCLC specimens (Figure 2). As classical criteria described above, 121 tumor specimens exhibited high-level MAC30 (55.5%) expression, whereas 97 sections were classified as low-level MAC30 expression (44.5%; Table 1).
Figure 2.
Representative immunohistochemical staining for MAC30 expression in NSCLC. A and a, Low expression of MAC30. B and b, High expression of MAC30. A and B, Original magnification, ×100; a and b, Original magnification, ×200. NSCLC indicates non–small cell lung cancer.
Table 1.
Association Between MAC30 Expression and Various Clinicopathological Features of Patients With NSCLC.
| Variables | No. (N = 218) | MAC30 Expression | P Value | |
|---|---|---|---|---|
| Low (n = 97) | High (n = 121) | |||
| Age (years) | .512 | |||
| <58 | 82 | 37 | 45 | |
| ≥58 | 136 | 60 | 76 | |
| Gender | .537 | |||
| Male | 121 | 53 | 68 | |
| Female | 97 | 44 | 53 | |
| Smoking status | .431 | |||
| Nonsmoker | 84 | 36 | 48 | |
| Smoker | 134 | 61 | 73 | |
| Histological type | .457 | |||
| SQCLC | 87 | 37 | 50 | |
| AC | 129 | 60 | 69 | |
| AS | 2 | 0 | 2 | |
| Tumor differentiation | .017 | |||
| Well | 33 | 15 | 18 | |
| Moderate | 69 | 42 | 27 | |
| Poor | 116 | 40 | 76 | |
| TNM stage | .013 | |||
| I | 62 | 41 | 21 | |
| II | 85 | 33 | 52 | |
| III | 71 | 23 | 48 | |
| Tumor classification | .102 | |||
| T1 + T2 | 90 | 36 | 54 | |
| T3 + T4 | 128 | 61 | 67 | |
| Lymph node metastasis | .012 | |||
| No | 82 | 51 | 31 | |
| Yes | 136 | 46 | 90 | |
Abbreviations: AC, adenocarcinoma; AS, adenosquamous; NSCLC, non–small cell lung cancer; SQCLC, squamous cell carcinomas; TNM, tumor–node–metastasis. Boldface values mean P < 0.05.
The correlation between MAC30 expression and clinicopathological features of patients with NSCLC is presented in Table 1. MAC30 expression was significantly associated with tumor differentiation, TNM stages, and lymph node metastasis. Further, patients with MAC30 overexpression showed poor tumor differentiation, lymph node metastasis, and higher TNM stage (P < .05). In contrast, no statistical difference was confirmed between MAC30 expression and patient age, smoking status, gender, histological type, and tumor classification.
MAC30 Expression on Prognosis of Tumor Differentiation
Published data already documented the close relationship between tumor differentiation and survival in patients with lung cancer.17 Patients with shorter survival were always with worse differentiated pathogenesis. So, it’s essential to investigate the biological indication of MAC30 on tumor differentiation, which affects clinical therapies and survival of patients with NSCLC. Among the 218 selected patients, 116 patients presented with poor tumor differentiation, whereas 69 cases with moderate differentiation and 33 patients with well differentiation. Among the patients with poorly differentiated pathogenesis, there were 89 cases with high-level MAC30 expression (76.7%). The univariate analysis showed that elevated MAC30 expression was associated with poor tumor differentiation (P < .05). Furthermore, a multivariate analysis clarified that apart from other clinicopathological parameters, overexpression of MAC30 was an independent predictor of poor tumor differentiation in patients with NSCLC (Table 2).
Table 2.
Risk Factors for Poor Tumor Differentiation in Patients With NSCLC.
| Variables | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | P Value | B | SE | 95% CI | P Value | |
| MAC30 | 1.161 | 0.415 | 0.975-3.013 | .015 | 1.324 | 0.663 | 1.175-2.883 | .008 |
| Age | 0.983 | 0.381 | 0.772-1.913 | .732 | ||||
| Gender | 0.875 | 0.423 | 1.024-3.847 | .772 | ||||
| Smoking status | 1.316 | 0.691 | 0.903-2.181 | .617 | ||||
| Tumor classification | 1.198 | 0.548 | 1.127-2.553 | .437 | ||||
| Lymph node metastasis | 1.003 | 0.519 | 0.861-2.261 | .551 | ||||
Abbreviations: B, the parameter estimator of association coefficient; CI, confidence interval; NSCLC, non–small cell lung cancer; SE: standard error. Boldface values mean P < 0.05.
MAC30 Expression Predicts OS and DFS of Patients With NSCLC
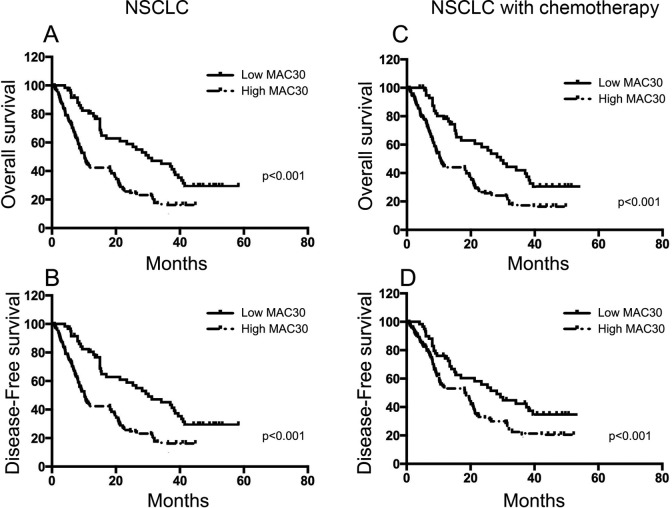
In our present study, we provided the significant evidence of the association between MAC30 and survival of patients with NSCLC via Kaplan-Meier analysis. Patients with higher MAC30 expression displayed shorter OS and DFS compared with patients with lower MAC30 expression (P < .05; Figure 3). Furthermore, the univariate and multivariate analyses indicated that besides tumor differentiation, TNM stages, and lymph node metastasis, MAC30 expression was an independent prognostic biomarker for OS and FDS in patients with NSCLC (Table 3). So, the data confirmed the correlation between elevated MAC30 expression and poor survival in patients with NSCLC.
Figure 3.
Kaplan-Meier survival curves for patients with NSCLC according to MAC30 expression. A and B, OS and DFS of patients with NSCLC showing high MAC30 expression compared with patients showing low MAC30 expression. C and D, OS and DFS of patients with NSCLC receiving only chemotherapy in high and low MAC30 expression groups. DFS indicates disease-free survival; NSCLC, non–small cell lung cancer; OS, overall survival. Boldface values mean P < 0.05.
Table 3.
Univariate and Multivariate Analysis of Prognostic Factors in Patients With NSCLC.
| Variables | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS | OS | DFS | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| MAC30 expression | 1.451 | 0.910-2.112 | .007 | 1.781 | 1.412-3.28 | .004 | 1.587 | 1.232-2.771 | .004 | 1.883 | 1.374-3.712 | .005 |
| Age (years) | 1.131 | 0.717-1.664 | .211 | 1.321 | 0.917-2.713 | .318 | ||||||
| Gender | 0.998 | 0.531-1.692 | .491 | 1.119 | 1.004-2.275 | .388 | ||||||
| Histological type | 1.241 | 0.741-1.793 | .442 | 1.418 | 1.021-2.73 | .591 | ||||||
| Smoking status | 1.113 | 0.641-1.803 | .273 | 1.219 | 0.975-2.509 | .331 | ||||||
| Tumor differentiation | 1.181 | 0.819-1.724 | .012 | 1.332 | 1.129-2.961 | .01 | 1.241 | 1.003-2.314 | .008 | 1.451 | 1.241-2.927 | .006 |
| TNM stage | 1.116 | 0.691-.1.703 | .017 | 1.471 | 1.219-3.028 | .008 | 1.337 | 1.231-2.446 | .013 | 1.401 | 1.336-2.749 | .01 |
| Tumor classification | 1.376 | 0.904-2.005 | .438 | 1.276 | 0.883-1.916 | .527 | ||||||
| Lymph node metastasis | 1.251 | 0.813-1.887. | .01 | 1.486 | 1.209-3.012 | .013 | 1.401 | 1.274-2.619 | .004 | 1.559 | 1.368-3.196 | .007 |
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NSCLC, non–small cell lung cancer; OS, overall survival; TNM, tumor–node–metastasis. Boldface values mean P < 0.05.
Expression of MAC30 With Postoperative Chemotherapy
A total of 164 patients receiving postoperative chemotherapy were selected in this study. As shown in Table 3, overexpression of MAC30 expression was presented in 93 (56.7%) patients, whereas 71 (43.3%) patients exhibited low MAC30 expression. Moreover, we also observed the significant difference in tumor differentiation, TNM stages, and lymph node metastasis between the 2 MAC30 groups (Table 4; P < .05). The univariate and multivariate analyses indicated that overexpression of MAC30 was an independent predictor of tumor differentiation in patients with NSCLC receiving postoperative chemotherapy (Table 5). In our present study, we also explored the association between MAC30 expression and prognosis of patients with adjuvant chemotherapy. As a result, a Kaplan-Meier test showed that patients with NSCLC having high level of MAC30 suffered from shorter OS and DFS (Figure 3A and B), whereas patients receiving chemotherapy with elevated MAC30 expression also showed lower OS and DFS (Figure 3C and D). So, it’s significant that patients with high-level MAC30 expression showed less reaction to chemotherapy compared to those with low-level MAC30 expression. Moreover, besides tumor differentiation, TNM stages, and lymph node metastasis, MAC30 expression was a valuable independent prognostic factor for both OS and DFS analyzed in patients receiving postoperative chemotherapy via univariate and multivariate tests (Table 6).
Table 4.
Association Between MAC30 Expression and Clinicopathological Variables of Patients With NSCLC Receiving Adjuvant Chemotherapy.
| Variables | No. (N = 164) | MAC30 Expression | P Value | |
|---|---|---|---|---|
| Low (n = 71) | High (n = 93) | |||
| Age (years) | .461 | |||
| <58 | 67 (40.8%) | 27 (38%) | 40 (43%) | |
| ≥58 | 97 (59.2%) | 44 (62%) | 53 (57%) | |
| Gender | .205 | |||
| Male | 104 (63.4%) | 45 (63.3%) | 59 (63.4%) | |
| Female | 60 (36.6%) | 26 (36.7%) | 34 (36.6%) | |
| Smoking status | .393 | |||
| Nonsmoker | 63 (38.4%) | 25 (35.2%) | 38 (40.9%) | |
| Smoker | 101 (61.6%) | 46 (64.8%) | 55 (59.1%) | |
| Histological type | .177 | |||
| SQCLC | 38 (23.2%) | 13 (18.3%) | 25 (26.9%) | |
| AC | 126 (76.8%) | 58 (81.7%) | 68 (73.1%) | |
| AS | ||||
| Tumor differentiation | .013 | |||
| Well | 27 (16.5%) | 13 (18.3%) | 14 (15.1%) | |
| Moderate | 53 (32.3%) | 34 (47.9%) | 19 (20.4%) | |
| Poor | 84 (51.2%) | 24 (33.8%) | 60 (64.5%) | |
| TNM stage | .008 | |||
| I | 31 (18.9%) | 17 (23.9%) | 14 (15.1%) | |
| II | 63 (38.4%) | 32 (45.1%) | 31 (33.3%) | |
| III | 70 (42.7%) | 22 (31%) | 48 (51.6%) | |
| Tumor classification | .229 | |||
| T1 + T2 | 61 (37.2%) | 27 (38%) | 34 (36.6%) | |
| T3 + T4 | 103 (62.8%) | 44 (62%) | 59 (63.4%) | |
| Lymph node metastasis | .006 | |||
| No | 36 (21.9%) | 24 (33.8%) | 12 (12.9%) | |
| Yes | 128 (78.1%) | 47 (66.2%) | 81 (87.1%) | |
Abbreviations: AC, adenocarcinoma; AS, adenosquamous; NSCLC, non–small cell lung cancer; SQCLC, squamous cell carcinomas; TNM, tumor–node–metastasis. Boldface values mean P < 0.05.
Table 5.
Risk Factors for Poor Tumor Differentiation in Patients With NSCLC Receiving Adjuvant Chemotherapy.
| Variables | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | P Value | B | SE | 95% CI | P Value | |
| MAC30 | 1.334 | 0.619 | 1.219-2.895 | .011 | 1.591 | 0.778 | 1.441-3.281 | .007 |
| Age | 1.108 | 0.411 | 0.802-1.885 | .482 | ||||
| Gender | 0.997 | 0.522 | 1.218-2.128 | .559 | ||||
| Smoking status | 1.291 | 0.642 | 1.003-2.318 | .429 | ||||
| Tumor classification | 1.331 | 0.717 | 1.109-2.835 | .297 | ||||
| Lymph node metastasis | 1.173 | 0.747 | 0.924-2.473 | .436 | ||||
Abbreviations: B, the parameter estimator of association coefficient; CI, confidence interval; NSCLC, non–small cell lung cancer; SE: standard error. Boldface values mean P < 0.05.
Table 6.
Univariate and Multivariate Analysis of Prognostic Factors in Patients With NSCLC Receiving Adjuvant Chemotherapy.
| Variables | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS | OS | DFS | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| MAC30 expression | 1.385 | 1.177-2.902 | .012 | 1.525 | 1.371-2.861 | .008 | 1.443 | 1.218-2.693 | .005 | 1.614 | 1.531-2.974 | .007 |
| Age (years) | 1.193 | 0.853-2.061 | .362 | 1.217 | 1.109-2.643 | .351 | ||||||
| Gender | 1.053 | 0.659-1.992 | .387 | 1.213 | 0.983-2.116 | .447 | ||||||
| Histological type | 0.994 | 0.615-1.936 | .212 | 1.125 | 1.008-2.362 | .275 | ||||||
| Smoking status | 1.238 | 0.881-2.492 | .336 | 1.401 | 1.114-2.793 | .472 | ||||||
| Tumor differentiation | 1.006 | 0.753-2.133 | .009 | 1.215 | 1.012-2.743 | .009 | 1.253 | 1.104-2.472 | .004 | 1.433 | 1.215-2.812 | .006 |
| TNM stage | 1.266 | 0.853-2.486 | .011 | 1.301 | 1.244-2.817 | .007 | 1.419 | 1.112-2.816 | .009 | 1.476 | 1.353-2.957 | .0003 |
| Tumor classification | 1.251 | 1.006-2.715 | .337 | 1.318 | 1.131-2.973 | .399 | ||||||
| Lymph node metastasis | 1.316 | 1.132-2.907 | .012 | 1.754 | 1.447-2.792 | .009 | 1.455 | 1.308-2.779 | .007 | 1.832 | 1.213-2.896 | .007 |
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NSCLC, non–small cell lung cancer; OS, overall survival; TNM, tumor–node–metastasis. Boldface values mean P < 0.05.
Discussion
Although investigations of molecule mechanisms in NSCLC were revealed, little improvement in survival was achieved.4 The study on lung cancer created an urgent demand for the valuable predictor of overall therapeutic effectiveness and survival in patients with NSCLC. Recently, several studies reported the opposite expressions of MAC30 in various human meningiomas.12–14 Consistent with the previous report showing the elevated MAC30 in patients with NSCLC,15 we confirmed the overexpression of MAC30 in patients with NSCLC from our present study. Obviously, MAC30 plays potential roles in the development and progression of NSCLC.
Actually, the role of MAC30-mediated tumor progression and invasion was explored with minor interruptions, since MAC30 was identified as a meningioma-associated protein.9 It has been reported that MAC30 expression increased in tumor and lymph node metastasis, while strong expression of MAC30 was related to a poor prognosis in patients with colon cancer.18 And other studies reported that overexpression of MAC30, which related to the development, invasiveness, and lymph node metastasis, might predict lymph nodal metastasis and poor prognosis in oral squamous cell carcinoma13 and epithelial ovarian cancer.19 Excitingly, the supportive research showing the relationship between MAC30 expression and cancer progression gave us a new perspective on MAC30 with lung cancer.
In our present study, MAC30 expression was significantly associated with tumor differentiation, TNM stages, and lymph node metastasis. Moreover, we observed the prognostic role of MAC30 overexpression in poor tumor differentiation, which could affect clinical chemotherapeutic response and survival of patients with NSCLC. Although Han et al also reported that MAC30 expression has close correlation with tumor differentiation,15 there’s no evidence of prognosis of MAC30 on tumor differentiation. In our opinion, the reason for the contrary outcomes might lie in the different number of patients enrolled and the different selected points. In our study, the median score of MAC30 expression in immunohistochemistry as the selected point was reached to divide the low and high MAC30 expression groups, which could maximally reduce the deviation. Notwithstanding, the current result in our study is consistent with the earlier work15 that patients with NSCLC having higher MAC30 expression were inclined to have a shorter OS. More importantly, in order to identify the implication of MAC30 on the development of NSCLC, we confirmed the correlation between MAC30 expression and DFS. As a result, our data showed that patients with NSCLC having high MAC30 expression also exhibited the significantly shorter DFS than that in cases with low MAC30 expression. From these current data, we conclude that high MAC30 expression, which could act as a valuable prognostic biomarker for poor tumor differentiation, short OS, and DFS, may ferment the awful prognosis of patients with NSCLC via mediating biological behavior of tumor cells.
To our knowledge, both fundamental and clinical studies have revealed that many molecules contribute to the various biological behaviors of malignant tumors including NSCLC. Based on the survival efficacy at 5 years of adjuvant chemotherapy in patients with NSCLC,20 it’s widely accepted that the current clinical issue in which adjuvant chemotherapy became a standard manner in NSCLC therapy had been described.21 To maximize the efficacy and minimize the side effects of adjuvant chemotherapy, new strategies based on a better understanding of molecular function in NSCLC is urgently needed. Recent research reported that MAC30 overexpression was certified to be associated with lymph node metastasis, which affected the treatment of chemotherapy in epithelial ovarian cancer.19 Indeed, our present study demonstrated the impact of MAC30 expression on postoperative chemotherapy in patients with NSCLC. In our study, MAC30 was significantly correlated with tumor differentiation, TNM stages, and lymph node metastasis but not associated with age, smoking status, tumor classification, and gender. Moreover, elevated MAC30 was shown as an independent prognostic factor of poor tumor differentiation, which influenced the response of chemotherapy in patients with NSCLC. Consistent with that found in all patients with NSCLC, the closed association further indicates the important stage of MAC30 in patients with NSCLC. As a general evaluation of therapeutic effect, OS and DFS in chemotherapeutic patients with increased MAC30 expression were curtailed, consistent with that in all patients with NSCLC. And the exploration of DFS analysis meticulously demonstrated the relationship between MAC30 expression and chemotherapeutic response. It’s more emphasized that the patients with stronger MAC30 expression, which indicated shorter OS and DFS, always showed the resistance to adjuvant chemotherapy followed by worse efficacy. Above all, as an independent prognostic biomarker of poor tumor differentiation, shorter OS, and DFS, overexpression of MAC30 is an unfavorable direction in patients with NSCLC receiving adjuvant chemotherapy.
By now, the biological mechanism of MAC30 in human malignancies is still unclear. With the evidence of conflicting expression,12–14 MAC30 might exhibit a distinct role in different organ tumors. Based on the inhibition of proliferation and mobility of human gastric cancer cells via suppressing AKT activation, MAC30 downregulation was a potential therapeutic approach for gastric carcinoma.22 A recent study suggested that as an important regulator in the progression of NSCLC,23 p53 mediates MAC30 expression levels.24 Also, MAC30 was regulated by BRCA1,25 another prognostic biomarker of NSCLC.26
Conclusion
In conclusion, we demonstrated that the overexpression of MAC30 was closely associated with poor tumor differentiation, high TNM stage, lymph node metastasis, and unfavorable prognosis in patients with NSCLC, especially in patients receiving chemotherapy. And MAC30 was an independent predictor for tumor differentiation, OS, and DFS in patients with NSCLC. More importantly, elevated MAC30 expression exhibited the worse response of adjuvant chemotherapy in patients with NSCLC. Our present study suggested the close relationship of MAC30 and prognosis of patients with NSCLC receiving chemotherapy. Further studies will be required to investigate the molecular function of MAC30 in patients with NSCLC.
Abbreviations
- AC
adenocarcinoma
- AS
adenosquamous
- B
the parameter estimator of association coefficient
- CI
confidence interval
- DFS
disease-free survival
- HR
hazard ratio
- mRNA
messenger RNA
- NSCLC
non–small cell lung cancer
- OS
overall survival
- OSQCLC
oral squamous cell carcinoma
- PBST
phosphate-buffered saline Tween solution
- SE
standard error
- SQCLC
squamous cell carcinomas
- TMEM97
transmembrane protein 97
- TNM
tumor–node–metastasis
Footnotes
Authors’ Note: HD, YS, and XL prepared the samples and carried out the data analysis mostly. XG, TM, and RC checked the data of patients. YF and HC designed the projects and wrote the paper. HD and XG contributed equally to the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant from Science Foundation of Health Department of Jiangsu Province in China (No. YG201408), grant from National Natural Science Foundation of China (No. 81500049), and grant from National Natural Science Foundation of China (No. 81470253).
References
- 1. William WN, Jr, Lin HY, Lee JJ, Lippman SM, Roth JA, Kim ES. Revisiting stage IIIB and IV non-small cell lung cancer: analysis of the surveillance, epidemiology, and end results data. Chest. 2009;136(3):701–709. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. [DOI] [PubMed] [Google Scholar]
- 3. Sun R, Liu Z, Wang L, et al. Overexpression of stathmin is resistant to paclitaxel treatment in patients with non-small cell lung cancer. Tumour Biol. 2015;36(9):7195–7204. [DOI] [PubMed] [Google Scholar]
- 4. Inomata M, Hayashi R, Tokui K, et al. Usefulness of the Palliative Prognostic Index in patients with lung cancer. Med Oncol. 2014;31(9):154. [DOI] [PubMed] [Google Scholar]
- 5. Banna GL, Lipari H, Nicolosi M, et al. A three-drug induction chemotherapy with gemcitabine, carboplatin, and paclitaxel for stage III non-small cell lung cancer. Med Oncol. 2013;30(2):533. [DOI] [PubMed] [Google Scholar]
- 6. Jouveshomme S, Canoui-Poitrine F, Le Thuaut A, Bastuji-Garin S. Results of platinum-based chemotherapy in unselected performance status (PS) 2 patients with advanced non-small cell lung cancer: a cohort study. Med Oncol. 2013;30(2):544. [DOI] [PubMed] [Google Scholar]
- 7. Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74(1):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitsudomi T, Suda K, Yatabe Y. Surgery for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol. 2013;10(4):235–244. [DOI] [PubMed] [Google Scholar]
- 9. Murphy M, Pykett MJ, Harnish P, Zang KD, George DL. Identification and characterization of genes differentially expressed in meningiomas. Cell Growth Differ. 1993;4(9):715–722. [PubMed] [Google Scholar]
- 10. Wilcox CB, Feddes GO, Willett-Brozick JE, Hsu LC, DeLoia JA, Baysal BE. Coordinate up-regulation of TMEM97 and cholesterol biosynthesis genes in normal ovarian surface epithelial cells treated with progesterone: implications for pathogenesis of ovarian cancer. BMC cancer. 2007;7:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malhotra K, Luehrsen KR, Costello LL, et al. Identification of differentially expressed mRNAs in human fetal liver across gestation. Nucleic Acids Res. 1999;27(3):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kayed H, Kleeff J, Ding J, et al. Expression analysis of MAC30 in human pancreatic cancer and tumors of the gastrointestinal tract. Histol Histopathol. 2004;19(4):1021–31. [DOI] [PubMed] [Google Scholar]
- 13. Yan BY, Wang DW, Zhu ZL, et al. Overexpression of MAC30 in the cytoplasm of oral squamous cell carcinoma predicts nodal metastasis and poor differentiation. Chemotherapy. 2010;56(6):424–428. [DOI] [PubMed] [Google Scholar]
- 14. Moparthi SB, Arbman G, Wallin A, et al. Expression of MAC30 protein is related to survival and biological variables in primary and metastatic colorectal cancers. Int J Oncol. 2007;30(1):91–95. [PubMed] [Google Scholar]
- 15. Han KY, Gu X, Wang HR, Liu D, Lv FZ, Li JN. Overexpression of MAC30 is associated with poor clinical outcome in human non-small-cell lung cancer. Tumour Biol. 2013;34(2):821–825. [DOI] [PubMed] [Google Scholar]
- 16. Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80(9):1803–1804. [DOI] [PubMed] [Google Scholar]
- 17. Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: a population-based cancer registry. J Thorac Oncol. 2013;8(9):1128–1135. [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZY, Zhao ZR, Adell G, et al. Expression of MAC30 in rectal cancers with or without preoperative radiotherapy. Oncology. 2006;71(3-4):259–265. [DOI] [PubMed] [Google Scholar]
- 19. Yang S, Li H, Liu Y, et al. Elevated expression of MAC30 predicts lymph node metastasis and unfavorable prognosis in patients with epithelial ovarian cancer. Med Oncol. 2013;30(1):324. [DOI] [PubMed] [Google Scholar]
- 20. Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. [DOI] [PubMed] [Google Scholar]
- 21. Pignon JP, Tribodet H, Scagliotti GV, et al. ; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. [DOI] [PubMed] [Google Scholar]
- 22. Xu XY, Zhang LJ, Yu YQ, et al. Down-regulated MAC30 expression inhibits proliferation and mobility of human gastric cancer cells. Cell Physiol Biochem. 2014;33(5):1359–1368. [DOI] [PubMed] [Google Scholar]
- 23. Fan X, Yu K, Wu J, Shao J, Zhu L, Zhang J. Correlation between squamous cell carcinoma of the lung and human papillomavirus infection and the relationship to expression of p53 and p16. Tumour Biol. 2015;36(4):3043–3049. [DOI] [PubMed] [Google Scholar]
- 24. Kannan K, Amariglio N, Rechavi G, et al. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20(18):2225–2234. [DOI] [PubMed] [Google Scholar]
- 25. Atalay A, Crook T, Ozturk M, Yulug IG. Identification of genes induced by BRCA1 in breast cancer cells. Biochem Biophys Res Commun. 2002;299(5):839–846. [DOI] [PubMed] [Google Scholar]
- 26. Zhao H, Zhang H, Du Y, Gu X. Prognostic significance of BRCA1, ERCC1, RRM1, and RRM2 in patients with advanced non-small cell lung cancer receiving chemotherapy. Tumour Biol. 2014;35(12):12679–12688. [DOI] [PubMed] [Google Scholar]