Structured Abstract
OBJECTIVE
To demonstrate that expectant observation of young infants with small adrenal masses would result in excellent event-free and overall survival (EFS and OS).
SUMMARY BACKGROUND DATA
Neuroblastoma is the most common malignant tumor in infants, and in young infants, 90% are located in the adrenal gland. Although surgical resection is standard therapy, multiple observations suggest that expectant observation could be a safe alternative for infants <6 months old who have small adrenal masses.
METHODS
A prospective study of infants less than six months of age with small adrenal masses and no evidence of spread beyond the primary tumor was performed at participating Children’s Oncology Group institutions. Parents could choose observation or immediate surgical resection. Serial abdominal sonograms and urinary VMA and HVA measurements were performed over a ninety-week interval. Infants experiencing a 50% increase in the volume of the mass or urine catecholamine values, or an increase in the HVA/VMA ratio >2 were referred for surgical resection.
RESULTS
87 eligible patients were enrolled, 83 elected observation and 4 chose immediate surgery. 16 observation patients ultimately had surgery; 8 had INSS stage 1 neuroblastoma, 2 had higher stage neuroblastoma (2B and 4S), 2 had low grade adrenocortical neoplasm, 2 had adrenal hemorrhage and 2 had extralobar pulmonary sequestration. The two patients with adrenocortical tumors were resected because of a >50% increase in tumor volume. The 3-year EFS for a neuroblastoma event was 97.7±2.2% within the entire cohort of patients (n=87). The 3-year overall survival was 100% with median follow-up of 3.2 years. 81% of patients on the observation arm were spared resection.
CONCLUSIONS
Expectant observation of infants <6 months old with small adrenal masses led to excellent EFS and OS while avoiding surgical intervention in a large majority of the patients.
INTRODUCTION
Neuroblastoma is the most common malignant tumor in infants. The majority of these are small, localized adrenal tumors at the time of diagnosis 1. Surgical resection is the standard treatment, with excellent overall results 2–8. Two lines of evidence suggest that expectant observation may decrease the need for resection. First, a high rate of spontaneous regression is observed in infants with neuroblastoma and several investigators have reported regression of localized prenatally diagnosed adrenal masses 9–11 and there is considerable evidence for spontaneous regression of tumors detected through population screening programs for neuroblastoma 12–16. Furthermore, several groups have reported spontaneous regression of larger tumors which were observed after diagnostic biopsy or partial resection 17,18.
In addition to these data supporting spontaneous regression, there is evidence that the morbidity and mortality associated with surgery may be greater than the risk of the tumor in an infant. Although no study has specifically investigated the risks of adrenal surgery in young infants, the available literature suggests that there are significant risks associated with resection of adrenal and non- adrenal abdominal neuroblastoma tumors in children under a year of age, with a trend toward higher risk in younger infants 5,19,20. Infants are also at a higher risk of anesthetic complications than are older children 21,22. The overall mortality for young infants undergoing adrenal surgery for tumor resection appears to be greater than or equal to 2% 5,23.
Previous observation trials suggest that tumor size and characteristics, as well as certain patterns of urine catecholamine metabolite (vanillylmandelic acid (VMA) and homovanillic acid (HVA)) secretion, are associated with a benign pattern of behavior and increased probability of spontaneous regression 13,15,16,24–26. We conducted a prospective trial of expectant observation of infants with small adrenal tumors identified either prenatally or within the first six months of life. The key feature of this protocol was close sonographic monitoring of tumor volume and catecholamine secretion with increased frequency of surveillance in those infants whose tumors enlarged in volume, had increased levels of urine VMA or HVA, or had an HVA:VMA ratio > 227. If these signs of tumor growth or de-differentiation persisted, the patient was referred for surgical resection.
METHODS
Study Enrollment and Eligibility Criteria
Patients could be enrolled on this protocol by any Children’s Oncology Group (COG) member institution (ClinicalTrials.org Identifier: NCT00445718) who obtained Institutional Review Board (local or centralized) and opened the study. The study was opened to enrollment on July 9, 2001, and reached accrual and was closed on January 8, 2010. Informed consent was given by the parent or legal guardian. The eligibility criteria were designed to select a group of infants with small adrenal masses without evidence of local extension beyond the gland or distant metastases, that is, International Neuroblastoma Staging System (INSS) stage 1 tumors.
The specific eligibility criteria were: (1) age ≤6 months on the date the mass was first identified and the date of enrollment must be <120 days after the first imaging study identifying the mass, if identified postnatally, or the patient must be <120 days of age if the first imaging study identifying the mass was prenatal; (2) a sonographically-identified adrenal mass which is ≤16 ml in volume (assuming an ellipsoid with volume V = (4/3)π(X/2)(Y/2)(Z/2) where X = maximum longitudinal diameter, Y= maximum transverse diameter and Z = maximum anterior –posterior diameter), corresponding to a sphere with a diameter of 3.1 cm if solid or ≦65 ml (corresponding to a sphere with diameter 5.0 cm) if the mass was at least 25% cystic, and did not cross the midline; (3) disease limited to the adrenal gland as demonstrated by abdominal computed tomography (CT) or magnetic resonance (MR) scan and MIBG (I131-meta-iodobenzylguanidine) scintiscan; and (4) no prior abdominal surgery or chemotherapy. In addition, a bone marrow biopsy was encouraged but not required. If such a biopsy was obtained, it was required that it be negative for tumor cells.
Treatment Protocol
At the time of enrollment, the parents or legal guardian were offered two possible treatment plans: expectant observation or immediate surgical resection. For those patients on the expectant observation arm, abdominal sonograms were performed at prescribed intervals over the 90-week observation period of the study (Table 1). All ultrasound studies included imaging of the liver with a high frequency linear transducer to assure optimal detection of small parenchymal nodules. In addition, all patients underwent CT or MR imaging of the abdomen at 6 and 42 weeks to assure that there was no progression beyond the adrenal gland. With rare exceptions, imaging studies were reviewed centrally by the study radiologist (C.E.B). Urinary samples were obtained at presentation and at the time of each subsequent ultrasound study, and analyzed for VMA and HVA using gas chromatography/mass spectroscopy (Table 1).
Table 1.
Follow-up Investigations during Observation Period
| Study Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up Investigation | 0 | 3 | 6 | 12 | 18 | 30 | 42 | 66 | 90 |
| Abdominal Sonogram | X | X | X | X | X | X | X | X | X |
| Urine VMA/HVA | X | X | X | X | X | X | X | X | X |
| Abdominal CT/MRI | X | X | X | ||||||
Expectant observation was terminated and the child was referred for surgical resection if the volume of the mass increased by 50% compared with the volume at the time of enrollment. If an increase in volume of the tumor was noted, but it was not of an adequate magnitude to mandate resection, then continued sonographic evaluation at three week intervals was performed until a stable volume was noted on two successive studies or the patient had surgery. After the mass had stabilized in volume on two consecutive three week studies, the next sonogram/urine VMA/HVA was at a six week interval and the following studies at the regularly scheduled times based on the original entry date. Urinary catecholamine levels, VMA and HVA, were obtained at presentation and at the time of each subsequent ultrasound study. If these metabolites showed an increase in value of greater than 50% above the initial baseline value (and actual value was abnormal) or if the VMA/HVA ratio decreased below 0.5 (and the actual HVA value was above normal), the time interval to the subsequent studies (sonograms and urine catecholamine sampling) was decreased to three weeks. Similar increased surveillance took place if the VMA or HVA level, having decreased to a new nadir level below the value at entry, subsequently increased 50% above the nadir, assuming that this value was above the upper limit of normal. If the elevated value did not return to the baseline/nadir value (or the upper limit of normal, whichever was greater) within twelve weeks or the VMA/HVA ratio did not rise above 0.5 within six weeks, the patient came off-observation and surgery was recommended without regard to the tumor volume. If there was no evidence of an adrenal mass on imaging at this time, it was recommended that the patient undergo CT or MR imaging of the neck, chest and abdomen as well as bone marrow aspiration/biopsy and bone scan to rule out occult disseminated disease. On the other hand, if the catecholamine values returned to baseline, subsequent studies took place six weeks later and then at the regularly scheduled times. These off-observation criteria are listed in Table 2.
Table 2.
Criteria for removal of a patient from the protocol-specified observation period
| Criterion | |
|---|---|
| Evidence of persistent tumor growth | A >50% increase in the volume of the mass, or |
| A >50% increase in either VMA or HVA, neither returns to baseline/nadir within 12 weeks, and the value is above the upper limit of normal for that metabolite, or | |
| The VMA/HVA <0.5 (and HVA greater than upper limit of normal) and does not increase above 0.5 within six weeks. | |
| Completion of the 90 week observation period | |
| Progression, secondary malignancy, or death | |
If there was a residual adrenal mass larger than two ml in volume (a 1.6 cm sphere) at the end of the observation period it was recommended that the mass be resected. If a patient completed the 90 week observation period without triggering resection due to an increase in tumor volume or catecholamine values, yet the VMA and/or HVA values remained elevated, any persistent adrenal mass was resected, regardless of volume. For patients whose parents opted for immediate surgery, data were collected regarding operative report, pathology report and initial diagnostic studies.
Statistical Considerations
There are an estimated 136 patients per year diagnosed with neuroblastoma prenatally or ≤6 months of age at COG member institutions. Based on prior work 6,10, we estimated that 90% of the tumors were adrenal in location and 30% would be INSS stage 1. In addition, we estimated that approximately one third of families would consent to protocol therapy, for an annual accrual rate of 14 patients. The actual accrual rate was approximately 11 patients/year during the interval of the study.
Event-free survival (EFS) and overall survival (OS) were estimated using the Kaplan-Meier method. For the purposes of this study, a “neuroblastoma event” (NE) was defined as progression or recurrence to an INSS stage ≥2 neuroblastoma after resection. For EFSNE, time to event was defined as the time from study enrollment until first occurrence of a NE, second malignancy, or death from any cause, or until the time of last contact if no event occurred. “Any malignancy event” (AME) was defined as the occurrence of NE or the finding of any non-neuroblastoma malignancy upon resection of the primary adrenal mass. For EFSAME, time to event was defined as the time from study enrollment until first occurrence of NE, AME, second malignancy, or death from any cause, or until the time of last contact if no event occurred. For OS, death was the only event considered. In addition, the resection-free survival (RFS) was estimated for those patients on the expectant observation arm, where the occurrence of a resection was the only event considered. The study was not designed or powered for subgroup comparisons of survival curves; log-rank tests were not performed, but 3-year estimates ± standard error have been presented.
Because this was a reduction in therapy study, sequential monitoring was performed to provide early detection of a reduction in EFS compared with the excellent results of legacy studies in which surgical resection had been the primary therapy for INSS stage 1 patients. An early stopping rule was planned to halt the study if the two-year EFS fell below an acceptable value of 95%. Sequential monitoring of need for surgical resection was also performed, whereby the study would be stopped if the rate of resection exceeded 75%.
Tests for proportions were based on the binomial distribution. P-values less than 0.05 were considered statistically significant.
RESULTS
A total of 97 infants were enrolled in the study, and 10 of these were subsequently determined to be ineligible: enrolled after surgical resection (5); the time interval was too long between diagnosis and enrollment (3); and informed consent issues (2). The remaining 87 eligible patients formed the analytic cohort for this report. Eighty three of these patients were enrolled on the observation arm of the study and 4 on the immediate surgery arm. For the observation arm, 56 patients completed observation and 27 discontinued observation (13 of whom were lost to follow-up during the 90 week observation period) (Table 3). Twenty-seven patients were diagnosed prenatally or on the first day of life; for patients diagnosed beyond this time, the median age at diagnosis was 14 days. The median age at enrollment was 42 days. The male: female ratio was 2.1. Twenty six percent of the patients had elevated urine catecholamine values at the time of enrollment, and 33% of the tumors were ≥25% cystic by volume. There was no significant correlation between the proportion of patients who completed the observation arm of the trial with: time of diagnosis (pre- or postnatal), gender, cystic or solid nature of the mass, or the urine catecholamine levels at the time of diagnosis (normal or elevated).
Table 3.
Characteristics of 87 eligible patients enrolled on COG study ANBL00P2
| Characteristic | Surgery Arm | Observation Arm | ||||
|---|---|---|---|---|---|---|
| Started Observation Period | Did not Complete Observation Period | Completed Observation Period | P-value* | |||
| Diagnostic Study: Prenatal vs. Postnatal | Prenatal | 2 | 27 | 8 | 19 (70%) | 0.8 |
| Postnatal | 1 | 44 | 15 | 29 (66%) | ||
| Unknown | 1 | 12 | 4 | 8 (67%) | NA | |
| Overall | 4 | 83 | 27 | 56 (67%) | NA | |
| Median Age (days) at Diagnosis (Postnatal only) | 86 | 14 | 5 | 17 | NA | |
| Median Age (days) at Study Entry | 32.5 | 42 | 2 | 3.5 | NA | |
| Gender | Male | 2 | 56 | 18 | 38 (68%) | 1.0 |
| Female | 2 | 27 | 9 | 18 (67%) | ||
| Nature of the Mass | ≥25% Cystic | 1 | 27 | 10 | 17 (63%) | 0.6 |
| Solid | 3 | 56 | 17 | 39 (70%) | ||
| VMA and/or HVA levels at Diagnosis | Normal | 1 | 58 | 16 | 42 (72%) | 0.2 |
| Elevated | 2 | 20 | 9 | 11 (55%) | ||
p-value - test of a difference in the proportion of patients who completed the observation period, by a characteristic (Timing of Diagnostic Study, gender, nature of the mass, and VMA/HVA levels at diagnosis)
The vast majority of the patients who completed the observation arm had a decrease in the volume of the adrenal mass. Among these 56 patients, 27 (48%) had no residual mass, 13 (23%) had a mass > 0 but ≤ 1 ml, eight (14%) had a mass > 1 but ≤ 2 ml, and eight (14%) had a mass > 2 ml. Thus, 71% had a residual mass ≤ 1 ml (including those with no discernible mass) and 86% had a residual mass ≤ 2 ml. Fifty four patients had VMA determinations at end observation, 50 (93%) of these had normal levels. Fifty six patients had HVA determinations at end observation, 53 (95%) of these had normal values.
Twenty patients underwent resection: four went to the immediate surgery arm, and 16 patients who were originally entered in the observation arm ultimately underwent a resection (Table 4). Fifteen patients in the latter group had resection during the 90 week observation period and one patient had surgery after completing the observation protocol. One of the 15 patients who had resection during observation was lost to follow up on the day of surgery-- the tumor histology was consistent with adrenal hemorrhage. Among the 16 observation arm patients who eventually had surgery, 10 patients had neuroblastoma, two patients had low-grade adrenal cortical neoplasms, two had adrenal hemorrhage or hematoma, and two had extralobar pulmonary sequestration. Nine of the neuroblastoma patients had signs of tumor growth or progressive disease and one patient underwent resection at the request of his/her parents. Among the nine patients with tumor growth or progression: a) seven had INSS stage 1 disease: six had surgery because of a greater than 50% increase in tumor volume and one because of a sustained increase in catecholamine secretion; and, b) two patients had progressive disease: one patient had liver involvement noted at the time of the first follow up ultrasound, this patient had stage 4S disease and was alive at 5.6 years follow up; the second patient went to surgery for a >50% increase in the volume of the mass and was found to have stage 2B disease (ipsilateral lymph node involvement), this patient was lost to follow up after 1.7 years without relapse or further progression. All of the nine neuroblastoma patients with tumor growth and/or progression had solid rather than cystic tumors.
Table 4.
Summary of Patients Who Underwent Resection
| Patient Number | Age at Enrollment (days) | Structural character | Diagnosis of NBL (INSS Stage) | Initial Treatment | COG Risk Group | Reason for Surgery | # Days Between Enrollment & Surgery | Outcome* |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | Cystic | Yes (Stage 1) | Surgery | Low | Immediate surgery arm | 2 | Alive w/o event |
| 2 | 8 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 421 | Alive w/o event |
| 3 | 40 | Cystic | No ACN | Observation Only | NA | >50% increase in volume | 25 | Alive w/o event |
| 4 | 76 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 12 | Lost to follow-up |
| 5 | 29 | Solid | No EPS | Observation Only | NA | >50% increase in volume | 794 | Alive w/o event |
| 6 | 28 | Solid | No Hematoma | Observation Only | NA | Elective surgery at parental request | 492 | Lost to follow-up |
| 7 | 33 | Cystic | Yes (Stage 1) | Surgery | Low | Immediate surgery arm | 1 | Alive w/o event |
| 8 | 36 | Solid | Yes (Stage 2B) | Observation Only | Low | >50% increase in volume | 58 | PD at time of resection; Lost to follow-up |
| 9 | 27 | Cystic | Yes (Stage 1) | Observation Only | Low | Elective surgery at parental request | 247 | Alive w/o event |
| 10 | 37 | Solid | Yes (Stage 4S) | Observation Only | Intermediate | Progressive disease or recurrence (Liver nodules) | 21 | PD at time of resection; Alive |
| 11 | 135 | Solid | Yes (Stage 1) | Surgery | Low | Immediate surgery arm | 2 | Alive w/o event |
| 12 | 28 | Solid | No ACN | Observation Only | NA | >50% increase in volume | 24 | Alive w/o event |
| 13 | 24 | Cystic | No Hematoma | Observation Only | NA | Persistent VMA/HVA < 0.5 | 71 | Lost to follow-up |
| 14 | 185 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 156 | Alive w/o event |
| 15 | 151 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 57 | Alive w/o event |
| 16 | 22 | Solid | No EPS | Observation Only | NA | Elective surgery at parental request | 142 | Alive w/o event |
| 17 | 94 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 86 | Alive w/o event |
| 18 | 50 | Solid | Yes (Stage 1) | Observation Only | Low | >50% increase in volume | 25 | Alive w/o event |
| 19 | 22 | Solid | Yes (Stage 1) | Observation Only | Low | > 50% increase in VMA or HVA | 47 | Alive w/o event |
| 20 | 27 | Solid | No EPS | Surgery | NA | Immediate surgery arm | 2 | Alive w/o event |
Immediate Surgery patients were only required to submit 1 month of follow-up data. (ACN, adrenal cortical neoplasm; EPS, extralobar pulmonary sequestration; PD, progressive disease)
Thirteen patients were lost to follow up during the 90 week observation period (Table 5). The mean observation period in this group was 342 days. Patient A had elective resection of the mass after 491 days of follow up and was found to have a resolving adrenal hemorrhage. The remaining patients were lost to follow up prior to any known tumor resection. In 12 of the 13 patients, the residual tumor volume was less than one ml at the time of the last submitted sonographic evaluation. In the 12 patients for whom data was available, 10 patients had normal urine catecholamine metabolite values at last evaluation.
Table 5.
Summary of Patients Who Were Lost to Follow Up During Observation Period
| Patient Identifier | Duration of Observation (days) | Tumor Volume at Enrollment (ml) | Tumor Volume at Last Report (ml) | Catecholamine Levels at Enrollment | Catecholamine Levels at Last Report | ||
|---|---|---|---|---|---|---|---|
| VMA | HVA | VMA | HVA | ||||
| A | 491 | 2.98 | 0.37 | Normal | Normal | Normal | Normal |
| B | 291 | 9.42 | 0.00 | Normal | Normal | Normal | Normal |
| C | 636 | 6.28 | 0.00 | Normal | Normal | Normal | Normal |
| D | 78 | 4.82 | 3.14 | Elevated | Normal | Normal | Elevated |
| E | 560 | 8.25 | 0.52 | Elevated | Normal | Normal | Normal |
| F | 645 | 17.39 | 0.73 | Normal | Normal | Normal | Normal |
| G | 293 | 23.18 | 0.00 | Normal | Normal | Normal | Normal |
| H | 180 | 13.48 | 0.77 | Normal | Normal | Not done | Not done |
| I | 225 | 0.57 | 0.00 | Normal | Normal | Normal | Normal |
| J | 465 | 3.36 | 0.00 | Normal | Elevated | Normal | Elevated |
| K | 79 | 2.92 | 0.00 | Normal | Normal | Normal | Normal |
| L | 160 | 20.69 | 0.68 | Normal | Normal | Normal | Normal |
| M | 349 | 2.54 | 0.00 | Normal | Normal | Normal | Normal |
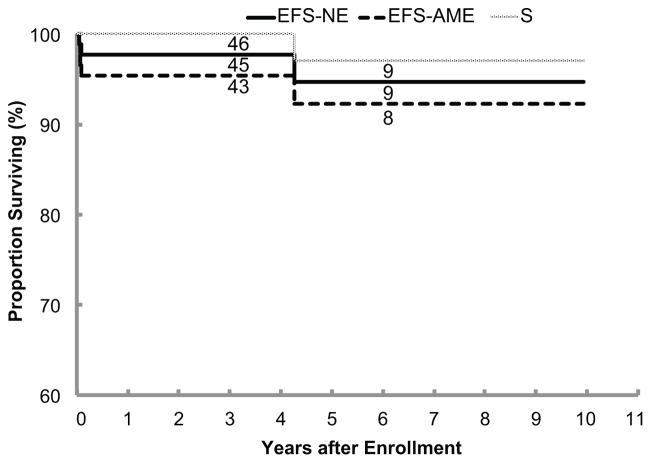
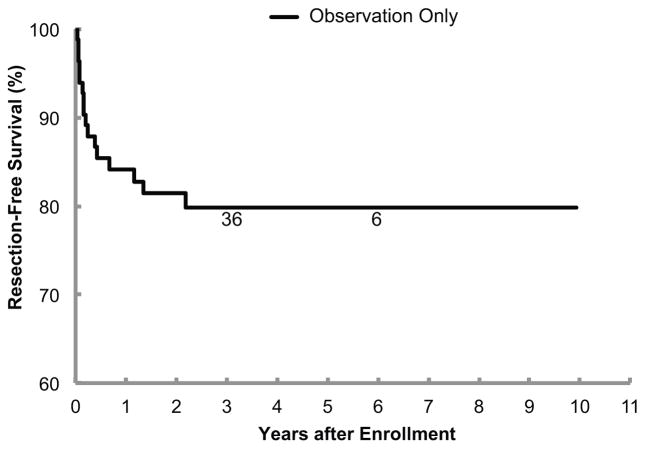
For the overall cohort (n=87), the 3-year EFSNE ± standard error was 97.7%±2.2%, the 3-year EFSAME was 95.3% ± 3.1%, and the 3-year OS was 100% (Figure 1). Note that the two low-grade adrenal cortical neoplasms and the two neuroblastoma progressions were the four events that factored into the 3-year EFSAME rate, since the two adrenal hemorrhage/hematomas and the two extralobar pulmonary sequestrations are benign and not considered malignancies. However, one patient with a solid tumor that completed the 90-week observation period without requiring resection died after 4 years due to end-stage renal disease due to a pre-existing Joubert syndrome with Dandy-Walker malformation. The patients without event had a median follow-up time of 3.2 years. Sixty-seven of the 83 (81%) observation patients were spared resection. The 3-year RFS rate was 79.8% ± 6.0% (Figure 2).
Figure 1.
Event-free survival for a “neuroblastoma event” (EFSNE), event-free survival for “any malignancy event” (EFSAME), and overall survival for 87 eligible patients. The number of patients at risk for event at the start of Years 3 and 6 appear along each curve. Note that the y-axis of the survival plots begins at 60%, not 0%.
Figure 2.
Resection-free survival (RFS) for 83 patients on the observation arm of the study. The number of patients at risk for event at the start of Years 3 and 6 appear along the curve. Note that the y-axis of the survival plots begins at 60%, not 0%.
Of the 83 patients in the observation arm: The 3-year EFSNE was 100% for patients with a cystic mass (n=27) and 96.4% ± 3.4% for patients with a solid mass (n=56). The 3-year RFS was 88.6% ± 8.6% for patients with a cystic mass (n=27) and 75.9% ± 7.6% for patients with a solid mass (n=56).
DISCUSSION
Neuroblastoma is a clinically heterogeneous disease. In infants, it often presents as a localized adrenal mass. While surgical resection is normally curative in these children, it can be associated with a significant morbidity and mortality. It is known from several single-institution studies that many of these tumors will regress spontaneously. Our hypothesis was that expectant observation, with resection limited to those tumors which exhibit persistent growth, would result in excellent survival while sparing the majority of infants from the need for surgical resection. The results of the study confirm this concept: the EFSNE, EFSAME, and OS were comparable to previous studies in which surgery, at times combined with chemotherapy, were used as the primary therapy for infants with stage 1 neuroblastoma 2,3,6–8,10,11,28. In fact, the two patients who had progression beyond stage 1 had low or intermediate risk disease and did not require further treatment.
Several studies have suggested that the burden of surgical treatment is greater than that of the disease in infants with localized neuroblastoma5,8,13,20,29. Given that four fifths of the patients in the present study were spared surgery, this approach represents a significant improvement in outcome for these children.
Our results are consistent with previous reports documenting the safety and effectiveness of expectant observation for localized infant neuroblastoma. Holgersen 9 reported spontaneous regression of adrenal masses detected on prenatal sonograms within the first 4 postnatal months. Similar results were obtained in a retrospective analysis of suprarenal masses detected either prenatally or in the first 120 days of life 11. Japanese investigators have conducted several prospective trials of expectant observation of presumed neuroblastoma tumors detected through mass screening for elevated urine VMA or HVA at 6 months of age 12–16,30. These studies used relatively similar eligibility criteria including tumor diameter <5 cm, Evans stage I or II, resectable mass without invasion of the spinal canal or involvement of the great vessels and urine VMA and HVA <50 μg/ mg creatinine. The rate of complete spontaneous regression ranged from 59% to 70%, and there were no recurrences among the observed patients. These findings were further extended in a prospective cooperative trial by the German Society of Pediatric Oncology and Hematology in which infants less than one year of age, with biopsy-proven, MYCN non-amplified, stage 1, 2 or 3 neuroblastoma underwent expectant observation unless tumor resection was judged to be low risk 18. Forty seven percent of these observed patients had some degree of regression, and in 17% it was complete, while 4% progressed to stage-4 disease.
In this study, we required a tumor diameter ≤3.1 cm for observation of solid masses and 5 cm for masses with greater than 25% cystic character by volume. The larger size limit for the cystic masses was based on identification of cystic tumors as a particularly benign subset of infant neuroblastomas 26,31,32. The EFSNE and RFS between those patients with a cystic mass appear to be similar to that of patients with a solid mass, in spite of allowing a larger volume at diagnosis for the former group.
We report the first North American cooperative group trial of expectant observation of a solid tumor in infants. Although the rate at which patients were lost to follow up was high, we argue that it is unlikely that that inclusion of these patients in the analysis would change the fundamental conclusions of our study. More than 90% of the lost patients had tumor volumes less than one ml at the time of last follow up, implying that these patients’ tumors were unlikely to increase in size after being lost.
This report supports the safety of expectant observation of small adrenal masses in young infants with stable or decreasing urine VMA and HVA. It is consistent with mounting evidence that size, stage, and catecholamine metabolite secretion pattern can be used to define a safe group for observation. Tumor diameter less than 5 cm and INSS stage 1 or 2 have been demonstrated as safe criteria in multiple observation studies 12–16,30. An upper limit on catecholamine secretion has been used in many protocols although it is not clear whether this adds safety or if it is simply a convenient way to exclude those children with tumors that are likely to grow rapidly and trigger resection after a short period of observation 12–16,33. Although the bulk of the available data demonstrating spontaneous regression is from infants diagnosed during the first 6 months of life, there is sufficient evidence that observation could be safely extended to infants less than one year with a standardized monitoring protocol 17,18,34. Furthermore, although the majority of neuroblastoma tumors arise within the adrenal gland in infants, statistical modeling suggests that the extra-adrenal subset of tumors have a higher probability of regression 35.
Expectant observation can be considered standard therapy for infants that fulfill the eligibility requirements of this study: solid adrenal tumor less than 3.1 cm (or cystic less than 5 cm), INSS stage 1, and younger than six months of age. We recommend extending these criteria to include all localized, non-infiltrative neuroblastoma tumors (corresponding to International Neuroblastoma Risk Group Staging System (INRGSS) stage L136) in infants less than one year of age. This further reduction in standard therapy should be tested in a prospective clinical trial.
Acknowledgments
Supported by grants from the National Institutes of Health (NIH-NCI, UO1 CA098543, U10 CA98413, and U10 CA98543).
The authors thank Michael LaQuaglia, M.D. and Julie Park, M.D. for advice and guidance with study conduct; Dina Willis and Lisa Clary for technical assistance; and all of the investigators and families who participated in this study.
References
- 1.Gutierrez JC, Fischer AC, Sola JE, et al. Markedly improving survival of neuroblastoma: a 30-year analysis of 1,646 patients. Pediatr Surg Int. 2007;23:637–646. doi: 10.1007/s00383-007-1933-7. [DOI] [PubMed] [Google Scholar]
- 2.Nitschke R, Smith EI, Shochat S, et al. Localized neuroblastoma treated by surgery: a Pediatric Oncology Group Study. J Clin Oncol. 1988;6:1271–1279. doi: 10.1200/JCO.1988.6.8.1271. [DOI] [PubMed] [Google Scholar]
- 3.De BB, Conte M, Mancini A, et al. Localized resectable neuroblastoma: results of the second study of the Italian Cooperative Group for Neuroblastoma. J Clin Oncol. 1995;13:884–893. doi: 10.1200/JCO.1995.13.4.884. [DOI] [PubMed] [Google Scholar]
- 4.Evans AE, Silber JH, Shpilsky A, et al. Successful management of low-stage neuroblastoma without adjuvant therapies: a comparison of two decades, 1972 through 1981 and 1982 through 1992, in a single institution. J Clin Oncol. 1996;14:2504–2510. doi: 10.1200/JCO.1996.14.9.2504. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda H, Suzuki N, Takahashi A, et al. Surgical treatment of neuroblastomas in infants under 12 months of age. J Pediatr Surg. 1998;33:1246–1250. doi: 10.1016/s0022-3468(98)90160-9. [DOI] [PubMed] [Google Scholar]
- 6.Alvarado CS, London WB, Look AT, et al. Natural history and biology of stage A neuroblastoma: a Pediatric Oncology Group Study. J Pediatr Hematol Oncol. 2000;22:197–205. doi: 10.1097/00043426-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.De BB, Mosseri V, Rubie H, et al. Treatment of localised resectable neuroblastoma. Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group. Br J Cancer. 2008;99:1027–1033. doi: 10.1038/sj.bjc.6604640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigliotti AR, Di CA, Sorrentino S, et al. Neuroblastoma in the newborn. A study of the Italian Neuroblastoma Registry. Eur J Cancer. 2009;45:3220–3227. doi: 10.1016/j.ejca.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Holgersen LO, Subramanian S, Kirpekar M, et al. Spontaneous resolution of antenatally diagnosed adrenal masses. J Pediatr Surg. 1996;31:153–155. doi: 10.1016/s0022-3468(96)90339-5. [DOI] [PubMed] [Google Scholar]
- 10.Acharya S, Jayabose S, Kogan SJ, et al. Prenatally diagnosed neuroblastoma. Cancer. 1997;80:304–310. doi: 10.1002/(sici)1097-0142(19970715)80:2<304::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Sauvat F, Sarnacki S, Brisse H, et al. Outcome of suprarenal localized masses diagnosed during the perinatal period: a retrospective multicenter study. Cancer. 2002;94:2474–2480. doi: 10.1002/cncr.10502. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Hanada R, Kikuchi A, et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J Clin Oncol. 1998;16:1265–1269. doi: 10.1200/JCO.1998.16.4.1265. [DOI] [PubMed] [Google Scholar]
- 13.Nishihira H, Toyoda Y, Tanaka Y, et al. Natural course of neuroblastoma detected by mass screening: s 5-year prospective study at a single institution. J Clin Oncol. 2000;18:3012–3017. doi: 10.1200/JCO.2000.18.16.3012. [DOI] [PubMed] [Google Scholar]
- 14.Yoneda A, Oue T, Imura K, et al. Observation of untreated patients with neuroblastoma detected by mass screening: a “wait and see” pilot study. Med Pediatr Oncol. 2001;36:160–162. doi: 10.1002/1096-911X(20010101)36:1<160::AID-MPO1039>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki T, Kohno S, Mimaya J, et al. Neuroblastoma detected by mass screening: the Tumor Board’s role in its treatment. Pediatr Surg Int. 2004;20:27–32. doi: 10.1007/s00383-003-1070-x. [DOI] [PubMed] [Google Scholar]
- 16.Oue T, Inoue M, Yoneda A, et al. Profile of neuroblastoma detected by mass screening, resected after observation without treatment: results of the Wait and See pilot study. J Pediatr Surg. 2005;40:359–363. doi: 10.1016/j.jpedsurg.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Kushner BH, Cheung NK, LaQuaglia MP, et al. Survival from locally invasive or widespread neuroblastoma without cytotoxic therapy. J Clin Oncol. 1996;14:373–381. doi: 10.1200/JCO.1996.14.2.373. [DOI] [PubMed] [Google Scholar]
- 18.Hero B, Simon T, Spitz R, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 19.Azizkhan RG, Shaw A, Chandler JG. Surgical complications of neuroblastoma resection. Surgery. 1985;97:514–517. [PubMed] [Google Scholar]
- 20.Barrette S, Bernstein ML, Leclerc JM, et al. Treatment complications in children diagnosed with neuroblastoma during a screening program. J Clin Oncol. 2006;24:1542–1545. doi: 10.1200/JCO.2005.04.4602. [DOI] [PubMed] [Google Scholar]
- 21.Tiret L, Nivoche Y, Hatton F, et al. Complications related to anaesthesia in infants and children. A prospective survey of 40240 anaesthetics. Br J Anaesth. 1988;61:263–269. doi: 10.1093/bja/61.3.263. [DOI] [PubMed] [Google Scholar]
- 22.Braz LG, Modolo NS, do NP, Jr, et al. Perioperative cardiac arrest: a study of 53,718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth. 2006;96:569–575. doi: 10.1093/bja/ael065. [DOI] [PubMed] [Google Scholar]
- 23.Rubie H, Hartmann O, Michon J, et al. N-Myc gene amplification is a major prognostic factor in localized neuroblastoma: results of the French NBL 90 study. Neuroblastoma Study Group of the Societe Francaise d’Oncologie Pediatrique. J Clin Oncol. 1997;15:1171–1182. doi: 10.1200/JCO.1997.15.3.1171. [DOI] [PubMed] [Google Scholar]
- 24.Hirata T, Tatara H, Zaizen Y, et al. Role of ultrasound in managing neuroblastoma detected by mass screening: a proposed ultrasonographic grading for children with neuroblastoma. J Clin Ultrasound. 1995;23:305–313. doi: 10.1002/jcu.1870230506. [DOI] [PubMed] [Google Scholar]
- 25.Richards ML, Gundersen AE, Williams MS. Cystic neuroblastoma of infancy. J Pediatr Surg. 1995;30:1354–1357. doi: 10.1016/0022-3468(95)90504-9. [DOI] [PubMed] [Google Scholar]
- 26.Kozakewich HP, Perez-Atayde AR, Donovan MJ, et al. Cystic neuroblastoma: emphasis on gene expression, morphology, and pathogenesis. Pediatr Dev Pathol. 1998;1:17–28. doi: 10.1007/s100249900003. [DOI] [PubMed] [Google Scholar]
- 27.Nishi M, Miyake H, Takeda T, et al. The relationship between homovanillic/vanillylmandelic acid ratios and prognosis in neuroblastoma. Oncol Rep. 1998;5:631–633. doi: 10.3892/or.5.3.631. [DOI] [PubMed] [Google Scholar]
- 28.Michalowski MB, Rubie H, Michon J, et al. Neonatal localized neuroblastoma: 52 cases treated from 1990 to 1999. Arch Pediatr. 2004;11:782–788. doi: 10.1016/j.arcped.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko M, Iwakawa M, Ikebukuro K, et al. Complete resection is not required in patients with neuroblastoma under 1 year of age. J Pediatr Surg. 1998;33:1690–1694. doi: 10.1016/s0022-3468(98)90611-x. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Kigasawa H, Kato K, et al. A prospective study of a long-term follow-up of an observation program for neuroblastoma detected by mass screening. Pediatr Blood Cancer. 2010;54:573–578. doi: 10.1002/pbc.22400. [DOI] [PubMed] [Google Scholar]
- 31.Ho PT, Estroff JA, Kozakewich H, et al. Prenatal detection of neuroblastoma: a ten-year experience from the Dana-Farber Cancer Institute and Children’s Hospital. Pediatrics. 1993;92:358–364. [PubMed] [Google Scholar]
- 32.Croitoru DP, Sinsky AB, Laberge JM. Cystic neuroblastoma. J Pediatr Surg. 1992;27:1320–1321. doi: 10.1016/0022-3468(92)90286-g. [DOI] [PubMed] [Google Scholar]
- 33.Fritsch P, Kerbl R, Lackner H, et al. “Wait and see” strategy in localized neuroblastoma in infants: an option not only for cases detected by mass screening. Pediatr Blood Cancer. 2004;43:679–682. doi: 10.1002/pbc.20126. [DOI] [PubMed] [Google Scholar]
- 34.Schilling FH, Spix C, Berthold F, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–1053. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 35.Nishi M, Miyake H, Takeda T, et al. A trial to discriminate spontaneous regression from non-regression cases during mass screening for neuroblastoma. Jpn J Clin Oncol. 1994;24:247–251. [PubMed] [Google Scholar]
- 36.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]