Abstract
PURPOSE
The identification of new therapies for high-risk (HR) hepatoblastoma (HB) is challenging. Children’s Oncology Group Study AHEP0731 included a HR stratum to explore the efficacy of novel agents. We report the response rate to vincristine/irinotecan and the outcome of high-risk HB patients.
PATIENTS AND METHODS
Patients with newly diagnosed metastatic HB or those with a serum alpha-fetoprotein (AFP) < 100 ng/ml were eligible. Patients received 2 cycles of vincristine (V) 1.5 mg/m2/day intravenously, on days 1 and 8 and irinotecan (I) 50 mg/m2/day, intravenously, on days 1-5. Patients were defined as responders if they had either a 30% decrease in tumor burden according to RECIST criteria or a 90% (> 1 log10) decline in the AFP level. Responders were to receive 2 additional cycles of VI intermixed with 6 cycles of cisplatin/doxorubicin/5-fluorouracil/vincristine (C5VD). Non-responders were to receive 6 cycles of C5VD alone.
RESULTS
Thirty-two patients (median age at diagnosis, 26 months, range: 11-159) were enrolled between September 2009 and February 2012. Fourteen of 30 evaluable patients were responders (RECIST and AFP = 6, RECIST only = 3, AFP only = 5). The median AFP decline following 2 cycles of VI for the entire group was 345,565 ng/ml (85%) of the initial AFP. Three-year event-free and overall survival was 49% (95% confidence interval-CI: 30-65%) and 62% (CI: 42-77%) respectively.
CONCLUSION
The combination of vincristine and irinotecan has substantial activity against HR hepatoblastoma. The ultimate impact of this regimen in improving outcomes of children with HR hepatoblastoma remains to be determined.
Keywords: Irinotecan, Hepatoblastoma, Metastatic, High-risk
Introduction
In children with hepatoblastoma (HB), those with metastatic disease or low serum alpha fetoprotein (AFP) at diagnosis have the worst prognosis with less than 50% event-free survival.1-4 Over the last several decades, the outcomes for these patients have not significantly improved.1
Chemotherapy, including cisplatin and doxorubicin, has resulted in increased survival for children with HB but these drugs are dose limited due to renal, auditory, and cardiac toxicities.5,6 Carboplatin, ifosfamide, and etoposide have also been used in various combinations with minimal improvements.7,8 Because of the rarity of HB, traditional phase I and phase II clinical trial mechanisms are challenging and to date have failed to identify novel effective agents for HB.9
Recently, the International Childhood Liver Tumors Strategy Group (SIOPEL) demonstrated improved survival in high-risk patients using an intensified schedule of cisplatin and doxorubicin.10 However, the significant toxicities associated with these two agents limit the cumulative doses that can be administered. Therefore, there continues to be a need to identify novel agents that can improve survival and minimize late effects for these high-risk patients.
Irinotecan is a topoisomerase I inhibitor with a wide range of antitumor activity in human tumor xenografts and in different dosing schedules and combinations, has been tested in several phase I and II pediatric trials.11,12 In these studies, one patient with HB had a complete response and another had a one-log reduction in AFP level and stable disease by imaging.13,14 Case reports have also suggested that irinotecan may be associated with a good response in patients with HB.15 Recently, a SIOPEL trial using single agent irinotecan administered in a 5 day × 2 week cycle demonstrated partial responses by AFP level or Response Evaluation Criteria In Solid Tumors (RECIST) in six of 24 patients with recurrent and refractory disease.16
The optimal method for assessing response to novel agents is debatable and perhaps somewhat controversial. Traditionally, RECIST criteria have been used but are limited to measuring tumors in a single dimension.17,18 Furthermore, radiographic response, or the lack thereof, does not necessarily correlate with intracellular events within the tumor. Ideally, biologic markers, such as AFP, should be a more reliable indicator of the efficacy of a novel agent or therapy. In a seminal paper by Van Tournout, serum AFP decline of 90% (1 log10) over an average of six cycles of therapy was shown to be associated with better outcome in a small cohort of patients with HB.19 However, since the initial publication over 20 years ago, little has been done to further investigate the power of AFP decline to predict outcome or to guide risk-based therapy.3,20
The Children’s Oncology Group (COG) Study for the Treatment of Children with All Stages of Hepatoblastoma, AHEP0731, included a stratum to explore the efficacy of innovative chemotherapy for high-risk patients. We report the response rate of two cycles of vincristine and irinotecan (VI) administered in an upfront window to newly diagnosed children with high-risk HB.
Methods
Study Patients
Patients with newly diagnosed, previously untreated HB were eligible for high-risk window chemotherapy if they had either metastatic disease or a serum AFP level of < 100 ng/ml at diagnosis, irrespective of stage. The National Cancer Institute, the Pediatric Central IRB, and the institutional review boards of the participating institutions approved the protocol. Informed consent was obtained for all patients prior to treatment.
Staging
Patients were staged for risk classification using the COG staging guidelines6 prior to the initiation of chemotherapy: stage IV, metastatic disease. Pretreatment extent of disease (PRETEXT)21 grouping was also performed at diagnosis and with any subsequent abdominal CT or MRI (Figure 1) and was used to guide the surgical management, but was not used for risk classification. All diagnostic and follow up imaging were centrally reviewed.
Figure 1.
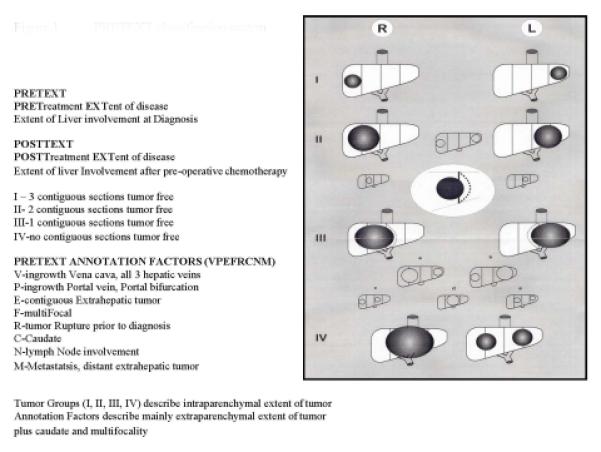
PRETEXT classification system
Chemotherapy
Patients received vincristine (V), 1.5 mg/m2/day (0.05 mg/kg/day) intravenously, on days 1 and 8 with irinotecan (I), 50 mg/m2/day (1.67 mg/kg for patients < 10 kg), intravenously over 90 minutes, days 1-5 in combination (Figure 2). Repeat imaging and AFP measurements were performed after 2 cycles to assess response to VI. Patients who were considered responders according to institutional assessment, received 2 additional cycles of VI intermixed with 6 cycles of cisplatin (100 mg/m2/dose or 3.3 mg/kg/dose for < 10 kg) intravenously over 6 hours on day 1/doxorubicin (30 mg/m2/dose or 1 mg/kg/dose for < 10 kg) intravenously over 15 minutes on days 1 and 2/ 5-fluorouracil (600 mg/m2/dose or 20 mg/kg/dose for < 10 kg) intravenous push on day 2/vincristine (1.5 mg/m2/day or 0.05 mg/kg/day) intravenous push on days 2, 9, and 16 (C5VD). Dexrazoxane (300 mg/m2/dose or 10 mg/kg/dose for patients less than 10 kg), intravenous push was given prior to doxorubicin during the last two cycles of C5VD. All cycles lasted 21 days. Patients who were non-responders received 6 cycles of C5VD without further VI.
Figure 2.
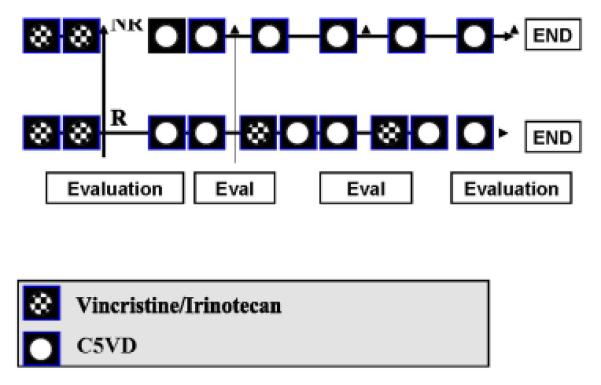
Treatment schema
Surgery
Study patients could have primary tumor resection at any point during therapy, either by surgical resection or total hepatectomy followed by orthotopic liver transplant (OLT). This was to optimally occur per protocol after cycle #7 of 10 in responders or after cycle #6 of 8 in non-responders. Metastatic lesions were to be addressed surgically at the discretion of treating physicians.
Pathology
Central pathologic review was performed for all biopsied and resected specimens (liver and/or lung) to assess tumor histology.
Toxicity
The individual occurrences of toxicities were graded according to National Cancer Institute Common Toxicity criteria v3/4 guidelines. The frequency of each toxicity type was quantified as the number of patients during a reporting period on which the toxicity of the relevant grade was reported.
Evaluation of Response
Baseline physical exams, organ function, AFP levels, and imaging studies, including a CT or MRI of the abdomen, ultrasound of the liver, and CT of the chest were performed prior to initiating therapy. Repeat imaging studies (CT or MRI of the abdomen) were performed after two cycles of VI and then after every 2 cycles of C5VD. AFP levels were obtained prior to beginning each cycle.
For enrollment, metastatic disease was determined by imaging assessment and/or biopsy at the treating institution. The definition of metastatic disease was the clinical impression of the treating institution on whether a pulmonary lesion was metastatic HB. For this trial, pulmonary lesions did not need to be biopsied or a certain size or number to be considered as disease. All patient responses were assessed by the institutional investigator and used to assign subsequent protocol therapy. RECIST and AFP response for the purposes of the efficacy assessment were obtained from central review. Two study radiologists (MM, AT) independently assessed imaging and determined overall response for all patients using RECIST criteria.18 Agreement on response assignment between the two radiologists was required for purposes of this study and discrepancies were resolved by consensus review. Response was also assessed using decline in serum AFP levels. The AFP decline during the initial window period was determined using the maximum AFP prior to the initiation of chemotherapy and the AFP prior to the initiation of cycle #3.
For the purpose of this study, a complete response (CR) was considered as the disappearance of all lesions and a normal AFP level. A partial response (PR) was considered as either: 1) a ≥ 30% decrease according to RECIST; or 2) a serum AFP level concentration decline of ≥ 90% (≥ 1 log10) after two VI cycles in the absence of disease progression.
Statistical Design and Analysis
In order to be considered for the evaluation of VI, patients were required to have measurable disease according to RECIST18 criteria at enrollment and AFP prior to the start of chemotherapy. The endpoint efficacy assessment was disease status at the end of two cycles of therapy. Patients were considered evaluable if the individual met eligibility criteria and received at least one dose of each agent in the combination being tested. Any evaluable patient who obtained a CR or PR during the first two cycles was considered a responder and this was the primary statistical endpoint. An increase in AFP alone was not considered evidence of progressive disease. All other evaluable patients were considered non-responders.
Patients were to be enrolled in two stages of 15 evaluable patients each. If, at the end of the first stage, four or fewer patients were considered responders or six or more patients experienced early progression, enrollment was to be halted with the conclusion that VI did not provide sufficient disease control. Early progression was defined to be increase in RECIST measureable disease size of 20% or greater or documentation of new lesions prior to the end of two cycles of VI. If 30 patients were enrolled and 14 or fewer patients demonstrated response, the data would not be considered consistent with a true response rate of 50%, the target described in the protocol as sufficient to warrant further development of VI.
Survival
Event free survival (EFS) was measured from the time of patient enrollment until the last follow-up or an analytic event was observed, whichever occurred first.22 Analytic events were: (1) progression of disease or occurrence of disease at new sites; (2) treatment failure defined as the presence of disease after planned chemotherapy; (3) death from any cause prior to disease progression; or (4) diagnosis of a second malignancy. Overall survival was defined from the time of enrollment until death from any cause or last follow-up, whichever came first.
Time of surgical excision of the primary tumor was identified as the cycle during which non-liver-transplant excision of the primary tumor or liver transplant of the primary tumor was attempted.
The proportion of patients event-free as a function of time since enrollment or the end of two cycles of VI, as appropriate, was estimated by the method of Kaplan and Meier.27 95% confidence intervals (CI) were estimated using the log-log transformation. Differences for risk for EFS event according to attained RECIST and attained AFP response were assessed using a two-sided logrank test28 from the end of cycle 2 evaluation. A p-value of 0.05 or less was to be considered indicative of a significant relationship.
Results
A total of 32 patients were enrolled on study between September 2009 and February 2012. Outcome current to June 30, 2014 was used in this analysis. One patient was deemed ineligible due to inadequate organ function. A second patient was deemed non-evaluable for window response because an accurate AFP level was not obtained prior to starting chemotherapy. The remaining 30 patients are the subject of this report. All patients had metastatic disease and no patient had an AFP < 100 ng/ml at diagnosis. The median age at diagnosis was 26 months (range: 11-159 months) (Table 1). There were 18 males and 12 females. The median AFP at diagnosis was 397,748 ng/ml (range: 36,300 – 5,036,000). The median duration of follow-up for EFS for those without an EFS-event was 38 months.
Table 1.
Characteristics of High-Risk Metastatic Hepatoblastoma
| Number (percent) | |
|---|---|
| Age | 11-159 months |
| < 12 months | 1 (3) |
| 1 -3 years | 21 (70) |
| 3-8 years | 7 (23) |
| > 8 years | 1 (3) |
|
| |
| Sex | |
| Male | 18 (60) |
| Female | 12 (40) |
|
| |
| PRETEXT | |
| I | |
| II | 9 (30) |
| III | 7 (23) |
| IV | 14 (47) |
|
| |
| Pathology | |
| SCU | 5 (18) |
| Non-SCU | 23 (82) |
|
Not evaluated at
diagnosis |
2 |
|
| |
| AFP | 397,748 ng/ml (36,300 – 5,036,000) |
| < 100 | |
| 100-1000 | 0 |
| ≥ 1,000-999,999 | 25 (83) |
| ≥ 1,000,000 | 5 (17) |
Response
Fourteen of 30 patients were responders according to study criteria (both RECIST and AFP=6, RECIST only=3, AFP only=5) (Table 2). Four patients demonstrated in increase in initial AFP from enrollment to the end of cycle 2. The median AFP decline following 2 cycles of VI for all patients was 85% (Figure 3). The median decline in RECIST following 2 cycles of VI was 18% (Figure 4). No patient had early progression. Only 9 patients (31%) had metastatic lesions considered measurable by RECIST. A detailed report of the characteristics, number, size, and response of pulmonary lesions will be reported separately.
Table 2.
Response of Patients to Vincristine/Irinotecan Window Therapy
| RECIST RESPONSDER |
AFP RESPONDER |
TOTAL RESPONDER |
|
|---|---|---|---|
| ≥ 30% RECIST RESPONSE ALONE |
3 | 3 | |
|
| |||
| ≥ 90% AFP DECLINE ALONE |
5 | 5 | |
|
| |||
| ≥ 30% RECIST AND ≥ 90% AFP DECLINE |
6 | 6 | 6 |
|
| |||
| TOTAL | 9 | 11 | 14 |
Figure 3.
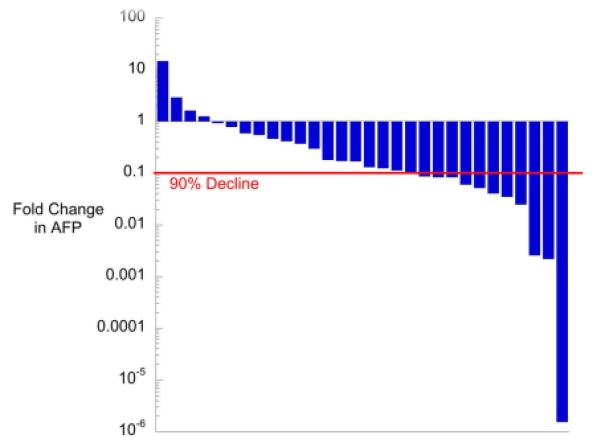
AFP Decline After 2 Cycles of VI Therapy Shown are the changes in AFP derived as [(initial measurement-final measurement)/initial measurement] plotted on a log-scale. The red line represents a 90% decline in alphafetoprotein between patient enrollment and the end of the 2 cycles of VI therapy. Ninety percent decline represents an alphafetoprotein response as defined in the protocol.
Figure 4.
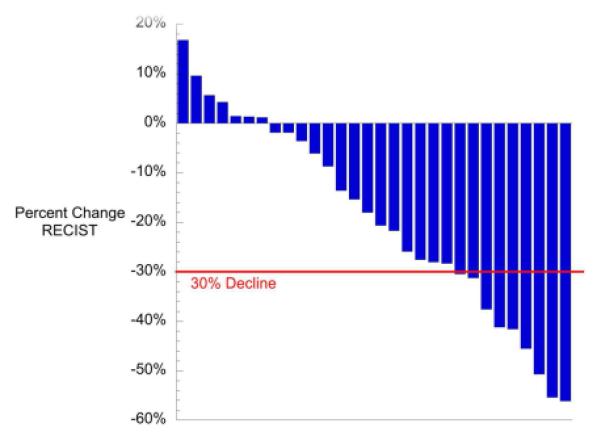
RECIST Tumor Measurements After 2 Cycles of VI The red line represents a 30% decline in RECIST measurement between patient enrollment and the end of the 2 cycles of VI therapy. Thirty percent decline represents a RECIST partial response.
AFP decline following the first two cycles of VI was not predictive of outcome (Figure 5). Patients who had a partial AFP response had three-year EFS of 36% (95% CI: 11%-63%) compared to 56% (95% CI: 31%-75%) in no1n2-responders (p=0.46). Response according to RECIST was also not predictive of outcome as responders had three-year EFS of 44% (95% CI: 14%-72%) compared to 51% (95% CI: 28%-70%) for non-responders (p=0.98). Of the seven patients that had an increase in tumor size after the two cycles of window therapy, all had less than a 20% increase which was the criteria for progressive disease. Only two of these patients had an increase in AFP level while the remaining patients had decreases in AFP ranging from 8-92%.
Figure 5.
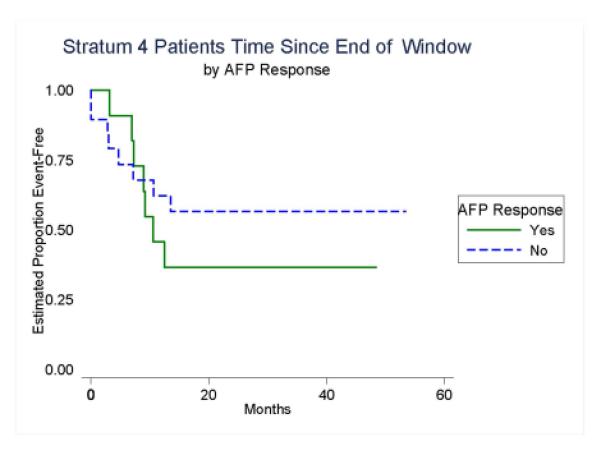
Event-Free Survival According to AFP Response After Two Cycles of VI
PRETEXT Risk Stratification and Surgery
All but one patient underwent an initial biopsy and three patients had an upfront biopsy of lung metastases performed. Twenty-one (70%) patients were able to undergo a tumor resection while on protocol therapy, including five who received a liver transplant (Table 3) and ten patients had a resection of pulmonary metastatic disease during protocol therapy (detailed results to be reported separately). PRETEXT staging was II (n=9), III (n=7), and IV (n=14). Fourteen patients (1 PRETEXT II, 3 PRETEXT III, and 10 PRETEXT 4) were downstaged in regards to their PRETEXT grouping prior to surgery, nine following cycle 2, four following cycle 4, and one following cycle 7.
Table 3.
Surgical Outcomes of Patients with Metastatic Hepatoblastoma
| Surgery | # (%) |
| Yes | 21 (70) |
| Resection | 16 (53) |
| Resection during cycles 3-4 | 2 |
| Resection during cycles 5-7 | 13 |
| Resection during cycles 8-10 | 1 |
| Transplant | 5 (17) |
| Transplant during cycles 5-7 | 2 |
| Transplant during cycles 8-10 | 3 |
| No | 9 (30) |
| Total | 30 |
Outcome
Three-year EFS for the 30 response-evaluable patients was 49% (95% CI: 30-65%) while three-year survival was 62% (95% CI: 42-77%)(Figure 6). Two patients died without relapse while on therapy, one patient had a cardiac arrest following a gastrointestinal procedure, and another patient died from post-resection complications. Thirteen (13) patients had disease recurrence. The sites of recurrence were pulmonary alone in 7 patients, liver alone in 1 patient, combined pulmonary and liver recurrence in 3 patients, 13 combined bone and liver in 1 patient and combined liver, bone and lymph nodes in one patient. The age distribution precluded any determination of differences in outcome.
Figure 6.
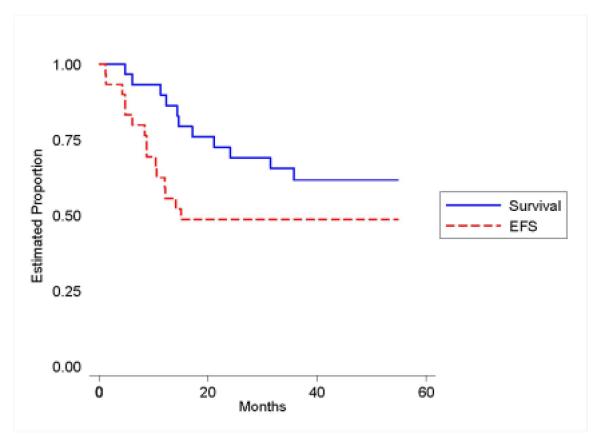
Event-Free and Overall Survival of Patients with Metastatic Hepatoblastoma
Pathology
Histology of the tumor at study enrollment was centrally reviewed in 28 of 30 patients. Tumors from five patients were noted on central review to have small cell undifferentiated (SCU) elements at diagnosis ranging from 1-10%. All retained INI1 immunohistochemical staining and will be detailed in a separate report.
Toxicities
The most common grade 3 and 4 toxicities during the first two cycles were diarrhea (n=8 patients), anorexia (7) and febrile neutropenia (7), nausea (6) and a documented infection occurred in 4 patients (Table 4). Neuropathy was only reported in one patient (3%). Fourteen patients (43%) had febrile neutropenia and an infection occurred in 10 patients during the first two cycles of C5VD.
Table 4.
Toxicities (Grade 3 and 4) of Patients with Metastatic Hepatoblastoma@
| Cycles 1-2 # (%) |
Cycles 3-4 # (%) |
Cycle 5-6/7*
# (%) |
Cycle 8-10*
# (%) |
|
|---|---|---|---|---|
|
Febrile
neutropenia |
7 (23) | 14 (50) | 7 (26) | 7 (33) |
|
| ||||
| Infection | 4 (13) | 8 (29) | 11 (41) | 10 (48) |
|
| ||||
| Diarrhea | 8 (26) | 3 (11) | 2 (7) | 2 (10) |
|
| ||||
| Vomiting | 4 (13) | 2 (7) | 4 (15) | 3 (14) |
|
| ||||
| Mucositis | 1 (3) | 2 (7) | 3 (11) | 2 (10) |
|
| ||||
| Anorexia | 7 (23) | 6 (21) | 5 (19) | 2 (10) |
|
| ||||
| Cardiac | 1(3) | 0 | 1 (4) | 2 (10) |
|
| ||||
| Ototoxicity | 0 | 1 (4) | 2 (7) | 1 (5) |
=Percentage of Evaluable Patients During That Reporting Period
= These cycles will include one cycle of VI and 2 cycles of C5VD in responders and 2 cycles of C5VD only in non responders
Discussion
The Treatment of Children with all Stages of Hepatoblastoma trial (AHEP0731) included a high-risk stratum to explore the utility of novel agents in an upfront therapeutic window. We observed a favorable response to vincristine/irinotecan (VI), in 14 of thirty patients (47%). Eleven patients (37%) had a 90% decline in AFP following the first two cycles of VI and were therefore considered AFP responders according to study criteria. However, an additional group of 15 patients had some decrease in AFP (7%-89%) in response to VI (Table 2/Figure 3) with an overall median decline of 85%. We believe that this demonstrates definitive activity of VI against HB in a group of untreated newly-diagnosed metastatic patients particularly when considering that the study criteria were based on an assessment tool previously used to evaluate outcome and not efficacy in patients who received a much longer course of treatment averaging 6 cycles of therapy.
A recent report from SIOPEL investigated the use of single agent irinotecan for patients with relapsed HB was stopped early due to meeting criteria for a positive treatment effect with 2 patients having a response observed by AFP decline only, one detected by RECIST only, and one patient who had both a RECIST and AFP response for an overall AFP response rate of 17% in this cohort of relapsed patients.16
The EFS of 49% may be better than what has been previously described, although it is less than a recent SIOPEL-4 report.10,23 Direct comparison of outcome data is difficult because of small patient numbers and that the primary study objective here was identification of novel agents with anti-tumor activity rather than outcome. We believe monitoring AFP levels by percent decline rather than logarithmically should be used going forward for simplicity.
Interestingly, no patient in this trial had a low AFP level at diagnosis. A small component (1-10%) of SCU was observed in 5 patients by central review at diagnosis.
VI treatment was well tolerated. Diarrhea was the most frequent complication occurring in 26% of patients, which is similar to other previously reported trials.26 While febrile neutropenia occurred in nearly 23% of patients, this toxicity during the VI cycles was less than seen during the C5VD cycles. Cardiac toxicity was observed in 2 patients but one case occurred in proximity to a gastrointestinal procedure not directly related to HB therapy and the other was during surgical resection.
This is one of the few trials in pediatric oncology that has used a biomarker as evidence of therapeutic activity. Some patients responded by RECIST only, some by AFP only, and some by both criteria, but AFP decline identified the largest number of responsive patients. The ease of AFP determination makes it a tool that could be used routinely to evaluate therapeutic efficacy. While only 11 patients met our predetermined definition of AFP response, 26 patients had some reduction in AFP following VI therapy.
These results highlight the importance of using the appropriate assessment tools to ensure that a potentially active regimen is not discarded. In this study, VI would be discarded based on our pre-determined criteria. However, a closer look at the data suggests that this regimen still holds promise and warrants further study. The 90% (1 log10) decline as a marker of therapeutic efficacy was based on Van Tournot’s paper from over 20 years ago that used AFP as a prognostic marker over a 6 cycle/18 week period. This trial used the 90% decline as a marker of efficacy over only 2 cycles. Perhaps our error was using AFP prognostic criteria to evaluate efficacy. SIOPEL trials have often used any AFP decline as an indicator of response. It is also important to note that RECIST response did not correlate with outcome either.
The role of VI in subsequent liver trials remains to be determined. Our study was not intended or powered to assess the impact on outcome and this should be studied through a randomized clinical trial. Due to the limitations on total deliverable cisplatin and doxorubicin, VI is attractive as a less toxic alternative. There are reports of VI use for maintenance therapy in patients with minimal residual disease.15 High-risk metastatic patient groups would seem like ideal patients to test the benefit of maintenance therapy.
In summary, while VI did not meet predetermined criteria for demonstrating sufficient disease control, a closer look at the percent decline in serum AFP levels demonstrated that VI does have activity in a large group of newly-diagnosed patients with high-risk HB. The results here suggest further exploration of this combination is warranted. The use of AFP as a biomarker is important for any subsequent study looking at patients with HB and further study is needed to evaluate how the percent decline of AFP can be best used to predict treatment efficacy.
ACKNOWLEDGEMENTS
The study team is indebted to Fran Laurie, Karina Rossi-Toole, Sandy Kessel, Richard Hanusik, Brandon Davis, and the rest of the staff of the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC) for collection of scans, compilation of data, and facilitation of central review sessions. We also would like to acknowledge Catherine Shannon, Alejandra Miranda, Megan Stahlman, and Judy Everett of the COG Operation office for countless hours of support in maintaining the operation of this trial.
RESEARCH SUPPORT: Research is supported by the Chair’s Grant U10 CA98543 and the Statistics and Data Center Grant U10 CA98413 of the Children’s Oncology Group and the Quality Assurance Review Center’s Grant U10 CA29511 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
Footnotes
Clinical Trials Registry: ClinicalTrials.gov Identifier: NCT00980460
CONFLICT OF INTEREST DISCLOSURE:
No potential conflict of interest relevant to this article was reported by any of the authors.
PREVIOUS PRESENTATION: None
AUTHOR CONTRIBUTION:
Study design, conduct, analysis and interpretation of the data: HM Katzenstein, MD Krailo, M McCarville, A Towbin, MH Malogolowkin, W Furman, M Finegold, S Ranganathan, S Dunn, M Langham, E McGahren, G Tiao, C Rodriguez-Galindo, RL Meyers
Drafting of the article: HM Katzenstein, MD Krailo
Final approval of the version to be published: HM Katzenstein, MD Krailo, M McCarville, A Towbin, MH Malogolowkin, W Furman, M Finegold, S Ranganathan, S Dunn, M Langham, E McGahren, G Tiao, C Rodriguez-Galindo, and RL Meyers
REFERENCES
- 1.Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. doi: 10.1097/MOP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 2.Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma--results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–25. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 3.Meyers RL, Rowland JR, Krailo M, et al. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;53:1016–22. doi: 10.1002/pbc.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ioris M, Brugieres L, Zimmermann A, et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer. 2008;44:545–50. doi: 10.1016/j.ejca.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Douglass EC, Green AA, Wrenn E, et al. Effective cisplatin (DDP) based chemotherapy in the treatment of hepatoblastoma. Med Pediatr Oncol. 1985;13:187–90. doi: 10.1002/mpo.2950130405. [DOI] [PubMed] [Google Scholar]
- 6.Ortega JA, Krailo MD, Haas JE, et al. Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol. 1991;9:2167–76. doi: 10.1200/JCO.1991.9.12.2167. [DOI] [PubMed] [Google Scholar]
- 7.Katzenstein HM, London WB, Douglass EC, et al. Treatment of unresectable and metastatic hepatoblastoma: a pediatric oncology group phase II study. J Clin Oncol. 2002;20:3438–44. doi: 10.1200/JCO.2002.07.400. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs J, Bode U, von Schweinitz D, et al. Analysis of treatment efficiency of carboplatin and etoposide in combination with radical surgery in advanced and recurrent childhood hepatoblastoma: a report of the German Cooperative Pediatric Liver Tumor Study HB 89 and HB 94. Klin Padiatr. 1999;211:305–9. doi: 10.1055/s-2008-1043805. [DOI] [PubMed] [Google Scholar]
- 9.Beaty O, 3rd, Berg S, Blaney S, et al. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2010;55:440–5. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol. 2013;14:834–42. doi: 10.1016/S1470-2045(13)70272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–24. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 12.Ma MK, Zamboni WC, Radomski KM, et al. Pharmacokinetics of irinotecan and its metabolites SN-38 and APC in children with recurrent solid tumors after protracted low-dose irinotecan. Clin Cancer Res. 2000;6:813–9. [PubMed] [Google Scholar]
- 13.Katzenstein HM, Rigsby C, Shaw PH, et al. Novel therapeutic approaches in the treatment of children with hepatoblastoma. J Pediatr Hematol Oncol. 2002;24:751–5. doi: 10.1097/00043426-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RD, Williams DM. Dramatic response of multiply relapsed hepatoblastoma to irinotecan (CPT-11) Med Pediatr Oncol. 2003;41:78–80. doi: 10.1002/mpo.10300. [DOI] [PubMed] [Google Scholar]
- 15.Qayed M, Powell C, Morgan ER, et al. Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer. 2010;54:761–3. doi: 10.1002/pbc.22408. [DOI] [PubMed] [Google Scholar]
- 16.Zsiros J, Brugieres L, Brock P, et al. Efficacy of irinotecan single drug treatment in children with refractory or recurrent hepatoblastoma--a phase II trial of the childhood liver tumour strategy group (SIOPEL) Eur J Cancer. 2012;48:3456–64. doi: 10.1016/j.ejca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–60. doi: 10.1016/j.ejca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck S, Eisenhauer A, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Van Tornout JM, Buckley JD, Quinn JJ, et al. Timing and magnitude of decline in alpha-fetoprotein levels in treated children with unresectable or metastatic hepatoblastoma are predictors of outcome: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:1190–7. doi: 10.1200/JCO.1997.15.3.1190. [DOI] [PubMed] [Google Scholar]
- 20.Purcell R, Childs M, Maibach R, et al. Potential biomarkers for hepatoblastoma: results from the SIOPEL-3 study. Eur J Cancer. 2012;48:1853–9. doi: 10.1016/j.ejca.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Aronson DC, Schnater JM, Staalman CR, et al. Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol. 2005;23:1245–52. doi: 10.1200/JCO.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL. Non-parametic estimation from incomplete observations. J American Statistical Association. 1958;53:457–481. MP. [Google Scholar]
- 23.Malogolowkin MH, Katzenstein H, Krailo MD, et al. Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol. 2006;24:2879–84. doi: 10.1200/JCO.2005.02.6013. [DOI] [PubMed] [Google Scholar]
- 24.Haas JE, Feusner JH, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer. 2001;92:3130–4. doi: 10.1002/1097-0142(20011215)92:12<3130::aid-cncr10115>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, et al. Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer. 2009;52:328–34. doi: 10.1002/pbc.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 28.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiely and Sons; New York: 2002. [Google Scholar]


