Abstract
Objective:
The biopsy needles currently used were designed for a transrectal biopsy and are known to experience significant deflection from the point of entry into the gland to the needle tip.
Methods:
Five designs were selected for testing: 18-gauge Bard, 15-gauge lancet tip needle with 12° vet-point cannula, and trocar tip needle with 12°, 15°, and 20° vet-point cannulas. The 15-gauge needle was designed to take a variable specimen sample between 20 and 60 mm, whereas the Bard needle specimen bed was fixed at 20 mm. The needles were bench tested on a spring-loaded platform and fired into gelatin matrix with modulus of elasticity similar to human prostate.
Results:
The Bard device with lancet tip needle deflected an average of 0.9 mm (range 0.3-1.3 mm) and 1.9° (range 0.6°-2.8°). Increasing needle diameter from 18-gauge Bard to 15-gauge variable with the same lancet tip needle design resulted in an average deflection across the 3 test lengths of 0.9 mm (range 0-2.0 mm) and 0.9° (range 0°-2.0°) with no significant difference. On the contrary, the use of the 3-point trocar tip needles with 12°, 15°, and 20° vet-point cannulas demonstrated significant reduction in the extent of deflection in both millimeters and degrees. There was no deflection at the 2- and 4-cm shots for both spring loads and preloads for the 3 vet tip angles tested. At 6 cm, the 20° vet tip performed the best.
Conclusion:
We proposed a mechanism that provides more accurate prostate sampling by combining a 3-point trocar tip on the needle with a 20° vet tip on the cutting cannula. Using the phantom, mimicking prostate gland tissue density, no deflection was revealed between 20- and 60-mm biopsy lengths, which should permit a straight sample in the majority of prostate glands and improve cancer localization for focal therapy planning.
Keywords: prostate cancer, transperineal template-mapping biopsy, deflection of biopsy needles, trocar tip needle, transrectal ultrasound
Introduction
Precise localization of cancerous regions within the prostate gland requires a biopsy needle that samples tissue without deflection. This requirement is critical if the biopsy information is used for planning targeted focal therapy (TFT) and magnetic resonance imaging (MRI)-guided biopsies.1,2 Transrectal biopsy needles are known to experience significant deflection from the point of entry into the gland to the distal end.3,4 If 3-dimensional (3D) representation of the biopsy site is going to be accurately utilized for planning TFT, the entry and resting positions of the biopsy needle should be the same.
Two methods for improving the accuracy of standard 12-core systematic TRUS (transrectal ultrasound)-guided biopsy are the transperineal template-mapping biopsy (TTMB) approach and MRI-targeted biopsy.5,6 Bittner et al demonstrated advantages of TTMB approach, which improves identification and location of cancer within the prostate compared to TRUS approach. They found cancer in 226 of 485 patients who underwent previously negative TRUS biopsy with subsequent TTMB as a confirmatory procedure, including 196 (86.7%) with clinically significant disease.7 Crawford et al investigated the clinicopathological correlation of TTMB with 3D reconstruction of RP specimens and demonstrated 72% of the TTMB cores were similar in grade to RP specimens and 80% accuracy in predicting laterality.8 These data proved the superiority of TTMB over standard TRUS-guided biopsy in accuracy of cancer detection. This is critical to selecting appropriate candidates for active surveillance versus focal or whole-gland therapies. Clearly, the more prospective studies comparing TTMB with RP specimens will further validate the accuracy of TTMB.
Although the transperineal prostate biopsy can significantly increase cancer detection rate compared to TRUS biopsy, there are concerns for overdetection of low-grade disease, the need for anesthesia, the number of biopsies required (a median of over 50), and the length of time the procedure takes. Investigators have turned to multiparametric MRI-targeted biopsy with the intention of only sampling clinically the significant cancers. The accuracy of needle placement for both biopsy methods is especially significant because TTPM or MRI-guided biopsy should enable the accurate collection of tissues from suspected tumor foci designated as regions of interest. Because the current biopsy needles have significant deflection, targets are often missed, requiring repeat attempts to secure the intended specimen. In addition, changing the length of the specimen bed from 20 to 60 mm might additionally enhance the accuracy by reducing the needle to take multiple biopsies along the same track. The focus of this study was to test needle deflection of various designs and lengths in order to improve the accuracy of needle placement and sampling quality during prostate biopsy.
Materials and Methods
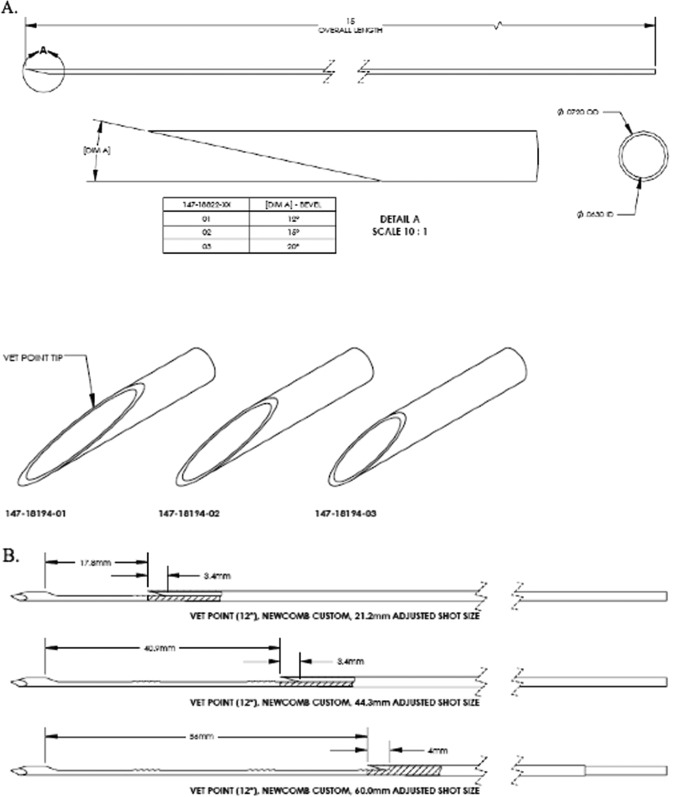
Five biopsy needle designs were selected for testing: 18-gauge (G) Bard (lancet tip), 15-G lancet tip needles with 12° vet-point cannula, and a 3-point trocar tip needle with 12°, 15°, and 20° vet-point cannulas (Figure 1A). The Bard system’s core length is fixed at 20 mm, whereas the 15-G needles and cannulas are designed to collect 20 to 60 mm core lengths (Figure 1B). The differences between the 18-G Bard and 15-G designs were needle diameter, 1.0 versus 1.5 mm; notch depth, 0.56 versus 0.76 mm; and core volume (at 20 mm) 0.00055 versus 0.0011 cm3. A 6-cm biopsy collected using 15-G needle has a core volume equal to 0.0033 cm3, 6 times that of the Bard needle. The designs were bench tested on a spring-loaded platform and fired into a gelatin matrix with modulus of elasticity similar to human prostate (gelatin at 4.0% by mass to water resulting in an average elastic modulus of 3.6 psi). Each 15-G design was tested at 3 biopsy lengths: 20, 40, and 60 mm. High-speed images of needle entry and deployed positions were captured and postprocessed to determine deflection distance and angle. Mean deflection was compared by paired T test (2-tailed).
Figure 1.
A, Vet tip design of cannula with 12°, 15°, and 20° angles. B, Three different shot lengths demonstrating vet-tip cannula over core bed with trocar tip needle.
Test Apparatus Preparation and Deflection Determination
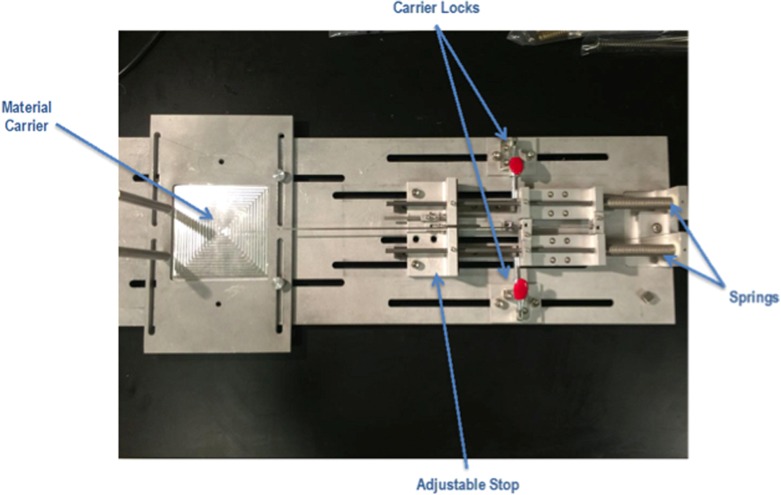
The needle and cannula were loaded into carriers on the test apparatus and locked in place with small L-brackets. The breadboard fixture was adjusted to the correct preload force and shot length, and the autotriggering mechanism was setup (Figure 2).
The gelatin block was placed into the material carrier and punctured with the tip of the needle.
High-speed images of firing and the final resting position of the needle inside the gelatin were captured. The gelatin block was replaced after firing the assembly 9 times (ie, 20, 40, and 60 mm shots for each spring).
Test images of the needle deployed with cannula retracted in the gelatin were isolated and imported into SOLIDWORKS.
A sketch line was drawn on the bottom of the cannula outside the gelatin. A second line was drawn tangent to the bottom of the needle beginning at the distal end (largest deflection).
The angle between the 2 line segments was measured. Linear deflection was determined using the measured angle and the sum of shot and tip lengths, 6 mm (ie, [shot length + 6 mm] × tangent [measured deflection angle]; Figures 3 and 4).
Figure 2.
Breadboard fixture for testing needle deflection.
Figure 3.

Test 16 demonstrating 2.0° deflection for the 15-G lancet tip needle with 15° vet tip fired 4 cm (see Table 1 for needle characteristics).
Figure 4.
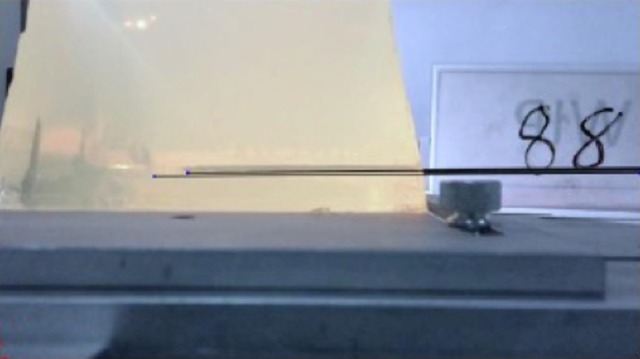
Test 88 demonstrating no deflection for 15-G Trocar tip needle with 20° vet tip fired 6.0 cm.
Results
The Bard device with lancet tip needle deflected an average of 0.9 mm (range: 0.3-1.3 mm) and an average of 1.9° (range: 0.6°-2.8°). Increasing the needle diameter from 18-G Bard to the 15-G variable while maintaining the lancet tip needle design resulted in an average deflection across the 3 test lengths of 0.9 mm (range: 0-2.0 mm) and 0.9° (range: 0°-2.0°). Statistical analysis demonstrated no significant difference between the Bard and variable designs (P = .671 and .064). However, a trocar tip needle design (3 points) significantly decreased deflection. No deflection was observed at 20 and 40 mm core lengths for the 3 vet-point cannula angles evaluated (12°, 15°, and 20°). At 60 mm, the 20° vet-point cannula performed the best with a spring rate of 2 lbs/in and a preload of 2.5 lbs (deflection 0°; Table 1).
Table 1.
Raw Data on 72 Test Firings.
| Needle | Cannula | Spring Number | Spring Rate (lbs/in) | Preload (lbs) | Shot Size (mm) | Test Number | Deflection (mm) | Deflection (°) |
|---|---|---|---|---|---|---|---|---|
| Bard | N/A | N/A | N/A | 20 | 1 | 0.3 | 0.6 | |
| 2 | 1.3 | 2.8 | ||||||
| 3 | NDa | ND | ||||||
| 4 | 1.2 | 2.7 | ||||||
| 5 | 1.3 | 2.8 | ||||||
| 6 | 0.9 | 2.0 | ||||||
| 7 | 0.5 | 1.0 | ||||||
| 8 | 0.7 | 1.6 | ||||||
| 9 | 0.9 | 2.0 | ||||||
| 10 | 0.9 | 1.9 | ||||||
| Lancet | Vet point 12° | Newcomb custom | 2 | 2.5 | 20 | 11 | 0 | 0 |
| 12 | 0 | 0 | ||||||
| 13 | 0 | 0 | ||||||
| 40 | 14 | 0.9 | 1.1 | |||||
| 15 | 1.2 | 1.5 | ||||||
| 16 | 1.6 | 2.0 | ||||||
| 60 | 17 | 0.9 | 0.8 | |||||
| 18 | 2.0 | 1.7 | ||||||
| 19 | 1.5 | 1.3 | ||||||
| Trocar | Vet point 12° | Newcomb custom | 2 | 2.5 | 20 | 37 | 0 | 0 |
| 38 | 0 | 0 | ||||||
| 39 | 0 | 0 | ||||||
| 40 | 40 | 0 | 0 | |||||
| 41 | 0 | 0 | ||||||
| 42 | 0 | 0 | ||||||
| 60 | 43 | 1 | 1.0 | |||||
| 44 | 1.3 | 1.1 | ||||||
| 45 | 0.0 | 0 | ||||||
| S-1277 | 3.2 | 3 | 20 | 46 | 0.0 | 0 | ||
| 47 | 0.0 | 0 | ||||||
| 48 | 0.0 | 0 | ||||||
| 40 | 49 | 0.0 | 0 | |||||
| 50 | 0.0 | 0 | ||||||
| 51 | 0.0 | 0 | ||||||
| 60 | 52 | 1.0 | 0.9 | |||||
| 53 | 0.5 | 0.4 | ||||||
| 54 | 1.7 | 1.5 | ||||||
| Trocar | Vet point 15° | Newcomb custom | 2 | 2.5 | 20 | 55 | 0 | 0 |
| 56 | 0 | 0 | ||||||
| 57 | 0 | 0 | ||||||
| 40 | 58 | 0 | 0 | |||||
| 59 | 0 | 0 | ||||||
| 60 | 0 | 0 | ||||||
| 60 | 61 | 1.4 | 1.2 | |||||
| 62 | 0 | 0 | ||||||
| 63 | 0 | 0 | ||||||
| S-1277 | 3.2 | 3 | 20 | 64 | 0 | 0 | ||
| 65 | 0 | 0 | ||||||
| 66 | 0 | 0 | ||||||
| 40 | 67 | 0 | 0 | |||||
| 68 | 0 | 0 | ||||||
| 69 | 0 | 0 | ||||||
| 60 | 70 | 0.7 | 0.6 | |||||
| 71 | NDa | ND | ||||||
| 72 | 0.8 | 0.7 | ||||||
| Trocar | Vet point 20° | Newcomb custom | 2 | 2.5 | 20 | 73 | 0 | 0 |
| 74 | 0 | 0 | ||||||
| 75 | 0 | 0 | ||||||
| 40 | 76 | 0 | 0 | |||||
| 77 | 0 | 0 | ||||||
| 78 | 0 | 0 | ||||||
| 60 | 79 | 0 | 0 | |||||
| 80 | 0 | 0 | ||||||
| 81 | 0 | 0 | ||||||
| S-1277 | 3.2 | 3 | 20 | 82 | 0 | 0 | ||
| 83 | 0 | 0 | ||||||
| 84 | 0 | 0 | ||||||
| 40 | 85 | 0 | 0 | |||||
| 86 | 0 | 0 | ||||||
| 87 | 0 | 0 | ||||||
| 60 | 88 | 0 | 0 | |||||
| 89 | 1.9 | 1.9 | ||||||
| 90 | 2 | 1.7 | ||||||
Abbreviations: N/A, not applicable; ND, no data.
aNo data resulting from poor image resolution or gelatin block distortion.
Comparison of the 3 trocar tip needles to the Bard needle demonstrated a significant reduction in the extent of deflection in both millimeters and degrees (Table 2). Figures 3 and 4 below illustrate the difference in deflection between the 15-G biopsy needles with lancet tip versus those with the trocar tip.
Table 2.
Comparison of Different Biopsy Needle Designs to the Bard Needle.
| Needle | Cannula | Number of Tests | Mean (mm) | Mean (°) | Difference (P Value) |
|---|---|---|---|---|---|
| Bard | Bard | 8 | 0.9 | 1.9 | Reference |
| Lancet point | Vet point, 12° | 9 | 0.9 | 0.9 | .671/.064 |
| Trocar | Vet point, 12° | 18 | 0.3 | 0.3 | .033/.002 |
| Trocar | Vet point, 15° | 17 | 0.2 | 0.1 | .013/.002 |
| Trocar | Vet point, 20° | 18 | 0.2 | 0.2 | .000/.000 |
Discussion
Conventional core biopsy devices are designed to collect 20-mm specimens, including the 18-G Bard used in this deflection study. This has been the standard of practice for 30 years. However, there is increasing interest in taking longer specimens when performing targeted TRUS MRI biopsies or a transperineal approach. In the former, anteriorly located lesions could be biopsied by sampling the entire prostate from posterior to anterior capsule, and in the latter, the full sagittal length of the gland could be biopsied as 1 specimen regardless of where the needle was positioned obliquely (lateral or midline). We designed a biopsy needle that can sample the prostate gland between 20 and 60 mm. A biopsy needle of this length is more prone to deflection between the entry point and the resting position, which could compromise sampling accuracy.
Recently, several studies have evaluated the modified needle shape with the purpose of optimizing image-guided prostate biopsy by minimizing the target error.9–11 For instance, Kuru et al tested trocar tip needles instead of beveled (or lancet) needles, which may significantly deviate away from the bevel.9 First, they performed stereotactic biopsies on 2 prostate phantoms with 3 randomly placed TRUS-visible lesions. Four stereotactic template biopsy cores from each lesion were sampled under TRUS guidance in a comparative setting—2 cores with standard beveled needles and 2 biopsies with novel trocar tip needles. As a study end point, the investigators calculated a procedural targeting error (PTE) between the virtually planned biopsy trajectory and the manually registered 3D needle position of every single biopsy core taken. The novel needle tip design demonstrated better performance with the absolute overall PTE as of 0.13 mm (standard deviation [SD], ± 0.15 mm), with the highest PTE in the sagittal plane (0.18 ± 0.16 mm), followed by the coronal (0.13 ± 0.17 mm) and axial (0.09 ± 0.05 mm) planes. Comparing the PTE of the novel trocar-shaped needles with conventional beveled needles, there was a statistically significant difference. In the axial plane, the PTE difference between the standard beveled needle and the trocar-shaper one was significant with P value of .03, however with insignificant overall P value of .47.
Secondly, the same group clinically evaluated the benefit of trocar point needles for image-guided prostate biopsy compared with standard beveled needles in 24 men who underwent MRI-targeted fusion-guided transrectal prostate biopsy (TPB).12 For this study as a primary end point, the authors use a scored scale (1 = worse to 5 = best) for operator using the following criteria: (1) accuracy of matching between planned and performed biopsy with PTE; (2) histologic quality of the sample; (3) elegance or the easiness to take the biopsy in proper time, planned position, and best histologic quality. The histologic sample quality was assessed by a blinded pathologist. As a result, the trocar point needles demonstrated a better performance with significantly increased (P < .05) scoring for accuracy and elegance rated by the urologist. Furthermore, the histologic quality scored by the pathologist was also superior. In addition, the experienced urologist achieved lower PTE with trocar-sharpened needles as opposed to trainees/residents.
Okamura et al used force modeling for needle insertion into silicone rubber phantom and soft tissue of bovine liver, which measured and modeled into 3 parts: (1) capsule stiffness, a nonlinear spring model; (2) friction, a modified Karnopp model; and (3) cutting, a constant for a given tissue. A bevel tip caused more needle bending and was more easily affected by tissue density variations compared to triangular and diamond tips.13
Blumenfeld et al researched needle placement accuracy of MRI-guided core needle biopsy of the prostate using 2 needle designs—symmetrical and asymmetrical beveled tip.11 A total of 10 biopsies in patients with prostate cancer were performed with 18-G core biopsy needle via a percutaneous transperineal approach. Needle placement error (NPE) was calculated by comparing the coordinates of preprocedural targets with the needle tip measured from the live intraprocedural coherent gradient echo images. The authors also analyzed possible causes of these errors by measuring displacement caused by needle deflection and needle susceptibility artifact shift in controlled phantom studies. The NPE due to misalignment of the needle template guide was also evaluated. The mean and SD of errors in targeted biopsies was 6.5 ± 3.5 mm. Phantom experiments showed a significant NPE due to needle deflection with an asymmetrically beveled tip (3.2-8.7 mm depending on the tissue type) but significantly smaller error with a symmetrical bevel (0.6-1.1 mm). Needle susceptibility artifacts observed a shift of 1.6 ± 0.4 mm from the true needle axis. Misalignment of the needle template guide contributed an error of 1.5 ± 0.3 mm. Finally, the NPE due to needle deflection was the most significant contributor to the error, especially for needles with an asymmetrical bevel.
Urologists can now target prostatic cancer lesions identifiable by advanced imaging such as 3D-TRUS or MRI. Therefore, achieving 100% accuracy in needle placement would be ideal. A study by Nakashima et al demonstrated that endorectal MRI is useful for predicting local extension, as well as tumor site and tumor size, of cancer foci greater than 10 mm in diameter, whereas MRI did not correlate significantly with the histological diameter of tumors smaller than 10 mm.14 A biopsy needle that takes a specimen along a straight path from entry to end position might improve the sensitivity of MRI-targeted biopsies.
Susil et al examined an accuracy of stereotactic-targeted biopsy in 4 clinical cases with intermediate and high-risk prostate cancer.15 They demonstrated a mean needle placement accuracy of 2.1 mm, with 95% of biopsies taken within 4.0 mm of their targets and a maximum of 4.4 mm. They used thick standard 14-G needles when in each case needle deflection was minimal but tissue deformation and dislocation were considerable. The limitation of this study was that the authors did not take superior–inferior (depth) error into account because they assumed that inaccuracy due to this error was minimal and not as significant as the transverse error. Furthermore, the superior–inferior discrepancy was significantly higher than the right/left or anterior/posterior discrepancy at the target locations.
The primary purpose of our study was to characterize needle deflection and confirm the output of the new 3DBiopsy system: the trocar point needle design combined with a vet-point cannula significantly reduces deflection over the more common, lancet point design. Part of the novelty of the 3D Biopsy needle is shot length up to 60 mm, which enables the user to sample the entire length of the gland, regardless of the approach (TRUS or TTMB). The longer shots could have resulted in greater total deflection when compared to standard, 20 mm lengths. Confirmation that excessive deflection does not occur at the upper bound of shot length was critical to ensuring patient safety and target accuracy. Although this study confirms that the combination of a 3-point trocar tip needle and a 20° vet tip cutting cannula performed the best, there are limitations to this investigation. Measuring absolute deflection proved difficult because of gelatin block distortion, minimal deflection of the trocar needle, and an inconsistent zero-deflection reference. The needle and cannula deflect over the entire length of the shaft and limitations of the camera field of view make identifying a true, zero-deflection reference along the axis difficult. Each of these limitations will be addressed during validation and verification testing in future phases.
We also hypothesized that the cutting angle of the cannula might affect deflection. Menghini and vet tip grinds on the cannula were evaluated for both specimen length consistency (data not reported) and deflection. To our knowledge, this is the first study to evaluate the dynamics between the cutting portion of the cannula and the tip of the needle. The data from this study suggest that a 20° vet tip on the cannula and a 3-point trocar had the least deflection (0 mm for the 3 test lengths of 20, 40 and 60 mm).
Testing in human tissue, which has varying degrees of density, will be required to confirm these favorable results. An ex vivo prostate model followed by then in vivo experience, where virtual guides can be compared to the actual needle position, will hopefully substantiate the value of using this new prostate biopsy needle.
Conclusion
We proposed a mechanism that provides more accurate prostate sampling by combining a 3-point trocar tip on the needle with a 20° vet tip on the cutting cannula. Testing in a 4% gel mimicking prostate gland tissue density confirmed no deflection between 20 and 60 mm biopsy lengths, which should permit a straight sample in the majority of prostate glands. This will allow positioning of the needle at the posterior of the gland and relying on the virtual needle guide to direct the biopsy directly into an anterior lesion when performing targeted MRI biopsy. For those wishing to perform a TTPM biopsy, especially when using visual targeting, a straight, nondeflecting biopsy may improve lesion location for purposes of focal therapy.
Abbreviations
- 3D
3-dimensional
- G
gauge
- MRI
magnetic resonance imaging
- ND
no data
- NPE
needle placement error
- PTE
procedural targeting error
- TFT
targeted focal therapy
- TPB
transrectal prostate biopsy
- TRUS
transrectal ultrasound
- TTMB
transperineal template-mapping biopsy
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stone, Crawford, Lucia, Smith own stock in 3DBiopsy, Inc. Stone is also officer in company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding is through 3DBiopsy, Inc
References
- 1. Ahmed HU, Akin O, Coleman JA, et al. Transatlantic Consensus Group on active surveillance and focal therapy for prostate cancer. BJU Int. 2012;109(11):1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanthabalan A, Emberton M, Ahmed HU. Biopsy strategies for selecting patients for focal therapy for prostate cancer. Curr Opin Urol. 2014;24(3):209–217. [DOI] [PubMed] [Google Scholar]
- 3. Fraggetta F, Pepe P, Improta G, Aragona F, Colecchia M. Prostate needle biopsy: what we do and what should be improved. Anal Quant Cytopathol Histpathol. 2013;35(3):130–138. [PubMed] [Google Scholar]
- 4. Acher P, Dooldeniya M. Prostate biopsy: will transperineal replace transrectal? BJU Int. 2013;112(5):533–534. [DOI] [PubMed] [Google Scholar]
- 5. Bostanci Y, Kazzazi A, Djavan B. Optimizing prostate biopsy. Minerva Urol Nefrol. 2012;64(4):233–243. [PubMed] [Google Scholar]
- 6. Berglund RK, Jones JS. Optimal prostate biopsy regimen. Curr Urol Rep. 2012;13(6):455–459. [DOI] [PubMed] [Google Scholar]
- 7. Bittner N, Merrick GS, Butler WM, Bennett A, Galbreath RW. Incidence and pathological features of prostate cancer detected on transperineal template guided mapping biopsy after negative transrectal ultrasound guided biopsy. J Urol. 2013;190(2):509–514. [DOI] [PubMed] [Google Scholar]
- 8. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73(7):778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuru TH, Simpfendorfer T, Roethke M, Hohenfellner M, Hadaschik BA. Improving accuracy in image-guided prostate biopsy by using trocar-sharpened needles. Urol Int. 2013;91(4):404–409. [DOI] [PubMed] [Google Scholar]
- 10. Simpfendorfer T, Kuru TH, Steinemann S, et al. Trocar-sharpened needles for image-guided prostate biopsy improve sample quality and performance: first clinical results. J Endourol. 2014;28(11):1384–1388. [DOI] [PubMed] [Google Scholar]
- 11. Blumenfeld P, Hata N, DiMaio S, et al. Transperineal prostate biopsy under magnetic resonance image guidance: a needle placement accuracy study. J Magn Reson Imaging. 2007;26(3):688–694. [DOI] [PubMed] [Google Scholar]
- 12. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190(4):1380–1386. [DOI] [PubMed] [Google Scholar]
- 13. Okamura AM, Simone C, O’Leary MD. Force modeling for needle insertion into soft tissue. IEEE Trans Biomed Eng. 2004;51(10):1707–1716. [DOI] [PubMed] [Google Scholar]
- 14. Nakashima J, Tanimoto A, Imai Y, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64(1):101–105. [DOI] [PubMed] [Google Scholar]
- 15. Susil RC, Camphausen K, Choyke P, et al. System for prostate brachytherapy and biopsy in a standard 1.5 T MRI scanner. Magn Reson Med. 2004;52(3):683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]